Longitudinal Monitoring of Mono- and Coinfections Involving Primary Porcine Reproductive Viruses (PCV2, PPV1, and PRRSV) as Well as Emerging Viruses (PCV3, PCV4, and nPPVs) in Primiparous and Multiparous Sows and Their Litters
Abstract
1. Introduction
2. Materials and Methods
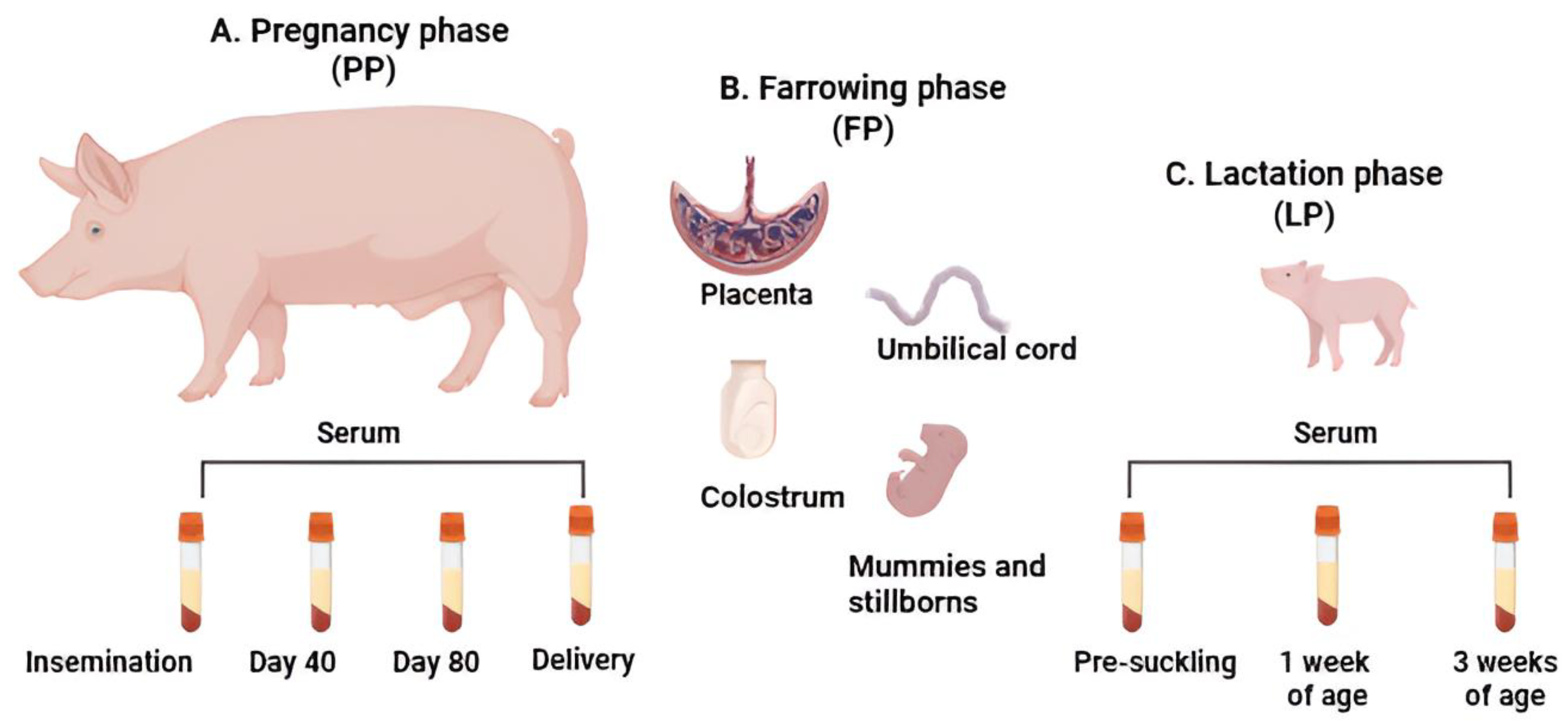
2.1. Herd Selection and Sample Collection
2.2. Serology
2.3. Processing Samples and Detecting Viruses
2.4. Sequencing of Identified Viruses and Phylogenetic Analysis
2.5. Histopathology
2.6. Statistical Analysis
3. Results
3.1. Reproductive Parameters
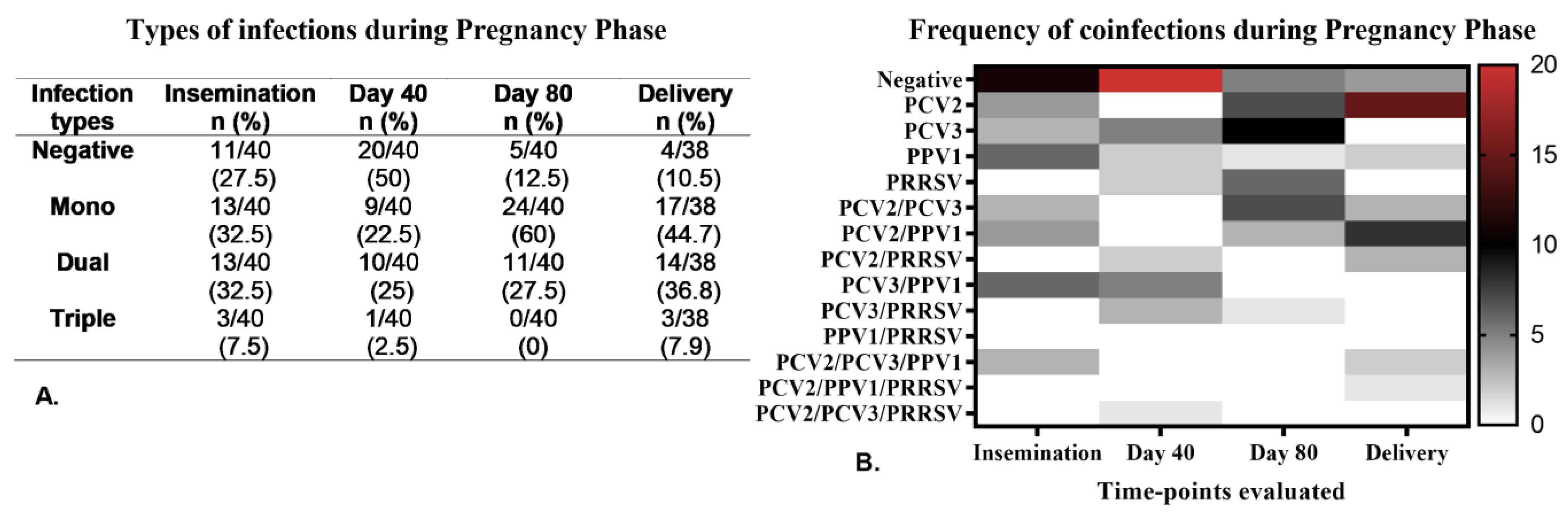
3.2. Viremia and Serological Assessments in Both Primiparous and Multiparous Sows Throughout the Pregnancy Phase
Coinfections Caused by Viruses During the Pregnancy Phase
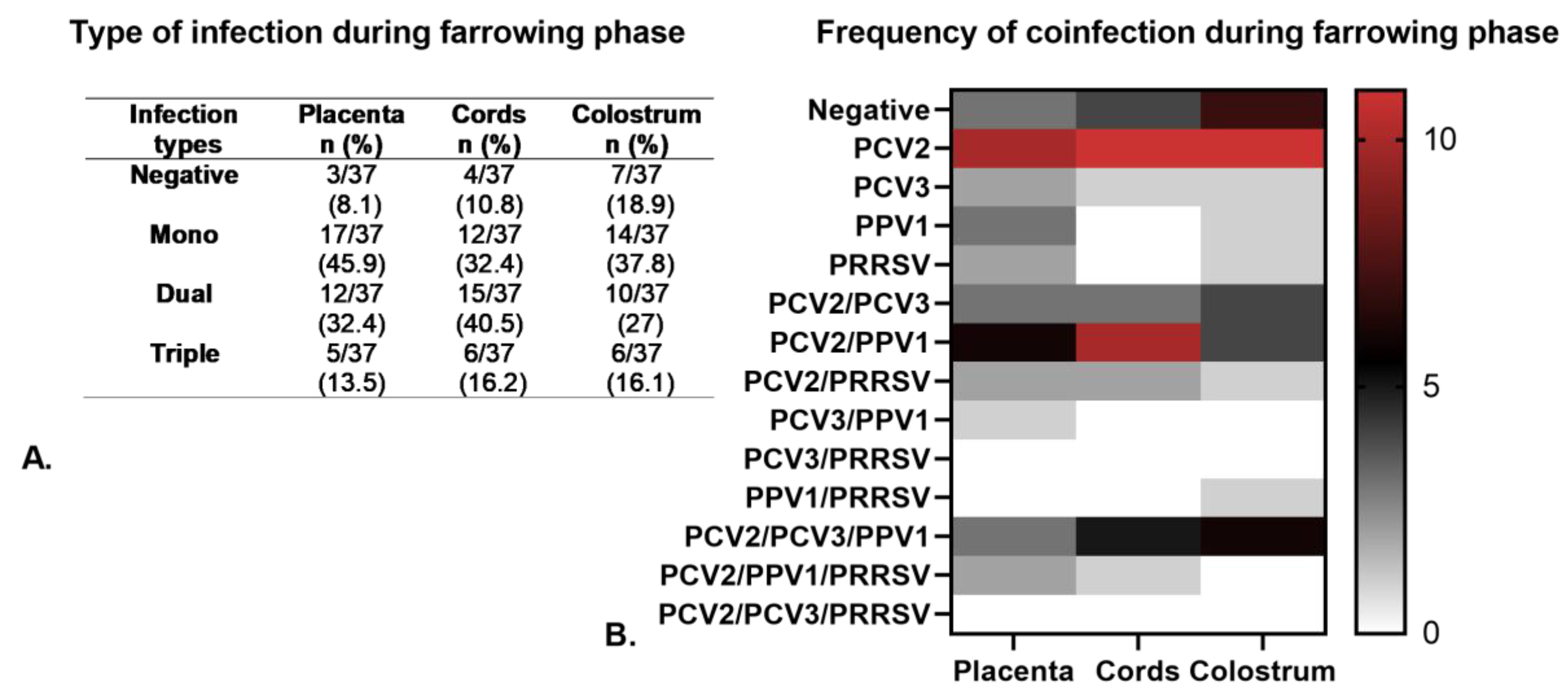
3.3. Viral Detection in Primiparous and Multiparous Sows During the Farrowing Phase
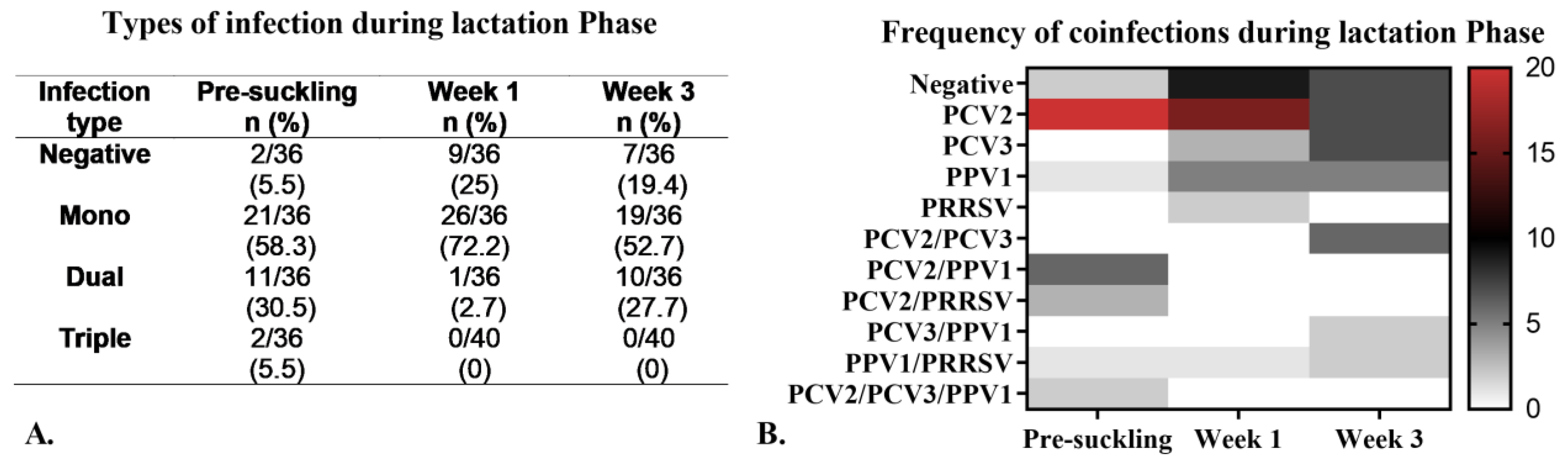
3.4. Detection of Viruses and Antibodies in Piglets During the Lactation Phase
Viral Coinfections During the Lactation Phase
3.5. Histopathological Examination of Tissues Collected During Delivery
3.6. Genetic Analysis of the Viruses
3.7. Proposed Diagnostic Criteria for Subclinical Porcine Reproductive Failure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PRF | Porcine reproductive failure |
| SMEDI | Stillbirth, mummification, embryonic death, and infertility |
| PCV2 | Porcine circovirus type 2 |
| PPV1 | Porcine parvovirus 1 |
| PRRSV | Porcine respiratory and reproductive syndrome |
| PCV3 | Porcine circovirus type 3 |
| PPV | Porcine parvovirus |
| H | Herd |
| PSs | Primiparous sows |
| MSs | Multiparous sows |
| PP | Pregnancy phase |
| FP | Farrowing phase |
| LP | Lactation phase |
| Abs | Antibodies |
| PBA | Piglet born alive |
| CRL | Crown-rump length |
| PWM | Preweaning mortality |
| nPPVs | Novel porcine parvovirus |
References
- Christianson, W.T. Stillbirths, mummies, abortions, and early embryonic death. Vet. Clin. N. Am. Food Anim. Pract. 1992, 8, 623–639. [Google Scholar] [CrossRef]
- Nathues, H.; Alarcon, P.; Rushton, J.; Jolie, R.; Fiebig, K.; Jimenez, M.; Geurts, V.; Nathues, C. Cost of porcine reproductive and respiratory syndrome virus at individual farm level-An economic disease model. Prev. Vet. Med. 2017, 142, 16–29. [Google Scholar] [CrossRef]
- Dunne, H.W.; Gobble, J.L.; Hokanson, J.F.; Kradel, D.C.; Bubash, G.R. Porcine reproductive failure associated with a newly identified “SMEDI” group of picorna viruses. Am. J. Vet. Res. 1965, 26, 1284–1297. [Google Scholar] [PubMed]
- Streck, A.F.; Truyen, U. Porcine Parvovirus. Curr. Issues Mol. Biol. 2020, 37, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Madson, D.M.; Opriessnig, T. Effect of porcine circovirus type 2 (PCV2) infection on reproduction: Disease, vertical transmission, diagnostics and vaccination. Anim. Health Res. Rev. 2011, 12, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Rossow, K.D. Porcine reproductive and respiratory syndrome. Vet. Pathol. 1998, 35, 1–20. [Google Scholar] [CrossRef]
