Evaluation of IgM, IgA, and IgG Antibody Responses Against PCV3 and PCV2 in Tissues of Aborted Fetuses from Late-Term Co-Infected Sows
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. ELISA
2.3. Real-Time PCR
2.4. Statistical Analysis
3. Results
3.1. PCV2 and PCV3 Detection in Sera from Aborted Sows
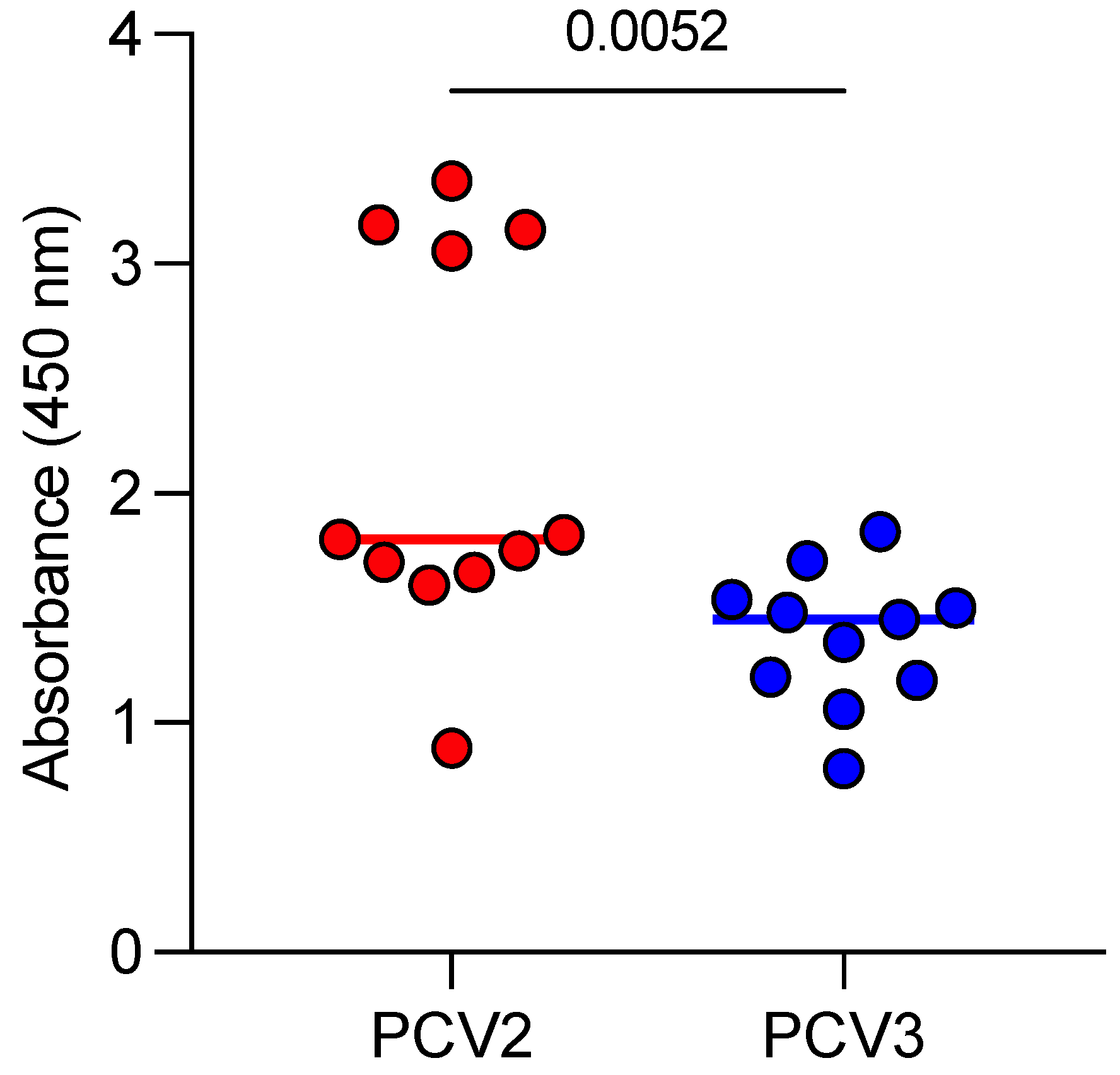
