Prevalence and Molecular Characterization of Cryptosporidium Species in Diarrheic Children in Cameroon
Abstract
1. Introduction
2. Materials and Methods
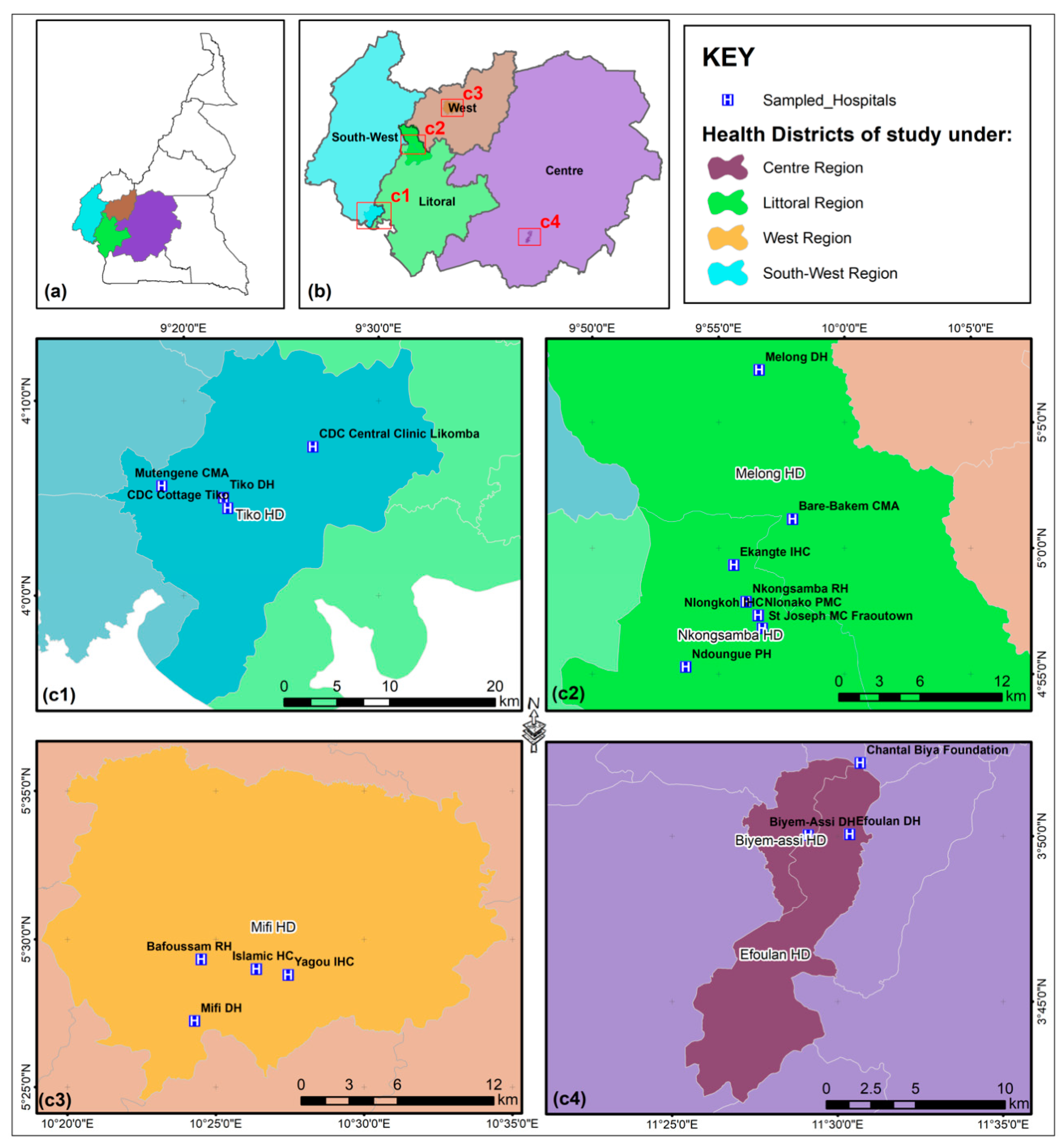
2.1. Sample Collection
2.2. Modified Ziehl Neelsen (MZN) Staining
2.3. DNA Extraction
2.4. SSU-rRNA Nested PCR
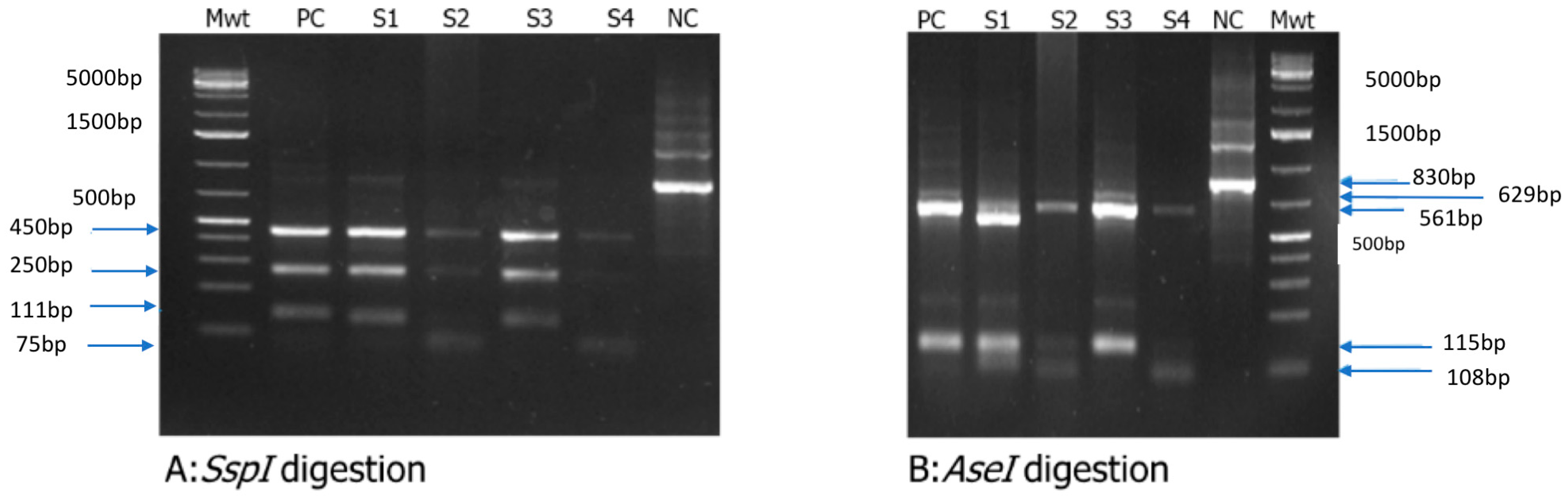
2.5. Restriction Fragment Length Polymorphism (RFLP) Analysis
2.6. Cryptosporidium GP60 Gene Amplification
2.7. DNA Sequencing and Phylogenetic Analysis
2.8. Statistical Analysis
3. Results
3.1. Demographic Data
3.2. Results of Microscopic Analysis
3.3. Results of PCR Analysis
3.4. Cryptosporidium spp. Genotyping
3.5. Cryptosporidium spp. Sub-Genotyping and Evolutionary Relationships of Taxa
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dillingham, R.A.; Lima, A.A.; Guerrant, R.L. Cryptosporidiosis: Epidemiology and impact. Microbes Infect. 2002, 4, 1059–1066. [Google Scholar] [CrossRef]
- Olson, M.E.; Handley, R.M.O.; Ralston, B.J.; Mcallister, T.A.; Thompson, R.C.A. Update on Cryptosporidium and Giardia infections in cattle. Trends Parasitol. 2004, 20, 185–191. [Google Scholar] [CrossRef]
- Ryan, U.; Zahedi, A.; Paparini, A. Cryptosporidium in humans and animals-a one health approach to prophylaxis. Parasite Immunol. 2016, 38, 535–547. [Google Scholar] [CrossRef]
- Sterling, C.R.; Arrowood, M.J. Cryptosporidia; Elsevier: Amsterdam, The Netherlands, 2020; Volume 14, p. 293. [Google Scholar]
- Current, W.L.; Garcia, L.S. Cryptosporidiosis. Clin. Lab. Med. 1991, 11, 873–895. [Google Scholar] [CrossRef]
- Garcia, L.S.; Lilly, E. Cryptosporidiosis: Clinical features and diagnosis. Crit. Rev. Clin. Lab. Sci. II 1989, 27, 439–460. [Google Scholar] [CrossRef]
- Feltus, D.C.; Giddings, C.W.; Schneck, B.L.; Monson, T.; Warshauer, D.; McEvoy, J.M. Evidence Supporting Zoonotic Transmission of Cryptosporidium spp. in Wisconsin. J. Clin. Microbiol. 2006, 44, 4303–4308. [Google Scholar] [CrossRef]
- Feng, Y.; Torres, E.; Li, N.; Wang, L.; Bowman, D.; Xiao, L. Population genetic characterisation of dominant Cryptosporidium parvum subtype IIaA15G2R1. Int. J. Parasitol. 2013, 43, 1141–1147. [Google Scholar] [CrossRef]
- Thomson, S.; Hamilton, C.A.; Hope, J.C.; Katzer, F.; Mabbott, N.A.; Morrison, L.J.; Innes, E.A. Bovine cryptosporidiosis: Impact, host-parasite interaction and control strategies. Vet. Res. 2017, 48, 42. [Google Scholar] [CrossRef]
- Amadi, B.; Mwiya, M.; Sianongo, S.; Payne, L.; Watuka, A.; Katubulushi, M.; Kelly, P. High dose prolonged treatment with nitazoxanide is not effective for cryptosporidiosis in HIV positive Zambian children: A randomised controlled trial. BMC Infect. Dis. 2009, 9, 195. [Google Scholar] [CrossRef]
