Venomous Cargo: Diverse Toxin-Related Proteins Are Associated with Extracellular Vesicles in Parasitoid Wasp Venom
Abstract
1. Introduction
2. Methods and Materials
2.1. Venom Gland Morphology
2.2. Annotation
2.3. Subcellular Localization Signals
2.4. Enrichment Analysis
2.5. Multiple Sequence Alignment
2.6. Venom Toxins
2.7. Bacterial ARTs
3. Results and Discussion
3.1. Venom Glands
3.2. Lb and Lh Venom Particles Share Proteomic Profiles
3.2.1. General Findings
3.2.2. Enrichment Analysis of Venom Particle Proteins and Localization Signals
3.2.3. Species-Unique Domains
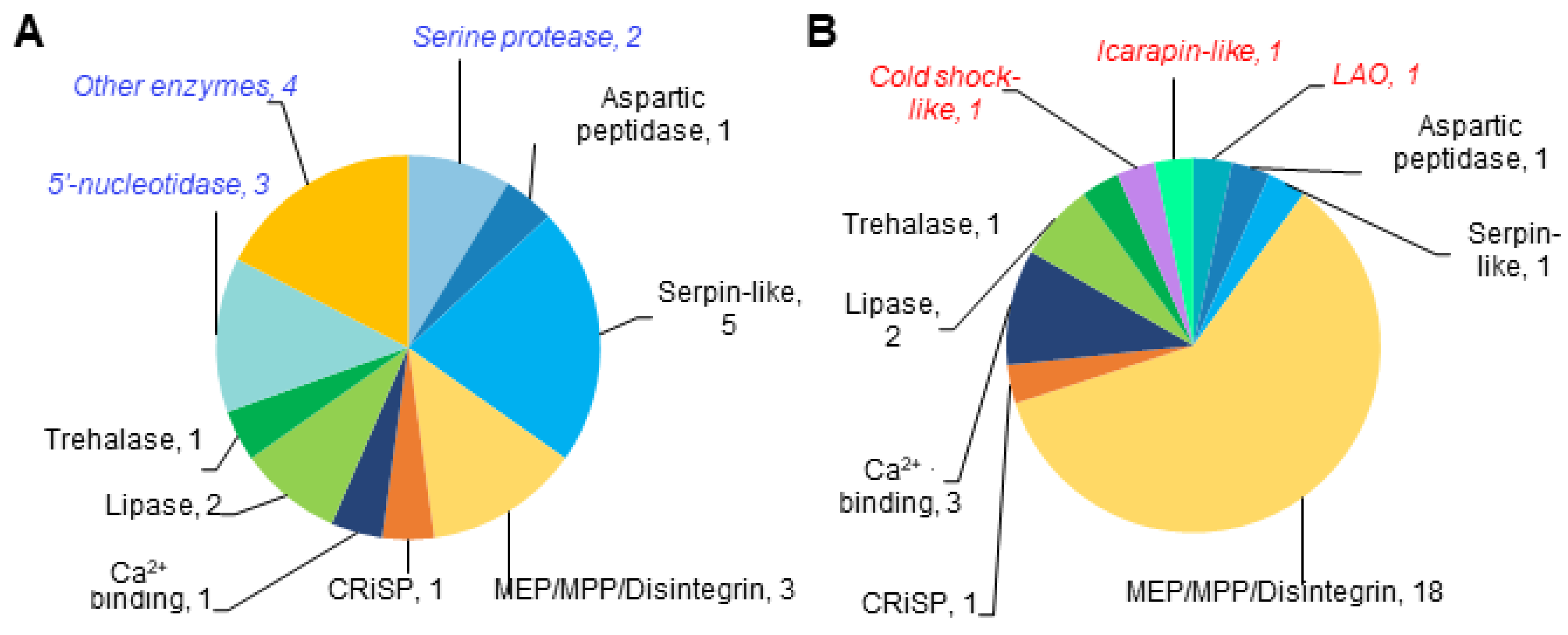
3.3. Many Toxins Are Present in Both Proteomes
3.3.1. Toxin-like Domains Are Present in Both Venom Particle Proteomes
3.3.2. Species-Unique Toxin-like Domains
3.4. Bacterial ART-like Proteins Are Present in the Venom Particle Proteomes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burke, G.R.; Sharanowski, B.J. Parasitoid wasps. Curr. Biol. 2024, 34, R483–R488. [Google Scholar] [CrossRef] [PubMed]
- Lue, C.H.; Buffington, M.L.; Scheffer, S.; Lewis, M.; Elliot, T.A.; Lindsey, A.R.I.; Driskell, A.; Jandova, A.; Kimura, M.T.; Carton, Y.; et al. DROP: Molecular voucher database for identification of Drosophila parasitoids. Mol. Ecol. Resour. 2021, 21, 2437–2454. [Google Scholar] [CrossRef]
- Kim-Jo, C.; Gatti, J.L.; Poirié, M. Drosophila cellular immunity against parasitoid wasps: A complex and time-dependent process. Front. Physiol. 2019, 10, 603. [Google Scholar] [CrossRef] [PubMed]
- Nappi, A.; Poirié, M.; Carton, Y. The role of melanization and cytotoxic side-products in the cellular immune responses of Drosophila against parasitic wasps. Adv. Parasitol. 2009, 70, 99–121. [Google Scholar] [PubMed]
- Salvia, R.; Cozzolino, F.; Scieuzo, C.; Grimaldi, A.; Franco, A.; Vinson, S.B.; Monti, M.; Falabella, P. Identification and functional characterization of Toxoneuron nigriceps ovarian proteins involved in the early suppression of host immune response. Insects 2022, 13, 144. [Google Scholar] [CrossRef]
- Strand, M.R.; Burke, G.R. Polydnaviruses: Evolution and function. Curr. Issues Mol. Biol. 2020, 34, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Rizki, R.M.; Rizki, T.M. Selective destruction of a host blood cell type by a parasitoid wasp. Proc. Natl. Acad. Sci. USA 1984, 81, 6154–6158. [Google Scholar] [CrossRef]
- Moreau, S.J.M.; Asgari, S. Venom proteins from parasitoid wasps and their biological functions. Toxins 2015, 7, 2385–2412. [Google Scholar] [CrossRef] [PubMed]
- Volkoff, A.-N.; Cusson, M. The unconventional viruses of ichneumonid parasitoid wasps. Viruses 2020, 12, 1170. [Google Scholar] [CrossRef]
- Drezen, J.-M.; Bézier, A.; Burke, G.R.; Strand, M.R. Bracoviruses, ichnoviruses, and virus-like particles from parasitoid wasps retain many features of their virus ancestors. Curr. Opin. Insect Sci. 2022, 49, 93–100. [Google Scholar] [CrossRef]
