Human Retinal Organoid Model of Ocular Toxoplasmosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Toxoplasma gondii
2.2. Human Retinal Organoids
2.3. Toxoplasma gondii Infection of Retinal Organoids
2.4. Immunohistochemistry
2.5. RNA Extraction and Reverse Transcription
2.6. Quantitative Polymerase Chain Reaction
2.7. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ESC | Embryonic stem cell |
| iPSC | Induced pluripotent stem cell |
| RT-qPCR | Reverse transcription-quantitative polymerase chain reaction |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FBS | Fetal bovine serum |
| FGF | Fibroblast growth factor |
| AUC | Area under the curve |
| IgG | Immunoglobulin |
| CCL2 | C-C motif chemokine ligand 2 |
| CXCL10 | C-X-C motif chemokine ligand 10 |
| CXCL8 | C-X-C motif chemokine ligand 8 |
| GAD2 | Glutamate decarboxylase 2 |
| GRA6 | T. gondii dense granule protein 6 |
| IL6 | Interleukin 6 |
| LHX1 | LIM homeobox 1 |
| MKI67 | Marker of proliferation Ki-67 |
| OTX2 | Orthodenticle homeobox 2 |
| PAX6 | Paired box 6 |
| PRKCA | Protein kinase c alpha |
| POU4F1 | POU class 4 homeobox 1 |
| RCVRN | Recoverin |
| RHO | Rhodopsin |
| RLBP1 | Retinaldehyde binding protein 1 |
| ROP16 | T. gondii rhoptry protein 16 |
| RPE65 | Retinoid isomerohydrolase RPE65 |
| RPLP0 | Ribosomal protein lateral stalk protein subunit P0 |
| SAG1 | T. gondii surface antigen 1 |
| VSX2 | Visual system homeobox 2 |
| YWHAZ | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta |
References
- Molan, A.; Nosaka, K.; Hunter, M.; Wang, W. Global status of Toxoplasma gondii infection: Systematic review and prevalence snapshots. Trop. Biomed. 2019, 36, 898–925. [Google Scholar]
- Akins, G.K.H.; Furtado, J.M.; Smith, J.R. Diseases caused by and behaviors associated with Toxoplasma gondii infection. Pathogens 2024, 13, 968. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.B.; Furtado, J.M.; Charng, J.; Franchina, M.; Matthews, J.M.; Molan, A.A.L.; Hunter, M.; Mackey, D.A.; Smith, J.R. Prevalence of toxoplasmic retinochoroiditis in an Australian adult population: A Community-Based Study. Ophthalmol. Retina 2022, 6, 963–968. [Google Scholar] [CrossRef]
- De Angelis, R.E.; Veronese Rodrigues, M.L.; Passos, A.D.C.; Bollela, V.R.; Freitas, E.S.M.S.; Vieira, B.R.; de Lucena, M.M.; Moralles, T.D.; de Morais Vicente, L.; de Melo Rocha, G.; et al. Frequency and visual outcomes of ocular toxoplasmosis in an adult Brazilian population. Sci. Rep. 2021, 11, 3420. [Google Scholar] [CrossRef]
- Butler, N.J.; Furtado, J.M.; Winthrop, K.L.; Smith, J.R. Ocular toxoplasmosis II: Clinical features, pathology and management. Clin. Exp. Ophthalmol. 2013, 41, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Aleixo, A.L.Q.d.C.; Curi, A.L.L.; Benchimol, E.I.; Amendoeira, M.R.R. Toxoplasmic retinochoroiditis: Clinical characteristics and visual outcome in a prospective study. PLoS Negl. Trop. Dis. 2016, 10, e0004685. [Google Scholar] [CrossRef] [PubMed]
- Arruda, S.; Vieira, B.R.; Garcia, D.M.; Araújo, M.; Simões, M.; Moreto, R.; Rodrigues, M.W.; Belfort, R.; Smith, J.R.; Furtado, J.M. Clinical manifestations and visual outcomes associated with ocular toxoplasmosis in a Brazilian population. Sci. Rep. 2021, 11, 3137. [Google Scholar] [CrossRef]
- Melamed, J.; Eckert, G.U.; Spadoni, V.S.; Lago, E.G.; Uberti, F. Ocular manifestations of congenital toxoplasmosis. Eye 2010, 24, 528–534. [Google Scholar] [CrossRef]
