The Prevalence of Pretreatment Drug Resistance and Transmission Networks Among Newly Diagnosed HIV-1-Infected Individuals in Nanning, Guangxi, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Laboratory Testing and Subtyping
2.3. Genotypic Resistance Analysis
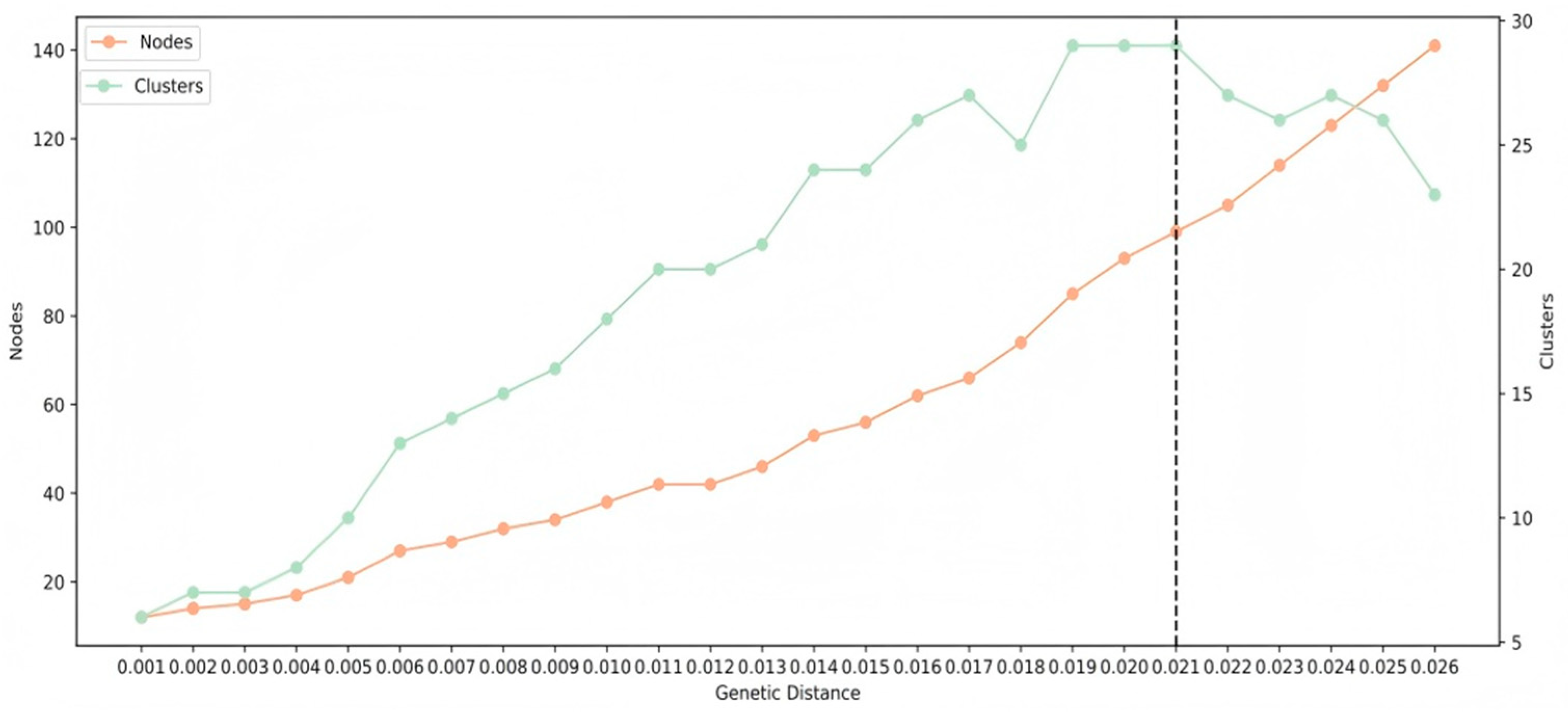
2.4. Genetic Network Inference
2.5. Statistical Analysis
3. Results
3.1. Socio-Demographic Characteristics and Clinical Characteristics of the Participants
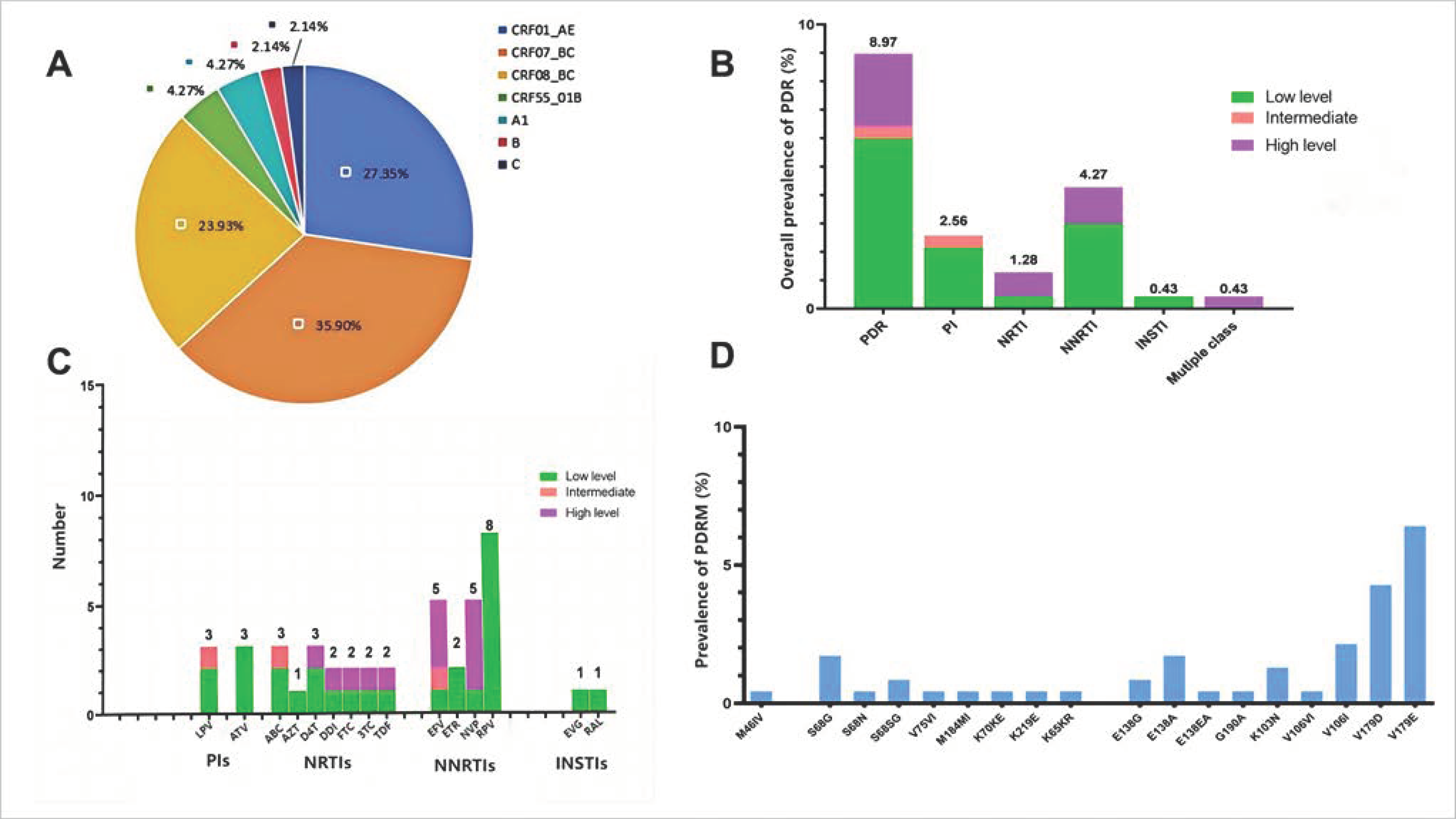
3.2. Prevalence and Patterns of PDR Across ARV Drug Classes
3.3. Distribution and Frequency of DRMs
3.4. PDR Transmission Within the HIV-1 Genetic Network
3.5. Factors Associated with PDR
3.6. Impact of PDR on the Response to ART After One-Year Follow-Up
4. Discussion
5. Conclusions
6. Limitation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HIV | Human immunodeficiency virus type 1 |
| PDR | Pretreatment drug resistance |
| DR | Drug resistance |
| ADR | Acquired drug resistance |
| DRM | Drug resistance mutation |
| WHO | World Health Organization |
| ART | Antiretroviral therapy |
| ARV | Antiretroviral |
| MSM | Men who have sex with men |
| VF | Virological failure |
| IF | Immunological failure |
| TCs | Transmission clusters |
| VL | Viral load |
| GD | Genetic distance |
| NNRTIs | Non-nucleoside reverse-transcriptase inhibitors |
| NRTIs | Nucleoside reverse-transcriptase inhibitors |
| PIs | Protease inhibitors |
| INSTIs | Integrase strand-transfer inhibitors |
| DRV | Darunavir |
| FPV | Fosamprenavir |
| IDV | Indinavir |
| LPV | Lopinavir |
| NFV | Nelfinavir |
| SQV | Saquinavir |
| TPV | Tipranavir |
| ABC | Abacavir |
| AZT | Zidovudine |
| D4T | Stavudine |
| DDI | Didanosine |
| FTC | Emtricitabine |
| 3TC | Lamivudine |
| TDF | Tenofovir |
| DOR | Doravirine |
| EFV | Efavirenz |
| ETR | Etravirine |
| NVP | Nevirapine |
| RPV | Rilpivirine |
| BIC | Bictegravir |
| CAB | Cabotegravir |
| DTG | Dolutegravir |
| EVG | Elvitegravir |
| RAL | Raltegravir |
| TMP/SMX | Trimethoprim/sulfamet |
| OR | Odds ratio |
| aOR | Adjusted odds ratio |
| HR | Hazard ratio |
| aHR | Adjusted hazard ratio |
| CI | Confidence interval |
| ART | Antiretroviral therapy |
| ARV | Antiretroviral |
Appendix A
| Variables | Total | PDR | OR (95%CI) | p | aOR (95%CI) | p |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Female | 182 (77.78%) | 13 (7.14%) | ||||
| Male | 52 (22.22%) | 8 (15.38%) | 2.36 (0.92, 6.06) | 0.073 | 1.7 (0.54, 5.35) | 0.362 |
| Age | ||||||
| <30 | 48 (20.51%) | 4 (8.33%) | ||||
| 30–39 | 53 (22.65%) | 2 (3.77%) | 0.43 (0.08, 2.47) | 0.345 | 0.25 (0.03, 1.91) | 0.181 |
| 40–49 | 35 (14.96%) | 6 (17.14%) | 2.28 (0.59, 8.77) | 0.232 | 1.41 (0.21, 9.51) | 0.722 |
