Repurposing COVID-19 Compounds (via MMV COVID Box): Almitrine and Bortezomib Induce Programmed Cell Death in Trypanosoma cruzi
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Culture Cells
2.3. Trypanocidal Activity
2.4. Cytotoxic Activity
2.5. Variations in ATP Levels
2.6. Mitochondrial Membrane Potential Disruption
2.7. Reactive Oxygen Species (ROS) Observation
2.8. Plasmatic Membrane Permeability
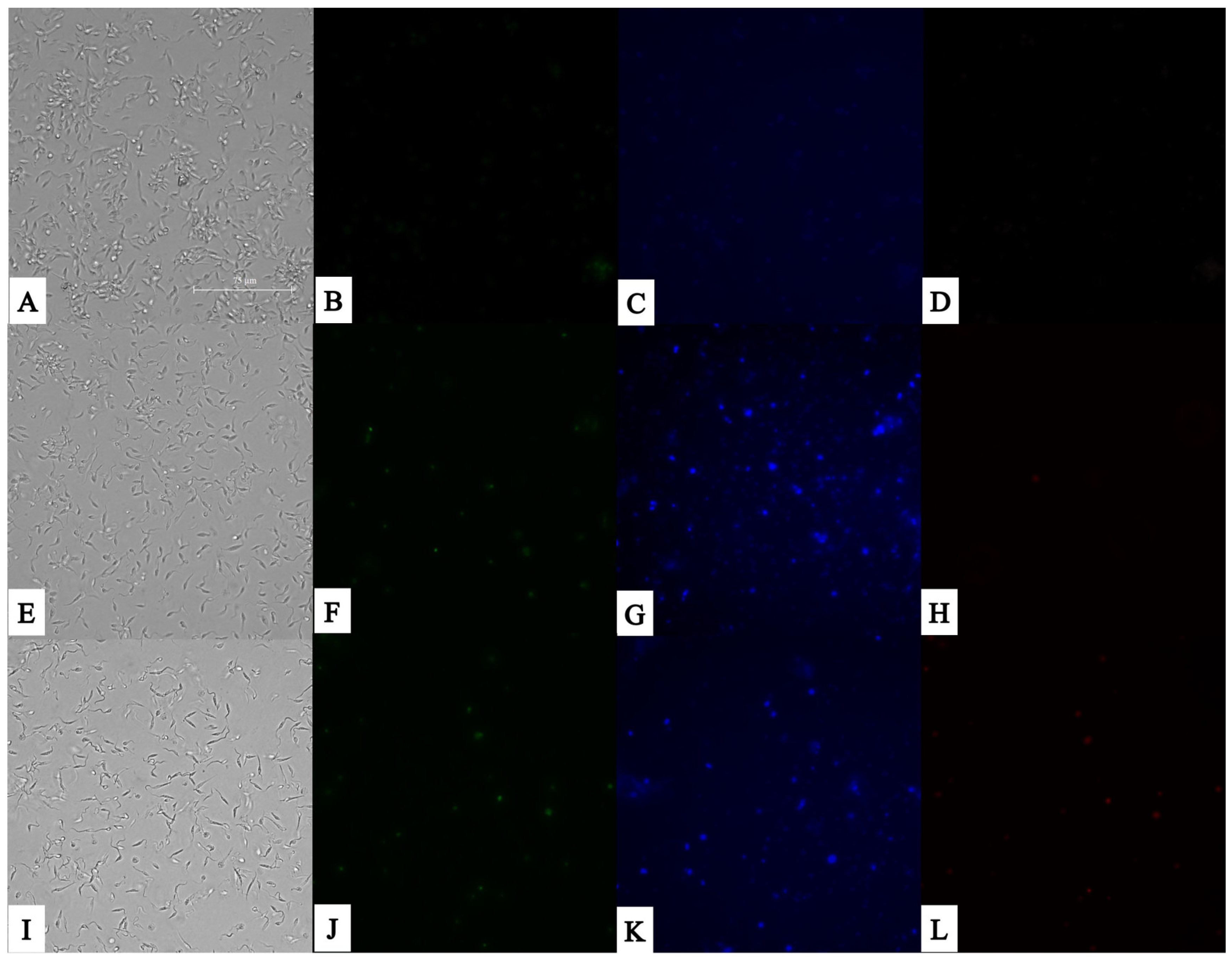
2.9. Chromatin Condensation Evaluation
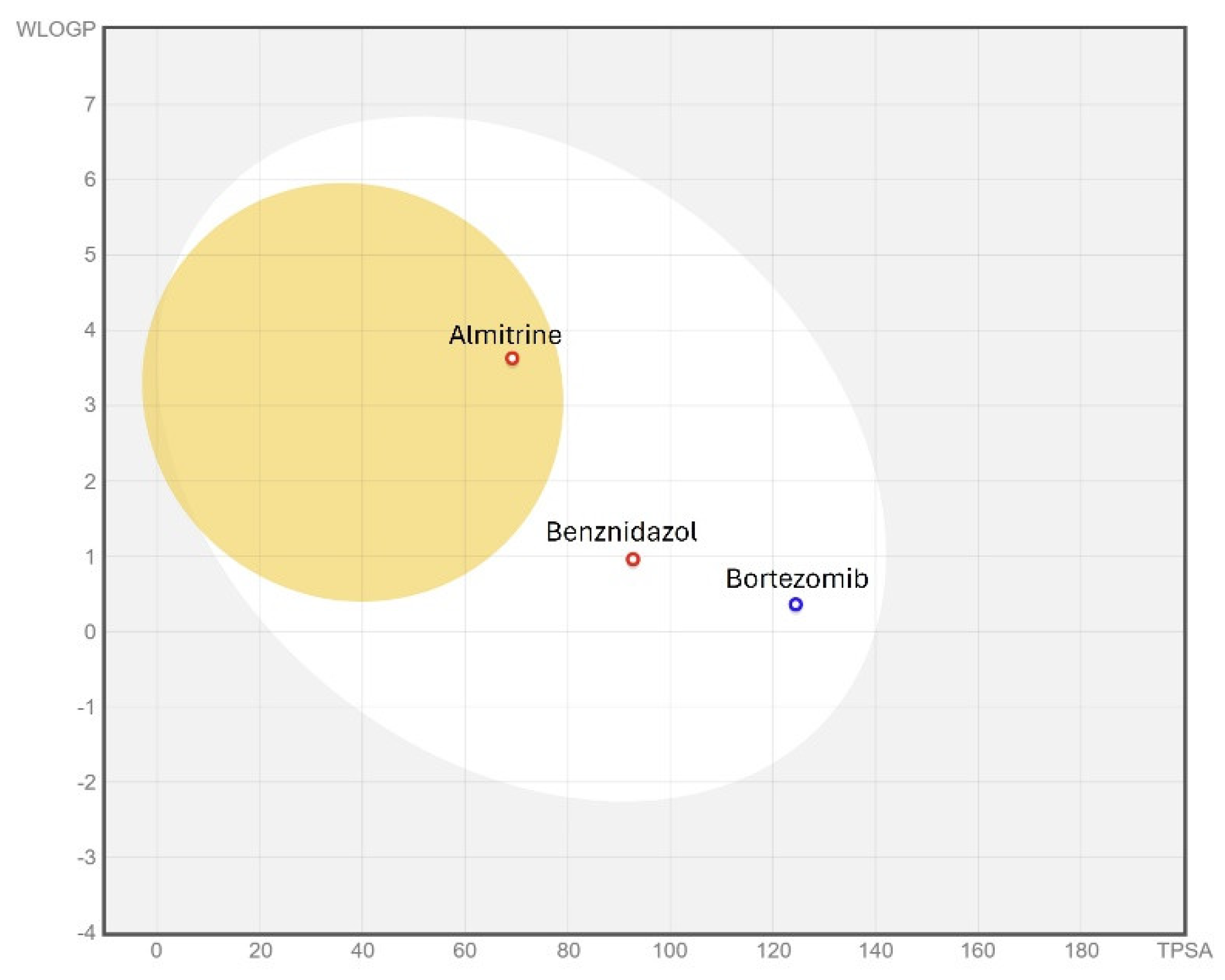
2.10. ADME-Tox Predictions
2.11. Statistical Analyses
3. Results
3.1. Trypanocidal and Cytotoxic Activity
3.2. Plasmatic Membrane Permeability and Chromatin Condensation Evaluation
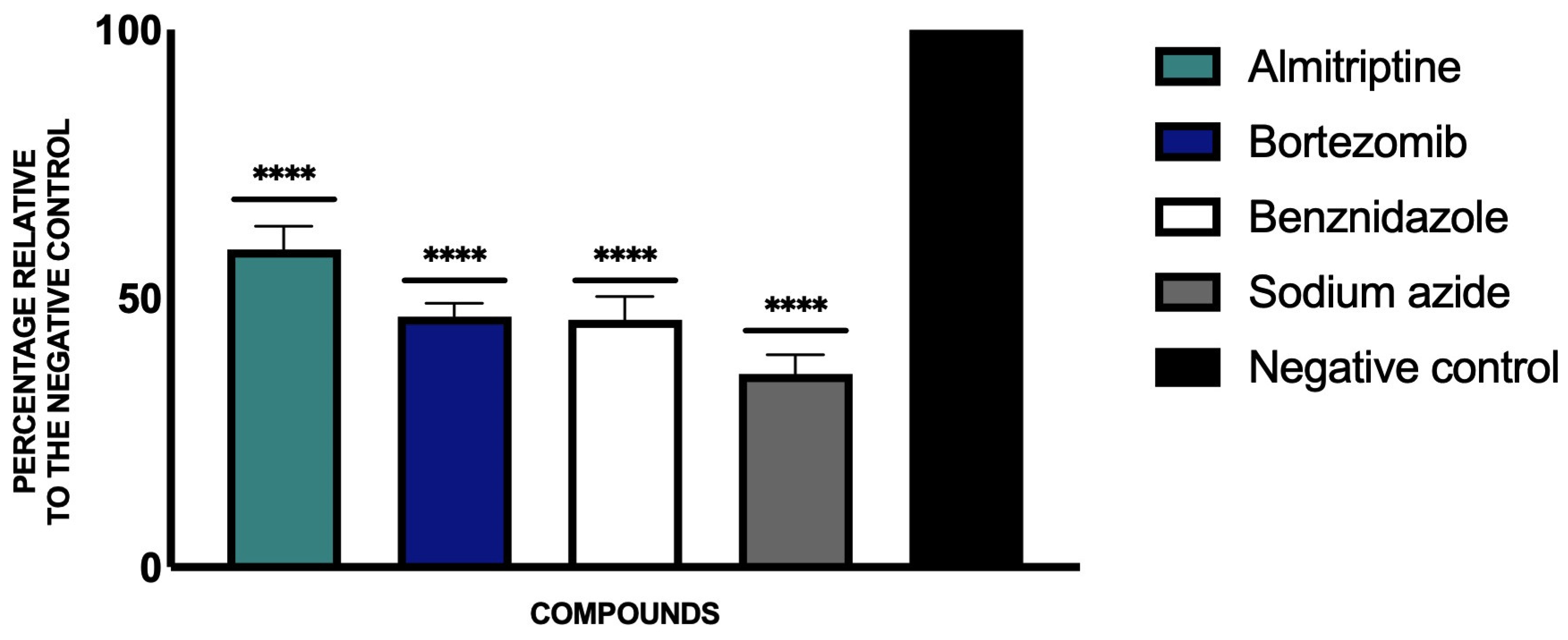
3.3. Variations in ATP Levels
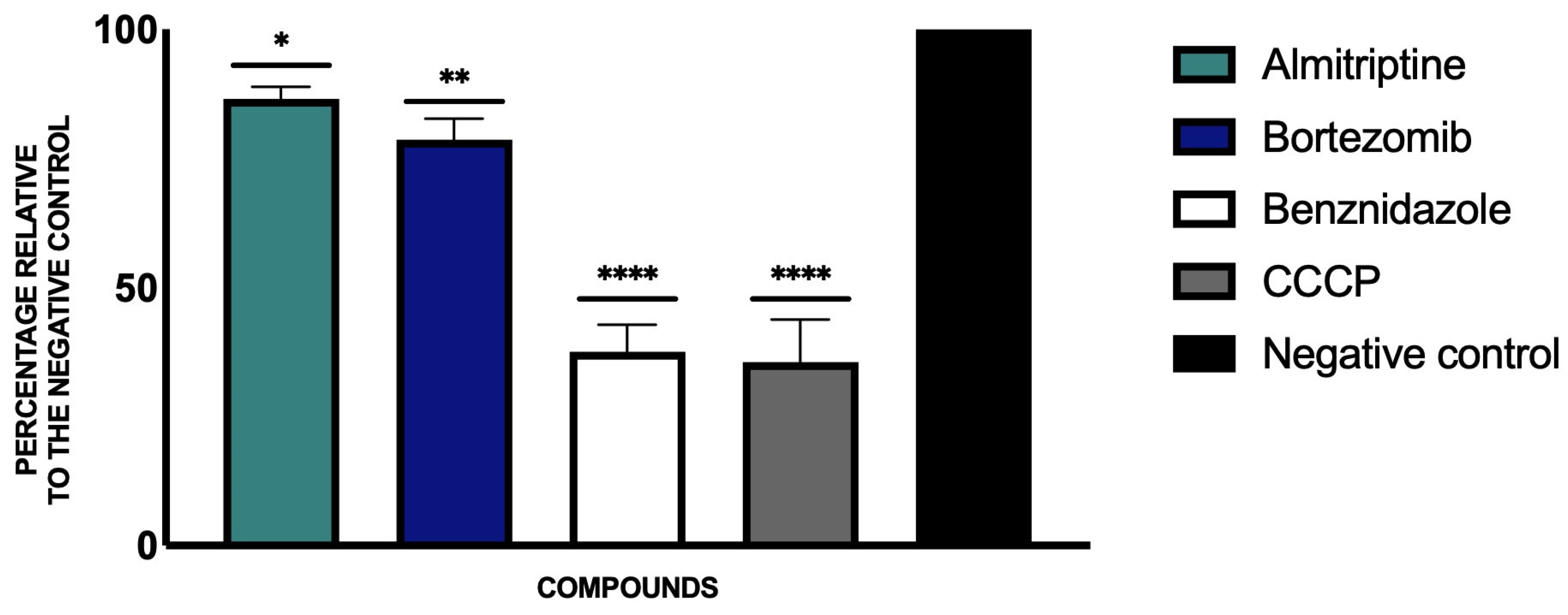
3.4. Mitochondrial Membrane Potential Disruption
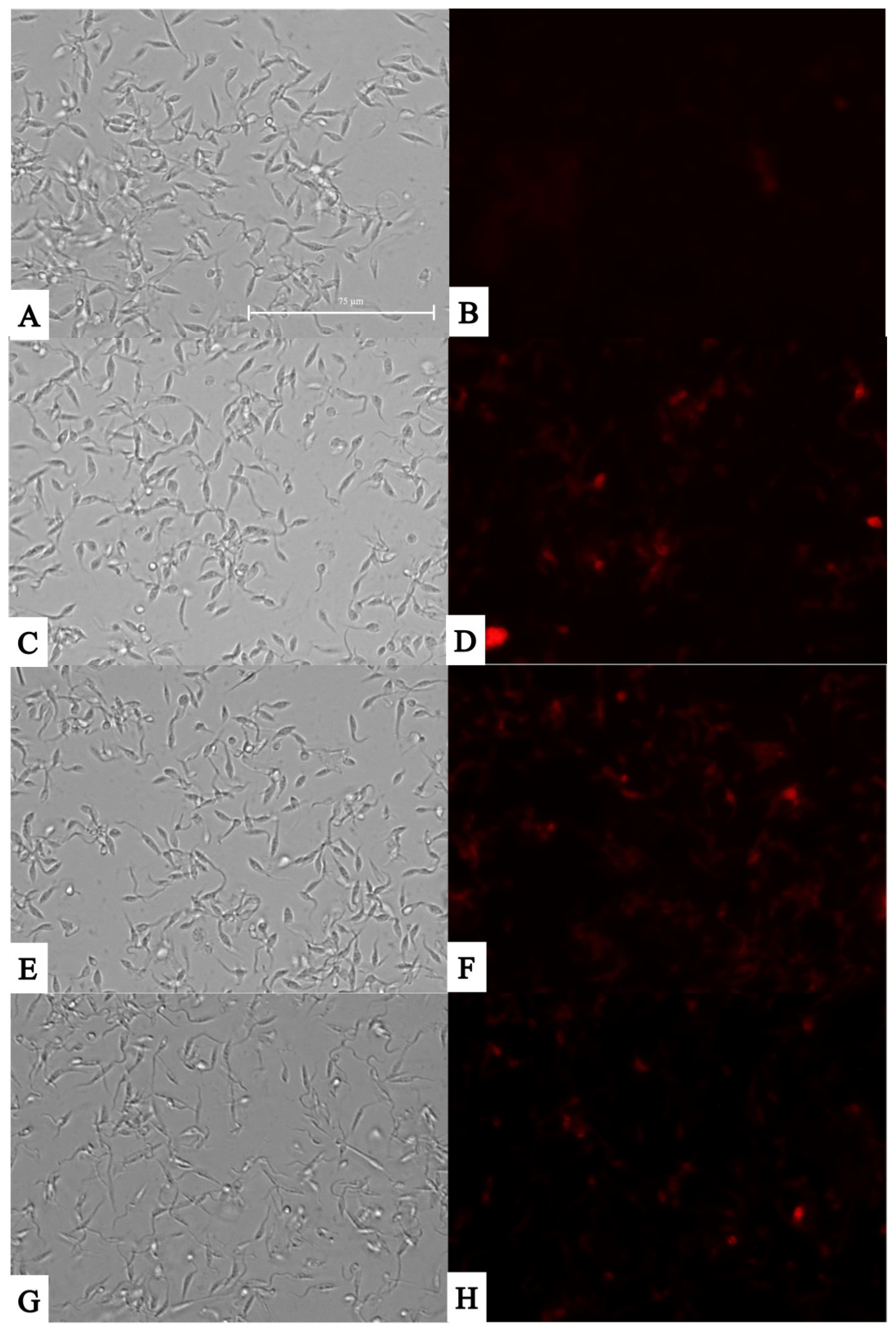
3.5. Reactive Oxygen Species (ROS) Observation
3.6. ADME/Tox Predictions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Chagas Disease (American Trypanosomiasis). Available online: https://www.who.int/health-topics/chagas-disease#tab=tab_1 (accessed on 18 September 2024).
- Bern, C. Chagas’ Disease. N. Engl. J. Med. 2015, 373, 456–466. [Google Scholar] [CrossRef]
- Rassi, A.; Rassi, A.; Marin-Neto, J.A. Chagas Disease. Lancet 2010, 375, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Jackson, Y.; Alirol, E.; Getaz, L.; Wolff, H.; Combescure, C.; Chappuis, F. Tolerance and Safety of Nifurtimox in Patients with Chronic Chagas Disease. Clin. Infect. Dis. 2010, 51, e69–e75. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.R.; Taylor, M.C.; Horn, D.; Kelly, J.M.; Cheeseman, I. A Mechanism for Cross-Resistance to Nifurtimox and Benznidazole in Trypanosomes. Proc. Natl. Acad. Sci. USA 2008, 105, 5022–5027. [Google Scholar] [CrossRef] [PubMed]
- Andrews, K.T.; Fisher, G.; Skinner-Adams, T.S. Drug Repurposing and Human Parasitic Protozoan Diseases. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 95–111. [Google Scholar] [CrossRef]
- Almeida-Paes, R.; de Andrade, I.B.; Ramos, M.L.M.; Rodrigues, M.V.d.A.; do Nascimento, V.A.; Bernardes-Engemann, A.R.; Frases, S. Medicines for Malaria Venture COVID Box: A Source for Repurposing Drugs with Antifungal Activity against Human Pathogenic Fungi. Mem. Inst. Oswaldo Cruz 2021, 116, e210207. [Google Scholar] [CrossRef] [PubMed]
- López-Arencibia, A.; Bethencourt-Estrella, C.J.; San Nicolás-Hernández, D.; Lorenzo-Morales, J.; Piñero, J.E. Anti-COVID Drugs (MMV COVID Box) as Leishmanicidal Agents: Unveiling New Therapeutic Horizons. Pharmaceuticals 2024, 17, 266. [Google Scholar] [CrossRef]
- Chao-Pellicer, J.; Arberas-Jiménez, I.; Sifaoui, I.; Piñero, J.E.; Lorenzo-Morales, J. Exploring Therapeutic Approaches against Naegleria Fowleri Infections through the COVID Box. Int. J. Parasitol. Drugs Drug Resist. 2024, 25, 100545. [Google Scholar] [CrossRef]
