Maternal HIV Infection and Antiretroviral Therapy in Pregnancy: Implications for Vertical Transmission, Fetal Safety, and Long-Term Infant Outcomes
Abstract
1. Introduction
2. Search Strategy
3. The Bridge Between HIV and Pregnancy
4. The Trialogue Between HIV, Antiretroviral Therapy, and the Child
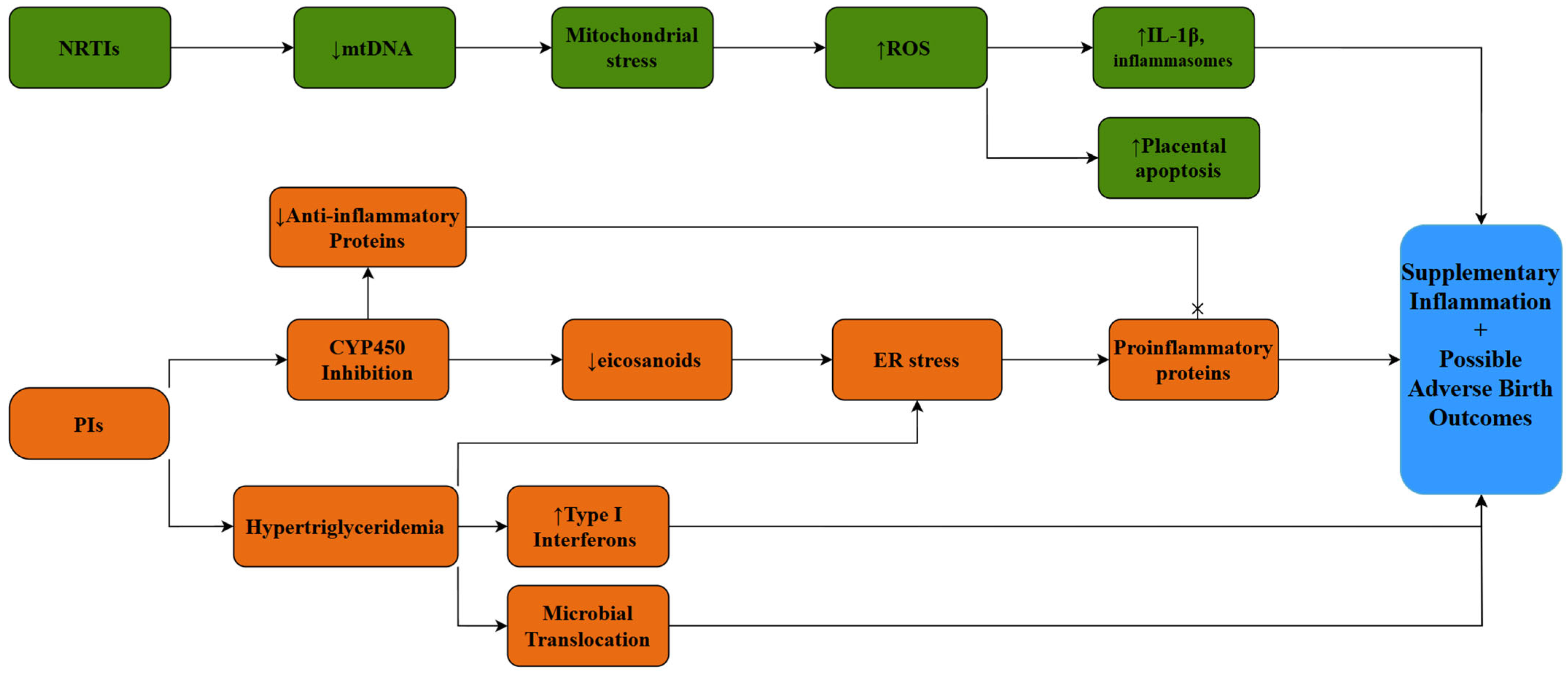
4.1. Metabolic Alterations
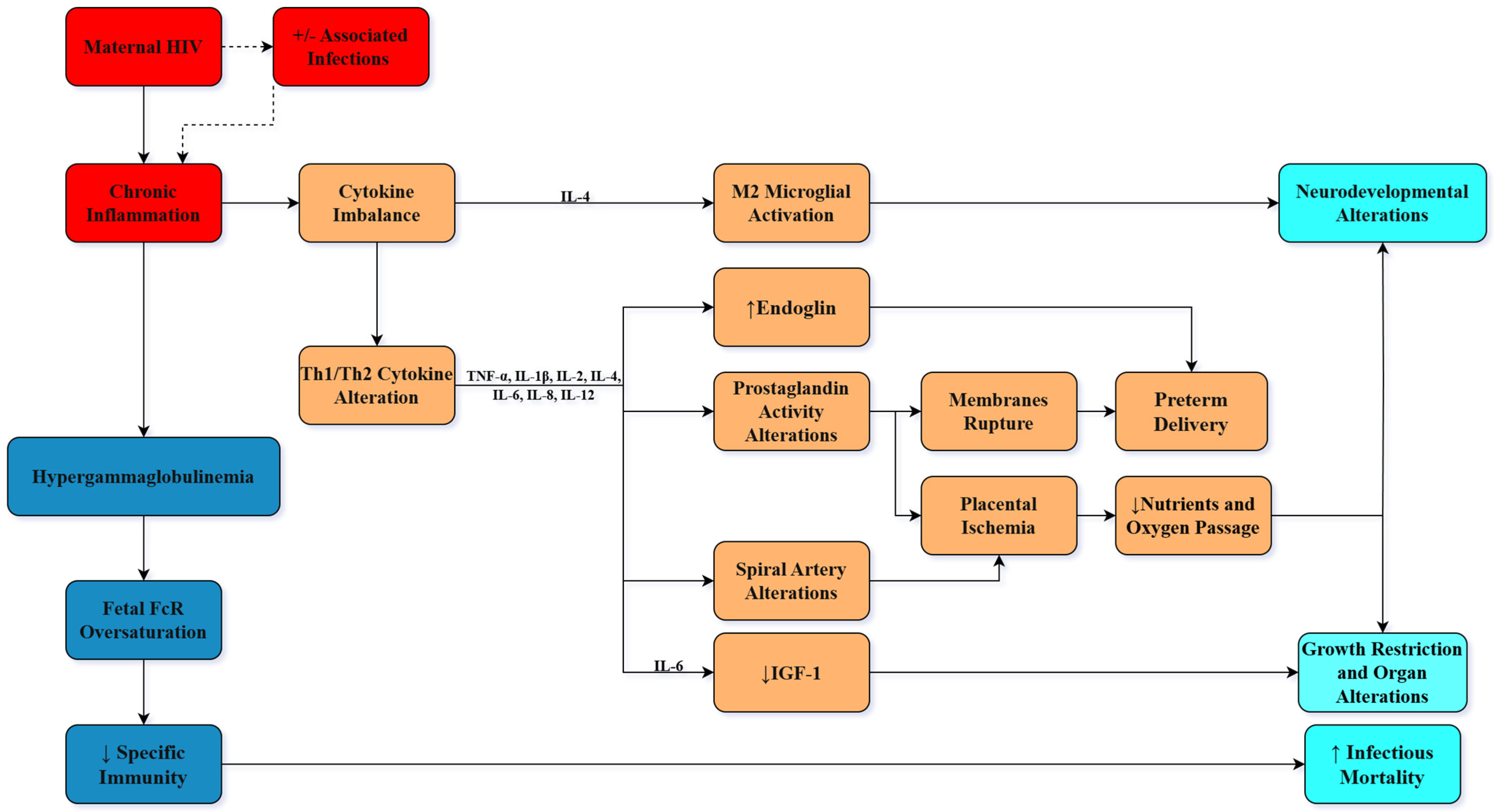
4.2. Immunological Changes
4.3. ART and the Placenta
4.4. ART and Pregnancy
5. Clinical Implications for Practice
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3TC | Lamivudine |
| AIDS | Acquired immunodeficiency syndrome |
| ART | Antiretroviral therapy |
| BHIVA | British HIV Association |
| BIC | Bictegravir |
| CCR5 | Chemokine (C-C motif) Receptor 5 |
| CD4+ | Cluster of Differentiation 4 Positive |
| CXCL10 | C-X-C Motif Chemokine Ligand 10 |
| CXCR4 | C-X-C Motif Chemokine Receptor 4 |
| CYP2B6 | Cytochrome P450 Enzyme 2B6 |
| CYP450 | Cytochrome P450 Enzyme System |
| CYP4A | Cytochrome P450 Enzyme 4A |
| DHHS | U.S. Department of Health and Human Services |
| DTG | Dolutegravir |
| ER | Endoplasmic reticulum |
| FTC | Emtricitabine |
| HEU | HIV-exposed uninfected (children) |
| HIV | Human immunodeficiency virus |
| IGF-1 | Insulin-like growth hormone |
| IL-1β | Interleukin-1 Beta |
| IL-2 | Interleukin-2 |
| IL-4 | Interleukin-4 |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| IL-12p70 | Interleukin-12p70 |
| INSTIs | Integrase strand transfer inhibitors |
| MTCT | Mother-to-child transmission |
| mtDNA | Mitochondrial DNA |
| NRTIs | Nucleoside reverse transcriptase inhibitors |
| PIs | Protease inhibitors |
| ROS | Reactive oxygen species |
| TAF | Tenofovir alafenamide |
| TDF | Tenofovir disoproxil fumarate |
| TNF-α | Tumor necrosis factor alpha |
| WHO | World Health Organization |
References
- Castillo-Chavez, C. Review of Recent Models of HIV/AIDS Transmission. In Applied Mathematical Ecology; Levin, S.A., Hallam, T.G., Gross, L.J., Eds.; Springer: Berlin/Heidelberg, Germany, 1989; pp. 253–262. ISBN 978-3-642-61317-3. [Google Scholar] [CrossRef]
- UNAIDS Report on the Global AIDS Epidemic 2012—World|ReliefWeb. Available online: https://reliefweb.int/report/world/unaids-report-global-aids-epidemic-2012 (accessed on 16 September 2024).