- Quicke, D.L.J.; Butcher, B.A. Review of venoms of non-polydnavirus carrying ichneumonoid wasps. Biology 2021, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Heavner, M.E.; Hudgins, A.D.; Rajwani, R.; Morales, J.; Govind, S. Harnessing the natural Drosophila-parasitoid model for integrating insect immunity with functional venomics. Curr. Opin. Insect Sci. 2014, 6, 61–67. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Poirié, M.; Colinet, D.; Gatti, J.-L. Insights into function and evolution of parasitoid wasp venoms. Curr. Opin. Insect Sci. 2014, 6, 52–60. [Google Scholar] [CrossRef]
- Schlenke, T.A.; Morales, J.; Govind, S.; Clark, A.G. Contrasting infection strategies in generalist and specialist wasp parasitoids of Drosophila melanogaster. PLoS Pathog. 2007, 3, 1486–1501. [Google Scholar] [CrossRef] [PubMed]
- Wan, B.; Goguet, E.; Ravallec, M.; Pierre, O.; Lemauf, S.; Volkoff, A.-N.; Gatti, J.-L.; Poirié, M. Venom atypical extracellular vesicles as interspecies vehicles of virulence factors involved in host specificity: The case of a Drosophila parasitoid wasp. Front. Immunol. 2019, 10, 1688. [Google Scholar] [CrossRef]
- Heavner, M.E.; Ramroop, J.; Gueguen, G.; Ramrattan, G.; Dolios, G.; Scarpati, M.; Kwiat, J.; Bhattacharya, S.; Wang, R.; Singh, S.; et al. Novel organelles with elements of bacterial and eukaryotic secretion systems weaponize parasites of Drosophila. Curr. Biol. 2017, 27, 2869–2877.e2866. [Google Scholar] [CrossRef]
- Wey, B.; Heavner, M.E.; Wittmeyer, K.T.; Briese, T.; Hopper, K.R.; Govind, S. Immune suppressive extracellular vesicle proteins of Leptopilina heterotoma are encoded in the wasp genome. G3 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rizki, R.M.; Rizki, T.M. Parasitoid virus-like particles destroy Drosophila cellular immunity. Proc. Natl. Acad. Sci. USA 1990, 87, 8388–8392. [Google Scholar] [CrossRef]
- Gueguen, G.; Rajwani, R.; Paddibhatla, I.; Morales, J.; Govind, S. VLPs of Leptopilina boulardi share biogenesis and overall stellate morphology with VLPs of the heterotoma clade. Virus Res. 2011, 160, 159–165. [Google Scholar] [CrossRef]
- Chiu, H.; Morales, J.; Govind, S. Identification and immuno-electron microscopy localization of p40, a protein component of immunosuppressive virus-like particles from Leptopilina heterotoma, a virulent parasitoid wasp of Drosophila. J. Gen. Virol. 2006, 87, 461–470. [Google Scholar] [CrossRef]
- Rizki, T.M.; Rizki, R.M. Parasitoid-induced cellular immune deficiency in Drosophila. Ann. N. Y Acad. Sci. 1994, 712, 178–194. [Google Scholar] [CrossRef] [PubMed]
- Wey, B. Insights into Leptopilina spp. immune-suppressive strategies using mixed-omics and molecular approaches. Ph.D. Thesis, CUNY Academic Works, New York, NY, USA, 2021. [Google Scholar]
- Colinet, D.; Schmitz, A.; Depoix, D.; Crochard, D.; Poirié, M. Convergent use of RhoGAP toxins by eukaryotic parasites and bacterial pathogens. PLoS Pathog. 2007, 3, e203. [Google Scholar] [CrossRef]
- Wan, B.; Poirié, M.; Gatti, J.-L. Parasitoid wasp venom vesicles (venosomes) enter Drosophila melanogaster lamellocytes through a flotillin/lipid raft-dependent endocytic pathway. Virulence 2020, 11, 1512–1521. [Google Scholar] [CrossRef] [PubMed]