| 50–59 | 37 (15.81%) | 3 (8.11%) | 0.97 (0.20, 4.63) | 0.97 | 0.54 (0.06, 5.34) | 0.6 |
| ≥60 | 61 (26.07%) | 6 (9.84%) | 1.2 (0.32, 4.52) | 0.788 | 0.59 (0.07, 4.93) | 0.629 |
| Region | ||||||
| Urban | 109 (46.58%) | 11 (10.09%) | ||||
| Suburban | 125 (53.42%) | 10 (8%) | 0.77 (0.32, 1.9) | 0.577 | 0.51 (0.17, 1.47) | 0.209 |
| Marital status | ||||||
| Unmarried | 108 (46.15%) | 8 (7.41%) | ||||
| Married or cohabiting | 96 (41.03%) | 10 (10.42%) | 1.45 (0.55, 3.85) | 0.451 | 0.84 (0.16, 4.29) | 0.834 |
| Divorced or widowed | 30 (12.82%) | 3 (10%) | 1.39 (0.34, 5.59) | 0.644 | 0.55 (0.07, 4.21) | 0.568 |
| Subtype | ||||||
| CRF01_AE | 64 (27.35%) | 6 (9.38%) | ||||
| CRF07_BC | 84 (35.9%) | 5 (5.95%) | 0.61 (0.18, 2.1) | 0.435 | 0.66 (0.17, 2.61) | 0.552 |
| CRF08_BC | 56 (23.93%) | 7 (12.5%) | 1.38 (0.44, 4.38) | 0.584 | 0.93 (0.24, 3.62) | 0.915 |
| Others | 30 (12.82%) | 3 (10%) | 1.07 (0.25, 4.62) | 0.924 | 0.7 (0.12, 3.97) | 0.684 |
| WHO clinical stage at initiation | ||||||
| 1 or 2 | 145 (61.97%) | 13 (8.97%) | ||||
| 3 or 4 | 89 (38.03%) | 8 (8.99%) | 1 (0.40, 2.52) | 0.995 | 0.56 (0.17, 1.85) | 0.339 |
| TMP SMX use at baseline | ||||||
| No | 189 (80.77%) | 15 (7.94%) | ||||
| Yes | 45 (19.23%) | 6 (13.33%) | 1.78 (0.65, 4.89) | 0.26 | 1.03 (0.18, 5.95) | 0.973 |
| Transmission route | ||||||
| Heterosexual | 166 (70.94%) | 20 (12.05%) | ||||
| Homosexual | 68 (29.06%) | 1 (1.47%) | 0.11 (0.01, 0.83) | 0.032 | 0.09 (0.01, 0.86) | 0.037 |
| Baseline CD4+ T cell count, cell/µL | ||||||
| <100 | 61 (26.07%) | 9 (14.75%) | ||||
| 100–200 | 43 (18.38%) | 2 (4.65%) | 0.28 (0.06, 1.38) | 0.118 | 0.25 (0.03, 2.06) | 0.199 |
| 200–349 | 66 (28.21%) | 4 (6.06%) | 0.37 (0.11, 1.28) | 0.117 | 0.29 (0.04, 2.13) | 0.223 |
| ≥349 | 64 (27.35%) | 6 (9.38%) | 0.6 (0.20, 1.79) | 0.359 | 0.38 (0.05, 3.16) | 0.371 |
| Baseline CD8+ T cell count | ||||||
| <1000 cells/μL | 139 (59.4%) | 15 (10.79%) | ||||
| ≥1000 cells/μL | 95 (40.6%) | 6 (6.32%) | 0.56 (0.21, 1.49) | 0.245 | 0.69 (0.19, 2.56) | 0.584 |
| Baseline CD4/CD8 ratio | ||||||
| <0.5 | 195 (83.33%) | 16 (8.21%) | ||||
| ≥0.5 | 39 (16.67%) | 5 (12.82%) | 1.65 (0.56, 4.79) | 0.361 | 1.8 (0.39, 8.35) | 0.45 |
| Baseline VL | ||||||
| <1000 copies/mL | 51 (21.79%) | 8 (15.69%) | ||||
| ≥1000 copies/mL | 183 (78.21%) | 13 (7.1%) | 0.41 (0.16, 1.05) | 0.064 | 0.49 (0.16, 1.45) | 0.197 |
| Variables | Total (N = 223) | VF | IF | ||||
|---|---|---|---|---|---|---|---|
| n = 45 | aHR [95%CI] | p | n = 47 | aHR [95%CI] | p | ||
| Group | |||||||
| No PDR | 174 (78.03%) | 37 (21.26%) | 37 (21.26%) | ||||
| Partly active ART | 29 (13%) | 5 (17.24%) | 1.33 [0.45, 3.97] | 0.608 | 5 (17.24%) | 1.44 [0.49, 4.18] | 0.506 |
| Fully active ART | 20 (8.97%) | 3 (15.00%) | 0.63 [0.16, 2.43] | 0.502 | 5 (25%) | 0.81 [0.28, 2.35] | 0.693 |