- Garweg, J.G.; Kieffer, F.; Mandelbrot, L.; Peyron, F.; Wallon, M. Long-term outcomes in children with congenital toxoplasmosis-a systematic review. Pathogens 2022, 11, 1187. [Google Scholar] [CrossRef]
- Yogeswaran, K.; Furtado, J.M.; Bodaghi, B.; Matthews, J.M.; Smith, J.R. Current practice in the management of ocular toxoplasmosis. Br. J. Ophthalmol. 2023, 107, 973. [Google Scholar] [CrossRef]
- Smith, J.R.; Ashander, L.M.; Arruda, S.L.; Cordeiro, C.A.; Lie, S.; Rochet, E.; Belfort, R., Jr.; Furtado, J.M. Pathogenesis of ocular toxoplasmosis. Prog. Retin. Eye Res. 2021, 81, 100882. [Google Scholar] [CrossRef] [PubMed]
- Lidgerwood, G.E.; Hewitt, A.W.; Pébay, A. Human pluripotent stem cells for the modelling of diseases of the retina and optic nerve: Toward a retina in a dish. Curr. Opin. Pharmacol. 2019, 48, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Eldred, K.C.; Reh, T.A. Human retinal model systems: Strengths, weaknesses, and future directions. Dev. Biol. 2021, 480, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Engelsberg, K.; Ehinger, B.; Ghosh, F. Early development of retinal subtypes in long-term cultures of human embryonic retina. Curr. Eye Res. 2008, 33, 185–191. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef]
- Nakano, T.; Ando, S.; Takata, N.; Kawada, M.; Muguruma, K.; Sekiguchi, K.; Saito, K.; Yonemura, S.; Eiraku, M.; Sasai, Y. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 2012, 10, 771–785. [Google Scholar] [CrossRef]
- Reichman, S.; Slembrouck, A.; Gagliardi, G.; Chaffiol, A.; Terray, A.; Nanteau, C.; Potey, A.; Belle, M.; Rabesandratana, O.; Duebel, J.; et al. Generation of storable retinal organoids and retinal pigmented epithelium from adherent human iPS cells in xeno-free and feeder-free conditions. Stem Cells 2017, 35, 1176–1188. [Google Scholar] [CrossRef]
- Cowan, C.S.; Renner, M.; De Gennaro, M.; Gross-Scherf, B.; Goldblum, D.; Hou, Y.; Munz, M.; Rodrigues, T.M.; Krol, J.; Szikra, T.; et al. Cell types of the human retina and its organoids at single-cell resolution. Cell 2020, 182, 1623–1640. [Google Scholar] [CrossRef]
- Sridhar, A.; Hoshino, A.; Finkbeiner, C.R.; Chitsazan, A.; Dai, L.; Haugan, A.K.; Eschenbacher, K.M.; Jackson, D.L.; Trapnell, C.; Bermingham-McDonogh, O.; et al. Single-cell transcriptomic comparison of human fetal retina, hPSC-derived retinal organoids, and long-term retinal cultures. Cell Rep. 2020, 30, 1644–1659. [Google Scholar] [CrossRef]
- Daniszewski, M.; Senabouth, A.; Liang, H.H.; Han, X.; Lidgerwood, G.E.; Hernández, D.; Sivakumaran, P.; Clarke, J.E.; Lim, S.Y.; Lees, J.G.; et al. Retinal ganglion cell-specific genetic regulation in primary open-angle glaucoma. Cell Genom. 2022, 2, 100142. [Google Scholar] [CrossRef]
- Gerdes, J.; Lemke, H.; Baisch, H.; Wacker, H.H.; Schwab, U.; Stein, H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 1984, 133, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- Wersto, R.P.; Herz, F.; Gallagher, R.E.; Koss, L.G. Cell cycle-dependent reactivity with the monoclonal antibody Ki-67 during myeloid cell differentiation. Exp. Cell Res. 1988, 179, 79–88. [Google Scholar] [CrossRef]
- Schlüter, C.; Duchrow, M.; Wohlenberg, C.; Becker, M.H.; Key, G.; Flad, H.D.; Gerdes, J. The cell proliferation-associated antigen of antibody Ki-67: A very large, ubiquitous nuclear protein with numerous repeated elements, representing a new kind of cell cycle-maintaining proteins. J. Cell Biol. 1993, 123, 513–522. [Google Scholar] [CrossRef]