- Kotloff, M.M.L.K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Sow, S.O.; Muhsen, K.; Nasrin, D.; Blackwelder, W.C.; Wu, Y.; Farag, T.H.; Panchalingam, S.; Sur, D.; Zaidi, A.K.M.; Faruque, A.S.G.; et al. The Burden of Cryptosporidium Diarrheal Disease among Children < 24 Months of Age in Moderate/High Mortality Regions of Sub-Saharan Africa and South Asia, Utilizing Data from the Global Enteric Multicenter Study (GEMS). PLoS Negl. Trop. Dis. 2016, 10, e0004729. [Google Scholar] [CrossRef]
- Checkley, W.; White, A.C.; Jaganath, D.; Arrowood, M.J.; Chalmers, R.M.; Chen, X.-M.; Fayer, R.; Griffiths, J.K.; Guerrant, R.L.; Hedstrom, L.; et al. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for Cryptosporidium. Lancet Infect. Dis. 2015, 15, 85–94. [Google Scholar] [CrossRef]
- Xiao, L.; Feng, Y. Zoonotic cryptosporidiosis. FEMS Immunol. Med. Microbiol. 2008, 52, 309–323. [Google Scholar] [CrossRef]
- Cho, M.-H.; Kim, A.-K.; Im, K.-I. Detection of Cryptosporidium oocysts from out-patients of the Severance Hospital Korea. Parasites Hosts Dis. 1993, 31, 193–199. [Google Scholar] [CrossRef]
- Garcia-R, J.C.; Hayman, D.T.S. A review and analysis of cryptosporidiosis outbreaks in New Zealand. Parasitology 2023, 150, 606–611. [Google Scholar] [CrossRef]
- Plutzer, J.; Kelen, K.; Varga, E.; Kucsera, I.; Reusz, G.; Szabó, A.J.; Fehér, Á.; Chalmers, R.M. First Cryptosporidium outbreak in Hungary, linked to a treated recreational water venue in 2015. Epidemiol. Infect. 2019, 147, e56. [Google Scholar] [CrossRef]
- Gharpure, R.; Perez, A.; Miller, A.D.; Wikswo, M.E.; Silver, R.; Hlavsa, M.C. Cryptosporidiosis outbreaks—United States, 2009–2017. Am. J. Transplant. 2019, 19, 2650–2654. [Google Scholar] [CrossRef]
- Mirdha, B.R. Evolving Patterns of Cryptosporidiosis: Issues and Implications in the Context of Public Health in India. Ann. Natl. Acad. Med. Sci. 2021, 57, 81–93. [Google Scholar] [CrossRef]
- O’Leary, J.K.; Sleator, R.D.; Lucey, B. Cryptosporidium spp. diagnosis and research in the 21st century. Food Waterborne Parasitol. 2021, 24, e00131. [Google Scholar] [CrossRef]
- Chalmers, R.M.; Smith, R.P.; Hadfield, S.J.; Elwin, K.; Giles, M. Zoonotic linkage and variation in Cryptosporidium parvum from patients in the United Kingdom. Parasitol. Res. 2011, 108, 1321–1325. [Google Scholar] [CrossRef]
- Vélez, J.; Velasquez, Z.; Silva, L.M.R.; Gärtner, U.; Failing, K.; Daugschies, A.; Mazurek, S.; Hermosilla, C.; Taubert, A. Metabolic signatures of Cryptosporidium parvum-infected hct-8 cells and impact of selected metabolic inhibitors on C. parvum infection under physioxia and hyperoxia. Biology 2021, 10, 60. [Google Scholar] [CrossRef]