- Colinet, D.; Schmitz, A.; Cazes, D.; Gatti, J.-L.; Poirié, M. The origin of intraspecific variation of virulence in an eukaryotic immune suppressive parasite. PLoS Pathog. 2010, 6, e1001206. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chiu, H.; Govind, S. Natural infection of D. melanogaster by virulent parasitic wasps induces apoptotic depletion of hematopoietic precursors. Cell Death Differ. 2002, 9, 1379–1381. [Google Scholar] [CrossRef] [PubMed]
- Ramroop, J.R.; Heavner, M.E.; Razzak, Z.H.; Govind, S. A parasitoid wasp of Drosophila employs preemptive and reactive strategies to deplete its host’s blood cells. PLoS Pathog. 2021, 17, e1009615. [Google Scholar] [CrossRef]
- Beer, K.B.; Wehman, A.M. Mechanisms and functions of extracellular vesicle release in vivo—What we can learn from flies and worms. Cell Adh Migr. 2017, 11, 135–150. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of exosome composition. Cell 2019, 177, 428–445.e418. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Lim, H.J.; Yoon, H.; Kim, H.; Kang, Y.-W.; Kim, J.-E.; Kim, O.Y.; Lee, E.-Y.; Twizere, J.-C.; Rak, J.; Kim, D.-K. Extracellular vesicle proteomes shed light on the evolutionary, interactive, and functional divergence of their biogenesis mechanisms. Front. Cell Dev. Biol. 2021, 9, 734950. [Google Scholar] [CrossRef]
- Heavner, M.E.; Gueguen, G.; Rajwani, R.; Pagan, P.E.; Small, C.; Govind, S. Partial venom gland transcriptome of a Drosophila parasitoid wasp, Leptopilina heterotoma, reveals novel and shared bioactive profiles with stinging Hymenoptera. Gene 2013, 526, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Small, C.; Paddibhatla, I.; Rajwani, R.; Govind, S. An introduction to parasitic wasps of Drosophila and the antiparasite immune response. J. Vis. Exp. 2012, 63, e3347. [Google Scholar]
- Di Giovanni, D.; Lepetit, D.; Guinet, B.; Bennetot, B.; Boulesteix, M.; Couté, Y.; Bouchez, O.; Ravallec, M.; Varaldi, J. A behavior-manipulating virus relative as a source of adaptive genes for Drosophila parasitoids. Mol. Biol. Evol. 2020, 1, 2791–2807. [Google Scholar] [CrossRef]
- Goecks, J.; Mortimer, N.T.; Mobley, J.A.; Bowersock, G.J.; Taylor, J.; Schlenke, T.A. Integrative approach reveals composition of endoparasitoid wasp venoms. PLoS ONE 2013, 8, e64125. [Google Scholar] [CrossRef] [PubMed]
- Käll, L.; Krogh, A.; Sonnhammer, E.L.L. A combined transmembrane topology and signal peptide prediction method. J. Mol. Biol. 2004, 338, 1027–1036. [Google Scholar] [CrossRef]
- Käll, L.; Krogh, A.; Sonnhammer, E.L.L. Advantages of combined transmembrane topology and signal peptide prediction—The Phobius web server. Nucleic Acids Res. 2007, 35, W429–W432. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H. Predicting secretory proteins with SignalP. Methods Mol. Biol. 2017, 1611, 59–73. [Google Scholar] [PubMed]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Sonnhammer, E.L.L.; von Heijne, G.; Krogh, A. A hidden Markov model for predicting transmembrane helices in protein sequences. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1998, 6, 175–182. [Google Scholar]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef]