- Faccini, S.; Barbieri, I.; Gilioli, A.; Sala, G.; Gibelli, L.R.; Moreno, A.; Sacchi, C.; Rosignoli, C.; Franzini, G.; Nigrelli, A. Detection and genetic characterization of Porcine circovirus type 3 in Italy. Transbound. Emerg. Dis. 2017, 64, 1661–1664. [Google Scholar] [CrossRef]
- Ruiz, A.; Saporiti, V.; Huerta, E.; Balasch, M.; Segalés, J.; Sibila, M. Exploratory Study of the Frequency of Detection and Tissue Distribution of Porcine Circovirus 3 (PCV-3) in Pig Fetuses at Different Gestational Ages. Pathogens 2022, 11, 118. [Google Scholar] [CrossRef]
- Dal Santo, A.C.; Cezario, K.C.; Bennemann, P.E.; Machado, S.A.; Martins, M. Full-genome sequences of porcine circovirus 3 (PCV3) and high prevalence in mummified fetuses from commercial farms in Brazil. Microb. Pathog. 2020, 141, 104027. [Google Scholar] [CrossRef]
- Faustini, G.; Tucciarone, C.M.; Franzo, G.; Donneschi, A.; Boniotti, M.B.; Alborali, G.L.; Drigo, M. Molecular Survey on Porcine Parvoviruses (PPV1-7) and Their Association with Major Pathogens in Reproductive Failure Outbreaks in Northern Italy. Viruses 2024, 16, 157. [Google Scholar] [CrossRef]
- Vargas-Bermudez, D.S.; Mogollon, J.D.; Franco-Rodriguez, C.; Jaime, J. The novel porcine parvoviruses: Current state of knowledge and their possible implications in clinical syndromes in pigs. Viruses 2023, 15, 2398. [Google Scholar] [CrossRef]
- Kim, J.; Jung, K.; Chae, C. Prevalence of porcine circovirus type 2 in aborted fetuses and stillborn piglets. Vet. Rec. 2004, 155, 489–492. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, Z.; Wang, W.; Tang, D.; Liang, H.; Liu, Z. Establishment and application of a multiplex PCR for rapid and simultaneous detection of six viruses in swine. J. Virol. Methods 2014, 208, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Saikumar, G. Porcine parvovirus- and porcine circovirus 2-associated reproductive failure and neonatal mortality in crossbred Indian pigs. Trop. Anim. Health Prod. 2010, 42, 515–522. [Google Scholar] [CrossRef]
- Eddicks, M.; Gründl, J.; Seifert, A.; Eddicks, L.; Reese, S.; Tabeling, R.; Swam, H.; Strutzberg-Minder, K.; Ritzmann, M.; Fux, R. Examination on the occurrence of coinfections in diagnostic transmittals in cases of stillbirth, mummification, embryonic death, and infertility (SMEDI) syndrome in germany. Microorganisms 2023, 11, 1675. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Camacho, L.A.; Vargas-Ruiz, A.; Marin-Flamand, E.; Ramírez-Álvarez, H.; Brown, C. A retrospective study of DNA prevalence of porcine parvoviruses in Mexico and its relationship with porcine circovirus associated disease. Microbiol. Immunol. 2020, 64, 366–376. [Google Scholar] [CrossRef]
- Kumar, N.; Sharma, S.; Barua, S.; Tripathi, B.N.; Rouse, B.T. Virological and immunological outcomes of coinfections. Clin. Microbiol. Rev. 2018, 31, 38. [Google Scholar] [CrossRef] [PubMed]
- Tramontana, S.; Bionaz, M.; Sharma, A.; Graugnard, D.E.; Cutler, E.A.; Ajmone-Marsan, P.; Hurley, W.L.; Loor, J.J. Internal controls for quantitative polymerase chain reaction of swine mammary glands during pregnancy and lactation. J. Dairy Sci. 2008, 91, 3057–3066. [Google Scholar] [CrossRef]