- Vélez, J.; Silva, L.M.R.; Kamena, F.; Daugschies, A.; Mazurek, S.; Taubert, A.; Hermosilla, C. The Oesophageal Squamous Cell Carcinoma Cell Line COLO-680N Fails to Support Sustained Cryptosporidium parvum Proliferation. Pathogens 2022, 11, 49. [Google Scholar] [CrossRef]
- Nguyen-Ho-bao, T.; Ambe, L.A.; Berberich, M.; Hermosilla, C.; Taubert, A.; Daugschies, A.; Kamena, F. Octaarginine Improves the Efficacy of Nitazoxanide against Cryptosporidium parvum. Pathogens 2022, 11, 653. [Google Scholar] [CrossRef]
- Anschütz, N.H.; Gerbig, S.; Ghezellou, P.; Silva, L.M.R.; Vélez, J.D.; Hermosilla, C.R.; Taubert, A.; Spengler, B. Mass Spectrometry Imaging of In Vitro Cryptosporidium parvum-Infected Cells and Host Tissue. Biomolecules 2023, 13, 1200. [Google Scholar] [CrossRef]
- Cui, Z.; Song, D.; Qi, M.; Zhang, S.; Wang, R.; Jian, F.; Ning, C.; Zhang, L. Revisiting the infectivity and pathogenicity of Cryptosporidium avium provides new information on parasitic sites within the host. Parasites Vectors 2018, 11, 514. [Google Scholar] [CrossRef]
- Alves, M.; Xiao, L.; Sulaiman, I.; Lal, A.A.; Matos, O.; Antunes, F. Subgenotype analysis of Cryptosporidium isolates from humans, cattle, and zoo ruminants in Portugal. J. Clin. Microbiol. 2003, 41, 2744–2747. [Google Scholar] [CrossRef]
- Šlapeta, J. Cryptosporidiosis and Cryptosporidium species in animals and humans: A thirty colour rainbow? Int. J. Parasitol. 2013, 43, 957–970. [Google Scholar] [CrossRef]
- Sréter, T.; Egyed, Z.; Szell, Z.; Kovacs, G.; Nikolausz, M.; Marialigeti, K.; Varga, I. Morphologic, host specificity, and genetic characterization of a European Cryptosporidium andersoni isolate. J. Parasitol. 2000, 86, 1244–1249. [Google Scholar] [CrossRef]
- Feng, Y.; Ryan, U.M.; Xiao, L. Genetic Diversity and Population Structure of Cryptosporidium. Trends Parasitol. 2018, 34, 997–1011. [Google Scholar] [CrossRef]
- Ryan, U.M.; Feng, Y.; Fayer, R.; Xiao, L. Taxonomy and molecular epidemiology of Cryptosporidium and Giardia—A 50 year perspective (1971–2021). Int. J. Parasitol. 2021, 51, 1099–1119. [Google Scholar] [CrossRef]
- Gatei, W.; Greensill, J.; Ashford, R.W.; Cuevas, L.E.; Parry, C.M.; Cunliffe, N.A.; Beeching, N.J.; Hart, C.A. Molecular analysis of the 18S rRNA gene of Cryptosporidium parasites from patients with or without human immunodeficiency virus infections living in Kenya, Malawi, Brazil, the United Kingdom, and Vietnam. J. Clin. Microbiol. 2003, 41, 1458–1462. [Google Scholar] [CrossRef]