- Nishina, S.; Kohsaka, S.; Yamaguchi, Y.; Handa, H.; Kawakami, A.; Fujisawa, H.; Azuma, N. PAX6 expression in the developing human eye. Br. J. Ophthalmol. 1999, 83, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Shaham, O.; Menuchin, Y.; Farhy, C.; Ashery-Padan, R. Pax6: A multi-level regulator of ocular development. Prog. Retin. Eye Res. 2012, 31, 351–376. [Google Scholar] [CrossRef]
- Marquardt, T.; Ashery-Padan, R.; Andrejewski, N.; Scardigli, R.; Guillemot, F.; Gruss, P. Pax6 is required for the multipotent state of retinal progenitor cells. Cell 2001, 105, 43–55. [Google Scholar] [CrossRef]
- Fuhrmann, S. Eye morphogenesis and patterning of the optic vesicle. Curr. Top. Dev. Biol. 2010, 93, 61–84. [Google Scholar] [CrossRef] [PubMed]
- Horsford, D.J.; Nguyen, M.T.; Sellar, G.C.; Kothary, R.; Arnheiter, H.; McInnes, R.R. Chx10 repression of Mitf is required for the maintenance of mammalian neuroretinal identity. Development 2005, 132, 177–187. [Google Scholar] [CrossRef]
- Nishida, A.; Furukawa, A.; Koike, C.; Tano, Y.; Aizawa, S.; Matsuo, I.; Furukawa, T. Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat. Neurosci. 2003, 6, 1255–1263. [Google Scholar] [CrossRef]
- Martínez-Morales, J.R.; Dolez, V.; Rodrigo, I.; Zaccarini, R.; Leconte, L.; Bovolenta, P.; Saule, S. OTX2 activates the molecular network underlying retina pigment epithelium differentiation. J. Biol. Chem. 2003, 278, 21721–21731. [Google Scholar] [CrossRef]
- Nadal-Nicolás, F.M.; Jiménez-López, M.; Sobrado-Calvo, P.; Nieto-López, L.; Cánovas-Martínez, I.; Salinas-Navarro, M.; Vidal-Sanz, M.; Agudo, M. Brn3a as a marker of retinal ganglion cells: Qualitative and quantitative time course studies in naïve and optic nerve–injured retinas. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3860–3868. [Google Scholar] [CrossRef] [PubMed]
- Badea, T.C.; Cahill, H.; Ecker, J.; Hattar, S.; Nathans, J. Distinct roles of transcription factors Brn3a and Brn3b in controlling the development, morphology, and function of retinal ganglion cells. Neuron 2009, 61, 852–864. [Google Scholar] [CrossRef]
- Poché, R.A.; Kwan, K.M.; Raven, M.A.; Furuta, Y.; Reese, B.E.; Behringer, R.R. Lim1 is essential for the correct laminar positioning of retinal horizontal cells. J. Neurosci. 2007, 27, 14099–14107. [Google Scholar] [CrossRef]
- Liu, W.; Wang, J.H.; Xiang, M. Specific expression of the LIM/homeodomain protein Lim-1 in horizontal cells during retinogenesis. Dev. Dyn. 2000, 217, 320–325. [Google Scholar] [CrossRef]
- Yan, W.; Peng, Y.-R.; van Zyl, T.; Regev, A.; Shekhar, K.; Juric, D.; Sanes, J.R. Cell atlas of the human fovea and peripheral retina. Sci. Rep. 2020, 10, 9802. [Google Scholar] [CrossRef]
- Haverkamp, S.; Haeseleer, F.; Hendrickson, A. A comparison of immunocytochemical markers to identify bipolar cell types in human and monkey retina. Vis. Neurosci. 2003, 20, 589–600. [Google Scholar] [CrossRef]
- Kolb, H.; Zhang, L.; Dekorver, L. Differential staining of neurons in the human retina with antibodies to protein kinase C isozymes. Vis. Neurosci. 1993, 10, 341–351. [Google Scholar] [CrossRef]
