Pregnant Women Chronically Infected by Toxoplasma gondii with Depressive Disorder: Differential Modulation of Pro-Inflammatory and Anti-Inflammatory Cytokines
Abstract
1. Introduction
2. Methods and Materials
2.1. Participants
2.2. Study Design
2.3. Assessment of T. gondii Seropositivity
2.4. Cytokine Measurements
2.5. Statistical Analysis
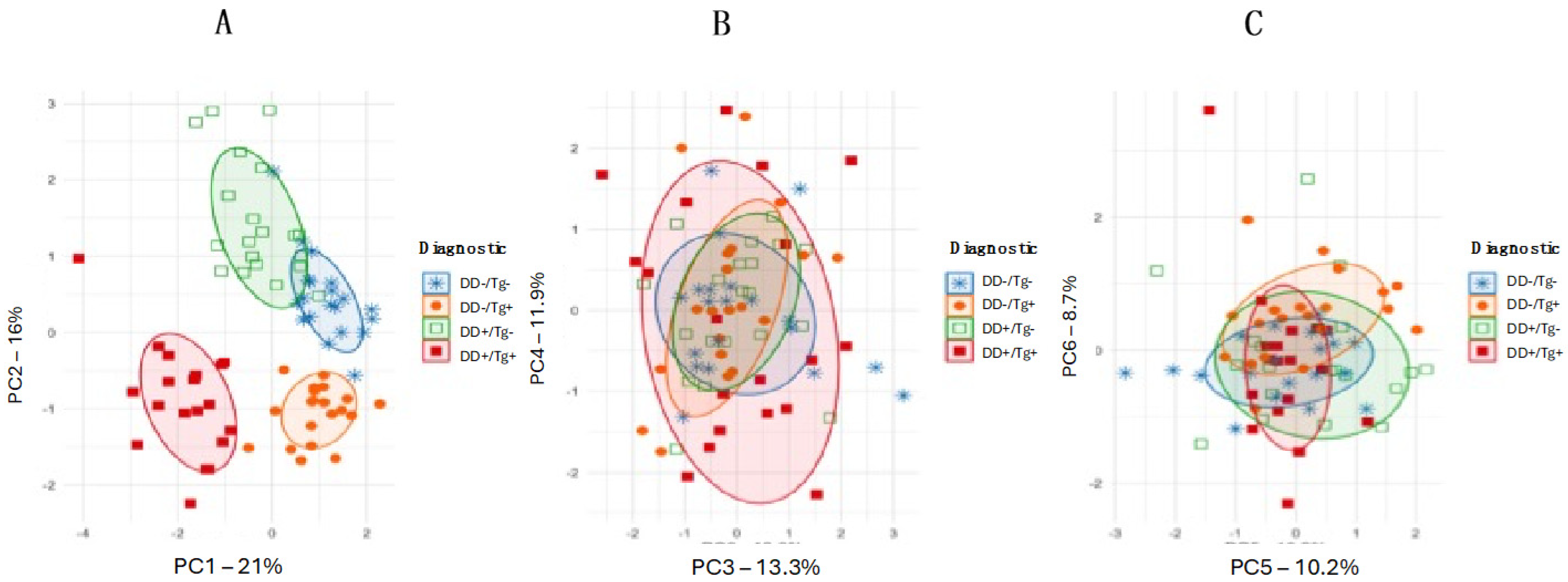
3. Results
3.1. Pregnant Women Present Significant Differences Among EPDS Scores from Experimental Versus Control Groups Without Previous Occurrence of Depression
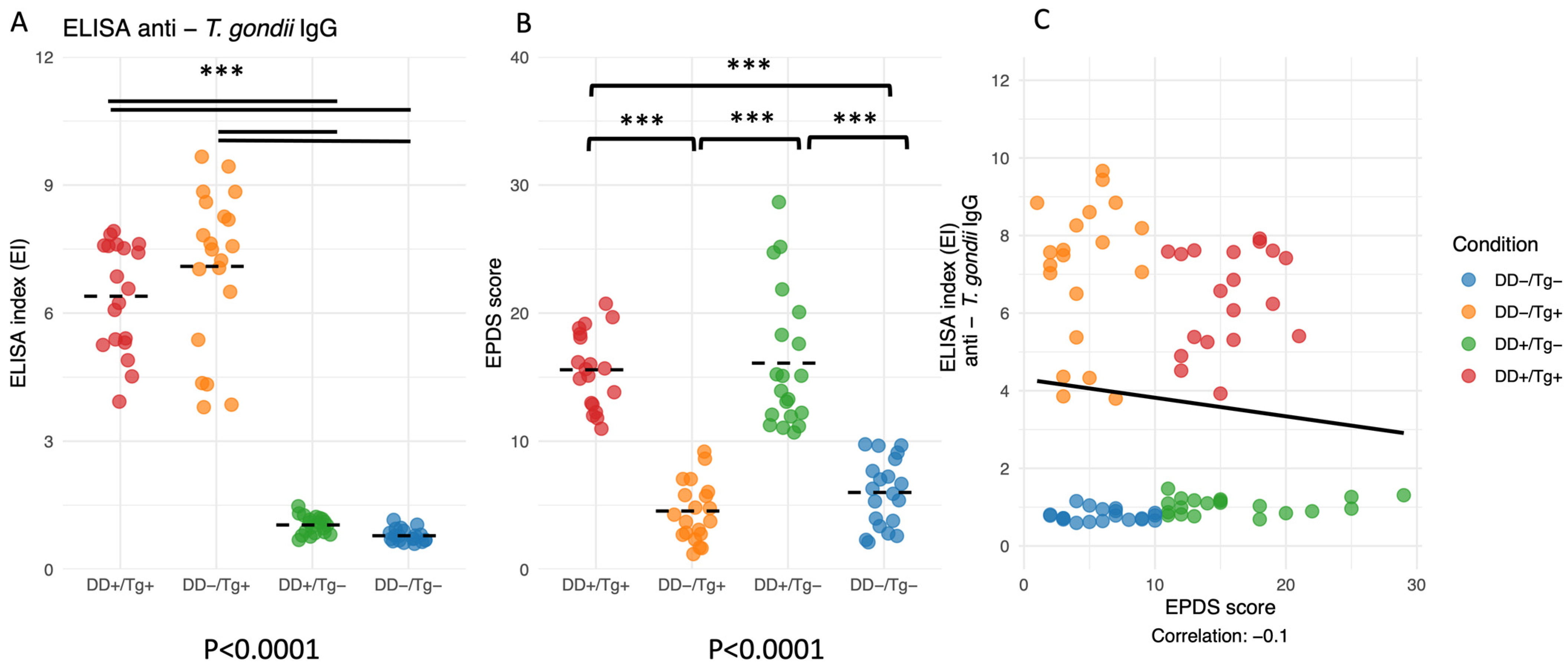
3.2. ELISA Indexes for IgG Antibodies to T. gondii and EPDS Scores Are Validated for Groups I, II, III, and IV of Patients, but No Correlation Was Found Between Both Parameters
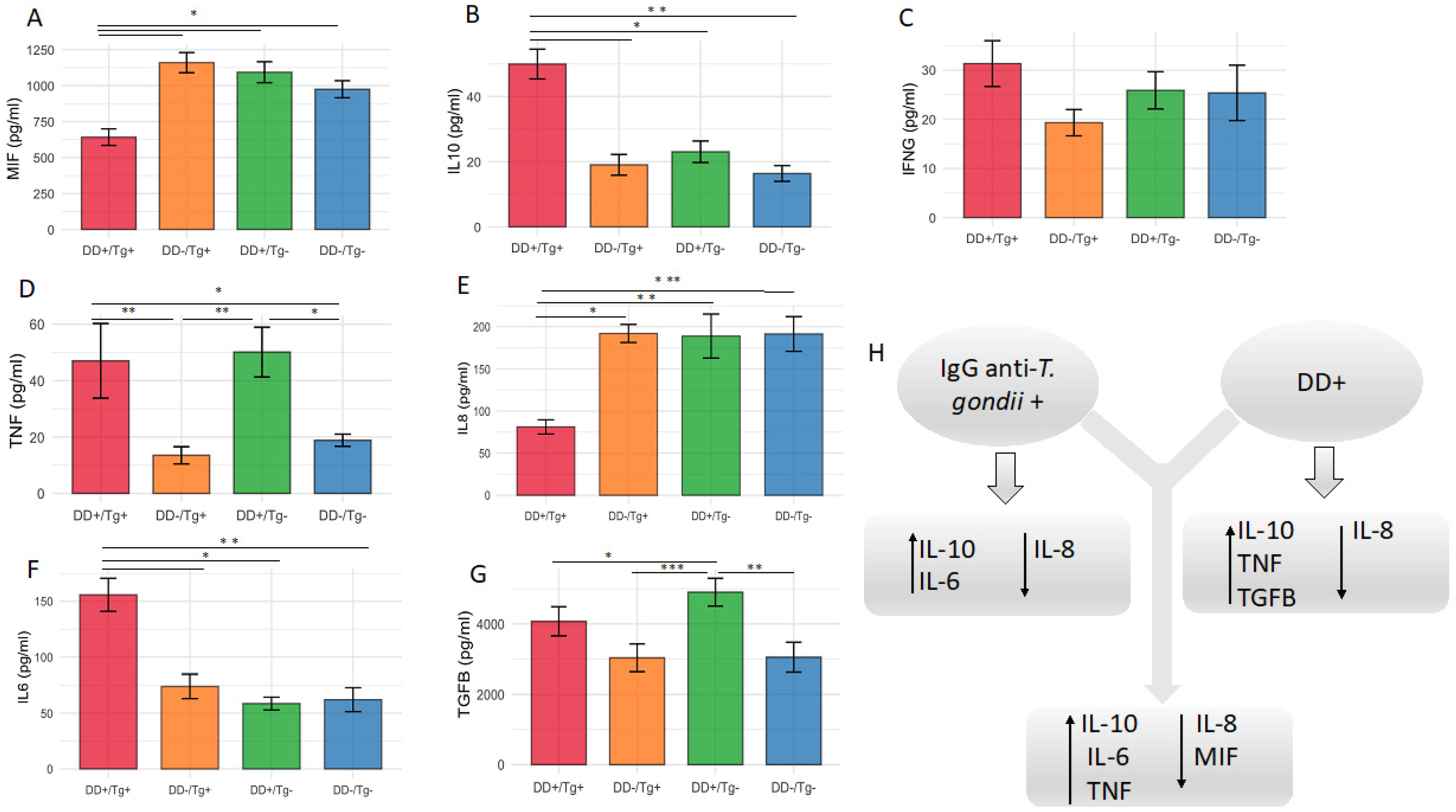
3.3. Higher Serum Levels of IL-10 and IL-6, but Lower Levels of IL-8, Cytokines Are Associated with Patients Chronically Infected by T. gondii
3.4. Higher Serum Levels of IL-10, TNF and TGF-β1, but Lower Levels of IL-8, Cytokines Are Associated with Patients Presenting Depressive Disorder
3.5. The Cytokine Level of MIF Is the Lowest for the Patients Presenting the Association of Infection by T. gondii and Depressive Disorder When Compared with All Other Groups of Patients
4. Discussion
5. Limitation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reddy, M.S. Depression: The disorder and the burden. Indian J. Psychol. Med. 2010, 32, 1–2. [Google Scholar] [CrossRef]
- Becker, M.; Weinberger, T.; Chandy, A.; Schmukler, S. Depression during pregnancy and postpartum. Curr. Psychiatry Rep. 2016, 18, 32. [Google Scholar] [CrossRef] [PubMed]
- Gelaye, B.; Rondon, M.B.; Araya, R.; Williams, M.A. Epidemiology of maternal depression, risk factors, and child outcomes in low-income and middle-income countries. Lancet Psychiatry 2016, 3, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.; De Mello, M.C.; Patel, V.; Rahman, A.; Tran, T.; Holton, S.; Holmes, W. Prevalence and determinants of common perinatal mental disorders in women in low- and lower-middle-income countries: A systematic review. Bull. World Health Organ. 2012, 90, 139G–149G. [Google Scholar] [PubMed]