- Peralta, R.H.S.; Velásquez, J.N.; Cunha, F.d.S.; Pantano, M.L.; Sodré, F.C.; da Silva, S.; Astudillo, O.G.; Peralta, J.M.; Carnevale, S. Genetic diversity of Cryptosporidium identified in clinical samples from cities in Brazil and Argentina. Mem. Inst. Oswaldo Cruz 2016, 111, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Takumi, K.; Cacciò, S.M.; Van Der Giessen, J.; Xiao, L.; Sprong, H. Hypothesis: Cryptosporidium genetic diversity mirrors national disease notification rate. Parasit. Vectors 2015, 8, 308. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.M.; Wilson, M.L.; Holland, R.E.; Meshnick, S.R.; Lal, A.A.; Xiao, L. Genetic diversity of Cryptosporidium spp. in cattle in Michigan: Implications for understanding the transmission dynamics. Parasitol. Res. 2003, 90, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Elmahallawy, E.K.; Sadek, H.A.; Aboelsoued, D.; Aloraini, M.A.; Alkhaldi, A.A.M.; Abdel-Rahman, S.M.; Bakir, H.Y.; Arafa, M.I.; Hassan, E.A.; Elbaz, E.; et al. Parasitological, Molecular, and Epidemiological Investigation of Cryptosporidium Infection Among Cattle and Buffalo Calves From Assiut Governorate, Upper Egypt: Current Status and Zoonotic Implications. Front. Vet. Sci. 2022, 9, 899854. [Google Scholar] [CrossRef]
- Bujila, I.; Troell, K.; Ögren, J.; Hansen, A.; Killander, G.; Agudelo, L.; Lebbad, M.; Beser, J. Cryptosporidium species and subtypes identified in human domestic cases through the national microbiological surveillance programme in Sweden from 2018 to 2022. BMC Infect. Dis. 2024, 24, 146. [Google Scholar] [CrossRef]
- Dąbrowska, J.; Sroka, J.; Cencek, T. Investigating Cryptosporidium spp. Using Genomic, Proteomic and Transcriptomic Techniques: Current Progress and Future Directions. Int. J. Mol. Sci. 2023, 24, 12867. [Google Scholar] [CrossRef]
- Berhanu, K.; Ayana, D.; Megersa, B.; Ashenafi, H.; Waktole, H. Cryptosporidium in human-animal-environment interphase at Adama and Asella areas of Oromia regional state, Ethiopia. BMC Vet. Res. 2022, 18, 402. [Google Scholar] [CrossRef]
- King, B.J.; Monis, P.T. Critical processes affecting Cryptosporidium oocyst survival in the environment. Parasitology 2007, 134, 309–323. [Google Scholar] [CrossRef]
- Zahedi, A.; Monis, P.; Aucote, S.; King, B.; Paparini, A.; Jian, F.; Yang, R.; Oskam, C.; Ball, A.; Robertson, I.; et al. Zoonotic Cryptosporidium species in animals inhabiting sydney water catchments. PLoS ONE 2016, 11, e0168169. [Google Scholar] [CrossRef]