- Hofmann, K.P.; Lamb, T.D. Rhodopsin, light-sensor of vision. Prog. Retin. Eye Res. 2023, 93, 101116. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, H.G.; Koch, K.W. Recoverin, a novel calcium-binding protein from vertebrate photoreceptors. Biochim. Biophys. Acta 1992, 1160, 63–66. [Google Scholar] [CrossRef]
- Kennedy, B.N.; Goldflam, S.; Chang, M.A.; Campochiaro, P.; Davis, A.A.; Zack, D.J.; Crabb, J.W. Transcriptional regulation of cellular retinaldehyde-binding protein in the retinal pigment epithelium. A role for the photoreceptor consensus element. J. Biol. Chem. 1998, 273, 5591–5598. [Google Scholar] [CrossRef]
- Xue, Y.; Shen, S.Q.; Jui, J.; Rupp, A.C.; Byrne, L.C.; Hattar, S.; Flannery, J.G.; Corbo, J.C.; Kefalov, V.J. CRALBP supports the mammalian retinal visual cycle and cone vision. J. Clin. Investig. 2015, 125, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Conley, S.M.; Naash, M.I. RPE65: Role in the visual cycle, human retinal disease, and gene therapy. Ophthalmic Genet. 2009, 30, 57–62. [Google Scholar] [CrossRef]
- Ma, J.S.; Sasai, M.; Ohshima, J.; Lee, Y.; Bando, H.; Takeda, K.; Yamamoto, M. Selective and strain-specific NFAT4 activation by the Toxoplasma gondii polymorphic dense granule protein GRA6. J. Exp. Med. 2014, 211, 2013–2032. [Google Scholar] [CrossRef]
- Yamamoto, M.; Standley, D.M.; Takashima, S.; Saiga, H.; Okuyama, M.; Kayama, H.; Kubo, E.; Ito, H.; Takaura, M.; Matsuda, T.; et al. A single polymorphic amino acid on Toxoplasma gondii kinase ROP16 determines the direct and strain-specific activation of Stat3. J. Exp. Med. 2009, 206, 2747–2760. [Google Scholar] [CrossRef]
- Butcher, B.A.; Fox, B.A.; Rommereim, L.M.; Kim, S.G.; Maurer, K.J.; Yarovinsky, F.; Herbert, D.R.; Bzik, D.J.; Denkers, E.Y. Toxoplasma gondii rhoptry kinase ROP16 activates STAT3 and STAT6 resulting in cytokine inhibition and arginase-1-dependent growth control. PLoS Pathog. 2011, 7, e1002236. [Google Scholar] [CrossRef] [PubMed]
- Lekutis, C.; Ferguson, D.J.; Grigg, M.E.; Camps, M.; Boothroyd, J.C. Surface antigens of Toxoplasma gondii: Variations on a theme. Int. J. Parasitol. 2001, 31, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Velge-Roussel, F.; Dimier-Poisson, I.; Buzoni-Gatel, D.; Bout, D. Anti-SAG1 peptide antibodies inhibit the penetration of Toxoplasma gondii tachyzoites into enterocyte cell lines. Parasitology 2001, 123, 225–233. [Google Scholar] [CrossRef]
- Ng, N.H.J.; Ghosh, S.; Bok, C.M.; Ching, C.; Low, B.S.J.; Chen, J.T.; Lim, E.; Miserendino, M.C.; Tan, Y.S.; Hoon, S.; et al. HNF4A and HNF1A exhibit tissue specific target gene regulation in pancreatic beta cells and hepatocytes. Nat. Commun. 2024, 15, 4288. [Google Scholar] [CrossRef]
- Eftekharian, M.M.; Ghafouri-Fard, S.; Noroozi, R.; Omrani, M.D.; Arsang-Jang, S.; Ganji, M.; Gharzi, V.; Noroozi, H.; Komaki, A.; Mazdeh, M.; et al. Cytokine profile in autistic patients. Cytokine 2018, 108, 120–126. [Google Scholar] [CrossRef]
- Hiratsuka, K.; Monkawa, T.; Akiyama, T.; Nakatake, Y.; Oda, M.; Goparaju, S.K.; Kimura, H.; Chikazawa-Nohtomi, N.; Sato, S.; Ishiguro, K.; et al. Induction of human pluripotent stem cells into kidney tissues by synthetic mRNAs encoding transcription factors. Sci. Rep. 2019, 9, 913. [Google Scholar] [CrossRef]
- Sobecki, M.; Mrouj, K.; Camasses, A.; Parisis, N.; Nicolas, E.; Llères, D.; Gerbe, F.; Prieto, S.; Krasinska, L.; David, A.; et al. The cell proliferation antigen Ki-67 organises heterochromatin. eLife 2016, 5, e13722. [Google Scholar] [CrossRef] [PubMed]