- Biratu, A.; Haile, D. Prevalence of antenatal depression and associated factors among pregnant women in Addis Ababa, Ethiopia: A cross-sectional study. Reprod. Health 2015, 12, 99. [Google Scholar] [CrossRef]
- Lima, M.D.O.P.; Tsunechiro, M.A.; Bonadio, I.C.; Murata, M. Depressive symptoms in pregnancy and associated factors: Longitudinal study. Acta Paul. Enferm. 2017, 30, 39–46. [Google Scholar] [CrossRef]
- Grote, N.K.; Bridge, J.A.; Gavin, A.R.; Melville, J.L.; Iyengar, S.; Katon, W.J. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch. Gen. Psychiatry 2010, 67, 1012–1024. [Google Scholar] [CrossRef]
- Sutterland, A.L.; Fond, G.; Kuin, A.; Koeter, M.W.J.; Lutter, R.; van Gool, T.; Yolken, R.; Szoke, A.; Leboyer, M.; de Haan, L. Beyond the association. Toxoplasma gondii in schizophrenia, bipolar disorder, and addiction: Systematic review and meta-analysis. Acta Psychiatr. Scand. 2015, 132, 161–179. [Google Scholar]
- Alvarado-Esquivel, C.; Urbina-Álvarez, J.D.; Estrada-Martínez, S.; Torres-Castorena, A.; Molotla-De-León, G.; Liesenfeld, O.; Dubey, J.P. Toxoplasma gondii infection and schizophrenia: A case control study in a low Toxoplasma seroprevalence Mexican population. Parasitol. Int. 2011, 60, 151–155. [Google Scholar]
- Groër, M.W.; Yolken, R.H.; Xiao, J.-C.; Beckstead, J.W.; Fuchs, D.; Mohapatra, S.S.; Seyfang, A.; Postolache, T.T. Prenatal depression and anxiety in Toxoplasma gondii positive women. Am. J. Obstet. Gynecol. 2011, 204, 433.e1–7. [Google Scholar] [CrossRef]
- Alvarado-Esquivel, C.; Sanchez-Anguiano, L.F.; Hernandez-Tinoco, J.; Berumen-Segovia, L.O.; Torres-Prieto, Y.E.; Estrada-Martinez, S.; Perez-Alamos, A.R.; Ortiz-Jurado, M.N.; Molotla-De-Leon, G.; Garcia, I.B.; et al. Toxoplasma gondii infection and mixed anxiety and depressive disorder: A case-control seroprevalence study in Durango, Mexico. J. Clin. Med. Res. 2016, 8, 519–523. [Google Scholar] [CrossRef]
- Kar, N.; Misra, B. Toxoplasma seropositivity and depression: A case report. BMC Psychiatry 2004, 4, 1. [Google Scholar]
- Hsu, P.C.; Groer, M.; Beckie, T. New findings: Depression, suicide, and Toxoplasma gondii infection. J. Am. Assoc. Nurse Pract. 2014, 26, 629–637. [Google Scholar] [CrossRef]