- Kleiboeker, S.B.; Schommer, S.K.; Lee, S.-M.; Watkins, S.; Chittick, W.; Polson, D. Simultaneous detection of North American and European porcine reproductive and respiratory syndrome virus using real-time quantitative reverse transcriptase-PCR. J. Vet. Diagn. Investig. 2005, 17, 165–170. [Google Scholar] [CrossRef]
- Opriessnig, T.; Prickett, J.R.; Madson, D.M.; Shen, H.-G.; Juhan, N.M.; Pogranichniy, R.R.; Meng, X.-J.; Halbur, P.G. Porcine circovirus type 2 (PCV2)-infection and re-inoculation with homologous or heterologous strains: Virological, serological, pathological and clinical effects in growing pigs. Vet. Res. 2010, 41, 31. [Google Scholar] [CrossRef]
- Song, C.; Zhu, C.; Zhang, C.; Cui, S. Detection of porcine parvovirus using a taqman-based real-time pcr with primers and probe designed for the NS1 gene. Virol. J. 2010, 7, 353. [Google Scholar] [CrossRef] [PubMed]
- Varela, A.P.M.; Loiko, M.R.; Andrade, J.D.S.; Tochetto, C.; Cibulski, S.P.; Lima, D.A.; Weber, M.N.; Roehe, P.M.; Mayer, F.Q. Complete genome characterization of porcine circovirus 3 recovered from wild boars in Southern Brazil. Transbound. Emerg. Dis. 2021, 68, 240–247. [Google Scholar] [CrossRef]
- Cui, J.; Biernacka, K.; Fan, J.; Gerber, P.F.; Stadejek, T.; Opriessnig, T. Circulation of Porcine Parvovirus Types 1 through 6 in Serum Samples Obtained from Six Commercial Polish Pig Farms. Transbound. Emerg. Dis. 2017, 64, 1945–1952. [Google Scholar] [CrossRef]
- Saekhow, P.; Mawatari, T.; Ikeda, H. Coexistence of multiple strains of porcine parvovirus 2 in pig farms. Microbiol. Immunol. 2014, 58, 382–387. [Google Scholar] [CrossRef]
- Xiao, C.-T.; Giménez-Lirola, L.G.; Halbur, P.G.; Opriessnig, T. Increasing porcine PARV4 prevalence with pig age in the U.S. pig population. Vet. Microbiol. 2012, 160, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.-T.; Giménez-Lirola, L.G.; Jiang, Y.-H.; Halbur, P.G.; Opriessnig, T. Characterization of a novel porcine parvovirus tentatively designated PPV5. PLoS ONE 2013, 8, e65312. [Google Scholar] [CrossRef]
- Xing, X.; Zhou, H.; Tong, L.; Chen, Y.; Sun, Y.; Wang, H.; Zhang, G. First identification of porcine parvovirus 7 in China. Arch. Virol. 2018, 163, 209–213. [Google Scholar] [CrossRef]
- Guo, Y.; Yan, G.; Chen, S.; Han, H.; Li, J.; Zhang, H.; Luo, S.; Liu, M.; Wu, Q.; Li, Q.; et al. Identification and genomic characterization of a novel porcine parvovirus in China. Front. Vet. Sci. 2022, 9, 1009103. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.-B.; Zhao, Y.; Cui, J.-T.; Zheng, H.-H.; Xu, T.; Hou, C.-Y.; Wang, Z.-Y.; Li, X.-S.; Zheng, L.-L.; Chen, H.-Y. Molecular detection and phylogenetic analysis of Porcine circovirus 4 in Henan and Shanxi Provinces of China. Transbound. Emerg. Dis. 2021, 68, 276–282. [Google Scholar] [CrossRef]
- Oliver-Ferrando, S.; Segalés, J.; López-Soria, S.; Callén, A.; Merdy, O.; Joisel, F.; Sibila, M. Evaluation of natural porcine circovirus type 2 (PCV2) subclinical infection and seroconversion dynamics in piglets vaccinated at different ages. Vet. Res. 2016, 47, 121. [Google Scholar] [CrossRef]