3.2. Anti-PCV2 and Anti-PCV3 Antibodies in the Sera of Aborted Sows
3.3. Viral DNA of PCV2 and PCV3 in Aborted Fetuses
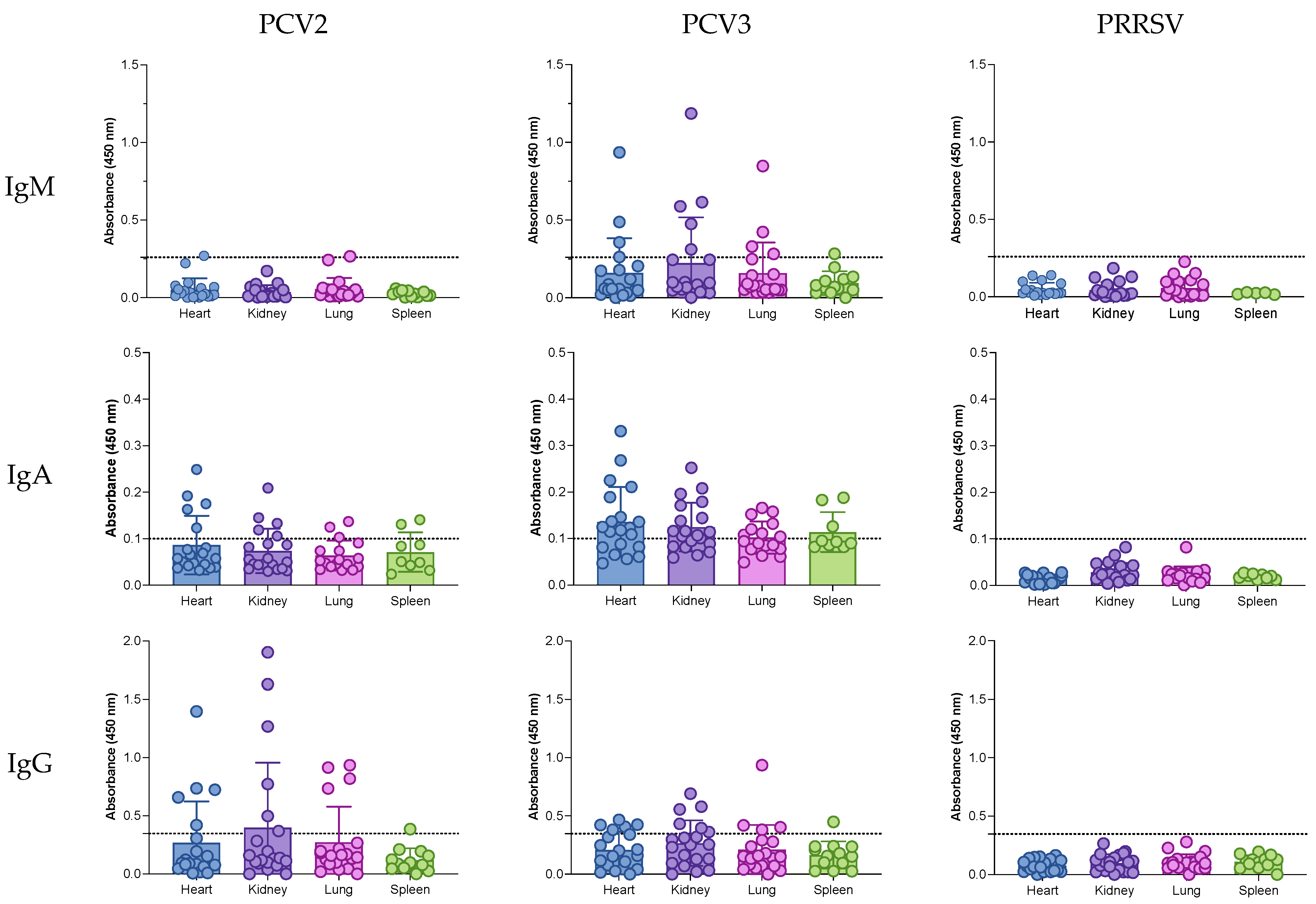
3.4. IgG, IgA, and IgM Antibodies in the Tissues of Aborted Fetuses
4. Discussion
- (i)
- The data may indicate a true intrauterine co-infection with PCV2 and PCV3.