- Gajewski, P.D.; Falkenstein, M.; Hengstler, J.G.; Golka, K. Toxoplasma gondii impairs memory in infected seniors. Brain Behav. Immun. 2014, 36, 193–199. [Google Scholar]
- Alvarado-Esquivel, C.; Liesenfeld, O.; Márquez-Conde, J.A.; Estrada-Martínez, S.; Dubey, J.P. Seroepidemiology of infection with Toxoplasma gondii in workers occupationally exposed to water, sewage, and soil in Durango, Mexico. J. Parasitol. 2010, 96, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Esquivel, C.; Campillo-Ruiz, F.; Liesenfeld, O. Seroepidemiology of infection with Toxoplasma gondii in migrant agricultural workers living in poverty in Durango, Mexico. Parasit. Vectors 2013, 6, 113. [Google Scholar] [CrossRef] [PubMed]
- Duffy, A.R.; Beckie, T.M.; Brenner, L.A.; Beckstead, J.W.; Seyfang, A.; Postolache, T.T.; Groer, M.W. Relationship between Toxoplasma gondii and mood disturbance in women veterans. Mil. Med. 2015, 180, 621–625. [Google Scholar] [CrossRef]
- Zhu, S. Psychosis may be associated with toxoplasmosis. Med. Hypotheses 2009, 73, 799–801. [Google Scholar] [CrossRef]
- Audet, M.C.; McQuaid, R.J.; Merali, Z.; Anisman, H. Cytokine variations and mood disorders: Influence of social stressors and social support. Front. Neurosci. 2014, 8, 416. [Google Scholar] [CrossRef]
- Del Grande, C.; Galli, L.; Schiavi, E.; Dell’Osso, L.; Bruschi, F. Is Toxoplasma gondii a trigger of bipolar disorder? Pathogens 2017, 6, E3. [Google Scholar] [CrossRef]
- Mammari, N.; Halabi, M.A.; Yaacoub, S.; Chlala, H.; Dardé, M.L.; Courtioux, B. Toxoplasma gondii modulates the host cell responses: An overview of apoptosis pathways. Biomed. Res. Int. 2019, 2019, 6152489. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Rosado, J.d.D.; Olguín, J.E.; Juárez-Avelar, I.; Saavedra, R.; Terrazas, L.I.; Robledo-Avila, F.H.; Vazquez-Mendoza, A.; Fernández, J.; Satoskar, A.R.; Partida-Sánchez, S.; et al. MIF Promotes classical activation and conversion of inflammatory Ly6Chigh monocytes into TipDCs during murine toxoplasmosis. Mediat. Inflamm. 2016, 2016, 9101762. [Google Scholar] [CrossRef] [PubMed]
- Park, E.A.; Han, I.H.; Kim, J.H.; Park, S.J.; Ryu, J.S.; Ahn, M.H. Production of inflammatory cytokines and nitric oxide by human mast cells incubated with Toxoplasma gondii lysate. Korean J. Parasitol. 2019, 57, 201–206. [Google Scholar] [CrossRef]