- Palinski, R.; Piñeyro, P.; Shang, P.; Yuan, F.; Guo, R.; Fang, Y.; Byers, E.; Hause, B.M. A Novel Porcine Circovirus Distantly Related to Known Circoviruses Is Associated with Porcine Dermatitis and Nephropathy Syndrome and Reproductive Failure. J. Virol. 2017, 91, e01879-16. [Google Scholar] [CrossRef] [PubMed]
- Gerber, P.F.; O’Neill, K.; Owolodun, O.; Wang, C.; Harmon, K.; Zhang, J.; Halbur, P.G.; Zhou, L.; Meng, X.-J.; Opriessnig, T. Comparison of commercial real-time reverse transcription-PCR assays for reliable, early, and rapid detection of heterologous strains of porcine reproductive and respiratory syndrome virus in experimentally infected or noninfected boars by use of different sample types. J. Clin. Microbiol. 2013, 51, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Kim, D.R. pcr: An R package for quality assessment, analysis and testing of qPCR data. PeerJ 2018, 6, e4473. [Google Scholar] [CrossRef]
- Segalés, J. Porcine circovirus type 2 (PCV2) infections: Clinical signs, pathology and laboratory diagnosis. Virus Res. 2012, 164, 10–19. [Google Scholar] [CrossRef]
- Saporiti, V.; Franzo, G.; Sibila, M.; Segalés, J. Porcine circovirus 3 (PCV-3) as a causal agent of disease in swine and a proposal of PCV-3 associated disease case definition. Transbound. Emerg. Dis. 2021, 68, 2936–2948. [Google Scholar] [CrossRef]
- Zimmerman, J.J.; Yoon, K.J.; Wills, R.W.; Swenson, S.L. General overview of PRRSV: A perspective from the United States. Vet. Microbiol. 1997, 55, 187–196. [Google Scholar] [CrossRef]
- Ladinig, A.; Wilkinson, J.; Ashley, C.; Detmer, S.E.; Lunney, J.K.; Plastow, G.; Harding, J.C.S. Variation in fetal outcome, viral load and ORF5 sequence mutations in a large scale study of phenotypic responses to late gestation exposure to type 2 porcine reproductive and respiratory syndrome virus. PLoS ONE 2014, 9, e96104. [Google Scholar] [CrossRef]
- Segalés, J.; Sibila, M. Revisiting porcine circovirus disease diagnostic criteria in the current porcine circovirus 2 epidemiological context. Vet. Sci. 2022, 9, 110. [Google Scholar] [CrossRef]
- Oropeza-Moe, M.; Oropeza Delgado, A.J.; Framstad, T. Porcine circovirus type 2 associated reproductive failure in a specific pathogen free (SPF) piglet producing herd in Norway: A case report. Porcine Health Manag. 2017, 3, 25. [Google Scholar] [CrossRef]
- Eddicks, M.; Beuter, B.; Stuhldreier, R.; Nolte, T.; Reese, S.; Sutter, G.; Ritzmann, M.; Fux, R. Cross-sectional study on viraemia and shedding of porcine circovirus type 2 in a subclinically infected multiplier sow herd. Vet. Rec. 2019, 184, 189. [Google Scholar] [CrossRef] [PubMed]
- West, K.H.; Bystrom, J.M.; Wojnarowicz, C.; Shantz, N.; Jacobson, M.; Allan, G.M.; Haines, D.M.; Clark, E.G.; Krakowka, S.; McNeilly, F.; et al. Myocarditis and abortion associated with intrauterine infection of sows with porcine circovirus 2. J. Vet. Diagn. Investig. 1999, 11, 530–532. [Google Scholar] [CrossRef] [PubMed]
- Oliver-Ferrando, S.; Segalés, J.; Sibila, M.; Díaz, I. Comparison of cytokine profiles in peripheral blood mononuclear cells between piglets born from Porcine circovirus 2 vaccinated and non-vaccinated sows. Vet. Microbiol. 2018, 214, 148–153. [Google Scholar] [CrossRef]