- Roy, A.; Ganguly, A.; BoseDasgupta, S.; Das, B.B.; Pal, C.; Jaisankar, P.; Majumder, H.K. Mitochondria-Dependent Reactive Oxygen Species-Mediated Programmed Cell Death Induced by 3,3’-Diindolylmethane through Inhibition of F0F1-ATP Synthase in Unicellular Protozoan Parasite Leishmania Donovani. Mol. Pharmacol. 2008, 74, 1292–1307. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Mukherjee, S.B.; Shaha, C. Hydrogen Peroxide Induces Apoptosis-like Death in Leishmania Donovani Promastigotes. J. Cell Sci. 2001, 114, 2461–2469. [Google Scholar] [CrossRef] [PubMed]
- Reece, S.E.; Pollitt, L.C.; Colegrave, N.; Gardner, A. The Meaning of Death: Evolution and Ecology of Apoptosis in Protozoan Parasites. PLoS Pathog. 2011, 7, e1002320. [Google Scholar] [CrossRef] [PubMed]
- Cartuche, L.; Sifaoui, I.; López-Arencibia, A.; Bethencourt-Estrella, C.J.; San Nicolás-Hernández, D.; Lorenzo-Morales, J.; Piñero, J.E.; Díaz-Marrero, A.R.; Fernández, J.J. Antikinetoplastid Activity of Indolocarbazoles from Streptomyces Sanyensis. Biomolecules 2020, 10, 657. [Google Scholar] [CrossRef] [PubMed]
- San Nicolás-Hernández, D.; Hernández-Álvarez, E.; Bethencourt-Estrella, C.J.; López-Arencibia, A.; Sifaoui, I.; Bazzocchi, I.L.; Lorenzo-Morales, J.; Jiménez, I.A.; Piñero, J.E. Multi-Target Withaferin-A Analogues as Promising Anti-Kinetoplastid Agents through the Programmed Cell Death. Biomed. Pharmacother. 2023, 164, 114879. [Google Scholar] [CrossRef] [PubMed]
- Zeouk, I.; Sifaoui, I.; López-Arencibia, A.; Reyes-Batlle, M.; Bethencourt-Estrella, C.J.; Bazzocchi, I.L.; Bekhti, K.; Lorenzo-Morales, J.; Jiménez, I.A.; Piñero, J.E. Sesquiterpenoids and Flavonoids from Inula Viscosa Induce Programmed Cell Death in Kinetoplastids. Biomed. Pharmacother. 2020, 130, 110518. [Google Scholar] [CrossRef] [PubMed]