- Davami, M.H.; Baharlou, R.; Vasmehjani, A.A.; Ghanizadeh, A.; Keshtkar, M.; Dezhkam, I.; Atashzar, M.R. Elevated IL-17 and TGF-β serum levels: A positive correlation between T-helper 17 cell-related pro-inflammatory responses with major depressive disorder. Basic Clin. Neurosci. 2016, 7, 137–142. [Google Scholar] [CrossRef]
- Zou, W.; Feng, R.; Yang, Y. Changes in the serum levels of inflammatory cytokines in antidepressant drug-naïve patients with major depression. PLoS ONE 2018, 13, e0197267. [Google Scholar] [CrossRef]
- Conboy, L.; Varea, E.; E Castro, J.; Sakouhi-Ouertatani, H.; Calandra, T.; A Lashuel, H.; Sandi, C. Macrophage migration inhibitory factor is critically involved in basal and fluoxetine-stimulated adult hippocampal cell proliferation and in anxiety, depression, and memory-related behaviors. Mol. Psychiatry 2011, 16, 533–547. [Google Scholar] [PubMed]
- Cox, J.L.; JHolden, M.; Sagovsky, R. Detection of Postnatal Depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry 1987, 150, 782–786. [Google Scholar]
- Santos, I.S.; Matijasevich, A.; Tavares, B.F.; Barros, A.J.D.; Botelho, I.P.; Lapolli, C.; da Silva Magalhães, P.V.; Barbosa, A.P.P.N.; Barros, F.C. Validation of the Edinburgh postnatal depression scale (EPDS) in a sample of mothers from the 2004 Pelotas Birth Cohort Study. Cad. Saude Publica 2007, 23, 2577–2588. [Google Scholar]
- Castro-E-Couto, T.; Brancaglion, M.Y.M.; Cardoso, M.N.; Protzner, A.B.; Garcia, F.D.; Nicolato, R.; Aguiar, R.A.L.P.; Leite, H.V.; Corrêa, H. What is the best tool for screening antenatal depression? J. Affect. Disord. 2015, 178, 12–17. [Google Scholar] [CrossRef]
- Bakker, M.; Veldkamp, C.L.S.; van den Akker, O.R.; van Assen, M.A.L.M.; Crompvoets, E.; Ong, H.H.; Wicherts, J.M. Recommendations in pre-registrations and internal review board proposals promote formal power analyses but do not increase sample size. PLoS ONE 2020, 15, e0236079. [Google Scholar] [CrossRef]
- Silva, D.A.O.; Silva, N.M.; Mineo, T.W.; Pajuaba Neto, A.A.; Ferro, E.A.; Mineo, J.R. Heterologous antibodies to evaluate the kinetics of the humoral immune response in dogs experimentally infected with Toxoplasma gondii RH strain. Vet. Parasitol. 2002, 107, 181–195. [Google Scholar] [CrossRef]