- Pleguezuelos, P.; Sibila, M.; Cuadrado, R.; López-Jiménez, R.; Pérez, D.; Huerta, E.; Llorens, A.M.; Núñez, J.I.; Segalés, J.; López-Soria, S. Exploratory field study on the effects of porcine circovirus 2 (PCV-2) sow vaccination at different physiological stages mimicking blanket vaccination. Porcine Health Manag. 2021, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Mak, C.K.; Yang, C.; Jeng, C.-R.; Pang, V.F.; Yeh, K.-S. Reproductive failure associated with coinfection of porcine circovirus type 2 and porcine reproductive and respiratory syndrome virus. Can. Vet. J. 2018, 59, 525–530. [Google Scholar]
- Dias, A.S.; Gerber, P.F.; Araújo, A.S.; Auler, P.A.; Gallinari, G.C.; Lobato, Z.I.P. Lack of antibody protection against Porcine circovirus 2 and Porcine parvovirus in naturally infected dams and their offspring. Res. Vet. Sci. 2013, 94, 341–345. [Google Scholar] [CrossRef]
- Oliver-Ferrando, S.; Segalés, J.; López-Soria, S.; Callén, A.; Merdy, O.; Joisel, F.; Sibila, M. Exploratory field study on the effect of Porcine circovirus 2 (PCV2) sow vaccination on serological, virological and reproductive parameters in a PCV2 subclinically infected sow herd. BMC Vet. Res. 2018, 14, 130. [Google Scholar] [CrossRef]
- Dvorak, C.M.T.; Yang, Y.; Haley, C.; Sharma, N.; Murtaugh, M.P. National reduction in porcine circovirus type 2 prevalence following introduction of vaccination. Vet. Microbiol. 2016, 189, 86–90. [Google Scholar] [CrossRef]
- Gerber, P.F.; Garrocho, F.M.; Lana, A.M.Q.; Lobato, Z.I.P. Serum antibodies and shedding of infectious porcine circovirus 2 into colostrum and milk of vaccinated and unvaccinated naturally infected sows. Vet. J. 2011, 188, 240–242. [Google Scholar] [CrossRef]
- Madson, D.M.; Patterson, A.R.; Ramamoorthy, S.; Pal, N.; Meng, X.J.; Opriessnig, T. Effect of porcine circovirus type 2 (PCV2) vaccination of the dam on PCV2 replication in utero. Clin. Vaccine Immunol. 2009, 16, 830–834. [Google Scholar] [CrossRef]
- Franzo, G.; Segalés, J. Porcine circovirus 2 genotypes, immunity and vaccines: Multiple genotypes but one single serotype. Pathogens 2020, 9, 1049. [Google Scholar] [CrossRef]
- Saporiti, V.; Martorell, S.; Cruz, T.F.; Klaumann, F.; Correa-Fiz, F.; Balasch, M.; Sibila, M.; Segalés, J. Frequency of Detection and Phylogenetic Analysis of Porcine circovirus3 (PCV-3) in Healthy Primiparous and Multiparous Sows and Their Mummified Fetuses and Stillborn. Pathogens 2020, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Bermúdez, D.S.; Vargas-Pinto, M.A.; Mogollón, J.D.; Jaime, J. Field infection of a gilt and its litter demonstrates vertical transmission and effect on reproductive failure caused by porcine circovirus type 3 (PCV3). BMC Vet. Res. 2021, 17, 150. [Google Scholar] [CrossRef] [PubMed]
- Klaumann, F.; Correa-Fiz, F.; Sibila, M.; Núñez, J.I.; Segalés, J. Infection dynamics of porcine circovirus type 3 in longitudinally sampled pigs from four Spanish farms. Vet. Rec. 2019, 184, 619. [Google Scholar] [CrossRef] [PubMed]
- Kedkovid, R.; Woonwong, Y.; Arunorat, J.; Sirisereewan, C.; Sangpratum, N.; Lumyai, M.; Kesdangsakonwut, S.; Teankum, K.; Jittimanee, S.; Thanawongnuwech, R. Porcine circovirus type 3 (PCV3) infection in grower pigs from a Thai farm suffering from porcine respiratory disease complex (PRDC). Vet. Microbiol. 2018, 215, 71–76. [Google Scholar] [CrossRef]