- Ubesie, A. Pediatric HIV/AIDS in Sub-Saharan Africa: Emerging Issues and Way Forward. Afr. Health Sci. 2012, 12, 297–304. [Google Scholar] [CrossRef][Green Version]
- Mother-to-Child Transmission of HIV. Available online: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/prevention/mother-to-child-transmission-of-hiv (accessed on 17 September 2024).
- HIV—Estimated Percentage of Pregnant Women Living with HIV Who Received Antiretrovirals for Preventing Mother-to-Child Transmission. Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/estimated-percentage-of-pregnant-women-living-with-hiv-who-received-antiretrovirals-for-preventing-mother-to-child-transmission (accessed on 25 May 2025).
- Preventing Perinatal Transmission of HIV|NIH. Available online: https://hivinfo.nih.gov/understanding-hiv/fact-sheets/preventing-perinatal-transmission-hiv (accessed on 17 September 2024).
- Preventing Mother to Child Transmission. Available online: https://www.unaids.org/en/resources/presscentre/featurestories/2016/october/20161024_EMotherToChildT (accessed on 17 September 2024).
- Botswana Leads the Way for High HIV Burden Country Certification on the Path to Eliminate Vertical HIV Transmission|UNAIDS. Available online: https://www.unaids.org/en/resources/presscentre/featurestories/2022/july/20220727_botswana-leads-way-eliminate-vertical-HIV-transmission (accessed on 8 August 2025).
- Evans, C.; Jones, C.E.; Prendergast, A.J. HIV-Exposed, Uninfected Infants: New Global Challenges in the Era of Paediatric HIV Elimination. Lancet Infect. Dis. 2016, 16, e92–e107. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.C.; Alimenti, A.M.; Singer, J.; Brophy, J.C.; Bitnun, A.; Samson, L.M.; Money, D.M.; Lee, T.C.K.; Lapointe, N.D.; Read, S.E.; et al. A National Review of Vertical HIV Transmission. AIDS 2012, 26, 757. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, R.L.; Kitch, D.; Ogwu, A.; Hughes, M.D.; Lockman, S.; Powis, K.; Souda, S.; Moffat, C.; Moyo, S.; McIntosh, K.; et al. HIV Transmission and 24-Month Survival in a Randomized Trial of HAART to Prevent MTCT during Pregnancy and Breastfeeding in Botswana (The Mma Bana Study). AIDS 2013, 27, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, A.; Tudor-Williams, G.; Jeena, P.; Burchett, S.; Goulder, P. International Perspectives, Progress, and Future Challenges of Paediatric HIV Infection. Lancet 2007, 370, 68–80. [Google Scholar] [CrossRef]
- Italian Multicentre Study. Epidemiology, Clinical Features, and Prognostic Factors of Paediatric HIV Infection. Lancet 1988, 2, 1043–1046. [Google Scholar] [CrossRef]
- European Collaborative Study. Risk Factors for Mother-to-Child Transmission of HIV-1. Lancet 1992, 339, 1007–1012. [Google Scholar] [CrossRef]
- Nesheim, S.R.; Lindsay, M.; Sawyer, M.K.; Mancao, M.; Lee, F.K.; Shaffer, N.; Jones, D.; Slade, B.A.; Ou, C.Y.; Nahmias, A. A Prospective Population-Based Study of HIV Perinatal Transmission. AIDS 1994, 8, 1293–1298. [Google Scholar] [CrossRef]
- Adjorlolo-Johnson, G.; De Cock, K.M.; Ekpini, E.; Vetter, K.M.; Sibailly, T.; Brattegaard, K.; Yavo, D.; Doorly, R.; Whitaker, J.P.; Kestens, L.; et al. Prospective Comparison of Mother-to-Child Transmission of HIV-1 and HIV-2 in Abidjan, Ivory Coast. JAMA 1994, 272, 462–466. [Google Scholar] [CrossRef]
- Datta, P.; Embree, J.E.; Kreiss, J.K.; Ndinya-Achola, J.O.; Braddick, M.; Temmerman, M.; Nagelkerke, N.J.; Maitha, G.; Holmes, K.K.; Piot, P. Mother-to-Child Transmission of Human Immunodeficiency Virus Type 1: Report from the Nairobi Study. J. Infect. Dis. 1994, 170, 1134–1140. [Google Scholar] [CrossRef]
- Temmerman, M.; Nyong’o, A.O.; Bwayo, J.; Fransen, K.; Coppens, M.; Piot, P. Risk Factors for Mother-to-Child Transmission of Human Immunodeficiency Virus-1 Infection. Am. J. Obstet. Gynecol. 1995, 172, 700–705. [Google Scholar] [CrossRef]
- Chouquet, C.; Richardson, S.; Burgard, M.; Blanche, S.; Mayaux, M.J.; Rouzioux, C.; Costagliola, D. Timing of Human Immunodeficiency Virus Type 1 (HIV-1) Transmission from Mother to Child: Bayesian Estimation Using a Mixture. Stat. Med. 1999, 18, 815–833. [Google Scholar] [CrossRef]
- World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach, 2nd ed.; World Health Organization: Geneva, Switzerland, 2016; Available online: https://www.who.int/publications/i/item/9789241549684 (accessed on 16 September 2024).
- Joint United Nations Programme on HIV/AIDS. Global Plan Towards the Elimination of New HIV Infections Among Children by 2015 and Keeping Their Mothers Alive; UNAIDS: Geneva, Switzerland, 2011; Available online: https://www.unaids.org/en/resources/documents/2011/20110609_JC2137_Global-Plan-Elimination-HIV-Children_en.pdf (accessed on 16 September 2024).