- Parlog, A.; Schluter, D.; Dunay, I.R. Toxoplasma gondii-induced neuronal alterations. Parasite Immunol. 2015, 37, 159–170. [Google Scholar] [CrossRef]
- McConkey, G.A.; Martin, H.L.; Bristow, G.C.; Webster, J.P. Toxoplasma gondii infection and behaviour—Location, location, location? J. Exp. Biol. 2013, 216, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.P.; McConkey, G.A. Toxoplasma gondii-altered host behaviour: Clues as to mechanism of action. Folia Parasitol. 2010, 57, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, E.E.; Nelson, K.; Rose, N.R.; Rhode, C.; Pillion, J.P.; Seaberg, E.; Talor, M.V.; Burek, L.; Eaton, W.; Duggan, A.; et al. Infection and thyroid autoimmunity: A seroepidemiologic study of TPOaAb. Autoimmunity 2009, 42, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.; Hall, R.; Othman, S.; Parkes, A.; Richards, C.; McCulloch, B.; Harris, B. The clinical spectrum of postpartum thyroid disease. QJM 1996, 89, 429–435. [Google Scholar] [CrossRef]
- Gaskell, E.A.; Smith, J.E.; Pinney, J.W.; Westhead, D.R.; McConkey, G.A. A unique dual activity amino acid hydroxylase in Toxoplasma gondii. PLoS ONE 2009, 4, e4801. [Google Scholar] [CrossRef]
- Skallova, A.; Kodym, P.; Frynta, D.; Flegr, J. The role of dopamine in Toxoplasma-induced behavioural alterations in mice: An ethological and ethopharmacological study. Parasitology 2006, 133, 525–535. [Google Scholar] [CrossRef]
- Sugden, K.; Moffitt, T.E.; Pinto, L.; Poulton, R.; Williams, B.S.; Caspi, A. Is Toxoplasma gondii infection related to brain and behavior impairments in humans? Evidence from a population-representative birth cohort. PLoS ONE 2016, 11, e0148435. [Google Scholar] [CrossRef]
- Alvarado-Esquivel, C.; Martínez-Martínez, A.L.; Sánchez-Anguiano, L.F.; Hernández-Tinoco, J.; Castillo-Orona, J.M.; Salas-Martínez, C.; Sifuentes-Álvarez, A.; Sandoval-Carrillo, A.A.; Salas-Pacheco, J.M.; Liesenfeld, O.; et al. Lack of association between Toxoplasma gondii exposure and depression in pregnant women: A case-control study. BMC Infect. Dis. 2017, 17, 190. [Google Scholar]
- Munoz, M.; Liesenfeld, O.; Heimesaat, M.O. Immunology of Toxoplasma gondii. Immunol. Rev. 2011, 240, 269–285. [Google Scholar] [PubMed]