- Forouzanfar, F.; Soleimannejad, M.; Soltani, A.; Sadat Mirsafaee, P.; Asgharzade, S. Retinoic acid and taurine enhance differentiation of the human bone marrow stem cells into cone photoreceptor cells and retinal ganglion cells. J. Cell. Biochem. 2021, 122, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Xu, Z.; Sun, H.; Luo, W.; Yan, Y.; Wang, J.; Guo, J.; Liu, Y.; Chen, S. Direct induction of functional neuronal cells from fibroblast-like cells derived from adult human retina. Stem Cell Res. 2017, 23, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Heppt, M.V.; Wessely, A.; Hornig, E.; Kammerbauer, C.; Graf, S.A.; Besch, R.; French, L.E.; Matthies, A.; Kuphal, S.; Kappelmann-Fenzl, M.; et al. HDAC2 is involved in the regulation of BRN3A in melanocytes and melanoma. Int. J. Mol. Sci. 2022, 23, 849. [Google Scholar] [CrossRef]
- Lie, S.; Rochet, E.; Segerdell, E.; Ma, Y.; Ashander, L.M.; Shadforth, A.M.A.; Blenkinsop, T.A.; Michael, M.Z.; Appukuttan, B.; Wilmot, B.; et al. Immunological molecular responses of human retinal pigment epithelial cells to infection with Toxoplasma gondii. Front. Immunol. 2019, 10, 708. [Google Scholar] [CrossRef]
- Selseleh, M.; Modarressi, M.H.; Mohebali, M.; Shojaee, S.; Eshragian, M.R.; Selseleh, M.; Azizi, E.; Keshavarz, H. Real-time RT-PCR on SAG1 and BAG1 gene expression during stage conversion in immunosuppressed mice infected with Toxoplasma gondii Tehran strain. Korean J. Parasitol. 2012, 50, 199–205. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, Research0034. [Google Scholar] [CrossRef]
- Han, Y.; Yang, L.; Lacko, L.A.; Chen, S. Human organoid models to study SARS-CoV-2 infection. Nat. Methods 2022, 19, 418–428. [Google Scholar] [CrossRef]
- Garcez, P.P.; Loiola, E.C.; Madeiro da Costa, R.; Higa, L.M.; Trindade, P.; Delvecchio, R.; Nascimento, J.M.; Brindeiro, R.; Tanuri, A.; Rehen, S.K. Zika virus impairs growth in human neurospheres and brain organoids. Science 2016, 352, 816–818. [Google Scholar] [CrossRef]
- Watanabe, Y.; Kimura, I.; Hashimoto, R.; Sakamoto, A.; Yasuhara, N.; Yamamoto, T.; Sato, K.; Takayama, K. Virological characterization of the 2022 outbreak-causing monkeypox virus using human keratinocytes and colon organoids. J. Med. Virol. 2023, 95, e28827. [Google Scholar] [CrossRef]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Correa Leite, P.E.; de Araujo Portes, J.; Pereira, M.R.; Russo, F.B.; Martins-Duarte, E.S.; Almeida Dos Santos, N.; Attias, M.; Barrantes, F.J.; Baleeiro Beltrão-Braga, P.C.; de Souza, W. Morphological and biochemical repercussions of Toxoplasma gondii infection in a 3D human brain neurospheres model. Brain Behav. Immun. Health 2021, 11, 100190. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.H.; Han, H.W.; Lee, S.E.; Hong, S.H.; Cho, S.H.; Kim, S.C.; Koo, S.K.; Kim, J.H. Modelling Toxoplasma gondii infection in human cerebral organoids. Emerg. Microbes Infect. 2020, 9, 1943–1954. [Google Scholar] [CrossRef]
- Luu, L.; Johnston, L.J.; Derricott, H.; Armstrong, S.D.; Randle, N.; Hartley, C.S.; Duckworth, C.A.; Campbell, B.J.; Wastling, J.M.; Coombes, J.L. An open-format enteroid culture system for interrogation of interactions between Toxoplasma gondii and the intestinal epithelium. Front. Cell. Infect. Microbiol. 2019, 9, 300. [Google Scholar] [CrossRef]