| Sex | |||||||
| Woman | 172 (77.13%) | 38 (22.09%) | 38 (22.09%) | ||||
| Man | 51 (22.87%) | 7 (13.73%) | 0.7 [0.28, 1.78] | 0.456 | 9 (17.65%) | 0.89 [0.35, 2.24] | 0.805 |
| Age | |||||||
| <30 | 45 (20.18%) | 14 (31.11%) | 12 (26.67%) | ||||
| 30–39 | 52 (23.32%) | 6 (11.54%) | 0.12 [0.04, 0.37] | <0.001 | 11 (21.15%) | 0.62 [0.23, 1.65] | 0.337 |
| 40–49 | 34 (15.25%) | 6 (17.65%) | 0.26 [0.07, 0.9] | 0.034 | 10 (29.41%) | 0.81 [0.27, 2.42] | 0.705 |
| 50–59 | 34 (15.25%) | 6 (17.65%) | 0.15 [0.04, 0.61] | 0.008 | 8 (23.53%) | 1.1 [0.32, 3.79] | 0.885 |
| ≥60 | 58 (26.01%) | 13 (22.41%) | 0.27 [0.07, 1.05] | 0.058 | 6 (10.34%) | 0.49 [0.13, 1.9] | 0.303 |
| Region | |||||||
| Urban | 105 (47.09%) | 22 (20.95%) | 25 (23.81%) | ||||
| Suburban | 118 (52.91%) | 23 (19.49%) | 0.69 [0.36, 1.32] | 0.259 | 22 (18.64%) | 0.71 [0.37, 1.37] | 0.311 |
| Marital status | |||||||
| Unmarried | 101 (45.29%) | 21 (20.79%) | 27 (26.73%) | ||||
| Married or cohabiting | 93 (41.7%) | 18 (19.35%) | 2.38 [0.77, 7.36] | 0.133 | 18 (19.35%) | 0.68 [0.25, 1.79] | 0.43 |
| Divorced or widowed | 29 (13%) | 6 (20.69%) | 1.81 [0.44, 7.47] | 0.412 | 2 (6.9%) | 0.19 [0.03, 1.04] | 0.055 |
| Subtype | |||||||
| CRF01_AE | 56 (25.11%) | 8 (14.29%) | 12 (21.43%) | ||||
| CRF07_BC | 82 (36.77%) | 22 (26.83%) | 1.3 [0.53, 3.22] | 0.57 | 16 (19.51%) | 0.62 [0.26, 1.48] | 0.28 |
| CRF08_BC | 56 (25.11%) | 12 (21.43%) | 1.04 [0.38, 2.89] | 0.936 | 14 (25%) | 1 [0.41, 2.46] | 0.999 |
| Others | 29 (13%) | 3 (10.34%) | 0.25 [0.06, 1.09] | 0.064 | 5 (17.24%) | 0.88 [0.26, 2.92] | 0.832 |
| WHO clinical stage | |||||||
| 1 or 2 | 139 (62.33%) | 32 (23.02%) | 28 (20.14%) | ||||
| 3 or 4 | 84 (37.67%) | 13 (15.48%) | 0.36 [0.16, 0.78] | 0.009 | 19 (22.62%) | 1.13 [0.56, 2.3] | 0.736 |
| TMP SMX use at baseline | |||||||
| No | 180 (80.72%) | 36 (20%) | 39 (21.67%) | ||||
| Yes | 43 (19.28%) | 9 (20.93%) | 0.29 [0.09, 0.98] | 0.046 | 8 (18.6%) | 0.4 [0.12, 1.38] | 0.146 |
| Transmission route | |||||||
| Heterosexual | 158 (70.85%) | 29 (18.35%) | 32 (20.25%) | ||||
| Homosexual | 65 (29.15%) | 16 (24.62%) | 1.49 [0.65, 3.4] | 0.348 | 15 (23.08%) | 0.76 [0.33, 1.75] | 0.521 |
| Initial ART regimen | |||||||
| EFV/ANV-based | 122 (54.71%) | 25 (20.49%) | 24 (19.67%) | ||||
| BIC-based | 45 (20.18%) | 9 (20%) | 0.93 [0.39, 2.23] | 0.873 | 15 (33.33%) | 2.37 [1.07, 5.25] | 0.034 |
| DTG-based | 38 (17.04%) | 8 (21.05%) | 1.06 [0.41, 2.71] | 0.909 | 7 (18.42%) | 1.34 [0.52, 3.41] | 0.544 |
| Lpv/r-based | 18 (8.07%) | 3 (16.67%) | 1.1 [0.31, 3.97] | 0.881 | 1 (5.56%) | 0.7 [0.08, 5.86] | 0.743 |
| ART regimen change | |||||||
| No | 166 (74.44%) | 30 (18.07%) | 31 (18.67%) | ||||
| Yes | 57 (25.56%) | 15 (26.32%) | 1.81 [0.89, 3.68] | 0.104 | 16 (28.07%) | 1.59 [0.74, 3.39] | 0.231 |