- Bethencourt-Estrella, C.J.; Nocchi, N.; López-Arencibia, A.; San Nicolás-Hernández, D.; Souto, M.L.; Suárez-Gómez, B.; Díaz-Marrero, A.R.; Fernández, J.J.; Lorenzo-Morales, J.; Piñero, J.E. Antikinetoplastid Activity of Sesquiterpenes Isolated from the Zoanthid Palythoa Aff. Clavata. Pharmaceuticals 2021, 14, 1095. [Google Scholar] [CrossRef]
- Nicolás-Hernández, D.S.; Rodríguez-Expósito, R.L.; López-Arencibia, A.; Bethencourt-Estrella, C.J.; Sifaoui, I.; Salazar-Villatoro, L.; Omaña-Molina, M.; Fernández, J.J.; Díaz-Marrero, A.R.; Piñero, J.E.; et al. Meroterpenoids from Gongolaria Abies-Marina against Kinetoplastids: In Vitro Activity and Programmed Cell Death Study. Pharmaceuticals 2023, 16, 476. [Google Scholar] [CrossRef] [PubMed]
- Bethencourt-Estrella, C.J.; Delgado-Hernández, S.; López-Arencibia, A.; San Nicolás-Hernández, D.; Tejedor, D.; García-Tellado, F.; Lorenzo-Morales, J.; Piñero, J.E. In Vitro Activity and Cell Death Mechanism Induced by Acrylonitrile Derivatives against Leishmania Amazonensis. Bioorg. Chem. 2022, 124, 105872. [Google Scholar] [CrossRef]
- Bethencourt-Estrella, C.J.; López-Arencibia, A.; Lorenzo-Morales, J.; Piñero, J.E. Global Health Priority Box: Discovering Flucofuron as a Promising Antikinetoplastid Compound. Pharmaceuticals 2024, 17, 554. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Finch, A.; Pillans, P. P-Glycoprotein and Its Role in Drug-Drug Interactions. Aust. Prescr. 2014, 37, 137–139. [Google Scholar] [CrossRef]