- Chu, H.; Chan, J.F.-W.; Yuen, T.T.-T.; Shuai, H.; Yuan, S.; Wang, Y.; Hu, B.; Yip, C.C.-Y.; Tsang, J.O.-L.; Huang, X.; et al. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: An observational study. Lancet Microbe 2020, 1, e14–e23. [Google Scholar] [CrossRef]
- Grimwood, J.; Mineo, J.R.; Kasper, L.H. Attachment of Toxoplasma gondii to host cells is host cell cycle dependent. Infect. Immun. 1996, 64, 4099–4104. [Google Scholar] [CrossRef] [PubMed]
- Lavine, M.D.; Arrizabalaga, G. Induction of mitotic S-phase of host and neighboring cells by Toxoplasma gondii enhances parasite invasion. Mol. Biochem. Parasitol. 2009, 164, 95–99. [Google Scholar] [CrossRef]
- Delair, E.; Monnet, D.; Grabar, S.; Dupouy-Camet, J.; Yera, H.; Brezin, A.P. Respective roles of acquired and congenital infections in presumed ocular toxoplasmosis. Am. J. Ophthalmol. 2008, 146, 851–855. [Google Scholar] [CrossRef]
- Dubey, J.P.; Murata, F.H.A.; Cerqueira-Cézar, C.K.; Kwok, O.C.H.; Villena, I. Congenital toxoplasmosis in humans: An update of worldwide rate of congenital infections. Parasitology 2021, 148, 1406–1416. [Google Scholar] [CrossRef]
- Black, M.W.; Boothroyd, J.C. Lytic cycle of Toxoplasma gondii. Microbiol. Mol. Biol. Rev. 2000, 64, 607–623. [Google Scholar] [CrossRef]
- Lüder, C.G.; Gross, U. Apoptosis and its modulation during infection with Toxoplasma gondii: Molecular mechanisms and role in pathogenesis. Curr. Top. Microbiol. Immunol. 2005, 289, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.F.; Matteson, D.M.; Tuaillon, N.; Suedekum, B.K.; Buggage, R.R.; Chan, C.C. Involvement of apoptosis and interferon-gamma in murine toxoplasmosis. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2031–2036. [Google Scholar]
- Hu, M.S.; Schwartzman, J.D.; Yeaman, G.R.; Collins, J.; Seguin, R.; Khan, I.A.; Kasper, L.H. Fas-FasL interaction involved in pathogenesis of ocular toxoplasmosis in mice. Infect. Immun. 1999, 67, 928–935. [Google Scholar] [CrossRef]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell. Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef]
- Smith, J.R.; Choi, D.; Chipps, T.J.; Pan, Y.; Zamora, D.O.; Davies, M.H.; Babra, B.; Powers, M.R.; Planck, S.R.; Rosenbaum, J.T. Unique gene expression profiles of donor-matched human retinal and choroidal vascular endothelial cells. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2676–2684. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, T.E.; Coelho-Dos-Reis, J.G.; Béla, S.R.; Carneiro, A.; Machado, A.S.; Cardoso, L.M.; Ribeiro, Á.L.; Dias, M.H.F.; Queiroz Andrade, G.M.; Vasconcelos-Santos, D.V.; et al. Early serum biomarker networks in infants with distinct retinochoroidal lesion status of congenital toxoplasmosis. Cytokine 2017, 95, 102–112. [Google Scholar] [CrossRef]
- Marino, A.P.; Dos Santos, L.I.; Henriques, P.M.; Roffe, E.; Vasconcelos-Santos, D.V.; Sher, A.; Jankovic, D.; Gomes, M.S.; Amaral, L.R.; Campi-Azevedo, A.C.; et al. Circulating inflammatory mediators as biomarkers of ocular toxoplasmosis in acute and in chronic infection. J. Leukoc. Biol. 2020, 108, 1253–1264. [Google Scholar] [CrossRef]
- Norose, K.; Kikumura, A.; Luster, A.D.; Hunter, C.A.; Harris, T.H. CXCL10 is required to maintain T-cell populations and to control parasite replication during chronic ocular toxoplasmosis. Investig. Ophthalmol. Vis. Sci. 2011, 52, 389–398. [Google Scholar] [CrossRef]