| Baseline CD4, cell/µL | |||||||
| <100 | 59 (26.46%) | 18 (30.51%) | 14 (23.73%) | ||||
| 100–200 | 42 (18.83%) | 6 (14.29%) | 0.11 [0.03, 0.36] | <0.001 | 2 (4.76%) | 0.09 [0.02, 0.5] | 0.006 |
| 200–349 | 61 (27.35%) | 7 (11.48%) | 0.06 [0.02, 0.2] | <0.001 | 10 (16.39%) | 0.19 [0.05, 0.66] | 0.009 |
| ≥349 | 61 (27.35%) | 14 (22.95%) | 0.24 [0.07, 0.82] | 0.023 | 21 (34.43%) | 0.3 [0.08, 1.08] | 0.065 |
| CD4/CD8 at baseline | |||||||
| <0.5 | 134 (60.09%) | 27 (20.15%) | 25 (18.66%) | ||||
| ≥0.5 | 89 (39.91%) | 18 (20.22%) | 0.72 [0.32, 1.62] | 0.425 | 22 (24.72%) | 1.84 [0.8, 4.25] | 0.15 |
| Baseline CD8 count | |||||||
| <1000 cells/μL | 185 (82.96%) | 39 (21.08%) | 32 (17.3%) | ||||
| ≥1000 cells/μL | 38 (17.04%) | 6 (15.79%) | 0.61 [0.21, 1.77] | 0.364 | 15 (39.47%) | 2.23 [0.91, 5.49] | 0.081 |
| Baseline HIV-1 RNA | |||||||
| <1000 copies/mL | 48 (21.52%) | 9 (18.75%) | 16 (33.33%) | ||||
| ≥1000 copies/mL | 175 (78.48%) | 36 (20.57%) | 2.71 [1.14, 6.44] | 0.024 | 31 (17.71%) | 0.58 [0.29, 1.18] | 0.134 |
References
- UNAIDS. Global HIV & AIDS Statistics—Fact Sheet. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 1 July 2024).
- Tang, M.W.; Shafer, R.W. HIV-1 antiretroviral resistance: Scientific principles and clinical applications. Drugs 2012, 72, e1–e25. [Google Scholar] [CrossRef] [PubMed]
- Bertagnolio, S.; Hermans, L.; Jordan, M.R.; Avila-Rios, S.; Iwuji, C.; Derache, A.; Delaporte, E.; Wensing, A.; Aves, T.; Borhan, A.S.M.; et al. Clinical Impact of Pretreatment Human Immunodeficiency Virus Drug Resistance in People Initiating Nonnucleoside Reverse Transcriptase Inhibitor-Containing Antiretroviral Therapy: A Systematic Review and Meta-analysis. J. Infect. Dis. 2021, 224, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Takem, E.N.; Coox, C.; Shang, J.; Ndongmo, C.; Dokubo, E.K. The association between HIV pretreatment drug resistance and virological outcomes in children and adults in sub-Saharan Africa: A systematic review and meta-analysis. PLoS ONE 2024, 19, e0300456. [Google Scholar] [CrossRef]
- Wittkop, L.; Günthard, H.F.; de Wolf, F.; Dunn, D.; Cozzi-Lepri, A.; de Luca, A.; Kücherer, C.; Obel, N.; von Wyl, V.; Masquelier, B.; et al. Effect of transmitted drug resistance on virological and immunological response to initial combination antiretroviral therapy for HIV (EuroCoord-CHAIN joint project): A European multicohort study. Lancet Infect. Dis. 2011, 11, 363–371. [Google Scholar] [CrossRef]
- World Health Organization. Surveillance of HIV Drug Resistance in Adults Initiating Antiretroviral Therapy (Pretreatment HIV Drug Resistance). Available online: https://www.who.int/publications/i/item/9789241507196 (accessed on 1 July 2024).