- Kleinertz, S.; Hermosilla, C.; Ziltener, A.; Kreicker, S.; Hirzmann, J.; Abdel-Ghaffar, F.; Taubert, A. Gastrointestinal parasites of free-living Indo-Pacific bottlenose dolphins (Tursiops aduncus) in the Northern Red Sea, Egypt. Parasitol. Res. 2014, 113, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Hermosilla, C.; Silva, L.M.R.; Navarro, M.; Taubert, A. Anthropozoonotic Endoparasites in Free-Ranging ‘Urban’ South American Sea Lions (Otaria flavescens). J. Vet. Med. 2016, 2016, 7507145. [Google Scholar] [CrossRef]
- Ebmer, D.; Navarrete, M.J.; Muñoz, P.; Flores, L.M.; Gärtner, U.; Brabec, J.; Poppert, S.; Taubert, A.; Hermosilla, C. Anthropozoonotic Parasites Circulating in Synanthropic and Pacific Colonies of South American Sea Lions (Otaria flavescens): Non-invasive Techniques Data and a Review of the Literature. Front. Mar. Sci. 2020, 7, 543829. [Google Scholar] [CrossRef]
- Xiao, L.; Morgan, U.M.; Limor, J.; Escalante, A.; Arrowood, M.; Shulaw, W.; Thompson, R.C.A.; Fayer, R.; Lal, A.A. Genetic Diversity within Cryptosporidium parvum and Related Cryptosporidium Species. Appl. Environ. Microbiol. 1999, 65, 3386–3391. [Google Scholar] [CrossRef]
- Leav, B.A.; Mackay, M.R.; Anyanwu, A.; Connor, R.M.O.; Cevallos, A.M.; Kindra, G.; Rollins, N.C.; Bennish, M.L.; Nelson, R.G.; Ward, H.D. Analysis of sequence diversity at the highly polymorphic Cpgp40/15 locus among Cryptosporidium isolates from human immunodeficiency virus-infected children in South Africa. Infect. Immun. 2002, 70, 3881–3890. [Google Scholar] [CrossRef]
- Waldron, L.S.; Ferrari, B.C.; Power, M.L. Glycoprotein 60 diversity in C. hominis and C. parvum causing human cryptosporidiosis in NSW, Australia. Exp. Parasitol. 2009, 122, 124–127. [Google Scholar] [CrossRef]
- Nkenfou, C.N.; Nana, C.T.; Payne, V.K. Intestinal Parasitic Infections in HIV Infected and Non-Infected Patients in a Low HIV Prevalence Region, West-Cameroon. PLoS ONE 2013, 8, e57914. [Google Scholar] [CrossRef] [PubMed]
- Tankoua-tchounda, R.; Nack, J.; Nchetnkou, C.M.; Tchankwé, D.L.K.; Lontsi-Demano, M.; Essangui, E.; Djimefo, A.K.T.; Lehman, L.G. Enteric Parasitosis of HIV-positive Patients in Cameroon: A Case of the Regional Hospital of Bafoussam (Western Region). Int. J. Trop. Dis. Heal. 2022, 43, 6–18. [Google Scholar] [CrossRef]
- Chia, P.N.; Ukaga, C.; Yongabi, K.A.; Nwoke, B.; Tih, P.M. Baseline Study on the Occurrence of Cryptosporidium Spp from Streams Water, After Torrential Rains in Bamenda, Cameroon. J. Biol. Agric. Health 2015, 4, 62–69. [Google Scholar]
- Eibach, D.; Krumkamp, R.; Al-Emran, H.M.; Sarpong, N.; Hagen, R.M.; Adu-Sarkodie, Y.; Tannich, E.; May, J. Molecular Characterization of Cryptosporidium spp. among Children in Rural Ghana. PLoS Negl. Trop. Dis. 2015, 9, e0003551. [Google Scholar] [CrossRef]