- Marinda, E.; Humphrey, J.H.; Iliff, P.J.; Mutasa, K.; Nathoo, K.J.; Piwoz, E.G.; Moulton, L.H.; Salama, P.; Ward, B.J.; ZVITAMBO Study Group. Child Mortality According to Maternal and Infant HIV Status in Zimbabwe. Pediatr. Infect. Dis. J. 2007, 26, 519. [Google Scholar] [CrossRef] [PubMed]
- Sutcliffe, C.G.; Scott, S.; Mugala, N.; Ndhlovu, Z.; Monze, M.; Quinn, T.C.; Cousens, S.; Griffin, D.E.; Moss, W.J. Survival from 9 Months of Age among HIV-Infected and Uninfected Zambian Children Prior to the Availability of Antiretroviral Therapy. Clin. Infect. Dis. 2008, 47, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Brahmbhatt, H.; Kigozi, G.; Wabwire-Mangen, F.; Serwadda, D.; Lutalo, T.; Nalugoda, F.; Sewankambo, N.; Kiduggavu, M.; Wawer, M.; Gray, R. Mortality in HIV-Infected and Uninfected Children of HIV-Infected and Uninfected Mothers in Rural Uganda. J. Acquir. Immune Defic. Syndr. 2006, 41, 504–508. [Google Scholar] [CrossRef]
- Spira, R.; Lepage, P.; Msellati, P.; Van De Perre, P.; Leroy, V.; Simonon, A.; Karita, E.; Dabis, F. Natural History of Human Immunodeficiency Virus Type 1 Infection in Children: A Five-Year Prospective Study in Rwanda. Mother-to-Child HIV-1 Transmission Study Group. Pediatrics 1999, 104, e56. [Google Scholar] [CrossRef]
- Schim van der Loeff, M.F.; Hansmann, A.; Awasana, A.A.; Ota, M.O.; O’Donovan, D.; Sarge-Njie, R.; Ariyoshi, K.; Milligan, P.; Whittle, H. Survival of HIV-1 and HIV-2 Perinatally Infected Children in The Gambia. AIDS 2003, 17, 2389–2394. [Google Scholar] [CrossRef]
- Filteau, S.; Baisley, K.; Chisenga, M.; Kasonka, L.; Gibson, R.S. CIGNIS Study Team. Provision of Micronutrient-Fortified Food from 6 Months of Age Does Not Permit HIV-Exposed Uninfected Zambian Children to Catch up in Growth to HIV-Unexposed Children: A Randomized Controlled Trial. J. Acquir. Immune Defic. Syndr. 2011, 56, 166–175. [Google Scholar] [CrossRef]
- McNally, L.M.; Jeena, P.M.; Gajee, K.; Thula, S.A.; Sturm, A.W.; Cassol, S.; Tomkins, A.M.; Coovadia, H.M.; Goldblatt, D. Effect of Age, Polymicrobial Disease, and Maternal HIV Status on Treatment Response and Cause of Severe Pneumonia in South African Children: A Prospective Descriptive Study. Lancet 2007, 369, 1440–1451. [Google Scholar] [CrossRef]
- Singh, H.K.; Gupte, N.; Kinikar, A.; Bharadwaj, R.; Sastry, J.; Suryavanshi, N.; Nayak, U.; Tripathy, S.; Paranjape, R.; Jamkar, A.; et al. High Rates of All-Cause and Gastroenteritis-Related Hospitalization Morbidity and Mortality among HIV-Exposed Indian Infants. BMC Infect. Dis. 2011, 11, 193. [Google Scholar] [CrossRef]
- Epalza, C.; Goetghebuer, T.; Hainaut, M.; Prayez, F.; Barlow, P.; Dediste, A.; Marchant, A.; Levy, J. High Incidence of Invasive Group B Streptococcal Infections in HIV-Exposed Uninfected Infants. Pediatrics 2010, 126, e631–e638. [Google Scholar] [CrossRef]
- Chilaka, V.N.; Konje, J.C. HIV in Pregnancy—An Update. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 256, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Jean, J.; Coll, A.; Monda, M.; Potter, J.; Jones, D. Perspectives on Safer Conception Practices and Preconception Counseling among Women Living with HIV. Health Care Women Int. 2016, 37, 1096–1118. [Google Scholar] [CrossRef]
- Camacho-Gonzalez, A.F.; Kingbo, M.-H.; Boylan, A.; Eckard, A.R.; Chahroudi, A.; Chakraborty, R. Missed Opportunities for Prevention of Mother-to-Child Transmission in the United States. AIDS 2015, 29, 1511–1515. [Google Scholar] [CrossRef]
- British HIV Association (BHIVA). BHIVA Guidelines for the Management of HIV in Pregnancy and the Postpartum Period 2025; British HIV Association: Hertfordshire, UK, 2025; Available online: https://bhiva.org/clinical-guideline/pregnancy-guidelines/ (accessed on 10 August 2025).
- Sorato, M.M.; Alemu, T.; Toma, A.; Paulos, G.; Mekonnen, S. Effect of HIV and substance use disorder comorbidity on the placenta, fetal and maternal health outcomes: Systematic review and meta-analysis protocol. BMJ Open 2024, 14, e083037. [Google Scholar] [CrossRef]
- Lazenby, G.B. Opportunistic infections in women with HIV AIDS. Clin. Obstet. Gynecol. 2012, 55, 927–937. [Google Scholar] [CrossRef]
- Stringer, E.M.; Kendall, M.A.; Lockman, S.; Campbell, T.B.; Nielsen-Saines, K.; Sawe, F.; Cu-Uvin, S.; Wu, X.; Currier, J.S. Pregnancy Outcomes among HIV-Infected Women Who Conceived on Antiretroviral Therapy. PLoS ONE 2018, 13, e0199555. [Google Scholar] [CrossRef]
- Dara, J.S.; Hanna, D.B.; Anastos, K.; Wright, R.; Herold, B.C. Low Birth Weight in Human Immunodeficiency Virus-Exposed Uninfected Infants in Bronx, New York. J. Pediatr. Infect. Dis. Soc. 2018, 7, e24–e29. [Google Scholar] [CrossRef]
- Dadabhai, S.; Gadama, L.; Chamanga, R.; Kawalazira, R.; Katumbi, C.; Makanani, B.; Dula, D.; Hua, N.; Lau, B.; Mallewa, M.; et al. Pregnancy Outcomes in the Era of Universal Antiretroviral Treatment in Sub-Saharan Africa (POISE Study). J. Acquir. Immune Defic. Syndr. 2019, 80, 7–14. [Google Scholar] [CrossRef]
- Jacobson, D.L.; Patel, K.; Siberry, G.K.; Van Dyke, R.B.; DiMeglio, L.A.; Geffner, M.E.; Chen, J.S.; McFarland, E.J.; Borkowsky, W.; Silio, M.; et al. Body fat distribution in perinatally HIV-infected and HIV-exposed but uninfected children in the era of highly active antiretroviral therapy: Outcomes from the Pediatric HIV/AIDS Cohort Study. Am. J. Clin. Nutr. 2011, 94, 1485–1495. [Google Scholar] [CrossRef]
- Tukei, V.J.; Hoffman, H.J.; Greenberg, L.; Thabelo, R.; Nchephe, M.; Mots’oane, T.; Masitha, M.; Chabela, M.; Mokone, M.; Mofenson, L.; et al. Adverse Pregnancy Outcomes Among HIV-Positive Women in the Era of Universal Antiretroviral Therapy Remain Elevated Compared With HIV-Negative Women. Pediatr. Infect. Dis. J. 2021, 40, 821. [Google Scholar] [CrossRef] [PubMed]
- Ekubagewargies, D.T.; Kassie, D.G.; Takele, W.W. Maternal HIV Infection and Preeclampsia Increased Risk of Low Birth Weight among Newborns Delivered at University of Gondar Specialized Referral Hospital, Northwest Ethiopia, 2017. Ital. J. Pediatr. 2019, 45, 7. [Google Scholar] [CrossRef] [PubMed]
- Ramokolo, V.; Goga, A.E.; Lombard, C.; Doherty, T.; Jackson, D.J.; Engebretsen, I.M. In Utero ART Exposure and Birth and Early Growth Outcomes Among HIV-Exposed Uninfected Infants Attending Immunization Services: Results From National PMTCT Surveillance, South Africa. Open Forum Infect. Dis. 2017, 4, ofx187. [Google Scholar] [CrossRef] [PubMed]