- Goldwaser, E.; Laurent, C.; Lagarde, N.; Fabrega, S.; Nay, L.; Villoutreix, B.O.; Jelsch, C.; Nicot, A.B.; Loriot, M.-A.; Miteva, M.A. Machine Learning-Driven Identification of Drugs Inhibiting Cytochrome P450 2C9. PLoS Comput. Biol. 2022, 18, e1009820. [Google Scholar] [CrossRef] [PubMed]
- Reigada, C.; Sayé, M.; Valera-Vera, E.; Miranda, M.R.; Pereira, C.A. Repurposing of Terconazole as an Anti Trypanosoma Cruzi Agent. Heliyon 2019, 5, e01947. [Google Scholar] [CrossRef] [PubMed]
- Menna-Barreto, R.F.S. Cell Death Pathways in Pathogenic Trypanosomatids: Lessons of (over)Kill. Cell Death Dis. 2019, 10, 93. [Google Scholar] [CrossRef]
- Bruchhaus, I.; Roeder, T.; Rennenberg, A.; Heussler, V.T. Protozoan Parasites: Programmed Cell Death as a Mechanism of Parasitism. Trends Parasitol. 2007, 23, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.; Hayden, P.J.; Howman, A.; Wheatley, K.; Coyne, I. Bortezomib for the Treatment of Multiple Myeloma. In Cochrane Database of Systematic Reviews; Scott, K., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2013. [Google Scholar]
- Muñoz, C.; San Francisco, J.; Gutiérrez, B.; González, J. Role of the Ubiquitin-Proteasome Systems in the Biology and Virulence of Protozoan Parasites. Biomed. Res. Int. 2015, 2015, 141526. [Google Scholar] [CrossRef]
- López-López, J.R.; Pérez-García, M.T.; Canet, E.; Gonzalez, C. Effects of Almitrine Bismesylate on the Ionic Currents of Chemoreceptor Cells from the Carotid Body. Mol. Pharmacol. 1998, 53, 330–339. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, B.R.; Ramos, A.B.d.S.B.; de Menezes, R.P.B.; Scotti, M.T.; Colombo, F.A.; Marques, M.J.; Reimão, J.Q. Repurposing the Medicines for Malaria Venture’s COVID Box to Discover Potent Inhibitors of Toxoplasma Gondii, and in Vivo Efficacy Evaluation of Almitrine Bismesylate (MMV1804175) in Chronically Infected Mice. PLoS ONE 2023, 18, e0288335. [Google Scholar] [CrossRef]