- Agba, A.A.; Aken’Ova, T.O.; Audu, P.A. Cryptosporidium infection among children attending some hospitals in Funtua Local Government Area, Katsina State, Nigeria. Zoologist 2018, 16, 1–5. [Google Scholar] [CrossRef]
- Gattan, H.S.; Alshammari, A.; Marzok, M.; Salem, M.; AL-Jabr, O.A.; Selim, A. Prevalence of Cryptosporidium infection and associated risk factors in calves in Egypt. Sci. Rep. 2023, 13, 17755. [Google Scholar] [CrossRef]
- Krumkamp, R.; Aldrich, C.; Maiga-Ascofare, O.; Mbwana, J.; Rakotozandrindrainy, N.; Borrmann, S.; Caccio, S.M.; Rakotozandrindrainy, R.; Adegnika, A.A.; A Lusingu, J.P.; et al. Transmission of Cryptosporidium Species Among Human and Animal Local Contact Networks in Sub-Saharan Africa: A Multicountry Study. Clin. Infect. Dis. 2021, 72, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Mulunda, N.R.; Hayashida, K.; Yamagishi, J.; Sianongo, S. Molecular characterization of Cryptosporidium spp. from patients with diarrhoea in Lusaka, Zambia. Parasite 2020, 27, 53. [Google Scholar] [CrossRef]
- Messa, A., Jr.; Köster, P.C.; Garrine, M.; Nhampossa, T.; Massora, S.; Cossa, A.; Bassat, Q.; Kotloff, K.; Levine, M.M.; Alonso, P.L.; et al. Molecular Characterisation of Cryptosporidium spp. in Mozambican Children Younger than 5 Years Enrolled in a Matched Case-Control Study on the Aetiology of Diarrhoeal Disease. Pathogens 2021, 10, 452. [Google Scholar] [CrossRef]
- Mbae, C.; Mulinge, E.; Waruru, A.; Ngugi, B.; Wainaina, J.; Kariuki, S. Genetic Diversity of Cryptosporidium in Children in an Urban Informal Settlement of Nairobi, Kenya. PLoS ONE 2015, 151, e0142055. [Google Scholar] [CrossRef] [PubMed]
- Lobos, E.; Weiss, N.; Karam, M.; Taylort, H.R.; Otresen, E.A.; Nutman, T.B. Specific and early marker of infection. Science 1991, 16, 12–15. [Google Scholar]
- Samra, N.A.; Thompson, P.N.; Jori, F.; Frean, J.; Poonsamy, B.; du Plessis, D.; Mogoye, B.; Xiao, L. Genetic Characterization of Cryptosporidium spp. in Diarrhoeic Children from Four Provinces in South Africa. Zoonoses Public Heal. 2012, 60, 154–159. [Google Scholar] [CrossRef]
- Akiyoshi, D.E.; Tumwine, J.K.; Bakeera-Kitaka, S.; Tzipori, S. Subtype Analysis of Cryptosporidium Isolates From Children in Uganda Subtype Analysis of Cryptosporidium Isolates From Children in Uganda. J. Parasitol. 2006, 92, 1097–1100. [Google Scholar] [CrossRef]
- Khan, A.A.; Shams, S.; Khan, S.; Khan, M.I.; Khan, S.; Ali, A. Modified ZN Staining Protocol V.2 Version 1 is Forked from mZN Staining Protocol. 2018. Available online: https://www.protocols.io/view/modified-zn-staining-protocol-tb2eiqe.pdf (accessed on 11 November 2024).