- Delicio, A.M.; Lajos, G.J.; Amaral, E.; Cavichiolli, F.; Polydoro, M.; Milanez, H. Adverse Effects in Children Exposed to Maternal HIV and Antiretroviral Therapy during Pregnancy in Brazil: A Cohort Study. Reprod. Health 2018, 15, 76. [Google Scholar] [CrossRef]
- Dos Reis, H.L.B.; da Silva Araujo, K.; Ribeiro, L.P.; Da Rocha, D.R.; Rosato, D.P.; Passos, M.R.L.; De Vargas, P.R.M. Preterm Birth and Fetal Growth Restriction in HIV-Infected Brazilian Pregnant Women. Rev. Inst. Med. Trop. Sao Paulo 2015, 57, 111–120. [Google Scholar] [CrossRef]
- Passini, R.; Cecatti, J.G.; Lajos, G.J.; Tedesco, R.P.; Nomura, M.L.; Dias, T.Z.; Haddad, S.M.; Rehder, P.M.; Pacagnella, R.C.; Costa, M.L.; et al. Brazilian Multicentre Study on Preterm Birth (EMIP): Prevalence and Factors Associated with Spontaneous Preterm Birth. PLoS ONE 2014, 9, e109069. [Google Scholar] [CrossRef]
- Santini-Oliveira, M.; Friedman, R.K.; Veloso, V.G.; Cunha, C.B.; Pilotto, J.H.; Marins, L.M.S.; João, E.C.; Torres, T.S.; Grinsztejn, B. Incidence of Antiretroviral Adverse Drug Reactions in Pregnant Women in Two Referral Centers for HIV Prevention of Mother-to-Child-Transmission Care and Research in Rio de Janeiro, Brazil. Braz. J. Infect. Dis. 2014, 18, 372–378. [Google Scholar] [CrossRef]
- Wei, S.; Evans, P.C.; Strijdom, H.; Xu, S. HIV Infection, Antiretroviral Therapy and Vascular Dysfunction: Effects, Mechanisms and Treatments. Pharmacol. Res. 2025, 217, 107812. [Google Scholar] [CrossRef]
- Shiau, S.; Jacobson, D.L.; Huo, Y.; Kacanek, D.; Yee, L.M.; Williams, D.B.; Haddad, L.B.; Serghides, L.; Powis, K.; Sperling, R.S.; et al. Unique Profile of Inflammation and Immune Activation in Pregnant People With HIV in the United States. J. Infect. Dis. 2023, 227, 720–730. [Google Scholar] [CrossRef]
- Lohman-Payne, B.; Gabriel, B.; Park, S.; Wamalwa, D.; Maleche-Obimbo, E.; Farquhar, C.; Bosire, R.K.; John-Stewart, G. HIV-Exposed Uninfected Infants: Elevated Cord Blood Interleukin 8 (IL-8) Is Significantly Associated with Maternal HIV Infection and Systemic IL-8 in a Kenyan Cohort. Clin. Transl. Med. 2018, 7, 26. [Google Scholar] [CrossRef]
- Han, V.X.; Patel, S.; Jones, H.F.; Nielsen, T.C.; Mohammad, S.S.; Hofer, M.J.; Gold, W.; Brilot, F.; Lain, S.J.; Nassar, N.; et al. Maternal Acute and Chronic Inflammation in Pregnancy Is Associated with Common Neurodevelopmental Disorders: A Systematic Review. Transl. Psychiatry 2021, 11, 71. [Google Scholar] [CrossRef]
- Ragsdale, H.B.; Kuzawa, C.W.; Borja, J.B.; Avila, J.L.; McDade, T.W. Regulation of Inflammation during Gestation and Birth Outcomes: Inflammatory Cytokine Balance Predicts Birth Weight and Length. Am. J. Human Biol. 2019, 31, e23245. [Google Scholar] [CrossRef]
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron 2012, 74, 691–705. [Google Scholar] [CrossRef]
- Lenz, K.M.; Nelson, L.H. Microglia and Beyond: Innate Immune Cells As Regulators of Brain Development and Behavioral Function. Front. Immunol. 2018, 9, 698. [Google Scholar] [CrossRef]
- Bilbo, S.D.; Schwarz, J.M. The Immune System and Developmental Programming of Brain and Behavior. Front. Neuroendocrinol. 2012, 33, 267–286. [Google Scholar] [CrossRef] [PubMed]
- Robinson-Agramonte, M.d.l.A.; Noris García, E.; Fraga Guerra, J.; Vega Hurtado, Y.; Antonucci, N.; Semprún-Hernández, N.; Schultz, S.; Siniscalco, D. Immune Dysregulation in Autism Spectrum Disorder: What Do We Know about It? Int. J. Mol. Sci. 2022, 23, 3033. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Ellis, S.E.; Ashar, F.N.; Moes, A.; Bader, J.S.; Zhan, J.; West, A.B.; Arking, D.E. Transcriptome Analysis Reveals Dysregulation of Innate Immune Response Genes and Neuronal Activity-Dependent Genes in Autism. Nat. Commun. 2014, 5, 5748. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, M.; Gouvêa, A.F.T.B.; Ono, E.; Succi, R.C.M.; Pahwa, S.; de Moraes-Pinto, M.I. Immune Development in HIV-Exposed Uninfected Children Born to HIV-Infected Women. Rev. Inst. Med. Trop. São Paulo 2017, 59, e30. [Google Scholar] [CrossRef]
- Sevenoaks, T.; Wedderburn, C.J.; Donald, K.A.; Barnett, W.; Zar, H.J.; Stein, D.J.; Naudé, P.J.W. Association of Maternal and Infant Inflammation with Neurodevelopment in HIV-Exposed Uninfected Children in a South African Birth Cohort. Brain Behav. Immun. 2021, 91, 65–73. [Google Scholar] [CrossRef]
- Valiukas, Z.; Tangalakis, K.; Apostolopoulos, V.; Feehan, J. Microglial Activation States and Their Implications for Alzheimer’s Disease. J. Prev. Alzheimer’s Dis. 2025, 12, 100013. [Google Scholar] [CrossRef]
- Morelli, S.S.; Mandal, M.; Goldsmith, L.T.; Kashani, B.N.; Ponzio, N.M. The Maternal Immune System during Pregnancy and Its Influence on Fetal|RRB. Res. Rep. Biol. 2015, 6, 171–189. [Google Scholar] [CrossRef]
- Hokello, J.; Tyagi, K.; Owor, R.O.; Sharma, A.L.; Bhushan, A.; Daniel, R.; Tyagi, M. New Insights into HIV Life Cycle, Th1/Th2 Shift during HIV Infection and Preferential Virus Infection of Th2 Cells: Implications of Early HIV Treatment Initiation and Care. Life 2024, 14, 104. [Google Scholar] [CrossRef] [PubMed]
- Neta, G.I.; von Ehrenstein, O.S.; Goldman, L.R.; Lum, K.; Sundaram, R.; Andrews, W.; Zhang, J. Umbilical Cord Serum Cytokine Levels and Risks of Small-for-Gestational-Age and Preterm Birth. Am. J. Epidemiol. 2010, 171, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Kadivnik, M.; Plečko, D.; Kralik, K.; Arvaj, N.; Wagner, J. Role of IL-6, IL-10 and TNFα Gene Variants in Preterm Birth. J. Clin. Med. 2024, 13, 2429. [Google Scholar] [CrossRef] [PubMed]
- Akoto, C.; Norris, S.A.; Hemelaar, J. Maternal HIV Infection Is Associated with Distinct Systemic Cytokine Profiles throughout Pregnancy in South African Women. Sci. Rep. 2021, 11, 10079. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Sáez, M.A.; Álvarez-Mon, M.A.; Torres-Carranza, D.; Álvarez-Mon, M.; Bujan, J.; García-Honduvilla, N.; Bravo, C.; et al. The Pivotal Role of the Placenta in Normal and Pathological Pregnancies: A Focus on Preeclampsia, Fetal Growth Restriction, and Maternal Chronic Venous Disease. Cells 2022, 11, 568. [Google Scholar] [CrossRef]