- Chen, H.; Hao, J.; Hu, J.; Song, C.; Zhou, Y.; Li, M.; Chen, J.; Liu, X.; Wang, D.; Xu, X.; et al. Pretreatment HIV Drug Resistance and the Molecular Transmission Network Among HIV-Positive Individuals in China in 2022: Multicenter Observational Study. JMIR Public Health Surveill. 2023, 9, e50894. [Google Scholar] [CrossRef]
- Kang, R.H.; Liang, S.J.; Ma, Y.L.; Liang, S.; Xiao, L.; Zhang, X.H.; Lu, H.Y.; Xu, X.Q.; Luo, S.B.; Sun, X.G.; et al. Pretreatment HIV drug resistance in adults initiating antiretroviral therapy in China, 2017. Infect. Dis. Poverty 2020, 9, 54. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, M.; Zhang, R.; Liu, L.; Shen, Y.; Wang, J.; Lu, H. Diversity of HIV-1 genotypes and high prevalence of pretreatment drug resistance in newly diagnosed HIV-infected patients in Shanghai, China. BMC Infect. Dis. 2019, 19, 313. [Google Scholar] [CrossRef]
- Zeng, R.; Ren, D.; Gong, X.; Wei, M.; Gao, L.; Yu, A.; Zhang, D.; Mi, Y.; Ma, P. HIV-1 Genetic Diversity and High Prevalence of Pretreatment Drug Resistance in Tianjin, China. AIDS Res. Hum. Retroviruses 2020, 36, 852–861. [Google Scholar] [CrossRef]
- WHO. HIV Drug Resistance Report 2021. Available online: https://www.who.int/publications/i/item/9789240038608 (accessed on 10 October 2024).
- Zuo, L.; Liu, K.; Liu, H.; Hu, Y.; Zhang, Z.; Qin, J.; Xu, Q.; Peng, K.; Jin, X.; Wang, J.H.; et al. Trend of HIV-1 drug resistance in China: A systematic review and meta-analysis of data accumulated over 17 years (2001–2017). EClinicalMedicine 2020, 18, 100238. [Google Scholar] [CrossRef]
- Yerly, S.; Calmy, A. Time to overcome pretreatment HIV drug resistance. Lancet Infect. Dis. 2018, 18, 239–240. [Google Scholar] [CrossRef]
- WHO. HIV Drug Resistance Strategy, 2021 Update. Available online: https://iris.who.int/handle/10665/343175 (accessed on 10 October 2024).
- Gandhi, R.T.; Bedimo, R.; Hoy, J.F.; Landovitz, R.J.; Smith, D.M.; Eaton, E.F.; Lehmann, C.; Springer, S.A.; Sax, P.E.; Thompson, M.A.; et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2022 Recommendations of the International Antiviral Society-USA Panel. JAMA 2023, 329, 63–84. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. HIV Drug Resistance—Brief Report 2024; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Lan, Y.; Li, L.; Xin, R.; Ling, X.; Deng, X.; Li, J.; Li, L.; Cai, W.; Li, F.; Hu, F. Drug Resistance to Integrase Strand-Transfer Inhibitors among HIV-1-Infected Adults in Guangdong, China. Pathogens 2022, 11, 1321. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.Q.; Lu, J.; Zhou, Y.; Shi, L.E.; Yuan, F.; Chen, J.S.; Xuan, Y.; Hu, H.Y.; Zhang, Z.; Xu, X.Q.; et al. Drug Resistance to HIV-1 Integrase Inhibitors among Treatment-naive Patients in Jiangsu, China. Biomed. Environ. Sci. 2021, 34, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yang, X.; Deng, X.; Wei, S.; Liu, J.; Ma, J.; Zhao, Q.; Huo, Y. Prevalence of integrase strand transfer inhibitor (INSTIs) resistance mutations in Henan Province, China (2018–2020). Infection 2021, 49, 1195–1202. [Google Scholar] [CrossRef]
- GROUP, W.B. Allocating Resources to Control HIV/AIDS in Guangxi Zhuang Autonomous Region: Potential Relevance for China. Available online: https://documents.worldbank.org/en/publication/documents-reports/documentdetail (accessed on 10 October 2024).
- Chen, H.; Luo, L.; Pan, S.W.; Lan, G.; Zhu, Q.; Li, J.; Zhu, J.; Chen, Y.; Shen, Z.; Ge, X.; et al. HIV Epidemiology and Prevention in Southwestern China: Trends from 1996–2017. Curr. HIV Res. 2019, 17, 85–93. [Google Scholar] [CrossRef]
- Ge, X.; Yang, W.; Zhu, Q.; Wu, X.; Shen, Z.; Zhu, J.; Lan, G.; Chen, H.; Meng, Q.; Zhou, X. Epidemiological characteristics of HIV/AIDS in Guangxi Zhuang autonomous region, 2010-2017. Zhonghua Liu Xing Bing Xue Za Zhi = Zhonghua Liuxingbingxue zazhi 2019, 40, 315–321. [Google Scholar]
- Chen, R.; Liang, B.; Wen, B.; Huang, G.; Ning, C.; Lao, C.; Jiang, J.; Liu, J.; Zhou, B.; Huang, J.; et al. No Difference in Prevalence of Transmitted Drug Resistance between Injection Drug Users and Non-Injection Drug Users: A Cross-Sectional Study among Antiretroviral Treatment-Naïve HIV Patients. Intervirology 2018, 61, 281–291. [Google Scholar] [CrossRef]
- Zhang, F.; Liang, B.; Liang, X.; Lin, Z.; Yang, Y.; Liang, N.; Yang, Y.; Liang, H.; Jiang, J.; Huang, J.; et al. Using Molecular Transmission Networks to Reveal the Epidemic of Pretreatment HIV-1 Drug Resistance in Guangxi, China. Front. Genet. 2021, 12, 688292. [Google Scholar] [CrossRef]
- Rhee, S.Y.; Clutter, D.; Hare, C.B.; Tchakoute, C.T.; Sainani, K.; Fessel, W.J.; Hurley, L.; Slome, S.; Pinsky, B.A.; Silverberg, M.J.; et al. Virological Failure and Acquired Genotypic Resistance Associated with Contemporary Antiretroviral Treatment Regimens. Open Forum Infect. Dis. 2020, 7, ofaa316. [Google Scholar] [CrossRef]
- Carr, A.; Mackie, N.E.; Paredes, R.; Ruxrungtham, K. HIV drug resistance in the era of contemporary antiretroviral therapy: A clinical perspective. Antivir. Ther. 2023, 28, 13596535231201162. [Google Scholar] [CrossRef]
- WHO. HIV Drug Resistance Report 2019. Available online: https://www.who.int/publications/i/item/WHO-CDS-HIV-19.21 (accessed on 10 October 2024).