- Savojardo, C.; Bruciaferri, N.; Tartari, G.; Martelli, P.L.; Casadio, R. DeepMito: Accurate prediction of protein sub-mitochondrial localization using convolutional neural networks. Bioinformatics 2019, 36, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Kalra, H.; Simpson, R.J.; Ji, H.; Aikawa, E.; Altevogt, P.; Askenase, P.; Bond, V.C.; Borràs, F.E.; Breakefield, X.; Budnik, V. Vesiclepedia: A compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012, 10, e1001450. [Google Scholar] [CrossRef] [PubMed]
- Pathan, M.; Fonseka, P.; Chitti, S.V.; Kang, T.; Sanwlani, R.; Van Deun, J.; Hendrix, A.; Mathivanan, S. Vesiclepedia 2019: A compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res. 2019, 47, D516–D519. [Google Scholar] [CrossRef]
- Pathan, M.; Keerthikumar, S.; Chisanga, D.; Alessandro, R.; Ang, C.-S.; Askenase, P.; Batagov, A.O.; Benito-Martin, A.; Camussi, G.; Clayton, A.; et al. A novel community driven software for functional enrichment analysis of extracellular vesicles data. J. Extracell. Vesicles 2017, 6, 1321455. [Google Scholar] [CrossRef] [PubMed]
- Di Tommaso, P.; Moretti, S.; Xenarios, I.; Orobitg, M.; Montanyola, A.; Chang, J.-M.; Taly, J.-F.; Notredame, C. T-Coffee: A web server for the multiple sequence alignment of protein and RNA sequences using structural information and homology extension. Nucleic Acids Res. 2011, 39, W13–W17. [Google Scholar] [CrossRef] [PubMed]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef] [PubMed]
- Jungo, F.; Bairoch, A. Tox-Prot, the toxin protein annotation program of the Swiss-Prot protein knowledgebase. Toxicon 2005, 45, 293–301. [Google Scholar] [CrossRef]
- Jungo, F.; Bougueleret, L.; Xenarios, I.; Poux, S. The UniProtKB/Swiss-Prot Tox-Prot program: A central hub of integrated venom protein data. Toxicon 2012, 60, 551–557. [Google Scholar] [CrossRef]
- Kaminski, K.; Ludwiczak, J.; Pawlicki, K.; Alva, V.; Dunin-Horkawicz, S. pLM-BLAST: Distant homology detection based on direct comparison of sequence representations from protein language models. Bioinformatics 2023, 39, btad579. [Google Scholar] [CrossRef]
- Huang, J.; Chen, J.; Fang, G.; Pang, L.; Zhou, S.; Zhou, Y.; Pan, Z.; Zhang, Q.; Sheng, Y.; Lu, Y.; et al. Two novel venom proteins underlie divergent parasitic strategies between a generalist and a specialist parasite. Nat. Commun. 2021, 12, 234. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A better web interface. Nucleic Acids Res. 2008, 36, W5–W9. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Holm, L.; Laiho, A.; Törönen, P.; Salgado, M. DALI shines a light on remote homologs: One hundred discoveries. Protein Sci. 2023, 32, e4519. [Google Scholar] [CrossRef] [PubMed]
- Schrodinger, LLC. The PyMol Molecular Graphics System, Version 3.0.2; Schrodinger, LLC: New York, NY, USA, 2010. [Google Scholar]
- Heinig, M.; Frishman, D. STRIDE: A web server for secondary structure assignment from known atomic coordinates of proteins. Nucleic Acids Res. 2004, 32, W500–W502. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Thornton, J.M. PDBsum extras: SARS-CoV-2 and AlphaFold models. Protein Sci. 2022, 31, 283–289. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Toda, A.; Tsurumura, T.; Yoshida, T.; Tsumori, Y.; Tsuge, H. Rho GTPase recognition by C3 exoenzyme based on C3-RhoA complex structure. J. Biol. Chem. 2015, 290, 19423–19432. [Google Scholar] [CrossRef]