- Sifaoui, I.; Rodríguez-Expósito, R.L.; Reyes-Batlle, M.; Sutak, R.; Piñero, J.E.; Lorenzo-Morales, J. Amoebicidal Effect of COVID Box Molecules against Acanthamoeba: A Study of Cell Death. Pharmaceuticals 2024, 17, 808. [Google Scholar] [CrossRef] [PubMed]
- Steverding, D.; Wang, X. Trypanocidal Activity of the Proteasome Inhibitor and Anti-Cancer Drug Bortezomib. Parasit. Vectors 2009, 2, 29. [Google Scholar] [CrossRef]
- Benaim, G.; Sanders, J.M.; Garcia-Marchán, Y.; Colina, C.; Lira, R.; Caldera, A.R.; Payares, G.; Sanoja, C.; Burgos, J.M.; Leon-Rossell, A.; et al. Amiodarone Has Intrinsic Anti-Trypanosoma ruzi Activity and Acts Synergistically with Posaconazole. J. Med. Chem. 2006, 49, 892–899. [Google Scholar] [CrossRef]
- Urbina, J.A.; Payares, G.; Sanoja, C.; Lira, R.; Romanha, A.J. In Vitro and in Vivo Activities of Ravuconazole on Trypanosoma Cruzi, the Causative Agent of Chagas Disease. Int. J. Antimicrob. Agents 2003, 21, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, R.; Bélec, L.; Louarn, F. Almitrine-Induced Peripheral Neuropathy and Weight Loss. J. Neurol. 1989, 236, 374. [Google Scholar] [CrossRef]
- Basco, L.K.; Le Bras, J. In Vitro Activity of Mitochondrial ATP Synthetase Inhibitors Against Plasmodium falciparum. J. Eukaryot. Microbiol. 1994, 41, 179–183. [Google Scholar] [CrossRef]
