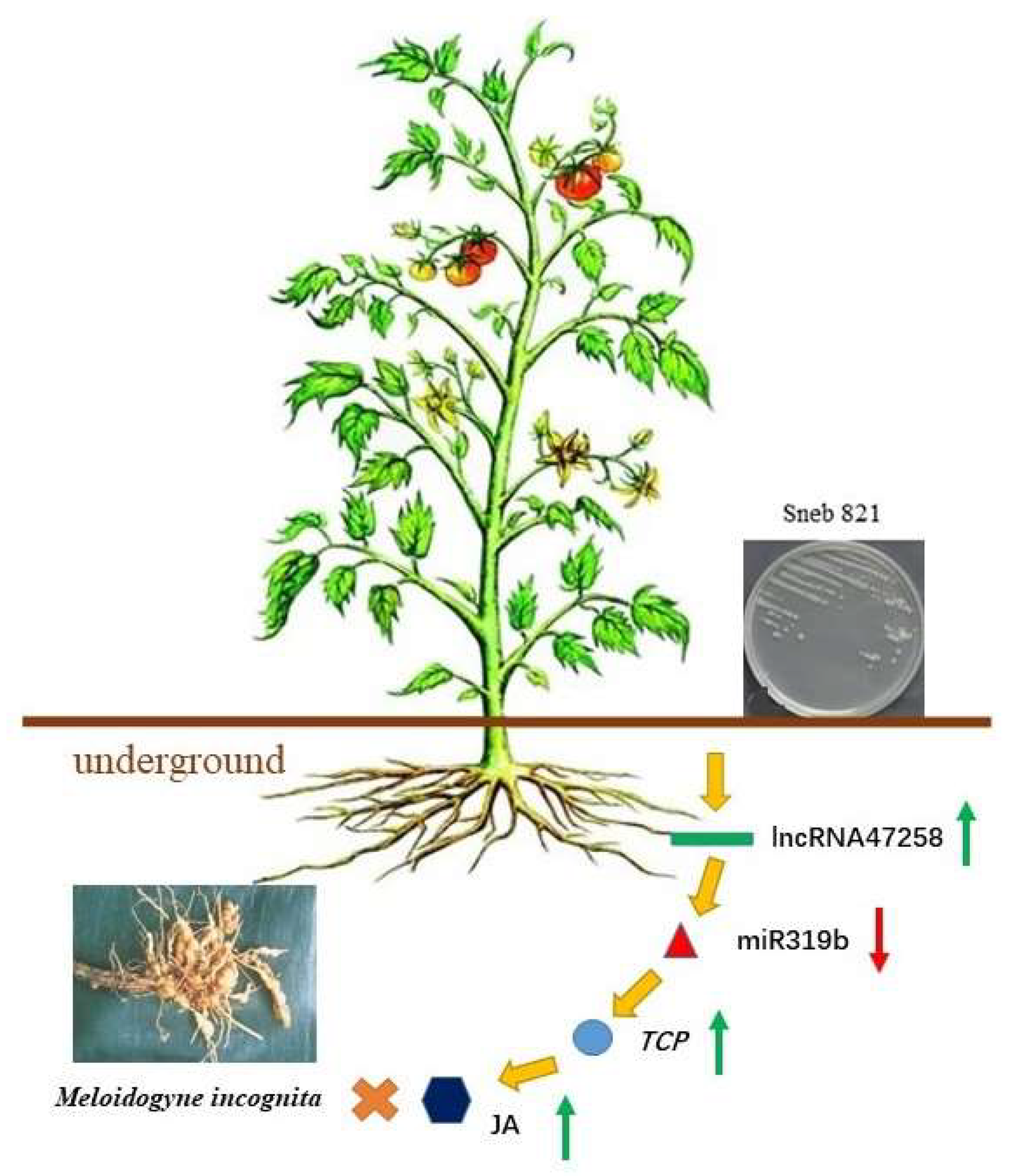
The Tomato lncRNA47258-miR319b-TCP Module in Biocontrol Bacteria Sneb821 Induced Plants Resistance to Meloidogyne incognita
Abstract
1. Introduction
2. Materials and Methods
2.1. Species, Plants, and Nematodes Tested
2.2. Materials for Test
2.3. Total RNA Extraction, Reverse Transcription cDNA, and qPCR Analysis of Tomato
2.4. Amplification of Target Fragments
2.5. E. coli Transformation and Culture of Tomato Hairy Roots
2.6. Determination of Jasmonic Acid Content
2.7. Nematode Infection and Enzyme Activity Detection
2.8. Histochemical Staining and Tomato GUS Staining Were Performed
2.9. Statistical Analysis of Data
3. Results
3.1. RT-PCR Validation of Differential lncRNA in Tomato
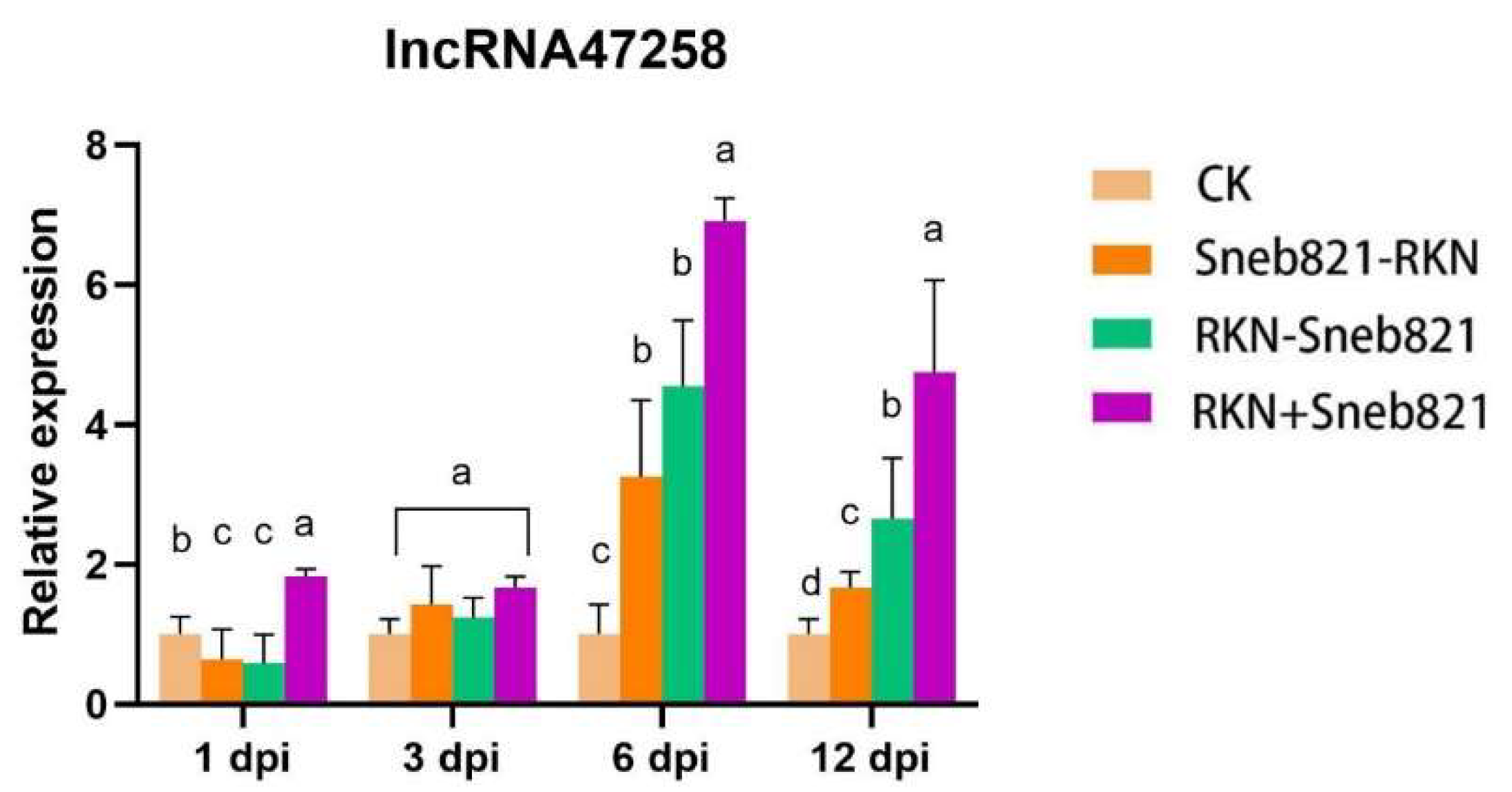
3.2. Expression Analysis of lncRNA47258 in Tomato
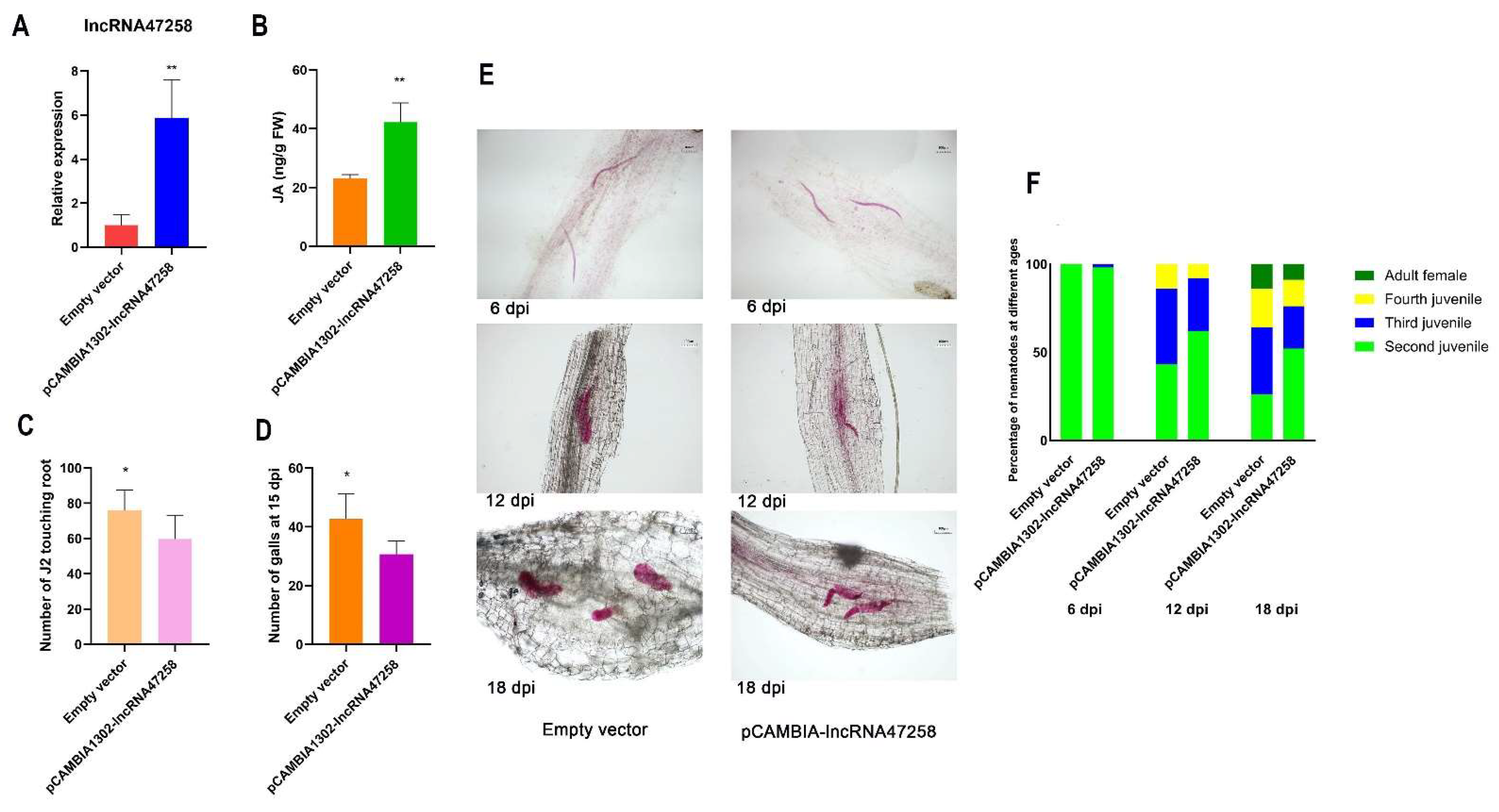
3.3. Construction of lncRNA47258 Overexpressed Tomato Plants
3.4. Overexpression of Tomato lncRNA47258 Inhibited the Infection and Development of M. incognita
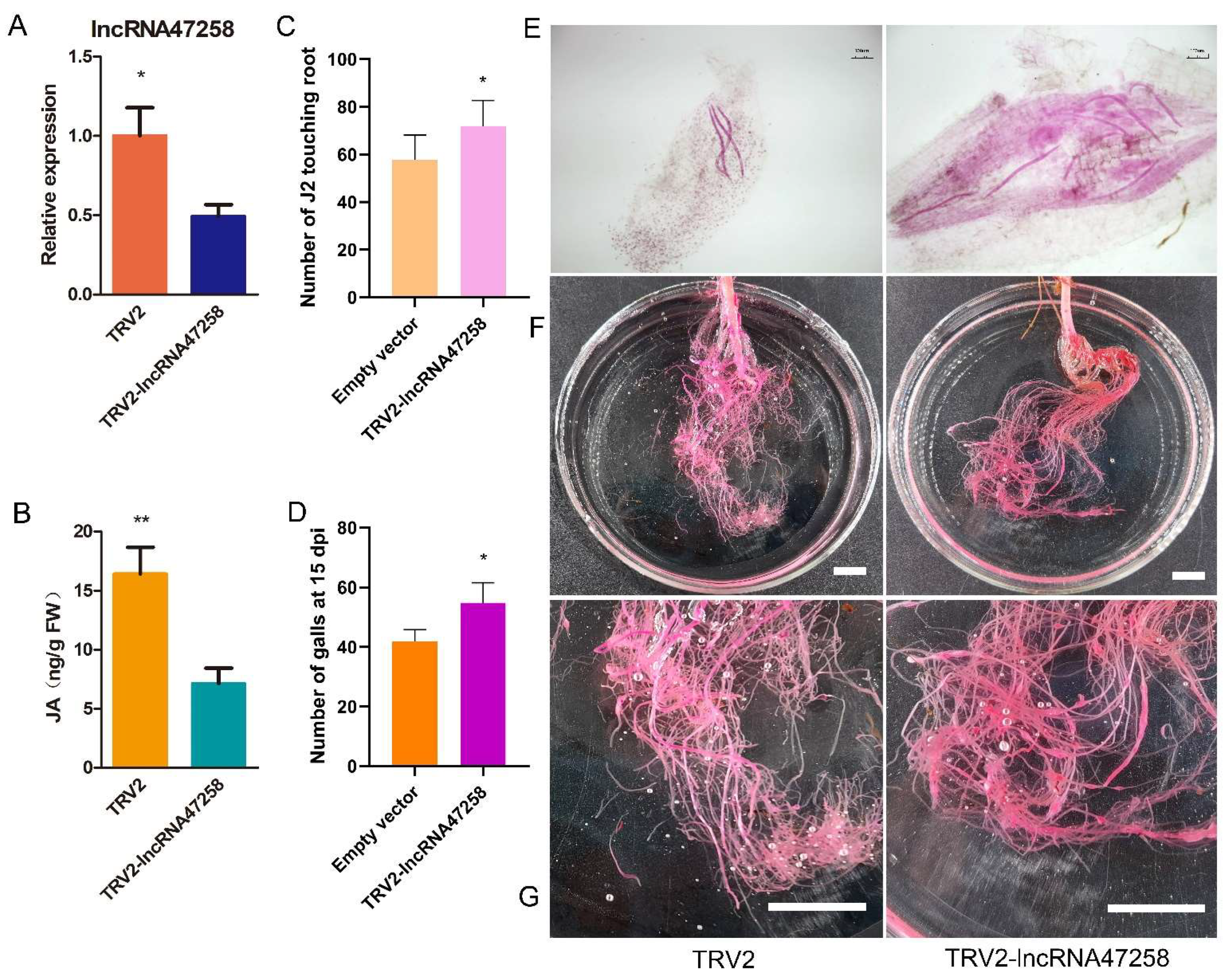
3.5. Construction of lncRNA47258 VIGS Vector in Tomato
3.6. Silencing lncRNA47258 Reduces the Resistance of Tomato to M. incognita
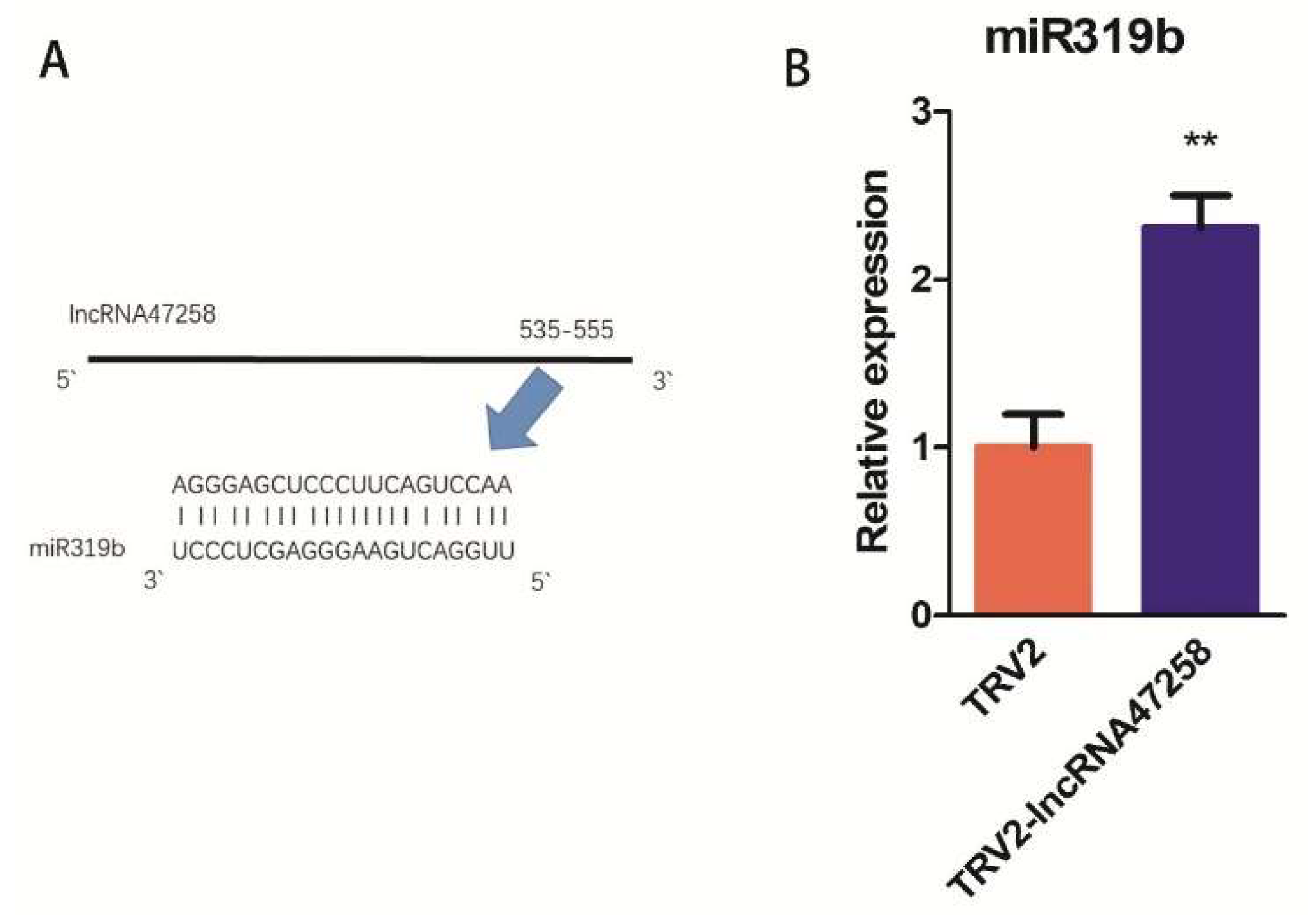