- Yoshida, T.; Tsuge, H. Common mechanism for target specificity of protein- and DNA-targeting ADP-ribosyltransferases. Toxins 2021, 13, 40. [Google Scholar] [CrossRef]
- Vogelsgesang, M.; Pautsch, A.; Aktories, K. C3 exoenzymes, novel insights into structure and action of Rho-ADP-ribosylating toxins. Naunyn Schmiedebergs Arch. Pharmacol. 2007, 374, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.; Chiu, H.; Oo, T.; Plaza, R.; Hoskins, S.; Govind, S. Biogenesis, structure, and immune-suppressive effects of virus-like particles of a Drosophila parasitoid, Leptopilina victoriae. J. Insect Physiol. 2005, 51, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Ferrarese, R.; Morales, J.; Fimiarz, D.; Webb, B.A.; Govind, S. A supracellular system of actin-lined canals controls biogenesis and release of virulence factors in parasitoid venom glands. J. Exp. Biol. 2009, 212, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- Labrosse, C.; Eslin, P.; Doury, G.; Drezen, J.M.; Poirié, M. Haemocyte changes in D. melanogaster in response to long gland components of the parasitoid wasp Leptopilina boulardi: A Rho-GAP protein as an important factor. J. Insect Physiol. 2005, 51, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.; Buffolo, F.; Schlotter, F.; Atkins, S.K.; Lee, L.H.; Halu, A.; Blaser, M.C.; Tsolaki, E.; Higashi, H.; Luther, K.; et al. Annexin A1-dependent tethering promotes extracellular vesicle aggregation revealed with single-extracellular vesicle analysis. Sci. Adv. 2020, 6, eabb1244. [Google Scholar] [CrossRef]
- Skryabin, G.O.; Komelkov, A.V.; Galetsky, S.A.; Bagrov, D.V.; Evtushenko, E.G.; Nikishin, I.I.; Zhordaniia, K.I.; Savelyeva, E.E.; Akselrod, M.E.; Paianidi, I.G.; et al. Stomatin is highly expressed in exosomes of different origin and is a promising candidate as an exosomal marker. J. Cell Biochem. 2021, 122, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Wang, J.; Deng, R.; Wang, X. Autographa californica multiple nucleopolyhedrovirus gene ac81 is required for nucleocapsid envelopment. Virus Res. 2016, 221, 47–57. [Google Scholar] [CrossRef]
- Ho, T.N.T.; Turner, A.; Pham, S.H.; Nguyen, H.T.; Nguyen, L.T.T.; Nguyen, L.T.; Dang, T.T. Cysteine-rich peptides: From bioactivity to bioinsecticide applications. Toxicon 2023, 230, 107173. [Google Scholar] [CrossRef]
- Arguelles, J.; Lee, J.; Cardenas, L.V.; Govind, S.; Singh, S. In silico analysis of a Drosophila parasitoid venom peptide reveals prevalence of the cation-polar-cation clip motif in knottin proteins. Pathogens 2023, 12, 143. [Google Scholar] [CrossRef]
- Chlastáková, A.; Kotál, J.; Beránková, Z.; Kaščáková, B.; Martins, L.A.; Langhansová, H.; Prudnikova, T.; Ederová, M.; Kutá Smatanová, I.; Kotsyfakis, M.; et al. Iripin-3, a new salivary protein isolated from Ixodes ricinus ticks, displays immunomodulatory and anti-hemostatic properties in vitro. Front. Immunol. 2021, 12, 626200. [Google Scholar] [CrossRef]