- Orchanian, S.B.; Still, K.; Harris, T.H.; Lodoen, M.B. Deficiency in astrocyte CCL2 production reduces neuroimmune control of Toxoplasma gondii infection. PLoS Pathog. 2024, 20, e1011710. [Google Scholar] [CrossRef]
- Harris, T.H.; Banigan, E.J.; Christian, D.A.; Konradt, C.; Tait Wojno, E.D.; Norose, K.; Wilson, E.H.; John, B.; Weninger, W.; Luster, A.D.; et al. Generalized Lévy walks and the role of chemokines in migration of effector CD8+ T cells. Nature 2012, 486, 545–548. [Google Scholar] [CrossRef]
- Strack, A.; Asensio, V.C.; Campbell, I.L.; Schlüter, D.; Deckert, M. Chemokines are differentially expressed by astrocytes, microglia and inflammatory leukocytes in Toxoplasma encephalitis and critically regulated by interferon-gamma. Acta Neuropathol. 2002, 103, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Sher, A.; Tosh, K.; Jankovic, D. Innate recognition of Toxoplasma gondii in humans involves a mechanism distinct from that utilized by rodents. Cell. Mol. Immunol. 2017, 14, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Harkin, J.; Peña, K.H.; Gomes, C.; Hernandez, M.; Lavekar, S.S.; So, K.; Lentsch, K.; Feder, E.M.; Morrow, S.; Huang, K.C.; et al. A highly reproducible and efficient method for retinal organoid differentiation from human pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2024, 121, e2317285121. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, T.; Onishi, A.; Matsuyama, T.; Masuda, T.; Ogino, Y.; Kageyama, M.; Takahashi, M.; Uchiumi, F. Rapid and efficient generation of mature retinal organoids derived from human pluripotent stem cells via optimized pharmacological modulation of Sonic hedgehog, activin A, and retinoic acid signal transduction. PLoS ONE 2024, 19, e0308743. [Google Scholar] [CrossRef]
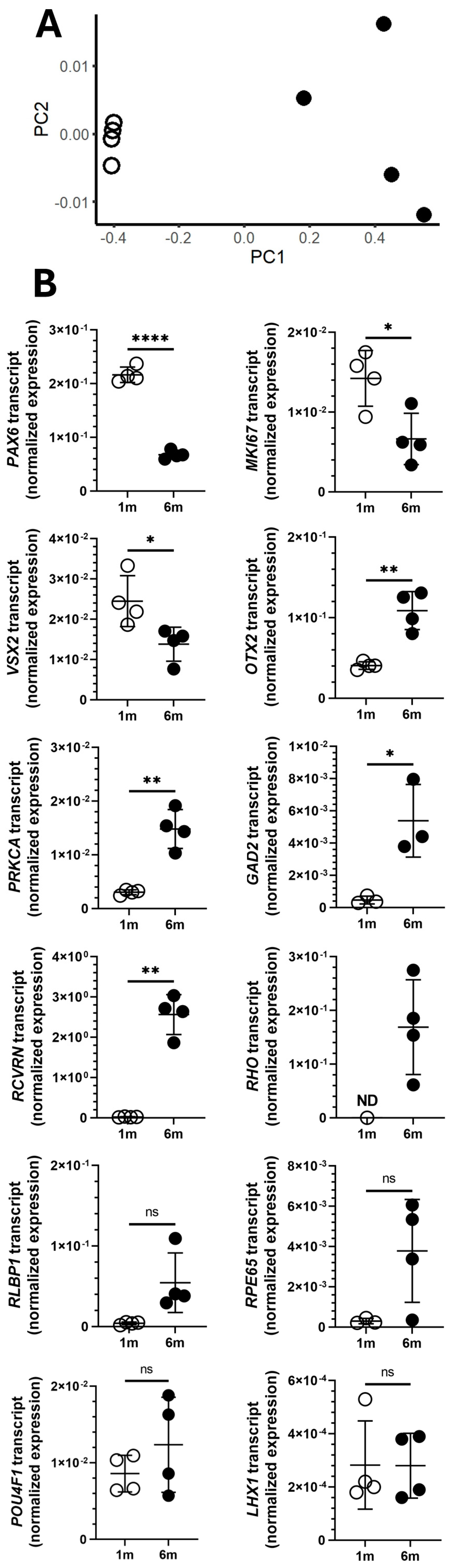
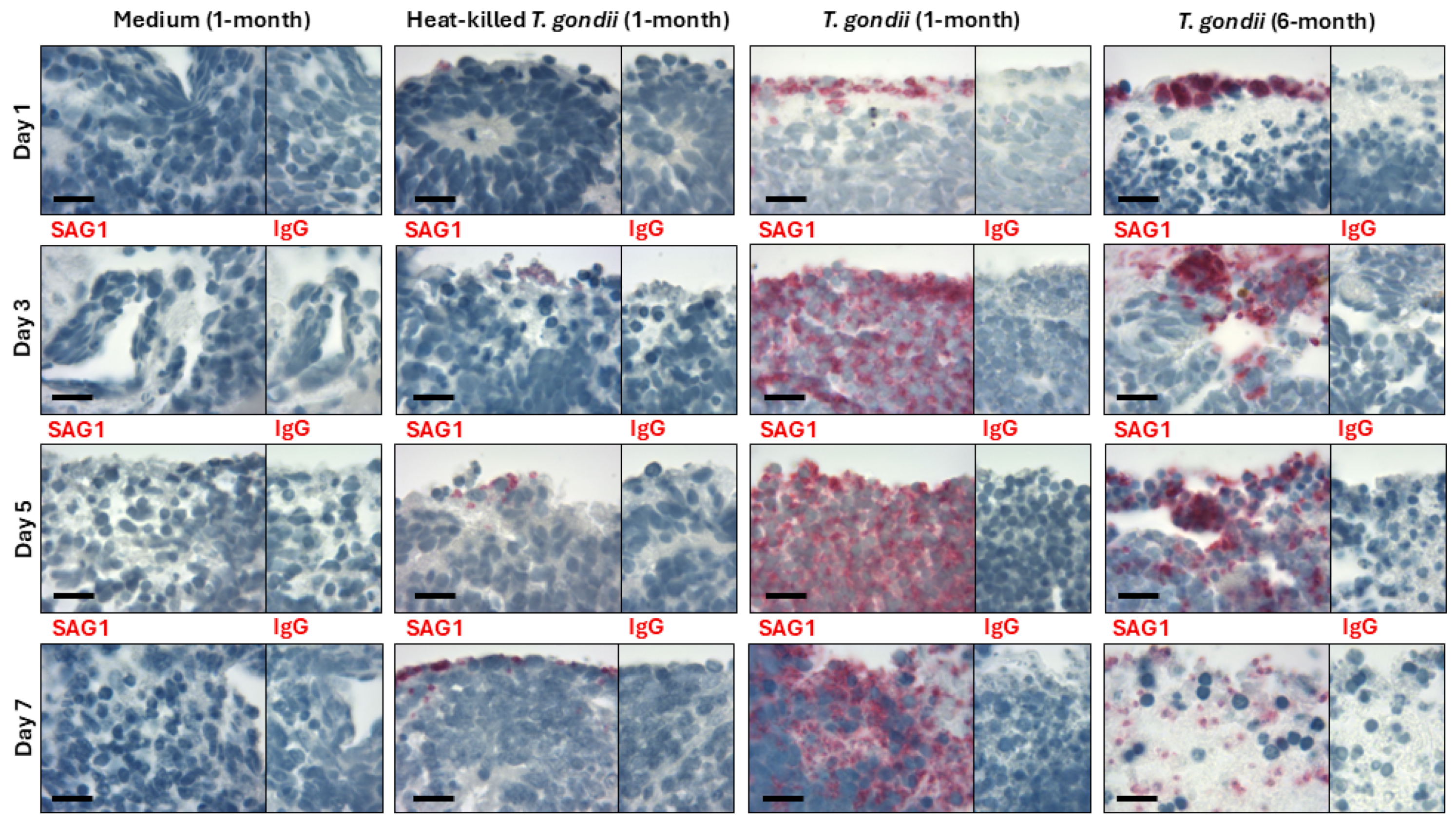
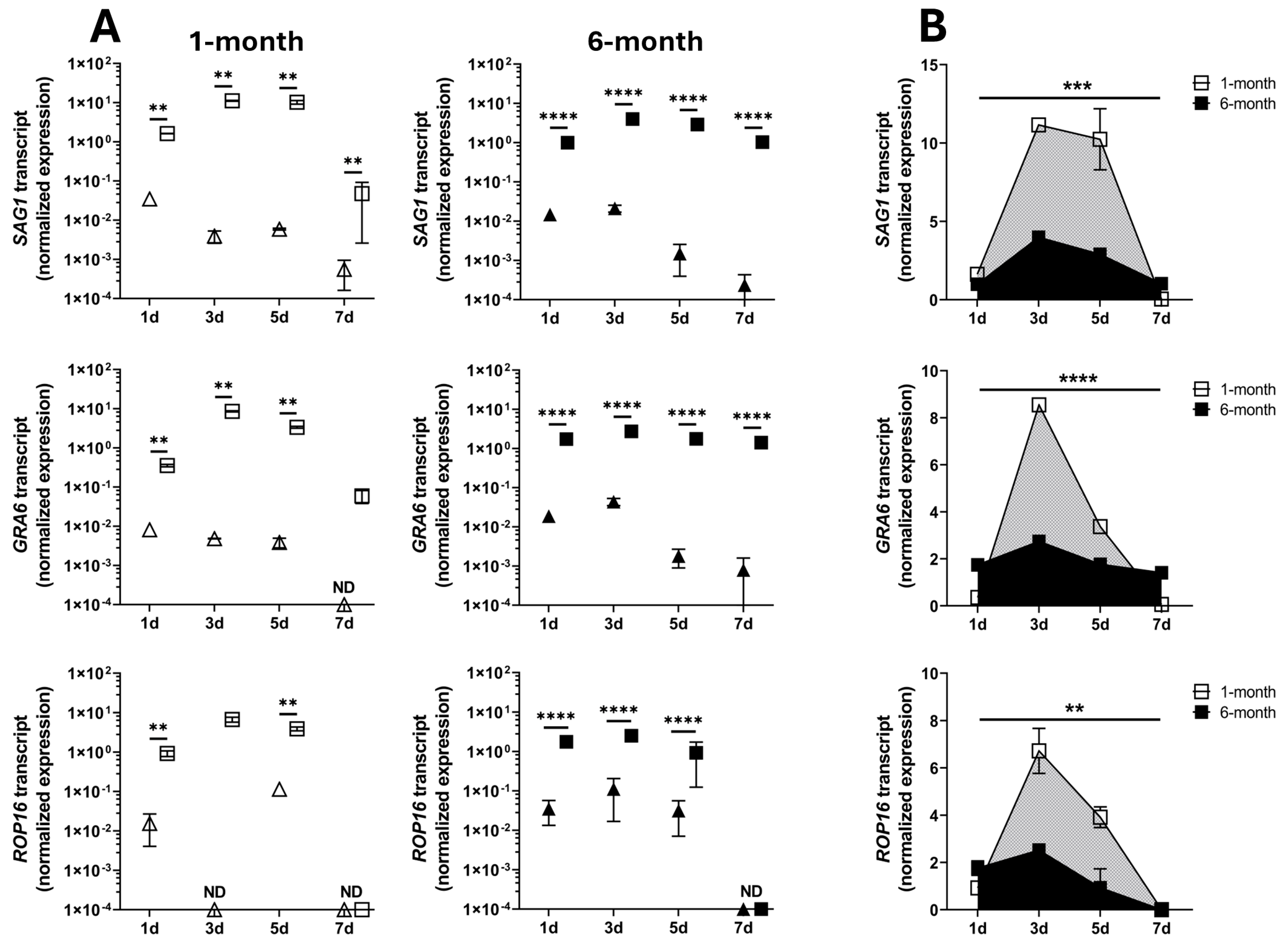
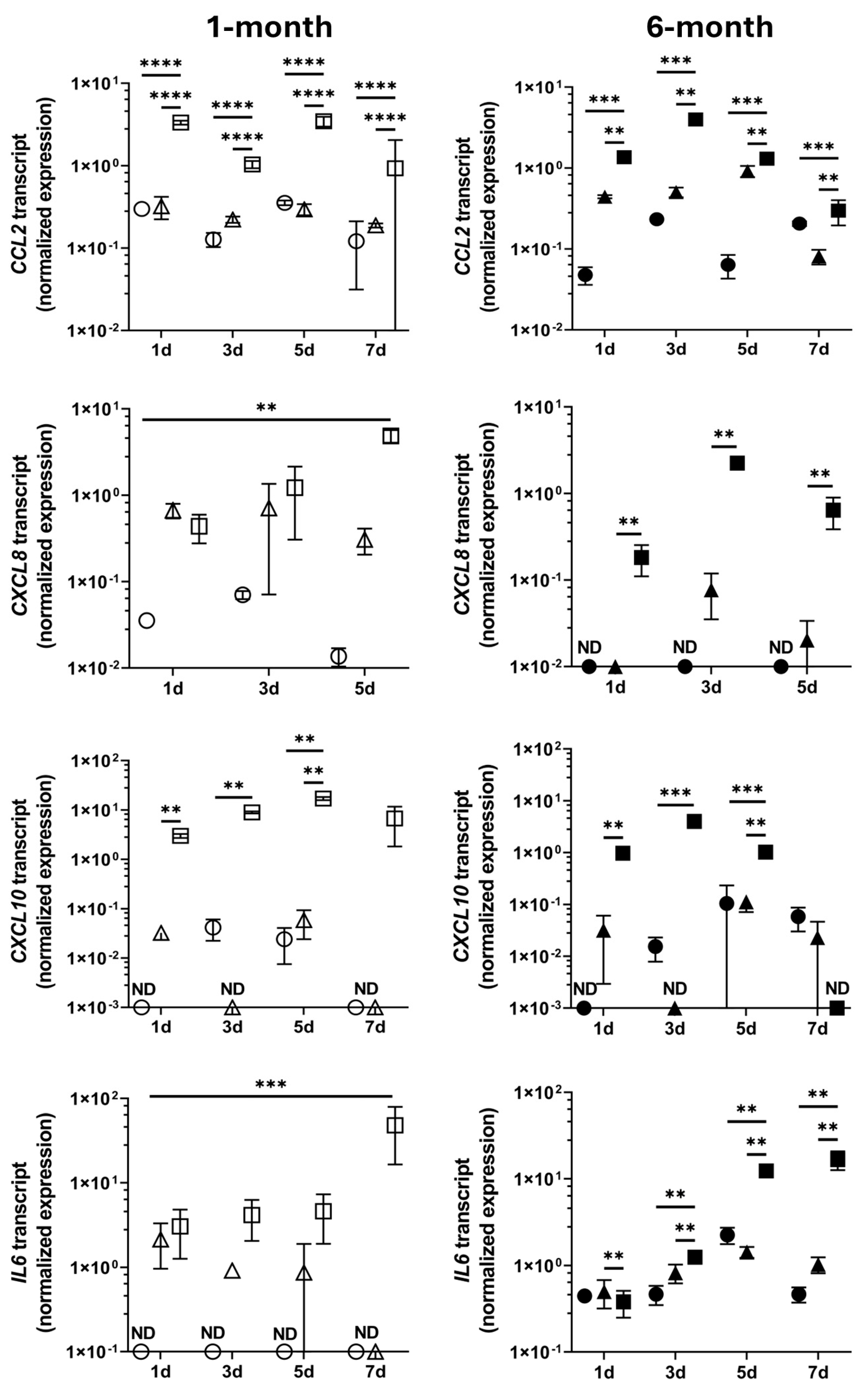
| Name | HGNC Symbol | Description | References |
|---|---|---|---|
| Human Cell Transcripts | |||
| Marker of proliferation ki-67 | MKI67 | Nuclear protein expressed in proliferating cells | [21,22,23] |
| Paired box 6 | PAX6 | Transcription factor involved in maintaining retinal progenitor cell multipotency | [24,25,26] |
| Visual system homeobox 2 | VSX2 | Transcription factor involved in retinal progenitor identity and proliferation | [27,28] |
| Orthodenticle homeobox 2 | OTX2 | Transcription factor involved in photoreceptor and retinal pigment epithelium differentiation | [29,30] |
| POU class 4 homeobox 1 | POU4F1 | Transcription factor expressed in developing and mature retinal ganglion cell subsets | [31,32] |
| LIM homeobox 1 | LHX1 | Transcription factor expressed in developing and mature horizontal cells | [33,34] |
| Glutamate decarboxylase 2 | GAD2 | Enzyme involved in gamma-aminobutyric acid synthesis in amacrine cells | [35] |
| Protein kinase c alpha | PRKCA | Serine/threonine kinase expressed bipolar cell subsets | [36,37] |
| Rhodopsin | RHO | Visual pigment protein expressed in rod photoreceptors | [38] |
| Recoverin | RCVRN | Calcium-binding protein expressed in rod and cone photoreceptors | [39] |
| Retinaldehyde binding protein 1 | RLBP1 | Visual cycle protein expressed in Müller cells and retinal pigment epithelial cells | [40,41] |
| Retinoid isomerohydrolase RPE65 | RPE65 | Visual cycle protein expressed in retinal pigment epithelial cells | [42] |
| Parasite Transcripts | |||