3.7. Verification of Target Sites of Tomato lncRNA47258 and Target Gene TCP (Solyc07g062681.1)
3.8. Construction of Tomato Plants Overexpressing TCP (Solyc07g062681.1), the Target Gene of lncRNA47258
3.9. Overexpression of Tomato TCP (Solyc07g062680.1) Inhibited the Infection and Development of M. incognita
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Catani, L.; Manachini, B.; Grassi, E.; Guidi, L.; Semprucci, F. Essential oils as nematicides in plant protection. Plants 2023, 12, 1418. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; Tomitaka, Y.; Abe, H.; Tsuda, S.; Futai, K.; Mizukubo, T. Expression profile of jasmonic acid-induced genes and the induced resistance against the root-knot nematode in tomato plants after foliar treatment with methyl jasmonate. J. Plant Physiol. 2011, 168, 1084–1097. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicki, A.T. The role of long non-coding RNA in transcriptional gene silencing. Curr. Opin. Plant Biol. 2012, 15, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hu, W.; Hao, J.; Lv, S.; Wang, C.; Tong, W.; Wang, Y.; Wang, Y.; Liu, X.; Ji, W. Genome-wide identification and functional prediction of novel and fungi-responsive lincRNAs in Triticum aestivum. BMC Genom. 2016, 17, 238–250. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, M.; Li, N.; Wang, H.; Qiu, P.; Pei, L.; Xu, Z.; Wang, T.; Gao, E.; Liu, J.; et al. Long noncoding RNAs involve in resistance to Verticillium dahliae, a fungal disease in cotton. Plant Biotechnol. J. 2018, 16, 1172–1185. [Google Scholar] [CrossRef]
- Qin, T.; Zhao, H.; Cui, P. A nucleus-localized long non-coding RNA enhances drought and salt stress tolerance. Plant Physiol. 2017, 175, 1321–1330. [Google Scholar] [CrossRef]
- Wu, J.G.; Yang, R.X.; Yang, Z.R. ROS accumulation and antiviral defence control by microRNA528 in rice. Nat. Plants 2017, 3, 16203. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.; Wang, Y.; Zhao, J.; Wang, Y. Gypsy retrotransposon-derived maize 1 lncRNA GARR2 modulates gibberellin response. Plant J. 2022, 110, 1433–1446. [Google Scholar] [CrossRef]
- Jiang, C.H.; Fan, Z.H.; Li, Z.J.; Niu, D.D.; Li, Y.; Zheng, M.Z.; Wang, Q.; Jin, H.L.; Guo, J.H. Bacillus cereus AR156 triggers induced systemic resistance against Pseudomonas syringae pv. tomato DC3000 by suppressing miR472 and activating CNLs-mediated basal immunity in Arabidopsis. Mol. Plant Pathol. 2020, 496, 1–17. [Google Scholar] [CrossRef]