- Leff-Gelman, P.; Mancilla-Herrera, I.; Flores-Ramos, M.; Cruz-Fuentes, C.; Reyes-Grajeda, J.P.; García-Cuétara, M.d.P.; Bugnot-Pérez, M.D.; Pulido-Ascencio, D.E. The immune system and the role of inflammation in perinatal depression. Neurosci. Bull. 2016, 32, 398–420. [Google Scholar] [CrossRef] [PubMed]
- Leff Gelman, P.; Flores-Ramos, M.; López-Martínez, M.; Fuentes, C.C.; Grajeda, J.P.R. Hypothalamic-pituitary-adrenal axis function during perinatal depression. Neurosci. Bull. 2015, 31, 338–350. [Google Scholar]
- Robinson, C.M.; Hale, P.T.; Carlin, J.M. The role of IFN-g and TNF-α-responsive regulatory elements in the synergistic induction of indoleamine dioxygenase. J. Interferon Cytokine Res. 2005, 25, 20–30. [Google Scholar]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar]
- Miranda, H.C.; Nunes, S.O.V.; Reiche, E.M.V.; Oda, J.M.M.; Watanabe, M.A.E. Higher than Normal Plasma Iinterleukin-6 Concentrations in Brazilian Patients with Mood Disorders. Braz. Arch. Biol. Technol. 2011, 54, 717–722. [Google Scholar]
- Christian, L.M.; Porter, K. Longitudinal Changes in Serum Proinflammatory Markers across Pregnancy and Postpartum: Effects of Maternal Body Mass Index. Cytokine 2014, 70, 134–140. [Google Scholar]
- Christian, L.M.; Franco, A.; Glaser, R.; Iams, J. Depressive symptoms are associated with elevated serum proinflammatory cytokines among pregnant women. Brain Behav. Immun. 2009, 23, 750–754. [Google Scholar] [CrossRef]
- Winkler, M. Role of cytokines and other inflammatory mediators. BJOG 2003, 110, 118–123. [Google Scholar]
- Bloom, J.; Al-Abed, Y. MIF: Mood Improving/Inhibiting Factor? J. Neuroinflamm. 2014, 11, 11. [Google Scholar] [CrossRef]
- Musil, R.; Schwarz, M.J.; Riedel, M.; Dehning, S.; Cerovecki, A.; Spellmann, I.; Arolt, V.; Müller, N. Elevated macrophage migration inhibitory factor and decreased transforming growth factor-beta levels in major depression—No influence of celecoxib treatment. J. Affect. Disord. 2011, 134, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Christian, L.; Franco, A.; Iams, J.; Sheridam, J.; Glaser, R. Depressive symptoms predict exaggerated inflammatory responses to an in vivo immune challenge among pregnant Women. Brain Behav. Immun. 2010, 24, 49–53. [Google Scholar] [PubMed]