- Krishna, U.; Bhalerao, S. Placental Insufficiency and Fetal Growth Restriction. J. Obstet. Gynaecol. India 2011, 61, 505–511. [Google Scholar] [CrossRef]
- Reisenberger, K.; Egarter, C.; Vogl, S.; Sternberger, B.; Kiss, H.; Husslein, P. The Transfer of Interleukin-8 across the Human Placenta Perfused in Vitro. Obstet. Gynecol. 1996, 87, 613–616. [Google Scholar] [CrossRef]
- Bermick, J.; Watson, S.; Lueschow, S.; McElroy, S. The Fetal Response to Maternal Inflammation Is Dependent upon Maternal IL-6 in a Murine Model. Cytokine 2023, 167, 156210. [Google Scholar] [CrossRef]
- Dahlgren, J.; Samuelsson, A.-M.; Jansson, T.; Holmäng, A. Interleukin-6 in the Maternal Circulation Reaches the Rat Fetus in Mid-Gestation. Pediatr. Res. 2006, 60, 147–151. [Google Scholar] [CrossRef]
- Chiesa, C.; Pacifico, L.; Natale, F.; Hofer, N.; Osborn, J. Fetal and Early Neonatal Interleukin-6 Response. Cytokine 2015, 76, 1–12. [Google Scholar] [CrossRef]
- Cooke, D.; Divall, S.; Radovick, S. Normal and Aberrant Growth in Children. In Williams Textbook of Endocrinology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 964–1073. [Google Scholar] [CrossRef]
- Klevebro, S.; Hellgren, G.; Hansen-Pupp, I.; Wackernagel, D.; Hallberg, B.; Borg, J.; Pivodic, A.; Smith, L.; Ley, D.; Hellström, A. Elevated Levels of IL-6 and IGFBP-1 Predict Low Serum IGF-1 Levels during Continuous Infusion of rhIGF-1/rhIGFBP-3 in Extremely Preterm Infants. Growth Horm. IGF Res. 2020, 50, 1–8. [Google Scholar] [CrossRef]
- Conroy, A.L.; McDonald, C.R.; Gamble, J.L.; Olwoch, P.; Natureeba, P.; Cohan, D.; Kamya, M.R.; Havlir, D.V.; Dorsey, G.; Kain, K.C. Altered Angiogenesis as a Common Mechanism Underlying Preterm Birth, Small for Gestational Age, and Stillbirth in Women Living with HIV. Am. J. Obstet. Gynecol. 2017, 217, 684.e1–684.e17. [Google Scholar] [CrossRef]
- Hernández, S.; Catalán-García, M.; Morén, C.; García-Otero, L.; López, M.; Guitart-Mampel, M.; Milisenda, J.; Coll, O.; Cardellach, F.; Gratacós, E.; et al. Placental Mitochondrial Toxicity, Oxidative Stress, Apoptosis, and Adverse Perinatal Outcomes in HIV Pregnancies Under Antiretroviral Treatment Containing Zidovudine. J. Acquir. Immune Defic. Syndr. 2017, 75, e113–e119. [Google Scholar] [CrossRef] [PubMed]
- Snijdewind, I.J.M.; Smit, C.; Godfried, M.H.; Bakker, R.; Nellen, J.F.J.B.; Jaddoe, V.W.V.; van Leeuwen, E.; Reiss, P.; Steegers, E.A.P.; van der Ende, M.E. Preconception Use of cART by HIV-Positive Pregnant Women Increases the Risk of Infants Being Born Small for Gestational Age. PLoS ONE 2018, 13, e0191389. [Google Scholar] [CrossRef] [PubMed]
- Favarato, G.; Townsend, C.L.; Bailey, H.; Peters, H.; Tookey, P.A.; Taylor, G.P.; Thorne, C. Protease Inhibitors and Preterm Delivery: Another Piece in the Puzzle. AIDS 2018, 32, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.M.; Brummel, S.S.; Britto, P.; Pilotto, J.H.; Masheto, G.; Aurpibul, L.; Joao, E.; Purswani, M.U.; Buschur, S.; Pierre, M.F.; et al. Adverse Pregnancy Outcomes Among Women Who Conceive on Antiretroviral Therapy. Clin. Infect. Dis. 2019, 68, 273–279. [Google Scholar] [CrossRef]
- Fowler, M.G.; Qin, M.; Fiscus, S.A.; Currier, J.S.; Flynn, P.M.; Chipato, T.; McIntyre, J.; Gnanashanmugam, D.; Siberry, G.K.; Coletti, A.S.; et al. Benefits and Risks of Antiretroviral Therapy for Perinatal HIV Prevention. N. Engl. J. Med. 2016, 375, 1726–1737. [Google Scholar] [CrossRef]
- Mesfin, Y.M.; Kibret, K.T.; Taye, A. Is Protease Inhibitors Based Antiretroviral Therapy during Pregnancy Associated with an Increased Risk of Preterm Birth? Systematic Review and a Meta-Analysis. Reprod. Health 2016, 13, 30. [Google Scholar] [CrossRef]
- Hemelaar, J.; Stanczuk, G.; Kirtley, S.; Mahler, A.; Tiwana, R.; Peebles, D.; Brocklehurst, P.; Townsend, C.; Gray, G.; Zash, R.; et al. Association of Nucleoside Reverse Transcriptase Inhibitors with Adverse Perinatal Outcomes in Pregnant Women Living with HIV: Systematic Review and Meta-Analysis. Clin. Microbiol. Infect. 2025, 31, 958–970. [Google Scholar] [CrossRef]
- Senise, J.F.; Castelo, A.; Martínez, M. Current Treatment Strategies, Complications and Considerations for the Use of HIV Antiretroviral Therapy during Pregnancy. AIDS Rev. 2011, 13, 198–213. [Google Scholar]
- Kourtis, A.P.; Wiener, J.; Kayira, D.; Chasela, C.; Ellington, S.R.; Hyde, L.; Hosseinipour, M.; van der Horst, C.; Jamieson, D.J. Health Outcomes of HIV-Exposed Uninfected African Infants. AIDS 2013, 27, 749–759. [Google Scholar] [CrossRef]
- Newell, M.-L.; Bunders, M.J. Safety of Antiretroviral Drugs in Pregnancy and Breastfeeding for Mother and Child. Curr. Opin. HIV AIDS 2013, 8, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.L.; Hazra, R.; Van Dyke, R.B.; Yildirim, C.; Crain, M.J.; Seage, G.R.; Civitello, L.; Ellis, A.; Butler, L.; Rich, K. Antiretroviral Exposure During Pregnancy and Adverse Outcomes in HIV-Exposed Uninfected Infants and Children Using a Trigger-Based Design: The SMARTT Study. AIDS 2016, 30, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.; Humphrey, J.H.; Ntozini, R.; Prendergast, A.J. HIV-Exposed Uninfected Infants in Zimbabwe: Insights into Health Outcomes in the Pre-Antiretroviral Therapy Era. Front. Immunol. 2016, 7, 190. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.; Leong, T.; Avery, A.; Castillo-Duran, M.; Bonilla, H.; Lebrecht, D.; Walker, U.; Storer, N.; Labbato, D.; Khaitan, A.; et al. Effects of in Utero Antiretroviral Exposure on Mitochondrial DNA Levels, Mitochondrial Function and Oxidative Stress. HIV Med. 2012, 13, 98–106. [Google Scholar] [CrossRef]
- McComsey, G.A.; Kang, M.; Ross, A.C.; Lebrecht, D.; Livingston, E.; Melvin, A.; Hitti, J.; Cohn, S.E.; Walker, U.A. Increased mtDNA Levels Without Change in Mitochondrial Enzymes in Peripheral Blood Mononuclear Cells of Infants Born to HIV-Infected Mothers on Antiretroviral Therapy. HIV Clin. Trials 2008, 9, 126–136. [Google Scholar] [CrossRef]
- Young, M.J. Off-Target Effects of Drugs That Disrupt Human Mitochondrial DNA Maintenance. Front. Mol. Biosci. 2017, 4, 74. [Google Scholar] [CrossRef]
- Eckard, A.R.; Kirk, S.E.; Hagood, N.L. Contemporary Issues in Pregnancy (and Offspring) in the Current HIV Era. Curr. HIV/AIDS Rep. 2019, 16, 492–500. [Google Scholar] [CrossRef]