- Cui, J.; Jiang, N.; Meng, J.; Yang, G.; Liu, W.; Zhou, X.; Ma, N.; Hou, X.; Luan, Y. LncRNA33732-respiratory burst oxidase module associated with WRKY1 in tomato-Phytophthora infestans interactions. Plant J. 2019, 97, 933–946. [Google Scholar] [CrossRef]
- Peng, T.; Liu, X.; Tian, F.; Xu, H.; Yang, F.; Chen, X.; Gao, X.; Lv, Y.; Li, J.; Pana, Y.; et al. Functional investigation of lncRNAs and target cytochrome P450 genes related to spirotetramat resistance in Aphis gossypii Glover. Pest Manag. Sci. 2022, 78, 1982–1991. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Qiu, K.; Sun, W.; Yang, T.; Wu, T.; Song, T.; Zhang, J.; Yao, Y.; Tian, J. A long non-coding RNA functions in high-light-induced anthocyanin accumulation in apple by activating ethylene synthesis. Plant Physiol. 2022, 189, 66–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, J.; Cheng, J.; Sun, Q.; Zhang, Y.; Liu, J.; Li, H.; Zhang, Z.; Wang, P.; Cai, C.; et al. lncRNA7 and lncRNA2 modulate cell wall defense genes to regulate cotton resistance to Verticillium wilt. Plant Physiol. 2022, 189, 264–284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shen, J.; Xu, Q.; Dong, J.; Song, L.; Wang, W.; Shen, F. Long noncoding RNA lncRNA354 functions as a competing endogenous RNA of miR160b to regulate ARF genes in response to salt stress in upland cotton. Plant Cell Environ. 