- (ii)
- The higher frequency of anti-PCV3 IgM compared to anti-PCV2 IgM suggests active PCV3 infection within the fetus.
- (iii)
- The higher frequency of anti-PCV3 IgA further supports ongoing PCV3 infection.
- (iv)
- An initial PCV2 infection, controlled by the vaccination program, may have reduced the virus load and, consequently, the induction of IgM. Some IgG antibodies could represent maternal transfer, especially if PCV2 infection altered the placenta.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Opriessnig, T.; Karuppannan, A.K.; Castro, A.; Xiao, C.T. Porcine circoviruses: Current status, knowledge gaps and challenges. Virus Res. 2020, 286, 198044. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Mai, J.; Yang, Y.; Xiao, C.T.; Wang, N. Current knowledge on epidemiology and evolution of novel porcine circovirus 4. Vet. Res. 2022, 53, 38. [Google Scholar] [CrossRef] [PubMed]
- Klaumann, F.; Correa-Fiz, F.; Franzo, G.; Sibila, M.; Núñez, J.I.; Segalés, J. Current Knowledge on Porcine circovirus 3 (PCV-3): A Novel Virus with a Yet Unknown Impact on the Swine Industry. Front. Vet. Sci. 2018, 5, 315. [Google Scholar] [CrossRef] [PubMed]
- Segalés, J.; Sibila, M. Revisiting Porcine Circovirus Disease Diagnostic Criteria in the Current Porcine Circovirus 2 Epidemiological Context. Vet. Sci. 2022, 9, 110. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Bermúdez, D.S.; Vargas-Pinto, M.A.; Mogollón, J.D.; Jaime, J. Field infection of a gilt and its litter demonstrates vertical transmission and effect on reproductive failure caused by porcine circovirus type 3 (PCV3). BMC Vet. Res. 2021, 17, 150. [Google Scholar] [CrossRef] [PubMed]
- Saporiti, V.; Valls, L.; Maldonado, J.; Perez, M.; Correa-Fiz, F.; Segalés, J.; Sibila, M. Porcine Circovirus 3 Detection in Aborted Fetuses and Stillborn Piglets from Swine Reproductive Failure Cases. Viruses 2021, 13, 264. [Google Scholar] [CrossRef]
- Saporiti, V.; Franzo, G.; Sibila, M.; Segalés, J. Porcine circovirus 3 (PCV-3) as a causal agent of disease in swine and a proposal of PCV-3 associated disease case definition. Transbound. Emerg. Dis. 2021, 68, 2936–2948. [Google Scholar] [CrossRef]
- Tassi Yunga, S.; Kayatani, A.K.; Fogako, J.; Leke, R.J.; Leke, R.G.; Taylor, D.W. Timing of the human prenatal antibody response to Plasmodium falciparum antigens. PLoS ONE 2017, 12, e0184571. [Google Scholar] [CrossRef]
- Silverstein, A.M.; Uhr, J.W.; Kraner, K.L.; Lukes, R.J. Fetal response to antigenic stimulus. II. Antibody production by the fetal lamb. J. Exp. Med. 1963, 117, 799–812. [Google Scholar] [CrossRef]
- Darbellay, J.; Cox, B.; Lai, K.; Delgado-Ortega, M.; Wheler, C.; Wilson, D.; Walker, S.; Starrak, G.; Hockley, D.; Huang, Y.; et al. Zika Virus Causes Persistent Infection in Porcine Conceptuses and may Impair Health in Offspring. EBioMedicine 2017, 25, 73–86. [Google Scholar] [CrossRef]
- Cho, J.; Han, S.C.; Ho Hwang, J.; Song, J. Characterization of immune development of fetal and early-life of minipigs. Int. Immunopharmacol. 2023, 120, 110310. [Google Scholar] [CrossRef] [PubMed]