- Reynolds, J.M.; El Bissati, K.; Brandenburg, J.; Günzl, A.; Mamoun, C. Ben Antimalarial Activity of the Anticancer and Proteasome Inhibitor Bortezomib and Its Analog ZL3B. BMC Clin. Pharmacol. 2007, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Jalovecka, M.; Hartmann, D.; Miyamoto, Y.; Eckmann, L.; Hajdusek, O.; O’Donoghue, A.J.; Sojka, D. Validation of Babesia Proteasome as a Drug Target. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 394–402. [Google Scholar] [CrossRef]

| Compound | Structure | Biological Activity Group | % of Inhibition |
|---|---|---|---|
| Cycloheximide | 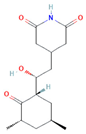 | Fungicidal agent (agriculture) | 61–70% |
| Almitrine | 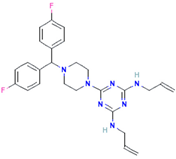 | Respiratory system agent | 61–70% |
| Bortezomib | 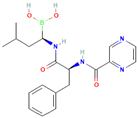 | Antitumor agent (multiple myeloma) | 61–70% |
| (-)-Anisomycin | 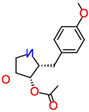 | Anti-infective agent (antibiotic) | 51–60% |
| Delanzomib |  | Antitumor agent (multiple myeloma) | 51–60% |
| Nitazoxanide | 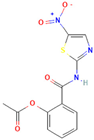 | Anti-infective agent (antiparasitic) | 61–70% |
| Manidipine | 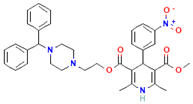 | Cardiovascular agent (antihypertensive) | 71–80% |
| SAX-187 |  | Nervous system agent (antipsychotic) | 71–80% |
| ABT-239 | 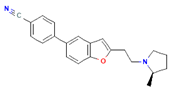 | Nervous system agent | 51–60% |
| Compound | IC50 Epimastigote Stage of T. cruzi | IC50 Amastigote Stage of T. cruzi | CC50 Murine Macrophages |
|---|---|---|---|
| Almitrine | 10.38 ± 0.42 | 1.62 ± 0.35 | >200 |