2021, 44, 3302–3321. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Y.; Ding, B.; Fei, Z. Comprehensive transcriptome analyses reveal that potato spindle tuber viroid triggers genome-wide changes in alternative splicing, inducible trans-acting activity of phased secondary small interfering RNAs and immune responses. J. Virol. 2017, 91, 217–247. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Y.; Jin, L.; Ling, X.; Liu, T.; Chen, T.; Ji, Y.; Yu, W.; Zhang, B. Re-analysis of long non-coding RNAs and prediction of circRNAs reveal their novel roles in susceptible tomato following TYLCV infection. BMC Plant Biol. 2018, 18, 104–106. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, C.; Wang, Z.; Yang, Z.; Chen, D.; Wu, Y.; Wu, Y. LncRNA expression profile and ceRNA analysis in tomato during flowering. PLoS ONE 2019, 14, e0210650. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, D.; Fan, H.Y.; Zhu, X.F.; Wang, Y.Y.; Liu, X.Y.; Duan, Y.X.; Xuan, Y.H.; Chen, L.J. Functional analysis of long non-coding RNAs reveal their novel roles in biocontrol of bacteria-induced tomato resistance to Meloidogyne incognita. Int. J. Mol. Sci. 2020, 21, 911. [Google Scholar] [CrossRef]