- Lv, S.; Lan, Y.; He, Y.; Li, Q.; Ling, X.; Li, J.; Li, L.; Guo, P.; Hu, F.; Cai, W.; et al. Pretreatment drug resistance among people living with HIV from 2018 to 2022 in Guangzhou, China. J. Med. Virol. 2024, 96, e29937. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; He, X.Q.; Deng, R.N.; Tang, S.Q.; Harypursat, V.; Lu, Y.Q.; He, K.; Huo, Q.; Yang, H.H.; Liu, Q.; et al. Pretreatment drug resistance in people living with HIV: A large retrospective cohort study in Chongqing, China. HIV Med. 2022, 23 (Suppl. S1), 95–105. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; He, Q.; Tang, K.; Huang, J.; Fang, N.; Xie, H.; Ma, J.; Zhu, Q.; Lan, G.; Liang, S. Drug resistance and influencing factors in HIV-1-infected individuals under antiretroviral therapy in Guangxi, China. J. Antimicrob. Chemother. 2024, 79, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Yu, B.; Li, Y.; Wang, Z.; Liu, M.; Ye, L.; Huang, Y.; Su, L.; Zhang, Y.; Api, L.; et al. Prevalence and Molecular Epidemiology of Transmitted Drug Resistance and Genetic Transmission Networks Among Newly Diagnosed People Living With HIV/AIDS in a Minority Area, China. Front. Public Health 2021, 9, 731280. [Google Scholar] [CrossRef]
- Gilks, C.F.; Crowley, S.; Ekpini, R.; Gove, S.; Perriens, J.; Souteyrand, Y.; Sutherland, D.; Vitoria, M.; Guerma, T.; De Cock, K. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet 2006, 368, 505–510. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, B.; Sheng, Z.; Ding, X.; Fan, Q.; Huang, G.; Guo, Z.; Zhong, P.; Liao, L.; Xing, H.; et al. Full-Spectrum Surveillance of Pre-Treatment HIV Drug Resistance in Southeastern China. Pharmaceuticals 2024, 17, 900. [Google Scholar] [CrossRef]
- Mazzuti, L.; Melengu, T.; Falasca, F.; Calabretto, M.; Cella, E.; Ciccozzi, M.; Mezzaroma, I.; Iaiani, G.; Spaziante, M.; d’Ettorre, G.; et al. Transmitted drug resistance mutations and trends of HIV-1 subtypes in treatment-naïve patients: A single-centre experience. J. Glob. Antimicrob. Resist. 2020, 20, 298–303. [Google Scholar] [CrossRef]
- Guidelines for Diagnosis and Treatment of AIDS in China (2024 Edition). Available online: https://rs.yiigle.com/cmaid/1501644 (accessed on 10 October 2024).
- Xu, X.; Luo, L.; Song, C.; Li, J.; Chen, H.; Zhu, Q.; Lan, G.; Liang, S.; Shen, Z.; Cao, Z.; et al. Survey of pretreatment HIV drug resistance and the genetic transmission networks among HIV-positive individuals in southwestern China, 2014–2020. BMC Infect. Dis. 2021, 21, 1153. [Google Scholar] [CrossRef]
- Clutter, D.S.; Jordan, M.R.; Bertagnolio, S.; Shafer, R.W. HIV-1 drug resistance and resistance testing. Infect. Genet. Evol. 2016, 46, 292–307. [Google Scholar] [CrossRef]
- Obeng, B.M.; Bonney, E.Y.; Asamoah-Akuoko, L.; Nii-Trebi, N.I.; Mawuli, G.; Abana, C.Z.; Sagoe, K.W.C. Transmitted drug resistance mutations and subtype diversity amongst HIV-1 sero-positive voluntary blood donors in Accra, Ghana. Virol. J. 2020, 17, 114. [Google Scholar] [CrossRef]
- Yang, L.L.; Li, Q.; Zhou, L.B.; Chen, S.Q. Meta-analysis and systematic review of the efficacy and resistance for human immunodeficiency virus type 1 integrase strand transfer inhibitors. Int. J. Antimicrob. Agents 2019, 54, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Liang, S.; Tang, K.; Huang, J.; He, Q.; Fang, N.; Xie, B.; Xie, X.; Wang, H.; Hu, Y.; et al. Disparity of HIV-1 Pretreatment Drug Resistance in Men Who Have Sex With Men and the Heterosexual Population in Guangxi, China. Open Forum Infect. Dis. 2023, 10, ofad016. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lan, G.; Shen, Z.; Vermund, S.H.; Zhu, Q.; Chen, Y.; Khoshnood, K.; Wu, Z.; Tang, Z. HIV and syphilis prevalence trends among men who have sex with men in Guangxi, China: Yearly cross-sectional surveys, 2008–2012. BMC Infect. Dis. 2014, 14, 367. [Google Scholar] [CrossRef]
- Pang, X.; Tang, K.; He, Q.; Huang, J.; Fang, N.; Zhou, X.; Zhu, Q.; Wu, X.; Shen, Z.; Liang, S. HIV drug resistance and HIV transmission risk factors among newly diagnosed individuals in Southwest China. BMC Infect. Dis. 2021, 21, 160. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, H.; Zhan, J.; Liu, J.; Li, Y.; Cai, W.; Liu, S.; Liang, N.; Lan, G. A Differentiated HIV Pre-Exposure Prophylaxis Delivery Model for High-Risk Groups in Nanning City, South China: Findings from a Pilot Program. AIDS Educ. Prev. 2024, 36, 428–445. [Google Scholar] [CrossRef]