- Parkinson, N.M.; Conyers, C.; Keen, J.; MacNicoll, A.; Smith, I.; Audsley, N.; Weaver, R. Towards a comprehensive view of the primary structure of venom proteins from the parasitoid wasp Pimpla hypochondriaca. Insect Biochem. Mol. Biol. 2004, 34, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, B.; Hu, J.; Yang, W.; Cao, Z.; Zhuo, R.; Li, W.; Wu, Y. SjAPI, the first functionally characterized Ascaris-type protease inhibitor from animal venoms. PLoS ONE 2013, 8, e57529. [Google Scholar] [CrossRef] [PubMed]
- Undheim, E.A.B.; Sunagar, K.; Herzig, V.; Kely, L.; Low, D.H.W.; Jackson, T.N.W.; Jones, A.; Kurniawan, N.; King, G.F.; Ali, S.A.; et al. A proteomics and transcriptomics investigation of the venom from the barychelid spider Trittame loki (brush-foot trapdoor). Toxins 2013, 5, 2488–2503. [Google Scholar] [CrossRef] [PubMed]
- Deshimaru, M.; Ichihara, M.; Hattori, T.; Koba, K.; Terada, S. Primary structure of brevilysin L4, an enzymatically active fragment of a disintegrin precursor from Gloydius halys brevicaudus venom. Toxicon 2005, 45, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Price, D.R.G.; Bell, H.A.; Hinchliffe, G.; Fitches, E.; Weaver, R.; Gatehouse, J.A. A venom metalloproteinase from the parasitic wasp Eulophus pennicornis is toxic towards its host, tomato moth (Lacanobia oleracae). Insect Mol. Biol. 2009, 18, 195–202. [Google Scholar] [CrossRef]
- Pantera, B.; Hoffman, D.R.; Carresi, L.; Cappugi, G.; Turillazzi, S.; Manao, G.; Severino, M.; Spadolini, I.; Orsomando, G.; Moneti, G.; et al. Characterization of the major allergens purified from the venom of the paper wasp Polistes gallicus. Biochim. et Biophys. Acta (BBA)—General. Subj. 2003, 1623, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Morrissette, J.; Krätzschmar, J.; Haendler, B.; el-Hayek, R.; Mochca-Morales, J.; Martin, B.M.; Patel, J.R.; Moss, R.L.; Schleuning, W.D.; Coronado, R.; et al. Primary structure and properties of helothermine, a peptide toxin that blocks ryanodine receptors. Biophys. J. 1995, 68, 2280–2288. [Google Scholar] [CrossRef]
- Junqueira-de-Azevedo, I.d.L.M.; Pertinhez, T.; Spisni, A.; Carreño, F.R.; Farah, C.S.; Ho, P.L. Cloning and expression of calglandulin, a new EF-hand protein from the venom glands of Bothrops insularis snake in E. coli. Biochim. Biophys. Acta 2003, 1648, 90–98. [Google Scholar] [CrossRef]
- Corrêa-Netto, C.; Junqueira-de-Azevedo, I.d.L.M.; Silva, D.A.; Ho, P.L.; Leitao-de-Araujo, M.; Alves, M.L.; Sanz, L.; Foguel, D.; Zingali, R.B.; Calvete, J.J. Snake venomics and venom gland transcriptomic analysis of Brazilian coral snakes, Micrurus altirostris and M. corallinus. J. Proteom. 2011, 74, 1795–1809. [Google Scholar] [CrossRef]
- Parkinson, N.M.; Conyers, C.M.; Keen, J.N.; MacNicoll, A.D.; Smith, I.; Weaver, R.J. cDNAs encoding large venom proteins from the parasitoid wasp Pimpla hypochondriaca identified by random sequence analysis. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2003, 134, 513–520. [Google Scholar] [CrossRef]