- Fallahi, S.; Rostami, A.; Shiadeh, M.N.; Behniafar, H.; Paktinat, S. An updated literature review on maternal-fetal and reproductive disorders of Toxoplasma gondii infection. J. Gynecol. Obstet. Hum. Reprod. 2018, 47, 133–140. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Group I DD+Tg+ (n = 19) | Group II DD−Tg+ (n = 20) | Group III DD+Tg− (n = 20) | Group IV DD−Tg− (n = 20) | p-Value |
|---|---|---|---|---|---|
| Maternal age (year) ± SD * | 31 ± 6.63 a | 30 ± 5.88 ab | 27 ± 5.90 ab | 26 ± 5.31 b | 0.0174 |
| Gestational age (weeks) ± SD | 29 ± 8.10 | 31 ± 8.50 | 32 ± 7.15 | 32 ± 7.75 | 0.1571 |
| EPDS score (score mean) ± SD * | 15.6 ± 2.9 a | 4.5 ± 2.3 b | 16.2 ± 5.5 a | 5.8 ± 2.8 b | 0.0001 |
| Marital status | |||||
| Married/living with a partner | 14 (74%) | 18 (90%) | 10 (50%) | 12 (60%) | 0.0359 |
| Single/separated/divorced/ living without a partner | 5 (26%) | 2 (10%) | 10 (50%) | 8 (40%) | |
| Degree of education | |||||
| Elementary/High school | 9 (47%) | 14 (70%) | 15 (75%) | 19 (95%) | 0.0099 |
| Undergraduate | 10 (53%) | 6 (30%) | 5 (25%) | 1 (5%) | |
| Occupancy | |||||
| Housewife | 9 (47%) | 7 (35%) | 10 (50%) | 6 (30%) | 0.5387 |
| Daily laborer/employed | 10 (53%) | 13 (65%) | 10 (50%) | 14 (70%) | |
| Average household income | |||||
| <minimum wage | 7 (37%) | 9 (45%) | 10 (50%) | 11 (55%) | 0.7326 |
| >minimum wage | 12 (63%) | 11 (55%) | 10 (50%) | 9 (45%) | |
| Parity | |||||
| Primiparous | 7 (37%) | 7 (35%) | 3 (15%) | 15 (75%) | 0.0009 |
| Multiparous | 12 (63%) | 13 (65%) | 17 (85%) | 5 (25%) | |
| Type of previous delivery | |||||
| Cesarean section | 5 (42%) | 5 (39%) | 6 (35%) | 3 (60%) | 0.8246 |
| Spontaneous labor | 7 (58%) | 8 (61%) | 11 (65%) | 2 (40%) | |
| Planed Pregnancy | |||||
| Yes | 5 (26%) | 9 (45%) | 5 (25%) | 10 (50%) | 0.2659 |
| No | 14 (74%) | 11 (55%) | 15 (75%) | 10 (50%) | |
| Chronic disease | |||||
| Yes | 3 (16%) | 4 (20%) | 5 (25%) | 6 (30%) | 0.8036 |
| No | 16 (84%) | 16 (80%) | 15 (75%) | 14 (70%) | |
| Previous depression | |||||
| Yes | 2 (10%) | 1 (5%) | 5 (25%) | 0 | 0.0419 |
| No | 17 (90%) | 19 (95%) | 15 (75%) | 20 (100%) | |