- Millar, C.; Moore, J.; Lowery, C.; McCorry, K.; Dooley, J. Successful PCR amplification of genomic DNA from Cryptosporidium parvum oocysts extracted from a human faecal sample: A rapid and simple method suited for outbreak analysis. Int. J. Hyg. Environ. Health 2001, 204, 191–194. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Yamada, K.D.; Tomii, K.; Katoh, K. Parallelization of MAFFT for large-scale multiple sequence alignments. Bioinformatics 2018, 34, 2490–2492. [Google Scholar] [CrossRef]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Okojokwu, O.J.; Ileigo, I.H.; Ezemuel, Y.S.; Oluyinka, O.O.; Akpakpan, E.E.; Kolawole, T.; Chima, N.J.; Anejo-Okopi, A.J. Molecular characterisation of Cryptosporidium species among Patients Presenting with Diarrhoea in Some Parts of Kaduna State, Nigeria. Am. J. Res. Commun. 2016, 4, 87–106. [Google Scholar]
- Robertson, L.J.; Johansen, Ø.H.; Kifleyohannes, T.; Efunshile, A.M.; Terefe, G. Cryptosporidium Infections in Africa—How Important Is Zoonotic Transmission? A Review of the Evidence. Front. Vet. Sci. 2020, 7, 575881. [Google Scholar] [CrossRef]
- Aldeyarbi, H.M.; El-Ezz, N.M.T.A.; Karanis, P. Cryptosporidium and cryptosporidiosis: The African perspective. Environ. Sci. Pollut. Res. 2016, 23, 13811–13821. [Google Scholar] [CrossRef] [PubMed]
- Squire, S.A.; Ryan, U. Cryptosporidium and Giardia in Africa: Current and future challenges. Parasit. Vectors 2017, 10, 195. [Google Scholar] [CrossRef]
- Appelbee, A.J.; Thompson, R.C.A.; Olson, M.E. Giardia and Cryptosporidium in mammalian wildlife—Current status and future needs. Trends Parasitol. 2005, 21, 370–376. [Google Scholar] [CrossRef]
- Lendner, M.; Daugschies, A. Cryptosporidium infections: Molecular advances. Parasitology 2014, 141, 1511–1532. [Google Scholar] [CrossRef]
- Grabbe, M.; Conejeros, I.; Velásquez, Z.D.; Hasheminasab, S.S.; Kamena, F.; Wehrend, A.; Gärtner, U.; Taubert, A.; Hermosilla, C.R. Cryptosporidium parvum-induced neutrophil extracellular traps in neonatal calves is a stage-independent process. Front. Vet. Sci. 2023, 10, 1256726. [Google Scholar] [CrossRef] [PubMed]
- Ryan, U.; Power, M. Cryptosporidium species in Australian wildlife and domestic animals. Parasitology 2012, 139, 1673–1688. [Google Scholar] [CrossRef] [PubMed]
- Morgan, U.M.; Sargent, K.D.; Deplazes, P.; Forbes, D.A.; Spano, F.; Hertzberg, H.; Elliot, A.; Thompson, R.C.A. Molecular characterization of Cryptosporidium from various hosts. Parasitology 1998, 117, 31–37. [Google Scholar] [CrossRef]
- Khalil, I.A.; Troeger, C.; Rao, P.C.; Blacker, B.F.; Brown, A.; Brewer, T.G.; Colombara, D.V.; De Hostos, E.L.; Engmann, C.; Guerrant, R.L.; et al. Articles Morbidity, mortality, and long-term consequences associated with diarrhoea from Cryptosporidium infection in children younger than 5 years: A meta-analyses study. Lancet Glob. Health 2016, 6, e758–e768. [Google Scholar] [CrossRef]
- Pantenburg, B.; Castellanos-Gonzalez, A.; Dann, S.M.; Connelly, R.L.; Lewis, D.E.; Ward, H.D.; White, A.C. Human CD8+ T cells clear Cryptosporidium parvum from infected intestinal epithelial cells. Am. J. Trop. Med. Hyg. 2010, 82, 600–607. [Google Scholar] [CrossRef]
- Haskins, B.E.; Gullicksrud, J.A.; Wallbank, B.A.; Dumaine, J.E.; Guérin, A.; Cohn, I.S.; O’Dea, K.M.; Pardy, R.D.; Merolle, M.I.; Shallberg, L.A.; et al. Dendritic cell-mediated responses to secreted Cryptosporidium effectors are required for parasite-specific CD8 (+) T cell responses. bioRxiv 2023, 2023, 553566. [Google Scholar] [CrossRef]
- Ross, R.; Hasheminasab, S.S.; Conejeros, I.; Gärtner, U.; Kamena, F.; Krueger, A.; Taubert, A.; Hermosilla, C.; Iv, G.U. Human dendritic cell interactions with the zoonotic parasite Cryptosporidium parvum result in activation and maturation. Front. Immunol. 2024, 15, 1388366. [Google Scholar] [CrossRef]