- Yang, F.; Ding, L.; Zhao, D.; Fan, H.Y.; Zhu, X.F.; Wang, Y.Y.; Liu, X.Y.; Duan, Y.X.; Xuan, Y.H.; Chen, L.J. Identification and functional analysis of tomato microRNAs in the biocontrol bacterium Pseudomonas putida induced plant resistance to Meloidogyne incognita. Phytopathology 2022, 112, 2372–2382. [Google Scholar] [CrossRef]
- Zhao, W.C.; Li, Z.L.; Fan, J.W.; Hu, C.L.; Yang, R.; Qi, X.; Chen, H.; Zhao, F.K.; Wang, S.H. Identification of jasmonic acid-associated microRNAs and characterization of the regulatory roles of the miR319/TCP4 module under root-knot nematode stress in tomato. J. Exp. Bot. 2015, 66, 4653–4667. [Google Scholar] [CrossRef]
- Wang, S.T.; Sun, X.L.; Hoshino, Y. MiR319 positively regulates cold tolerance by targeting OsPCF6 and OsTCP21 in rice. PLoS ONE 2014, 9, e91357. [Google Scholar]
- Wang, J.; Yu, W.; Yang, Y. Genome-wide analysis of tomato long non-coding RNAs and identification as endogenous target mimic for microRNA in response to TYLCV infection. Sci. Rep. 2015, 5, 16946. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Meng, J.; Cui, J.; Sun, G.; Luan, Y. Function identification of miR482b, a negative regulator during tomato resistance to Phytophthora infestans. Hortic. Res. 2018, 5, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Cui, J.; Shi, Y.S.; Yang, G.L.; Zhou, X.X.; Hou, X.X.; Meng, J.; Luan, Y.S. Tomato lncRNA23468 functions as a competing endogenous RNA to modulate NBS-LRR genes by decoying miR482b in the tomato-Phytophthora infestans interaction. Hortic. Res. 2019, 6, 28–40. [Google Scholar] [CrossRef]
- Cui, J.; Luan, Y.; Jiang, N.; Bao, H.; Meng, J. Comparative transcriptome analysis between resistant and susceptible tomato allows the identification of lncRNA16397 conferring resistance to Phytophthora infestans by co-expressing glutaredoxin. Plant J. 2017, 89, 577–589. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, Y.F.; Feng, Y.Z.; He, H.; Lian, J.P.; Yang, Y.W.; Lei, M.Q.; Zhang, Y.C.; Chen, Y.Q. Transcriptional landscape of pathogen-responsive lncRNAs in rice unveils the role of ALEX1 in jasmonate pathway and disease resistance. Plant Biotechnol. J. 2020, 18, 679–690. [Google Scholar] [CrossRef]
- Fan, C.L.; Hu, L.N.; Zhang, Z. Jasmonic acid mediates tomato’s response to root knot nematodes. J. Plant Growth Regul. 2015, 34, 196–205. [Google Scholar] [CrossRef]