- Smith, R.L.; Tan, J.M.E.; Jonker, M.J.; Jongejan, A.; Buissink, T.; Veldhuijzen, S.; van Kampen, A.H.C.; Brul, S.; van der Spek, H. Beyond the Polymerase-γ Theory: Production of ROS as a Mode of NRTI-Induced Mitochondrial Toxicity. PLoS ONE 2017, 12, e0187424. [Google Scholar] [CrossRef]
- Rodriguez, N.R.; Fortune, T.; Hegde, E.; Weinstein, M.P.; Keane, A.M.; Mangold, J.F.; Swartz, T.H. Oxidative Phosphorylation in HIV-1 Infection: Impacts on Cellular Metabolism and Immune Function. Front. Immunol. 2024, 15, 1360342. [Google Scholar] [CrossRef]
- Barret, B.; Tardieu, M.; Rustin, P.; Lacroix, C.; Chabrol, B.; Desguerre, I.; Dollfus, C.; Mayaux, M.-J.; Blanche, S.; French Perinatal Cohort Study Group. Persistent Mitochondrial Dysfunction in HIV-1-Exposed but Uninfected Infants: Clinical Screening in a Large Prospective Cohort. AIDS 2003, 17, 1769–1785. [Google Scholar] [CrossRef]
- Zha, B.S.; Wan, X.; Zhang, X.; Zha, W.; Zhou, J.; Wabitsch, M.; Wang, G.; Lyall, V.; Hylemon, P.B.; Zhou, H. HIV Protease Inhibitors Disrupt Lipid Metabolism by Activating Endoplasmic Reticulum Stress and Inhibiting Autophagy Activity in Adipocytes. PLoS ONE 2013, 8, e59514. [Google Scholar] [CrossRef]
- Timmons, T.; Shen, C.; Aldrovandi, G.; Rollie, A.; Gupta, S.K.; Stein, J.H.; Dubé, M.P. Microbial Translocation and Metabolic and Body Composition Measures in Treated and Untreated HIV Infection. AIDS Res. Hum. Retroviruses 2014, 30, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Chu, Y.; Wang, Y. HIV Protease Inhibitors: A Review of Molecular Selectivity and Toxicity. HIV AIDS 2015, 7, 95–104. [Google Scholar] [CrossRef]
- Schoeman, J.C.; Moutloatse, G.P.; Harms, A.C.; Vreeken, R.J.; Scherpbier, H.J.; Van Leeuwen, L.; Kuijpers, T.W.; Reinecke, C.J.; Berger, R.; Hankemeier, T.; et al. Fetal Metabolic Stress Disrupts Immune Homeostasis and Induces Proinflammatory Responses in Human Immunodeficiency Virus Type 1– and Combination Antiretroviral Therapy–Exposed Infants. J. Infect. Dis. 2017, 216, 436–446. [Google Scholar] [CrossRef] [PubMed]
- West, A.P.; Khoury-Hanold, W.; Staron, M.; Tal, M.C.; Pineda, C.M.; Lang, S.M.; Bestwick, M.; Duguay, B.A.; Raimundo, N.; MacDuff, D.A.; et al. Mitochondrial DNA Stress Primes the Antiviral Innate Immune Response. Nature 2015, 520, 553–557. [Google Scholar] [CrossRef]
- Hernàndez, S.; Morén, C.; López, M.; Coll, O.; Cardellach, F.; Gratacós, E.; Miró, Ò.; Garrabou, G. Perinatal Outcomes, Mitochondrial Toxicity and Apoptosis in HIV-Treated Pregnant Women and in-Utero-Exposed Newborn. AIDS 2012, 26, 419. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Endoplasmic Reticulum Stress and the Inflammatory Basis of Metabolic Disease. Cell 2010, 140, 900–917. [Google Scholar] [CrossRef]
- Harijith, A.; Ebenezer, D.L.; Natarajan, V. Reactive Oxygen Species at the Crossroads of Inflammasome and Inflammation. Front. Physiol. 2014, 5, 352. [Google Scholar] [CrossRef]
- Hadden, D.R.; McLaughlin, C. Normal and Abnormal Maternal Metabolism during Pregnancy. Semin. Fetal Neonatal Med. 2009, 14, 66–71. [Google Scholar] [CrossRef]
- White, M.R.; Yates, D.T. Dousing the Flame: Reviewing the Mechanisms of Inflammatory Programming during Stress-Induced Intrauterine Growth Restriction and the Potential for ω-3 Polyunsaturated Fatty Acid Intervention. Front. Physiol. 2023, 14, 1250134. [Google Scholar] [CrossRef]
- Raghupathy, R.; Al-Azemi, M.; Azizieh, F. Intrauterine Growth Restriction: Cytokine Profiles of Trophoblast Antigen-Stimulated Maternal Lymphocytes. Clin. Dev. Immunol. 2012, 2012, 734865. [Google Scholar] [CrossRef]
- Kirmse, B.; Hobbs, C.V.; Peter, I.; Laplante, B.; Caggana, M.; Kloke, K.; Raymond, K.; Summar, M.; Borkowsky, W. Abnormal Newborn Screens and Acylcarnitines in HIV-Exposed and ARV-Exposed Infants. Pediatr. Infect. Dis. J. 2013, 32, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Morén, C.; Noguera-Julián, A.; Garrabou, G.; Rovira, N.; Catalán, M.; Bañó, M.; Guitart-Mampel, M.; Tobías, E.; Hernández, S.; Cardellach, F.; et al. Mitochondrial Disturbances in HIV Pregnancies. AIDS 2015, 29, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Meliț, L.E.; Mărginean, C.O.; Mărginean, C.D.; Săsăran, M.O. The Peculiar Trialogue between Pediatric Obesity, Systemic Inflammatory Status, and Immunity. Biology 2021, 10, 512. [Google Scholar] [CrossRef]
- Mărginean, C.O.; Meliţ, L.E.; Ghiga, D.V.; Mărginean, M.O. Early Inflammatory Status Related to Pediatric Obesity. Front. Pediatr. 2019, 7, 241. [Google Scholar] [CrossRef]
- Baroncelli, S.; Galluzzo, C.M.; Orlando, S.; Mphwere, R.; Kavalo, T.; Luhanga, R.; Amici, R.; Floridia, M.; Andreotti, M.; Ciccacci, F.; et al. Immunoglobulin G Passive Transfer from Mothers to Infants: Total IgG, IgG Subclasses and Specific Antipneumococcal IgG in 6-Week Malawian Infants Exposed or Unexposed to HIV. BMC Infect. Dis. 2022, 22, 342. [Google Scholar] [CrossRef]
- Abu-Raya, B.; Smolen, K.K.; Willems, F.; Kollmann, T.R.; Marchant, A. Transfer of Maternal Antimicrobial Immunity to HIV-Exposed Uninfected Newborns. Front. Immunol. 2016, 7, 338. [Google Scholar] [CrossRef]
- Martinez, D.R.; Fong, Y.; Li, S.H.; Yang, F.; Jennewein, M.F.; Weiner, J.A.; Harrell, E.A.; Mangold, J.F.; Goswami, R.; Seage, G.R.; et al. Fc Characteristics Mediate Selective Placental Transfer of IgG in HIV-Infected Women. Cell 2019, 178, 190–201.e11. [Google Scholar] [CrossRef]
- Eke, A.C.; Olagunju, A.; Best, B.M.; Mirochnick, M.; Momper, J.D.; Abrams, E.; Penazzato, M.; Cressey, T.R.; Colbers, A. Innovative Approaches for Pharmacology Studies in Pregnant and Lactating Women: A Viewpoint and Lessons from HIV. Clin. Pharmacokinet. 2020, 59, 1185–1194. [Google Scholar] [CrossRef]
- Cerveny, L.; Murthi, P.; Staud, F. HIV in Pregnancy: Mother-to-Child Transmission, Pharmacotherapy, and Toxicity. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166206. [Google Scholar] [CrossRef] [PubMed]
- Mouafo, L.C.M.; Dambaya, B.; Ngoufack, N.N.; Nkenfou, C.N. Host Molecular Factors and Viral Genotypes in the Mother-to-Child HIV-1 Transmission in Sub-Saharan Africa. J. Public. Health Afr. 2017, 8, 594. [Google Scholar] [CrossRef] [PubMed]