- Gerber, P.F.; Garrocho, F.M.; Lana, A.M.; Lobato, Z.I. Fetal infections and antibody profiles in pigs naturally infected with porcine circovirus type 2 (PCV2). Can. J. Vet. Res. Rev. Can. Rech. Vet. 2012, 76, 38–44. [Google Scholar]
- Saha, D.; Lefebvre, D.J.; Van Doorsselaere, J.; Atanasova, K.; Barbé, F.; Geldhof, M.; Karniychuk, U.U.; Nauwynck, H.J. Pathologic and virologic findings in mid-gestational porcine foetuses after experimental inoculation with PCV2a or PCV2b. Vet. Microbiol. 2010, 145, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, R.E., Jr.; Nauwynck, H.J.; McNeilly, F.; Allan, G.M.; Pensaert, M.B. Porcine circovirus 2 infection in swine foetuses inoculated at different stages of gestation. Vet. Microbiol. 2001, 83, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Del Pozo Sacristán, R.; Van Renne, N.; Huang, L.; Decaluwe, R.; Michiels, A.; Rodriguez, A.L.; Rodríguez, M.J.; Durán, M.G.; Declerk, I.; et al. Anti-porcine circovirus type 2 (PCV2) antibody placental barrier leakage from sow to fetus: Impact on the diagnosis of intra-uterine PCV2 infection. Virol. Sin. 2014, 29, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Cobos, À.; Ruiz, A.; Pérez, M.; Llorens, A.; Huerta, E.; Correa-Fiz, F.; Lohse, R.; Balasch, M.; Segalés, J.; Sibila, M. Experimental Inoculation of Porcine Circovirus 3 (PCV-3) in Pregnant Gilts Causes PCV-3-Associated Lesions in Newborn Piglets that Persist until Weaning. Transbound. Emerg. Dis. 2023, 2023, 5270254. [Google Scholar] [CrossRef]
- Vargas-Bermudez, D.S.; Mogollón, J.D.; Jaime, J. The Prevalence and Genetic Diversity of PCV3 and PCV2 in Colombia and PCV4 Survey during 2015–2016 and 2018–2019. Pathogens 2022, 11, 633. [Google Scholar] [CrossRef] [PubMed]
- Visuthsak, W.; Woonwong, Y.; Thanantong, N.; Poolperm, P.; Boonsoongnern, A.; Ratanavanichrojn, N.; Jirawattanapong, P.; Soda, N.; Kaminsonsakul, T.; Phuttapatimok, S.; et al. PCV3 in Thailand: Molecular epidemiology and relationship with PCV2. Transbound. Emerg. Dis. 2021, 68, 2980–2989. [Google Scholar] [CrossRef] [PubMed]
- Serena, M.S.; Cappuccio, J.A.; Barrales, H.; Metz, G.E.; Aspitia, C.G.; Lozada, I.; Perfumo, C.J.; Quiroga, M.A.; Piñeyro, P.; Echeverría, M.G. First detection and genetic characterization of porcine circovirus type 3 (PCV3) in Argentina and its association with reproductive failure. Transbound. Emerg. Dis. 2021, 68, 1761–1766. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, Y.H.; Tian, R.B.; Hou, C.Y.; Li, X.S.; Zheng, L.L.; Wang, L.Q.; Chen, H.Y. Prevalence and genetic analysis of porcine circovirus type 2 (PCV2) and type 3 (PCV3) between 2018 and 2020 in central China. Infect. Genet. Evol. 2021, 94, 105016. [Google Scholar] [CrossRef]
- Hernández, J.; Henao-Díaz, Y.A.; Reséndiz-Sandoval, M.; Cota-Valdez, A.; Mata-Haro, V.; Gimenez-Lirola, L.G. Dynamics of PCV2 and PCV3 in the Serum and Oral Fluids of Pigs After PCV2 Vaccination in a Commercial Farm. Vaccines 2024, 12, 1318. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, A.; Miłek, D.; Bąska, P.; Stadejek, T. Does porcine circovirus type 3 (PCV3) interfere with porcine circovirus type 2 (PCV2) vaccine efficacy? Transbound. Emerg. Dis. 2019, 66, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