- Gupta, R.; Mfarrej MF, B.; Xhemali, B.; Khan, A.; Nadeem, H.; Ahmad, F. Metabolic responses of plants to Meloidogyne species parasitism: A review on molecular events and functions. J. King Saud Univ.-Sci. 2023, 36, 103083. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Wu, X.; Chen, L.; Qi, M. The Tomato lncRNA47258-miR319b-TCP Module in Biocontrol Bacteria Sneb821 Induced Plants Resistance to Meloidogyne incognita. Pathogens 2025, 14, 256. https://doi.org/10.3390/pathogens14030256
Yang F, Wu X, Chen L, Qi M. The Tomato lncRNA47258-miR319b-TCP Module in Biocontrol Bacteria Sneb821 Induced Plants Resistance to Meloidogyne incognita. Pathogens. 2025; 14(3):256. https://doi.org/10.3390/pathogens14030256
Chicago/Turabian StyleYang, Fan, Xiaoxiao Wu, Lijie Chen, and Mingfang Qi. 2025. "The Tomato lncRNA47258-miR319b-TCP Module in Biocontrol Bacteria Sneb821 Induced Plants Resistance to Meloidogyne incognita" Pathogens 14, no. 3: 256. https://doi.org/10.3390/pathogens14030256
APA StyleYang, F., Wu, X., Chen, L., & Qi, M. (2025). The Tomato lncRNA47258-miR319b-TCP Module in Biocontrol Bacteria Sneb821 Induced Plants Resistance to Meloidogyne incognita. Pathogens, 14(3), 256. https://doi.org/10.3390/pathogens14030256