- Wang, L.; Hong, C.; Chen, L.; John, S.A.; Simoni, J.M.; Wong, F.Y.; Velloza, J.; Holloway, I.W. Engagement Along the PrEP Care Continuum Among Men Who Have Sex with Men in China: A Systematic Review and Meta-analysis. AIDS Behav. 2024, 28, 3270–3282. [Google Scholar] [CrossRef]
- Gibas, K.M.; van den Berg, P.; Powell, V.E.; Krakower, D.S. Drug Resistance During HIV Pre-Exposure Prophylaxis. Drugs 2019, 79, 609–619. [Google Scholar] [CrossRef]
- Murchu, E.O.; Marshall, L.; Teljeur, C.; Harrington, P.; Hayes, C.; Moran, P.; Ryan, M. Oral pre-exposure prophylaxis (PrEP) to prevent HIV: A systematic review and meta-analysis of clinical effectiveness, safety, adherence and risk compensation in all populations. BMJ Open 2022, 12, e048478. [Google Scholar] [CrossRef]
- Hofstra, L.M.; Sauvageot, N.; Albert, J.; Alexiev, I.; Garcia, F.; Struck, D.; Van de Vijver, D.; Åsjö, B.; Beshkov, D.; Coughlan, S.; et al. Transmission of HIV Drug Resistance and the Predicted Effect on Current First-line Regimens in Europe. Clin. Infect. Dis. 2016, 62, 655–663. [Google Scholar] [CrossRef]
- Lai, C.C.; Hung, C.C.; Chen, M.Y.; Sun, H.Y.; Lu, C.L.; Tseng, Y.T.; Chang, S.F.; Su, Y.C.; Liu, W.C.; Hsieh, C.Y.; et al. Trends of transmitted drug resistance of HIV-1 and its impact on treatment response to first-line antiretroviral therapy in Taiwan. J. Antimicrob. Chemother. 2012, 67, 1254–1260. [Google Scholar] [CrossRef]
- Sui, H.; Gui, T.; Jia, L.; Guo, W.; Han, J.; Liu, Y.; Bao, Z.; Li, H.; Li, J.; Li, L. Different frequencies of drug resistance mutations among HIV-1 subtypes circulating in China: A comprehensive study. PLoS ONE 2014, 9, e91803. [Google Scholar] [CrossRef]
- Chen, M.; Ma, Y.; Chen, H.; Dai, J.; Dong, L.; Yang, C.; Li, Y.; Luo, H.; Zhang, R.; Jin, X.; et al. HIV-1 genetic transmission networks among men who have sex with men in Kunming, China. PLoS ONE 2018, 13, e0196548. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C. Phylogeographic analyses reveal a crucial role of Xinjiang in HIV-1 CRF07_BC and HCV 3a transmissions in Asia. PLoS ONE 2011, 6, e23347. [Google Scholar] [CrossRef]
- Yuan, R.; Cheng, H.; Chen, L.S.; Zhang, X.; Wang, B. Prevalence of different HIV-1 subtypes in sexual transmission in China: A systematic review and meta-analysis. Epidemiol. Infect. 2016, 144, 2144–2153. [Google Scholar] [CrossRef]
- Li, D.; Li, S.; Liu, Y.; Gao, Y.; Yu, M.; Yang, X.; Li, Q.; Jiang, S.; Zhou, Z.; Zhang, Z.; et al. HIV incidence among men who have sex with men in Beijing: A prospective cohort study. BMJ Open 2012, 2, e001829. [Google Scholar] [CrossRef]
- Li, Z.; Liao, L.; Feng, Y.; Zhang, J.; Yan, J.; He, C.; Xu, W.; Ruan, Y.; Xing, H.; Shao, Y. Trends of HIV subtypes and phylogenetic dynamics among young men who have sex with men in China, 2009–2014. Sci. Rep. 2015, 5, 16708. [Google Scholar] [CrossRef]
- Li, D.; Chen, H.; Li, H.; Ma, Y.; Dong, L.; Dai, J.; Jin, X.; Yang, M.; Zeng, Z.; Sun, P.; et al. HIV-1 pretreatment drug resistance and genetic transmission network in the southwest border region of China. BMC Infect. Dis. 2022, 22, 741. [Google Scholar] [CrossRef]
- Larder, B. Mechanisms of HIV-1 drug resistance. AIDS 2001, 15 (Suppl. S5), S27–S34. [Google Scholar] [CrossRef]

| Variables | Total | No PDR | Partly Active ART | Fully Ineffective ART | p * |
|---|---|---|---|---|---|
| Total | 234 (100.00) | 184 (78.63) | 29 (12.39) | 21 (8.97) | |
| Sex | |||||
| Man | 182 (77.78) | 150 (81.52) | 19 (65.52) | 13 (61.9) | 0.029 |
| Woman | 52 (22.22) | 34 (18.48) | 10 (34.48) | 8 (38.1) | |
| Age | |||||
| <30 | 48 (20.51) | 40 (21.74) | 4 (13.79) | 4 (19.05) | 0.161 |
| 30–39 | 53 (22.65) | 41 (22.28) | 10 (34.48) | 2 (9.52) | |
| 40–49 | 35 (14.96) | 27 (14.67) | 2 (6.9) | 6 (28.57) | |
| 50–59 | 37 (15.81) | 32 (17.39) | 2 (6.9) | 3 (14.29) | |
| ≥60 | 61 (26.07) | 44 (23.91) | 11 (37.93) | 6 (28.57) | |
| Region | |||||
| Urban | 125 (53.42) | 99 (53.8) | 16 (55.17) | 10 (47.62) | 0.848 |
| Suburban | 109 (46.58) | 85 (46.2) | 13 (44.83) | 11 (52.38) | |