- Wilkinson, M.C.; Nightingale, D.J.H.; Harrison, R.A.; Wagstaff, S.C. Isolation and characterization of renin-like aspartic-proteases from Echis ocellatus venom. Toxicon 2017, 137, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Flores, M.P.; Fritzen, M.; Reis, C.V.; Chudzinski-Tavassi, A.M. Losac, a factor X activator from Lonomia obliqua bristle extract: Its role in the pathophysiological mechanisms and cell survival. Biochem. Biophys. Res. Commun. 2006, 343, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Flores, M.P.; Furlin, D.; Ramos, O.H.P.; Balan, A.; Konno, K.; Chudzinski-Tavassi, A.M. Losac, the first hemolin that exhibits procogulant activity through selective factor X proteolytic activation. J. Biol. Chem. 2011, 286, 6918–6928. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.Z.; Tablante, A.; Bartoli, F.; Beguin, S.; Hemker, H.C.; Apitz-Castro, R. Expression of biological activity of draculin, the anticoagulant factor from vampire bat saliva, is strictly dependent on the appropriate glycosylation of the native molecule. Biochim. Biophys. Acta 1998, 1425, 291–299. [Google Scholar] [CrossRef]
- Rokyta, D.R.; Lemmon, A.R.; Margres, M.J.; Aronow, K. The venom-gland transcriptome of the eastern diamondback rattlesnake (Crotalus adamanteus). BMC Genom. 2012, 13, 312. [Google Scholar] [CrossRef]
- Grunwald, T.; Bockisch, B.; Spillner, E.; Ring, J.; Bredehorst, R.; Ollert, M.W. Molecular cloning and expression in insect cells of honeybee venom allergen acid phosphatase (Api m 3). J. Allergy Clin. Immunol. 2006, 117, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.R.; Weimer, E.T.; Sakell, R.H.; Schmidt, M. Sequence and characterization of honeybee venom acid phosphatase. J. Allergy Clin. Immunol. 2005, 115, S107. [Google Scholar] [CrossRef]
- Calvete, J.J.; Fasoli, E.; Sanz, L.; Boschetti, E.; Righetti, P.G. Exploring the venom proteome of the western diamondback rattlesnake, Crotalus atrox, via snake venomics and combinatorial peptide ligand library approaches. J. Proteome Res. 2009, 8, 3055–3067. [Google Scholar] [CrossRef]
- Blank, S.; Seismann, H.; Bockisch, B.; Braren, I.; Cifuentes, L.; McIntyre, M.; Rühl, D.; Ring, J.; Bredehorst, R.; Ollert, M.W. Identification, recombinant expression, and characterization of the 100 kDa high molecular weight Hymenoptera venom allergens Api m 5 and Ves v 3. J. Immunol. 2010, 184, 5403–5413. [Google Scholar] [CrossRef]
- Raibekas, A.A.; Massey, V. Primary structure of the snake venom L-amino acid oxidase shows high homology with the mouse B cell interleukin 4-induced Fig1 protein. Biochem. Biophys. Res. Commun. 1998, 248, 476–478. [Google Scholar] [CrossRef]
- Murakawa, M.; Jung, S.K.; Iijima, K.; Yonehara, S.; Apoptosis-inducing protein. AIP, from parasite-infected fish induces apoptosis in mammalian cells by two different molecular mechanisms. Cell Death Differ. 2001, 8, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Wang, X.; Zhang, X.-S.; Grigoriu, S.; Page, R.; Peti, W.; Wood, T.K. Escherichia coli toxin/antitoxin pair MqsR/MqsA regulate toxin CspD. Environ. Microbiol. 2010, 12, 1105–1121. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K.; Zheng, W.; Crooke, E.; Wang, Y.-H.; Inouye, M. CspD, a novel DNA replication inhibitor induced during the stationary phase in Escherichia coli. Mol. Microbiol. 