- Marinda, E.T.; Moulton, L.H.; Humphrey, J.H.; Hargrove, J.W.; Ntozini, R.; Mutasa, K.; Levin, J. In Utero and Intra-Partum HIV-1 Transmission and Acute HIV-1 Infection during Pregnancy: Using the BED Capture Enzyme-Immunoassay as a Surrogate Marker for Acute Infection. Int. J. Epidemiol. 2011, 40, 945–954. [Google Scholar] [CrossRef]
- Taha, T.E.; James, M.M.; Hoover, D.R.; Sun, J.; Laeyendecker, O.; Mullis, C.E.; Kumwenda, J.J.; Lingappa, J.R.; Auvert, B.; Morrison, C.S.; et al. Association of Recent HIV Infection and In-Utero HIV-1 Transmission. AIDS 2011, 25, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Minkoff, H. Human Immunodeficiency Virus Infection in Pregnancy. Obstet. Gynecol. 2003, 101, 797–810. [Google Scholar] [CrossRef]
- Al-Husaini, A.M. Role of Placenta in the Vertical Transmission of Human Immunodeficiency Virus. J. Perinatol. 2009, 29, 331–336. [Google Scholar] [CrossRef]
- Vidricaire, G.; Gauthier, S.; Tremblay, M.J. HIV-1 Infection of Trophoblasts Is Independent of Gp120/CD4 Interactions but Relies on Heparan Sulfate Proteoglycans. J. Infect. Dis. 2007, 195, 1461–1471. [Google Scholar] [CrossRef]
- Arias, R.A.; Muñoz, L.D.; Muñoz-Fernández, M.A. Transmission of HIV-1 Infection between Trophoblast Placental Cells and T-Cells Take Place via an LFA-1-Mediated Cell to Cell Contact. Virology 2003, 307, 266–277. [Google Scholar] [CrossRef]
- Vidricaire, G.; Tardif, M.R.; Tremblay, M.J. The Low Viral Production in Trophoblastic Cells Is Due to a High Endocytic Internalization of the Human Immunodeficiency Virus Type 1 and Can Be Overcome by the Pro-Inflammatory Cytokines Tumor Necrosis Factor-Alpha and Interleukin-1. J. Biol. Chem. 2003, 278, 15832–15841. [Google Scholar] [CrossRef]
- Tang, Y.; Woodward, B.O.; Pastor, L.; George, A.M.; Petrechko, O.; Nouvet, F.J.; Haas, D.W.; Jiang, G.; Hildreth, J.E.K. Endogenous Retroviral Envelope Syncytin Induces HIV-1 Spreading and Establishes HIV Reservoirs in Placenta. Cell Rep. 2020, 30, 4528–4539.e4. [Google Scholar] [CrossRef]
- Derrien, M.; Faye, A.; Dolcini, G.; Chaouat, G.; Barré-Sinoussi, F.; Menu, E. Impact of the Placental Cytokine-Chemokine Balance on Regulation of Cell-Cell Contact-Induced Human Immunodeficiency Virus Type 1 Translocation across a Trophoblastic Barrier In Vitro. J. Virol. 2005, 79, 12304–12310. [Google Scholar] [CrossRef]
- Panel on Treatment of HIV During Pregnancy and Prevention of Perinatal Transmission. Recommendations for the Use of Antiretroviral Drugs During Pregnancy and Interventions to Reduce Perinatal HIV Transmission in the United States. In Recommendations for the Use of Antiretroviral Drugs During Pregnancy and Interventions to Reduce Perinatal HIV Transmission in the United States; U.S. Department of Health and Human Services: Washington, DC, USA; Available online: https://clinicalinfo.hiv.gov/en/guidelines/perinatal/recommendations-arv-drugs-pregnancy-what-start-art-never-received (accessed on 12 August 2025).
- Lytvyn, L.; Siemieniuk, R.A.; Dilmitis, S.; Ion, A.; Chang, Y.; Bala, M.M.; Manja, V.; Mirza, R.; Rodriguez-Gutierrez, R.; Mir, H.; et al. Values and Preferences of Women Living with HIV Who Are Pregnant, Postpartum or Considering Pregnancy on Choice of Antiretroviral Therapy during Pregnancy. BMJ Open 2017, 7, e019023. [Google Scholar] [CrossRef]
- Puthanakit, T.; Thepnarong, N.; Chaithongwongwatthana, S.; Anugulruengkitt, S.; Anunsittichai, O.; Theerawit, T.; Ubolyam, S.; Pancharoen, C.; Phanuphak, P. Intensification of Antiretroviral Treatment with Raltegravir for Pregnant Women Living with HIV at High Risk of Vertical Transmission. J. Virus Erad. 2018, 4, 61–65. [Google Scholar] [CrossRef]
- O’Kelly, B.; Murtagh, R.; Lambert, J.S. Therapeutic Drug Monitoring of HIV Antiretroviral Drugs in Pregnancy: A Narrative Review. Ther. Drug Monit. 2020, 42, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Shamsuddin, H.; Raudenbush, C.L.; Sciba, B.L.; Zhou, Y.-P.; Mast, T.C.; Greaves, W.L.; Hanna, G.J.; Leong, R.; Straus, W. Evaluation of Neural Tube Defects (NTDs) After Exposure to Raltegravir During Pregnancy. J. Acquir. Immune Defic. Syndr. 2019, 81, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Jeantils, V.; Messaouden, H.; Carbillon, L.; Hosp, J.V.; France, B. Pregnancy and a Regimen Containing Raltegravir: A Pilot Study on the Materno Foetal Safety. In Proceedings of the 53rd Intersciecne Conference on Antimicrobial Agents and Chemotherapy (ICAAC) 2013, Denver, CO, USA, 10–13 September 2013; Available online: https://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=cc7c2e8e-d70c-4d53-b850-d62af2c1fde6&cKey=340e21de-ed1e-41e9-8808-3eb3fc60fa2f&mKey=%7b7DD36E88-52C3-4FF1-A5DF-1D00766558B8%7d (accessed on 23 September 2024).
- Watts, D.H.; Stek, A.; Best, B.M.; Wang, J.; Capparelli, E.V.; Cressey, T.R.; Aweeka, F.; Lizak, P.; Kreitchmann, R.; Burchett, S.K.; et al. Raltegravir Pharmacokinetics during Pregnancy. J. Acquir. Immune Defic. Syndr. 2014, 67, 375–381. [Google Scholar] [CrossRef]
- Wohl, D.A.; Dumond, J.B.; Blevins, S.; Pittard, D.; Ragan, D.; Wang, R.; Massengale, K.; Walsh, K.; Floris-Moore, M.; Eron, J.J.; et al. Raltegravir Pharmacokinetics in Treatment-Naive Patients Is Not Influenced by Race: Results from the Raltegravir Early Therapy in African-Americans Living with HIV (REAL) Study. Antimicrob. Agents Chemother. 2013, 57, 784–788. [Google Scholar] [CrossRef]
- Blonk, M.I.; Colbers, A.P.H.; Hidalgo-Tenorio, C.; Kabeya, K.; Weizsäcker, K.; Haberl, A.E.; Moltó, J.; Hawkins, D.A.; van der Ende, M.E.; Gingelmaier, A.; et al. Raltegravir in HIV-1-Infected Pregnant Women: Pharmacokinetics, Safety, and Efficacy. Clin. Infect. Dis. 2015, 61, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, N.; Best, B.M.; Wang, J.; Capparelli, E.V.; Stek, A.; Barr, E.; Buschur, S.L.; Acosta, E.P.; Smith, E.; Chakhtoura, N.; et al. Dolutegravir Pharmacokinetics in Pregnant and Postpartum Women Living with HIV. AIDS 2018, 32, 729–737. [Google Scholar] [CrossRef]
- Bollen, P. A Comparison of the Pharmacokinetics of Dolutegravir in Pregnancy and Postpartum. In Proceedings of the 18th International Workshop on Clinical Pharmacology of Antiviral Therapy, Chicago, IL, USA, 14–16 June 2017; Reported by Jules Levin. Available online: https://www.natap.org/2017/Pharm/Pharm_34.htm (accessed on 5 February 2025).