- Yao, L.; Li, C.; Wang, J.; Cheng, Y.; Ghonaim, A.H.; Sun, Q.; Yu, X.; Niu, W.; Fan, S.; He, Q. Development of an indirect immunofluorescence assay for PCV3 antibody detection based on capsid protein. Anim. Dis. 2021, 1, 11. [Google Scholar] [CrossRef]
- Cobos, À.; Ruiz, A.; Pérez, M.; Llorens, A.; Huerta, E.; Correa-Fiz, F.; Lohse, R.; Balasch, M.; Segalés, J.; Sibila, M. Experimental Inoculation of Porcine Circovirus 3 (PCV-3) in Pregnant Gilts Causes PCV-3-Associated Lesions in Newborn Piglets that Persist until Weaning. Transbound. Emerg. Dis. 2023, 2023, 1–14. [Google Scholar] [CrossRef]
- Sanchez, R.E.; Nauwynck, H.J.; McNeilly, F.; Allan, G.M.; Pensaert, M.B. Porcine circovirus 2 infection in swine foetuses inoculated at different stages of gestation. Vet. Microbiol. 2001, 83, 169–176. [Google Scholar] [CrossRef]
- Zhai, S.-L.; Zhou, X.; Zhang, H.; Hause, B.M.; Lin, T.; Liu, R.; Chen, Q.-L.; Wei, W.-K.; Lv, D.-H.; Wen, X.-H.; et al. Comparative epidemiology of porcine circovirus type 3 in pigs with different clinical presentations. Virol. J. 2017, 14, 222. [Google Scholar] [CrossRef]
- Woźniak, A.; Miłek, D.; Bąska, P.; Stadejek, T. Does porcine circovirus type 3 (PCV3) interfere with porcine circovirus type 2 (PCV2) vaccine efficacy? Transbound. Emerg. Dis. 2019, 66, 1454–1461. [Google Scholar] [CrossRef]
- Vargas-Bermudez, D.S.; Campos, F.S.; Bonil, L.; Mogollon, D.; Jaime, J. First detection of porcine circovirus type 3 in Colombia and the complete genome sequence demonstrates the circulation of PCV3a1 and PCV3a2. Vet. Med. Sci. 2019, 5, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Bermudez, D.S.; Mogollón, J.D.; Jaime, J. The Prevalence and Genetic Diversity of PCV3 and PCV2 in Colombia and PCV4 Survey during 2015-2016 and 2018-2019. Pathogens 2022, 11, 633. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Bermudez, D.S.; Diaz, A.; Polo, G.; Mogollon, J.D.; Jaime, J. Infection and Coinfection of Porcine-Selected Viruses (PPV1 to PPV8, PCV2 to PCV4, and PRRSV) in Gilts and Their Associations with Reproductive Performance. Vet. Sci. 2024, 11, 185. [Google Scholar] [CrossRef]
- Vargas-Bermudez, D.S.; Gil-Silva, A.C.; Naranjo-Ortíz, M.F.; Mogollón, J.D.; Gómez-Betancur, J.F.; Estrada, J.F.; Aldaz, Á.; Garzón-González, H.; Angulo, J.; Foss, D.; et al. Detection of PCV2d in Vaccinated Pigs in Colombia and Prediction of Vaccine T Cell Epitope Coverage against Circulating Strains Using EpiCC Analysis. Vaccines 2024, 12, 1119. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.-Y.; Zhang, L.-H.; Zhang, Y.-H.; Cui, J.-T.; Zhao, L.; Zheng, L.-L.; Chen, H.-Y. Phylogenetic analysis of porcine circovirus 4 in Henan Province of China: A retrospective study from 2011 to 2021. Transbound. Emerg. Dis. 2022, 69, 1890–1901. [Google Scholar] [CrossRef]
- Nguyen, V.-G.; Do, H.-Q.; Huynh, T.-M.-L.; Park, Y.-H.; Park, B.-K.; Chung, H.-C. Molecular-based detection, genetic characterization and phylogenetic analysis of porcine circovirus 4 from Korean domestic swine farms. Transbound. Emerg. Dis. 2022, 69, 538–548. [Google Scholar] [CrossRef]