| Marital status | |||||
| Unmarried | 108 (46.15) | 90 (48.91) | 10 (34.48) | 8 (38.1) | 0.588 |
| Married/cohabiting | 96 (41.03) | 71 (38.59) | 15 (51.72) | 10 (47.62) | |
| Divorced/widowed | 30 (12.82) | 23 (12.5) | 4 (13.79) | 3 (14.29) | |
| HIV Subtype | |||||
| CRF01_AE | 64 (27.35) | 53 (28.8) | 5 (17.24) | 6 (28.57) | 0.002 |
| CRF07_BC | 84 (35.9) | 71 (38.59) | 8 (27.59) | 5 (23.81) | |
| CRF08_BC | 56 (23.93) | 44 (23.91) | 5 (17.24) | 7 (33.33) | |
| Others | 30 (12.82) | 16 (8.7) | 11 (37.93) | 3 (14.29) | |
| WHO clinical stage at initiation | |||||
| 1 or 2 | 145 (61.97) | 113 (61.41) | 19 (65.52) | 13 (61.9) | 0.914 |
| 3 or 4 | 89 (38.03) | 71 (38.59) | 10 (34.48) | 8 (38.1) | |
| TMP SMX use at baseline | |||||
| No | 189 (80.77) | 149 (80.98) | 25 (86.21) | 15 (71.43) | 0.42 |
| Yes | 45 (19.23) | 35 (19.02) | 4 (13.79) | 6 (28.57) | |
| Transmission route | |||||
| HET | 166 (70.94) | 123 (66.85) | 23 (79.31) | 20 (95.24) | 0.014 |
| HOM | 68 (29.06) | 61 (33.15) | 6 (20.69) | 1 (4.76) | |
| Initial ART regimen | |||||
| EFV/ANV-based | 128 (54.70) | 104 (56.52) | 19 (65.52) | 5 (23.81) | 0.015 |
| BIC-based | 48 (20.51) | 40 (21.74) | 2 (6.90) | 6 (28.57) | |
| DTG-based | 40 (17.09) | 30 (16.3) | 4 (13.79) | 6 (28.57) | |
| LPV/r-based | 18 (7.69) | 10 (5.43) | 4 (13.79) | 4 (19.05) | |
| ART regimen change | |||||
| No | 174 (74.36) | 138 (75) | 18 (62.07) | 18 (85.71) | 0.153 |
| Yes | 60 (25.64) | 46 (25.00) | 11 (37.93) | 3 (14.29) | |
| Baseline CD4+ T cell count, cell/µL | |||||
| <100 | 61 (26.07) | 46 (25.00) | 6 (20.69) | 9 (42.86) | 0.187 |
| 100–200 | 43 (18.38) | 33 (17.93) | 8 (27.59) | 2 (9.52) | |
| 200–349 | 66 (28.21) | 51 (27.72) | 11 (37.93) | 4 (19.05) | |
| ≥349 | 64 (27.35) | 54 (29.35) | 4 (13.79) | 6 (28.57) | |
| Baseline CD8+ T cell count | |||||
| <1000 cells/μL | 139 (59.4) | 105 (57.07) | 19 (65.52) | 15 (71.43) | 0.345 |
| ≥1000 cells/μL | 95 (40.6) | 79 (42.93) | 10 (34.48) | 6 (28.57) | |
| Baseline CD4/CD8 ratio | |||||
| <0.5 | 195 (83.33) | 153 (83.15) | 26 (89.66) | 16 (76.19) | 0.447 |
| ≥0.5 | 39 (16.67) | 31 (16.85) | 3 (10.34) | 5 (23.81) | |
| Baseline VL | |||||
| <1000 copies/mL | 51 (21.79) | 35 (19.02) | 8 (27.59) | 8 (38.10) | 0.097 |
| ≥1000 copies/mL | 183 (78.21) | 149 (80.98) | 21 (72.41) | 13 (61.90) | |
| Variables | Total | VF | IF | ||||
|---|---|---|---|---|---|---|---|
| n (%) | aHR (95CI) | p-Value | n (%) | aHR (95CI) | p-Value | ||
| Group | 234 (100.00) | 45 (19.23) | 47 (20.09) | ||||
| No PDR (reference) | 174 (78.03) | 37 (21.26) | 1 | - | 37 (21.26) | 1 | - |
| Partly active ART | 29 (13.00) | 5 (17.24) | 1.33 (0.45, 3.97) | 0.608 | 5 (17.24) | 1.44 (0.49, 4.18) | 0.506 |
| Fully ineffective ART | 20 (8.97) | 3 (15.00) | 0.63 (0.16, 2.43) | 0.502 | 5 (25) | 0.81 (0.28, 2.35) | 0.693 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Q.; Li, Y.; Huang, T.; Wei, L.; He, J.; Huang, Y.; Mo, G.; Qin, J.; Tao, C.; Huang, X.; et al. The Prevalence of Pretreatment Drug Resistance and Transmission Networks Among Newly Diagnosed HIV-1-Infected Individuals in Nanning, Guangxi, China. Pathogens 2025, 14, 336. https://doi.org/10.3390/pathogens14040336
Su Q, Li Y, Huang T, Wei L, He J, Huang Y, Mo G, Qin J, Tao C, Huang X, et al. The Prevalence of Pretreatment Drug Resistance and Transmission Networks Among Newly Diagnosed HIV-1-Infected Individuals in Nanning, Guangxi, China. Pathogens. 2025; 14(4):336. https://doi.org/10.3390/pathogens14040336
Chicago/Turabian StyleSu, Qiuqian, Yanjun Li, Ting Huang, Liangjia Wei, Jinfeng He, Yumei Huang, Guidan Mo, Jiao Qin, Chunxing Tao, Xinju Huang, and et al. 2025. "The Prevalence of Pretreatment Drug Resistance and Transmission Networks Among Newly Diagnosed HIV-1-Infected Individuals in Nanning, Guangxi, China" Pathogens 14, no. 4: 336. https://doi.org/10.3390/pathogens14040336
APA StyleSu, Q., Li, Y., Huang, T., Wei, L., He, J., Huang, Y., Mo, G., Qin, J., Tao, C., Huang, X., Ye, L., Liang, H., Liang, B., & Huang, J. (2025). The Prevalence of Pretreatment Drug Resistance and Transmission Networks Among Newly Diagnosed HIV-1-Infected Individuals in Nanning, Guangxi, China. Pathogens, 14(4), 336. https://doi.org/10.3390/pathogens14040336