| Age (Years) | No. of Parities | No. of Aborted Pigs | No. of Pigs Born Alive | No. of Vaccinations Against PCV2 | |
|---|---|---|---|---|---|
| Sow 1 | 1.4 | 2 | 15 | 3 | 3 |
| Sow 2 | 2.5 | 5 | 2 | 15 | 6 |
| Sow 3 | 1.8 | 3 | 2 | 16 | 4 |
| Sow 4 | 2.5 | 5 | 3 | 17 | 6 |
| Sow 5 | 1.4 | 2 | 3 | 14 | 3 |
| Sow 6 | 1.4 | 2 | 5 | 5 | 3 |
| Sow 7 | 1.8 | 3 | 2 | 16 | 4 |
| Sow 8 | 1.4 | 2 | 2 | 18 | 3 |
| Sow 9 | 2.5 | 5 | 1 | 12 | 6 |
| Sow 10 | 1.0 | 1 | 2 | 8 | 2 |
| Sow 11 | 2.5 | 5 | 3 | 15 | 6 |
| Sow 1 | Sow 2 | Sow 3 | Sow 4 | Sow 5 | Sow 6 | Sow 7 | Sow 8 | Sow 9 | Sow 10 | Sow 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PCV2 | 30.7 | Neg | 32.0 | 32.0 | 32.0 | Neg | Neg | Neg | Neg | Neg | Neg |
| PCV3 | Neg | Neg | 32.0 | 27.5 | 28.8 | 31.5 | 32.0 | 33.4 | 31.29 | 29.55 | 33.25 |
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | F11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PCV2 | 9.56 | 8.86 | 14.54 | 7.81 | 9.54 | 26.81 | 27.5 | 15.26 | 30.79 | 29.23 | 28.75 |
| PCV3 | 12.89 | 11.43 | 27.45 | 12.15 | 12.6 | 12.56 | 9.74 | 16.43 | 16.29 | 11.4 | 16.83 |
| Heart | Kidney | Lung | Spleen | |
|---|---|---|---|---|
| IgM | ||||
| PCV2 | 5 (1 of 20) | 0 | 10 (2 of 20) | 0 |
| PCV3 | 20 (4 of 20) | 35 (7 of 20) | 25 (5 of 20) | 7.1 (1 of 14) |
| IgA | ||||
| PCV2 | 26.3 (5 of 19) | 25 (5 of 20) | 17.4 (3 of 17) | 22.2 (2 of 9) |
| PCV3 | 65 (13 of 20) | 65 (13 of 20) | 47 (8 of 17) | 33.3 (3 of 9) |
| IgG | ||||
| PCV2 | 25 (5 of 20) | 30 (6 of 20) | 20 (4 of 20) | 7.7 (1 of 13) |
| PCV3 | 30 (6 of 20) | 30 (6 of 20) | 20 (4 of 20) | 7.7 (1 of 13) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, J.; Henao-Díaz, A.; Reséndiz-Sandoval, M.; Ramírez-Morán, J.; Cota-Valdez, A.; Mata-Haro, V.; Giménez-Lirola, L.G. Evaluation of IgM, IgA, and IgG Antibody Responses Against PCV3 and PCV2 in Tissues of Aborted Fetuses from Late-Term Co-Infected Sows. Pathogens 2025, 14, 198. https://doi.org/10.3390/pathogens14020198
Hernández J, Henao-Díaz A, Reséndiz-Sandoval M, Ramírez-Morán J, Cota-Valdez A, Mata-Haro V, Giménez-Lirola LG. Evaluation of IgM, IgA, and IgG Antibody Responses Against PCV3 and PCV2 in Tissues of Aborted Fetuses from Late-Term Co-Infected Sows. Pathogens. 2025; 14(2):198. https://doi.org/10.3390/pathogens14020198
Chicago/Turabian StyleHernández, Jesús, Alexandra Henao-Díaz, Mónica Reséndiz-Sandoval, Joana Ramírez-Morán, Angel Cota-Valdez, Verónica Mata-Haro, and Luis G. Giménez-Lirola. 2025. "Evaluation of IgM, IgA, and IgG Antibody Responses Against PCV3 and PCV2 in Tissues of Aborted Fetuses from Late-Term Co-Infected Sows" Pathogens 14, no. 2: 198. https://doi.org/10.3390/pathogens14020198
APA StyleHernández, J., Henao-Díaz, A., Reséndiz-Sandoval, M., Ramírez-Morán, J., Cota-Valdez, A., Mata-Haro, V., & Giménez-Lirola, L. G. (2025). Evaluation of IgM, IgA, and IgG Antibody Responses Against PCV3 and PCV2 in Tissues of Aborted Fetuses from Late-Term Co-Infected Sows. Pathogens, 14(2), 198. https://doi.org/10.3390/pathogens14020198