- Saporiti, V.; Valls, L.; Maldonado, J.; Perez, M.; Correa-Fiz, F.; Segalés, J.; Sibila, M. Porcine Circovirus 3 Detection in Aborted Fetuses and Stillborn Piglets from Swine Reproductive Failure Cases. Viruses 2021, 13, 264. [Google Scholar] [CrossRef]
- Maldonado, J.; Segalés, J.; Martínez-Puig, D.; Calsamiglia, M.; Riera, P.; Domingo, M.; Artigas, C. Identification of viral pathogens in aborted fetuses and stillborn piglets from cases of swine reproductive failure in Spain. Vet. J. 2005, 169, 454–456. [Google Scholar] [CrossRef]
- Noguera, M.; Vela, A.; Kraft, C.; Chevalier, M.; Goutebroze, S.; de Paz, X.; Kunze, M.; Rathkjen, P.; Schacht, E.; Garcia-Morante, B. Effects of three commercial vaccines against porcine parvovirus 1 in pregnant gilts. Vaccine 2021, 39, 3997–4005. [Google Scholar] [CrossRef]
- Oravainen, J.; Hakala, M.; Rautiainen, E.; Veijalainen, P.; Heinonen, M.; Tast, A.; Virolainen, J.V.; Peltoniemi, O.A.T. Parvovirus antibodies in vaccinated gilts in field conditions--results with HI and ELISA tests. Reprod. Domest. Anim. 2006, 41, 91–93. [Google Scholar] [CrossRef]
- Gava, D.; Souza, C.K.; Mores, T.J.; Argenti, L.E.; Streck, A.F.; Canal, C.W.; Bortolozzo, F.P.; Wentz, I. Dynamics of vanishing of maternally derived antibodies of Ungulate protoparvovirus 1 suggests an optimal age for gilts vaccination. Trop. Anim. Health Prod. 2017, 49, 1085–1088. [Google Scholar] [CrossRef] [PubMed]
- Damm, B.I.; Friggens, N.C.; Nielsen, J.; Ingvartsen, K.L.; Pedersen, L.J. Factors affecting the transfer of porcine parvovirus antibodies from sow to piglets. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2002, 49, 487–495. [Google Scholar] [CrossRef]
- Zeeuw, E.J.L.; Leinecker, N.; Herwig, V.; Selbitz, H.J.; Truyen, U. Study of the virulence and cross-neutralization capability of recent porcine parvovirus field isolates and vaccine viruses in experimentally infected pregnant gilts. J. Gen. Virol. 2007, 88, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Bermudez, D.S.; Prandi, B.A.; de Souza, U.J.B.; Durães-Carvalho, R.; Mogollón, J.D.; Campos, F.S.; Roehe, P.M.; Jaime, J. Molecular Epidemiology and Phyloevolutionary Analysis of Porcine Parvoviruses (PPV1 through PPV7) Detected in Replacement Gilts from Colombia. Int. J. Mol. Sci. 2024, 25, 10354. [Google Scholar] [CrossRef]
- Holtkamp, D.J.; Polson, D.D.; Torremorell, M.; Morrison, B.; Classen, D.M.; Becton, L.; Henry, S.; Rodibaugh, M.T.; Rowland, R.R.; Snelson, H.; et al. American Association of Swine Veterinarians; United States Department of Agriculture PRRS-Coordinated Agricultural Project [Terminology for classifying the porcine reproductive and respiratory syndrome virus (PRRSV) status of swine herds]. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 2011, 39, 101–112. [Google Scholar]
- Karniychuk, U.U.; Nauwynck, H.J. Pathogenesis and prevention of placental and transplacental porcine reproductive and respiratory syndrome virus infection. Vet. Res. 2013, 44, 95. [Google Scholar] [CrossRef]