| Dense granule protein 6 | GRA6 | T. gondii virulence factor and activator of host NFAT4 | [43] |
| Rhoptry protein 16 | ROP16 | T. gondii virulence factor and activator of host STAT3 | [44,45] |
| Surface antigen 1 | SAG1 | T. gondii host cell attachment and invasion protein | [46,47] |
| Gene Transcript | Primer Sequences | Size (bp) | NCBI Accession Number |
|---|---|---|---|
| CCL2 † | FWD: 5′-GCATGAAAGTCTCTGCCGC-3′ REV: 5′-GGACACTTGCTGCTGGTGATT-3′ | 181 | NM_002982.4 |
| CXCL10 † | FWD: 5′-CCTATCTTTCTGACTCTAAGTGGC-3′ REV: 5′-ACGTGGACAAAATTGGCTTG-3′ | 148 | NM_001565.4 |
| CXCL8 † | FWD: 5′-CCAGGAAGAAACCACCGGAA-3′ REV: 5′-TCTCAGCCCTCTTCAAAAACTTC-3′ | 336 | NM_000584.4 |
| GAD2 [48] | FWD: 5′-GCGTGGAGAGGGCCAACTCTG-3′ REV: 5′-CCCGGTAGTCCCCTTTGCCC-3′ | 251 | NM_000818.3 |
| GRA6 † | FWD: 5′-AAGCAGACCCCTTCGGAAAC-3′ REV: 5′-TCTTCAGCTAACGAGTCGCC-3′ | 119 | - |
| IL6 [49] | FWD: 5′-ATGCAATAACCACCCCTGACC-3′ REV: 5′-CCATGCTACATTTGCCGAAGAG-3′ | 160 | NM_000600.5 |
| LHX1 [50] | FWD: 5′-ATGCAACCTGACCGAGAAGT-3′ REV: 5′-CAGGTCGCTAGGGGAGATG-3′ | 121 | NM_005568.5 |
| MKI67 [51] | FWD: 5′-TGACCCTGATGAGAAAGCTCAA-3′ REV: 5′-CCCTGAGCAACACTGTCTTTT-3′ | 141 | NM_002417.5 |
| OTX2 [52] | FWD: 5′-CATGAGGCTGTAAGTTCCAC-3′ REV: 5′-TTGTTTGGAGGTGCAAAGTC-3′ | 126 | NM_021728.4 |
| PAX6 [53] | FWD: 5′-TAAGGATGTTGAACGGGCAG-3′ REV: 5′-TGGTATTCTCTCCCCCTCCT-3′ | 126 | NM_000280.6 |
| PRKCA [52] | FWD: 5′-CCCGACACTGATGACCCCA-3′ REV: 5′-AAGTCCATAGAGCAGTGACCC-3′ | 99 | NM_002737.3 |
| POU4F1 [54] | FWD: 5′-TTTCCTCCACCCATTCTCTG-3′ REV: 5′-ACCCCAGTCCTCAAGGCTA-3′ | 62 | NM_006237.4 |
| RCVRN [52] | FWD: 5′-GGGACCATCAGCAAGAAT-3′ REV: 5′-GATCTTCTCGGCTCGCTTT-3′ | 123 | NM_002903.3 |
| RHO [52] | FWD: 5′-GTCCAGGTACATCCCCG-3′ REV: 5′-ACGAACATGTAGATGACAA-3′ | 102 | NM_000539.3 |
| RLBP1 † | FWD: 5′-CCTCTCTAGTCGGGACAAGTAT-3′ REV: 5′-TCATCTCAAGCAGCCCTTTC-3′ | 607 | NM_000326.4 |
| ROP16 † | FWD: 5′-GAGCCAATACGGTCTGCCAT-3′ REV: 5′-CACAGCTCGCTGGAAGAGAA-3′ | 187 | - |
| RPE65 † | FWD: 5′-CTGGAGCCTGAAGTTCTCTTT-3′ REV: 5′-AAGATGGGTTCTGATGGGTATG-3′ | 209 | NM_000329.2 |
| RPLP0 [55] | FWD: 5′-GCAGCATCTACAACCCTGAA-3′ REV: 5′-GCAGATGGATCAGCCAAGAA-3′ | 235 | NM_053275.3 |
| SAG1 [56] | FWD: 5′-GCTGTAACATTGAGCTCCTTGASTTCCTG-3′ REV: 5′-CCGGAACAGTACTGATTGTTGTCTTGAG-3′ | 350 | - |
| VSX2 [53] | FWD: 5′-ACCAGACCAAGAAACGGAAG-3′ REV: 5′-GGGTAGTGGGCTTCGTTG-3′ | 97 | NM_182894.3 |
| YWHAZ [57] | FWD: 5′-ACTTTTGGTACATTGTGGCTTCAA-3′ REV: 5′-CCGCCAGGACAAACCAGTAT-3′ | 98 | NM_003406.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashander, L.M.; Lidgerwood, G.E.; Lumsden, A.L.; Furtado, J.M.; Pébay, A.; Smith, J.R. Human Retinal Organoid Model of Ocular Toxoplasmosis. Pathogens 2025, 14, 286. https://doi.org/10.3390/pathogens14030286
Ashander LM, Lidgerwood GE, Lumsden AL, Furtado JM, Pébay A, Smith JR. Human Retinal Organoid Model of Ocular Toxoplasmosis. Pathogens. 2025; 14(3):286. https://doi.org/10.3390/pathogens14030286
Chicago/Turabian StyleAshander, Liam M., Grace E. Lidgerwood, Amanda L. Lumsden, João M. Furtado, Alice Pébay, and Justine R. Smith. 2025. "Human Retinal Organoid Model of Ocular Toxoplasmosis" Pathogens 14, no. 3: 286. https://doi.org/10.3390/pathogens14030286
APA StyleAshander, L. M., Lidgerwood, G. E., Lumsden, A. L., Furtado, J. M., Pébay, A., & Smith, J. R. (2025). Human Retinal Organoid Model of Ocular Toxoplasmosis. Pathogens, 14(3), 286. https://doi.org/10.3390/pathogens14030286