| Alcohol consumption | |||||
| Yes | 1 (5%) | 2 (10%) | 2 (10%) | 2 (10%) | 0.9999 |
| No | 18 (95%) | 18 (90%) | 18 (90%) | 18 (90%) | |
| Smoking | |||||
| Yes | 0 | 2 (10%) | 3 (15%) | 0 | 0.1514 |
| No | 19 (100%) | 18 (90%) | 17 (85%) | 20 (100%) | |
| Negative life events | |||||
| Yes | 6 (31%) | 3 (15%) | 10 (50%) | 5 (25%) | 0.1094 |
| No | 13 (69%) | 17 (85%) | 10 (50%) | 15 (75%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salomão Lopes, C.; Carvalho, R.J.V.; da Silva, T.L.; Barros, H.L.S.; Costa, L.V.S.; Mota, D.C.A.M.; Barbosa, B.F.; Vieira, L.S.; de Araújo, T.M.; Costa, A.R.; et al. Pregnant Women Chronically Infected by Toxoplasma gondii with Depressive Disorder: Differential Modulation of Pro-Inflammatory and Anti-Inflammatory Cytokines. Pathogens 2025, 14, 330. https://doi.org/10.3390/pathogens14040330
Salomão Lopes C, Carvalho RJV, da Silva TL, Barros HLS, Costa LVS, Mota DCAM, Barbosa BF, Vieira LS, de Araújo TM, Costa AR, et al. Pregnant Women Chronically Infected by Toxoplasma gondii with Depressive Disorder: Differential Modulation of Pro-Inflammatory and Anti-Inflammatory Cytokines. Pathogens. 2025; 14(4):330. https://doi.org/10.3390/pathogens14040330
Chicago/Turabian StyleSalomão Lopes, Carolina, Ricardo José Victal Carvalho, Tamires Lopes da Silva, Heber Leão Silva Barros, Lucas Vasconcelos Soares Costa, Danielly Christine Adriani Maia Mota, Bellisa Freitas Barbosa, Luan Souza Vieira, Talyene Marques de Araújo, Alírio Resende Costa, and et al. 2025. "Pregnant Women Chronically Infected by Toxoplasma gondii with Depressive Disorder: Differential Modulation of Pro-Inflammatory and Anti-Inflammatory Cytokines" Pathogens 14, no. 4: 330. https://doi.org/10.3390/pathogens14040330
APA StyleSalomão Lopes, C., Carvalho, R. J. V., da Silva, T. L., Barros, H. L. S., Costa, L. V. S., Mota, D. C. A. M., Barbosa, B. F., Vieira, L. S., de Araújo, T. M., Costa, A. R., Awoyinka, R. O., Mineo, T. W. P., Diniz, A. L. D., & Mineo, J. R. (2025). Pregnant Women Chronically Infected by Toxoplasma gondii with Depressive Disorder: Differential Modulation of Pro-Inflammatory and Anti-Inflammatory Cytokines. Pathogens, 14(4), 330. https://doi.org/10.3390/pathogens14040330