- Kintu, K.; Malaba, T.R.; Nakibuka, J.; Papamichael, C.; Colbers, A.; Byrne, K.; Seden, K.; Hodel, E.M.; Chen, T.; Twimukye, A.; et al. Dolutegravir versus Efavirenz When Starting HIV Therapy in Late Pregnancy: A Randomised Controlled Trial. Lancet HIV 2020, 7, e332–e339. [Google Scholar] [CrossRef]
- On Potential Safety Issue Affecting Women Living With HIV Using Dolutegravir at the Time of Conception; U. S. Department of State: Washington, DC, USA, 2018.
- Mofenson, L.; Vannappagari, V.; Scheuerle, A.; Baugh, B.; Beckerman, K.; Betman, H.; Chakhtoura, N.; Dominguez, K.; Pikis, A.; Santanello, N.; et al. Periconceptional Antiretroviral Exposure and Central Nervous System (CNS) and Neural Tube Birth Defects—Data from Antiretroviral Pregnancy Registry (APR). In Proceedings of the 10th IAS Conference on HIV Science, Mexico City, MX, USA, 21–24 July 2019. Reviews in Antiviral Therapy & Infectious Diseases 7. [Google Scholar]
- Zash, R.; Holmes, L.; Diseko, M.; Jacobson, D.L.; Brummel, S.; Mayondi, G.; Isaacson, A.; Davey, S.; Mabuta, J.; Mmalane, M.; et al. Neural-Tube Defects and Antiretroviral Treatment Regimens in Botswana. N. Engl. J. Med. 2019, 381, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.F.M.; Kim, A.; Jalil, E.M.; Fernandes Fonseca, F.; Shepherd, B.E.; Veloso, V.G.; Rick, F.; Ribeiro, R.; Pimenta, M.C.; Beber, A.; et al. Dolutegravir and Pregnancy Outcomes in Women on Antiretroviral Therapy in Brazil: A Retrospective National Cohort Study. Lancet HIV 2021, 8, e33–e41. [Google Scholar] [CrossRef]
- Appendix B: Dolutegravir (Tivicay, Tivicay PD)—Safety and Toxicity in Pregnancy|NIH. Available online: https://clinicalinfo.hiv.gov/en/guidelines/perinatal/safety-toxicity-arv-agents-integrase-inhibitors-dolutegravir-tivicay (accessed on 27 May 2025).
- Holt, L.M.; Short, W.R.; Momplaisir, F.; Hyun, E.; McKinney, J.; Lugo Morales, A.; Duque, A.; Druyan, B.; Ndubizu, C.; Duthely, L.; et al. Bictegravir Use During Pregnancy: A Multicenter Retrospective Analysis Evaluating HIV Viral Suppression and Perinatal Outcomes. Clin. Infect. Dis. 2024, 79, 1258–1261. [Google Scholar] [CrossRef]
- Zhang, H.; Hindman, J.T.; Lin, L.; Davis, M.; Shang, J.; Xiao, D.; Avihingsanon, A.; Arora, P.; Palaparthy, R.; Girish, S.; et al. A Study of the Pharmacokinetics, Safety, and Efficacy of Bictegravir/Emtricitabine/Tenofovir Alafenamide in Virologically Suppressed Pregnant Women with HIV. AIDS 2024, 38, F1–F9. [Google Scholar] [CrossRef]
- Olivero, R.; Williams, P.L.; Sawyer, G.; Yee, L.M.; Patel, K.; Hernandez-Diaz, S.; Powis, K.; Paul, M.; Chadwick, E.G.; Pediatric HIV/AIDS Cohort Study and the HOPE study. Birth Outcomes Following Bictegravir Exposure during Pregnancy. AIDS 2025, 39, 381–386. [Google Scholar] [CrossRef]
- Appendix B: Bictegravir—Safety and Toxicity in Pregnancy|NIH. Available online: https://clinicalinfo.hiv.gov/en/guidelines/perinatal/safety-toxicity-arv-agents-integrase-inhibitors-bictegravir (accessed on 27 May 2025).
- Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach. Available online: https://www.who.int/publications/i/item/9789240031593 (accessed on 13 August 2025).
| Drug | Key Benefits | Potential Risks | Summary of Findings |
|---|---|---|---|
| Raltegravir | Rapid viral load suppression; Safe in all trimesters; No fetal malformations reported | High pharmacokinetic variability | Suppresses viral load < 400 copies/mL in 92% of pregnant women |
| Dolutegravir | Stronger resistance barrier; Faster viral suppression; WHO and BHIVA recommended as 1st-line therapy | Early concerns over neural tube defects in the 1st trimester | >90% achieved VL < 50 copies/mL at delivery; newer evidence confirms safety in early pregnancy |
| Bictegravir | High virological efficacy; Good safety profile per emerging data; DHHS recommends as 1st-line therapy | Limited pregnancy-specific data; Not yet widely adopted | Similar suppression and safety to dolutegravir |
| ART Class | Representative Drugs | Adverse Fetal Outcomes | Notes |
|---|---|---|---|
| Nucleoside Reverse Transcriptase Inhibitors (NRTIs) | Zidovudine, Lamivudine, Abacavir, Tenofovir | Mitochondrial dysfunction (Decreases mitochondrial DNA content, increases reactive oxygen species production) Growth restriction Preterm birth Low birth weight | Zidovudine interferes with mitochondrial γ-DNA polymerase, leading to impaired replication and oxidative stress |
| Protease Inhibitors (PIs) | Lopinavir/ritonavir, Atazanavir | Preterm birth Small for gestational age Low birth weight Hypertriglyceridemia | Lopinavir/ritonavir linked to increased prematurity risk, especially when antiretroviral therapy is initiated at conception |
| Integrase Strand Transfer Inhibitors (INSTIs) | Raltegravir, Dolutegravir, Bictegravir | (Early concerns) Neural tube defects with dolutegravir Overall favorable profile High efficacy in viral suppression | Dolutegravir linked to neural tube defects in early reports, but recent data support its safety |
| Guideline | BHIVA (2025) | DHHS (2025) | WHO (2021) |
|---|---|---|---|
| First-line regimens in pregnancy | INSTI: DTG Backbone: TDF + FTC | INSTI: BIC Backbone: TAF + FTC | INSTI: DTG Backbone: TDF + 3TC/FTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fleșeriu, T.; Meliț, L.E.; Mărginean, C.O.; Pop, A.V.; Văsieșiu, A.-M. Maternal HIV Infection and Antiretroviral Therapy in Pregnancy: Implications for Vertical Transmission, Fetal Safety, and Long-Term Infant Outcomes. Pathogens 2025, 14, 818. https://doi.org/10.3390/pathogens14080818
Fleșeriu T, Meliț LE, Mărginean CO, Pop AV, Văsieșiu A-M. Maternal HIV Infection and Antiretroviral Therapy in Pregnancy: Implications for Vertical Transmission, Fetal Safety, and Long-Term Infant Outcomes. Pathogens. 2025; 14(8):818. https://doi.org/10.3390/pathogens14080818
Chicago/Turabian StyleFleșeriu, Tudor, Lorena Elena Meliț, Cristina Oana Mărginean, Adrian Vlad Pop, and Anca-Meda Văsieșiu. 2025. "Maternal HIV Infection and Antiretroviral Therapy in Pregnancy: Implications for Vertical Transmission, Fetal Safety, and Long-Term Infant Outcomes" Pathogens 14, no. 8: 818. https://doi.org/10.3390/pathogens14080818
APA StyleFleșeriu, T., Meliț, L. E., Mărginean, C. O., Pop, A. V., & Văsieșiu, A.-M. (2025). Maternal HIV Infection and Antiretroviral Therapy in Pregnancy: Implications for Vertical Transmission, Fetal Safety, and Long-Term Infant Outcomes. Pathogens, 14(8), 818. https://doi.org/10.3390/pathogens14080818