| Bortezomib | 30.35 ± 1.30 | 2.79 ± 0.28 | >250 |
| ABT239 | 80.11 ± 0.91 | - | 68.48 ± 2.57 |
| Benznidazole | 6.92 ± 0.77 | 2.67 ± 0.39 | 399.91 ± 1.4 |
| Compound | SI Epimastigote Stage of T. cruzi | SI Amastigote Stage of T. cruzi |
|---|---|---|
| Almitrine | >19.3 | >123.5 |
| Bortezomib | >8.2 | >89.6 |
| ABT239 | 0.9 | - |
| Benznidazole | 57.8 | 149.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bethencourt-Estrella, C.J.; López-Arencibia, A.; Lorenzo-Morales, J.; Piñero, J.E. Repurposing COVID-19 Compounds (via MMV COVID Box): Almitrine and Bortezomib Induce Programmed Cell Death in Trypanosoma cruzi. Pathogens 2025, 14, 127. https://doi.org/10.3390/pathogens14020127
Bethencourt-Estrella CJ, López-Arencibia A, Lorenzo-Morales J, Piñero JE. Repurposing COVID-19 Compounds (via MMV COVID Box): Almitrine and Bortezomib Induce Programmed Cell Death in Trypanosoma cruzi. Pathogens. 2025; 14(2):127. https://doi.org/10.3390/pathogens14020127
Chicago/Turabian StyleBethencourt-Estrella, Carlos J., Atteneri López-Arencibia, Jacob Lorenzo-Morales, and José E. Piñero. 2025. "Repurposing COVID-19 Compounds (via MMV COVID Box): Almitrine and Bortezomib Induce Programmed Cell Death in Trypanosoma cruzi" Pathogens 14, no. 2: 127. https://doi.org/10.3390/pathogens14020127
APA StyleBethencourt-Estrella, C. J., López-Arencibia, A., Lorenzo-Morales, J., & Piñero, J. E. (2025). Repurposing COVID-19 Compounds (via MMV COVID Box): Almitrine and Bortezomib Induce Programmed Cell Death in Trypanosoma cruzi. Pathogens, 14(2), 127. https://doi.org/10.3390/pathogens14020127