- Ajjampur, S.S.R.; Gladstone, B.P.; Selvapandian, D.; Muliyil, J.P.; Ward, H.; Kang, G. Molecular and spatial epidemiology of cryptosporidiosis in children in a semiurban community in South India. J. Clin. Microbiol. 2007, 45, 915–920. [Google Scholar] [CrossRef]
- Cama, V.A.; Ross, J.M.; Crawford, S.; Kawai, V.; Chavez-Valdez, R.; Vargas, D.; Vivar, A.; Ticona, E.; Ñavincopa, M.; Williamson, J.; et al. Differences in clinical manifestations among Cryptosporidium species and subtypes in HIV-infected persons. J. Infect. Dis. 2007, 196, 684–691. [Google Scholar] [CrossRef]
- Xiao, L. Molecular epidemiology of cryptosporidiosis: An update. Exp. Parasitol. 2010, 124, 80–89. [Google Scholar] [CrossRef]


| Regions n (%) | |||||
|---|---|---|---|---|---|
| Variables | Center | Southwest | Littoral | West | Overall n (%) |
| Age | |||||
| <5 yrs | 248 (91.18) | 150 (46.88) | 179 (80.27) | 189 (62.17) | 766 (68.45) |
| 5–10 yrs | 24 (8.82) | 170 (53.12) | 44 (19.73) | 115 (37.83) | 353 (31.55) |
| Gender | |||||
| Female | 119 (43.75) | 174 (54.37) | 95 (42.60) | 136 (44.74) | 524 (46.83) |
| Male | 153 (56.25) | 146 (45.62) | 128 (57.40) | 168 (55.26) | 595 (53.17) |
| Duration of Diarrhea (days) | |||||
| <2 | 61 (22.43) | 98 (30.63) | 97 (43.50) | 107 (35.20) | 363 (32.44) |
| >2 | 211 (77.57) | 222 (69.38) | 126 (56.50) | 197 (64.80) | 756 (67.56) |
| Education of mother | |||||
| Primary | 30 (11.03) | 100 (31.25) | 46 (20.63) | 52 (17.11) | 228 (20.38) |
| Secondary | 242 (88.97) | 209 (65.31) | 139 (62.33) | 241 (79.28) | 831 (74.26) |
| Tertiary | 0 | 11 (3.44) | 38 (17.04) | 11 (3.62) | 60 (5.36) |
| Variables | Number Positive | Total Examined | Prevalence (%) | |
|---|---|---|---|---|
| Age | ||||
| <5 yrs | 94 | 766 | 12.27 | |
| 5–10 yrs | 38 | 353 | 10.76 | |
| Gender | ||||
| Female | 66 | 524 | 12.60 | |
| Male | 66 | 595 | 11.09 | |
| Duration of Diarrhea (days) | ||||
| <2 | 38 | 363 | 10.47 | |
| >2 | 94 | 756 | 12.43 | |
| Education of Mother | ||||
| Primary | 24 | 228 | 10.53 | |
| Secondary | 102 | 831 | 12.27 | |
| Tertiary | 6 | 60 | 10.00 | |
| Overall Prevalence | ||||
| Number of positive | Total Examined | Prevalence | Lcl | Ucl |
| 132 | 1119 | 11.8 | 10.03 | 13.82 |
| (Result = “Positive”) | ||||
| Predictors | Odds Ratios | CI | p | |
| (Intercept) | 0.23 | 0.12–0.43 | <0.001 | |
| Age [5–10 yrs] | 0.83 | 0.55–1.24 | 0.365 | |
| Gender [Male] | 0.83 | 0.57–1.20 | 0.317 | |
| Duration of Diarrhea [>2] | 1.23 | 0.83–1.86 | 0.321 | |
| Education [Secondary] | 1.28 | 0.81–2.11 | 0.308 | |
| Education [Tertiary] | 0.98 | 0.35–2.41 | 0.968 | |
| Observations | 1119 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sone, B.; Ambe, L.A.; Ampama, M.N.; Ajohkoh, C.; Che, D.; Nguinkal, J.A.; Taubert, A.; Hermosilla, C.; Kamena, F. Prevalence and Molecular Characterization of Cryptosporidium Species in Diarrheic Children in Cameroon. Pathogens 2025, 14, 287. https://doi.org/10.3390/pathogens14030287
Sone B, Ambe LA, Ampama MN, Ajohkoh C, Che D, Nguinkal JA, Taubert A, Hermosilla C, Kamena F. Prevalence and Molecular Characterization of Cryptosporidium Species in Diarrheic Children in Cameroon. Pathogens. 2025; 14(3):287. https://doi.org/10.3390/pathogens14030287
Chicago/Turabian StyleSone, Bertrand, Lum Abienwi Ambe, Mireille Nguele Ampama, Constance Ajohkoh, Desmond Che, Julien Alban Nguinkal, Anja Taubert, Carlos Hermosilla, and Faustin Kamena. 2025. "Prevalence and Molecular Characterization of Cryptosporidium Species in Diarrheic Children in Cameroon" Pathogens 14, no. 3: 287. https://doi.org/10.3390/pathogens14030287
APA StyleSone, B., Ambe, L. A., Ampama, M. N., Ajohkoh, C., Che, D., Nguinkal, J. A., Taubert, A., Hermosilla, C., & Kamena, F. (2025). Prevalence and Molecular Characterization of Cryptosporidium Species in Diarrheic Children in Cameroon. Pathogens, 14(3), 287. https://doi.org/10.3390/pathogens14030287