2001, 39, 1572–1584. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.-L.; Li, H.; Wong, K.-K.K.; Jiang, T.; Shaw, P.-C. Location and reduction of icarapin antigenicity by site specific coupling to polyethylene glycol. Protein Pept. Lett. 2012, 19, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Mikolčević, P.; Hloušek-Kasun, A.; Ahel, I.; Mikoč, A. ADP-ribosylation systems in bacteria and viruses. Comput. Struct. Biotechnol. J. 2021, 19, 2366–2383. [Google Scholar] [CrossRef] [PubMed]
- Simon, N.C.; Aktories, K.; Barbieri, J.T. Novel bacterial ADP-ribosylating toxins: Structure and function. Nat. Rev. Microbiol. 2014, 12, 599–611. [Google Scholar] [CrossRef]
- Aktories, K. Bacterial protein toxins that modify host regulatory GTPases. Nat. Rev. Microbiol. 2011, 9, 487–498. [Google Scholar] [CrossRef]
- Laing, S.; Unger, M.; Koch-Nolte, F.; Haag, F. ADP-ribosylation of arginine. Amino Acids 2011, 41, 257–269. [Google Scholar] [CrossRef]
- Hottiger, M.O.; Hassa, P.O.; Luscher, B.; Schuler, H.; Koch-Nolte, F. Toward a unified nomenclature for mammalian ADP-ribosyltransferases. Trends Biochem. Sci. 2010, 35, 208–219. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef]
- Nagendra, K.; Bakkannavar, S.M.; Bhat, V.R.; Sirur, F.M. A review on snake venom extracellular vesicles: Past to present. Toxicon 2024, 244, 107772. [Google Scholar]
- Gill, S.; Catchpole, R.; Forterre, P. Extracellular membrane vesicles in the three domains of life and beyond. FEMS Microbiol. Rev. 2019, 43, 273–303. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Regev-Rudzki, N. Biogenesis of extracellular vesicles from the pathogen perspective: Transkingdom strategies for delivering messages. Curr. Opin. Cell Biol. 2024, 88, 102366. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Forterre, P. Origin of life: LUCA and extracellular membrane vesicles (EMVs). Int. J. Astrobiol. 2016, 15, 7–15. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, J.; Li, M.Z.; Wey, B.; Mumtaz, M.; Ramroop, J.R.; Singh, S.; Govind, S. Venomous Cargo: Diverse Toxin-Related Proteins Are Associated with Extracellular Vesicles in Parasitoid Wasp Venom. Pathogens 2025, 14, 255. https://doi.org/10.3390/pathogens14030255
Chou J, Li MZ, Wey B, Mumtaz M, Ramroop JR, Singh S, Govind S. Venomous Cargo: Diverse Toxin-Related Proteins Are Associated with Extracellular Vesicles in Parasitoid Wasp Venom. Pathogens. 2025; 14(3):255. https://doi.org/10.3390/pathogens14030255
Chicago/Turabian StyleChou, Jennifer, Michael Z. Li, Brian Wey, Mubasshir Mumtaz, Johnny R. Ramroop, Shaneen Singh, and Shubha Govind. 2025. "Venomous Cargo: Diverse Toxin-Related Proteins Are Associated with Extracellular Vesicles in Parasitoid Wasp Venom" Pathogens 14, no. 3: 255. https://doi.org/10.3390/pathogens14030255
APA StyleChou, J., Li, M. Z., Wey, B., Mumtaz, M., Ramroop, J. R., Singh, S., & Govind, S. (2025). Venomous Cargo: Diverse Toxin-Related Proteins Are Associated with Extracellular Vesicles in Parasitoid Wasp Venom. Pathogens, 14(3), 255. https://doi.org/10.3390/pathogens14030255