- Unterweger, C.; Kreutzmann, H.; Buenger, M.; Klingler, E.; Auer, A.; Rümenapf, T.; Truyen, U.; Ladinig, A. Litters of Various-Sized Mummies (LVSM) and Stillborns after Porcine Reproductive and Respiratory Syndrome Virus Type 1 Infection-A Case Report. Vet. Sci. 2023, 10, 494. [Google Scholar] [CrossRef]
- Olanratmanee, E.; Wongyanin, P.; Thanawongnuwech, R.; Tummaruk, P. Prevalence of porcine reproductive and respiratory syndrome virus detection in aborted fetuses, mummified fetuses and stillborn piglets using quantitative polymerase chain reaction. J. Vet. Med. Sci. 2015, 77, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Alkhamis, M.A.; Perez, A.M.; Murtaugh, M.P.; Wang, X.; Morrison, R.B. Applications of bayesian phylodynamic methods in a recent U.S. porcine reproductive and respiratory syndrome virus outbreak. Front. Microbiol. 2016, 7, 67. [Google Scholar] [CrossRef]
- Osorio, F.A.; Galeota, J.A.; Nelson, E.; Brodersen, B.; Doster, A.; Wills, R.; Zuckermann, F.; Laegreid, W.W. Passive transfer of virus-specific antibodies confers protection against reproductive failure induced by a virulent strain of porcine reproductive and respiratory syndrome virus and establishes sterilizing immunity. Virology 2002, 302, 9–20. [Google Scholar] [CrossRef]
- Kim, S.-C.; Kim, J.-H.; Kim, J.-Y.; Park, G.-S.; Jeong, C.-G.; Kim, W.-I. Prevalence of porcine parvovirus 1 through 7 (PPV1-PPV7) and co-factor association with PCV2 and PRRSV in Korea. BMC Vet. Res. 2022, 18, 133. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.A.; Bratanich, A.; Clark, E.G.; Allan, G.; Meehan, B.; Haines, D.M.; Harding, J.; West, K.H.; Krakowka, S.; Konoby, C.; et al. Coinfection by porcine circoviruses and porcine parvovirus in pigs with naturally acquired postweaning multisystemic wasting syndrome. J. Vet. Diagn. Investig. 2000, 12, 21–27. [Google Scholar] [CrossRef]
- Salogni, C.; Lazzaro, M.; Giacomini, E.; Giovannini, S.; Zanoni, M.; Giuliani, M.; Ruggeri, J.; Pozzi, P.; Pasquali, P.; Boniotti, M.B.; et al. Infectious agents identified in aborted swine fetuses in a high-density breeding area: A three-year study. J. Vet. Diagn. Investig. 2016, 28, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Serena, M.S.; Dibárbora, M.; Olivera, V.; Metz, G.E.; Aspitia, C.G.; Pereda, A.; Echeverría, M.G.; Cappuccio, J. Evidence of porcine circovirus type 2 and co-infection with ungulate protoparvovirus 1 (porcine parvovirus) in mummies and stillborn piglets in subclinically infected farm. Infect. Genet. Evol. 2021, 89, 104735. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, G.; Piscopo, N.; Pagnini, U.; Esposito, L.; Montagnaro, S. Detection of selected pathogens in reproductive tissues of wild boars in the Campania region, southern Italy. Acta Vet. Scand. 2024, 66, 9. [Google Scholar] [CrossRef] [PubMed]
- Pescador, C.A.; Bandarra, P.M.; Castro, L.A.; Antoniassi, N.A.B.; Ravazzolo, A.P.; Sonne, L.; Cruz, C.E.F.; Driemeier, D. Co-infection by porcine circovirus type 2 and porcine parvovirus in aborted fetuses and stillborn piglets in southern Brazil. Pesq. Vet. Bras. 2007, 27, 425–429. [Google Scholar] [CrossRef]
- Yue, F.; Cui, S.; Zhang, C.; Yoon, K.-J. A multiplex PCR for rapid and simultaneous detection of porcine circovirus type 2, porcine parvovirus, porcine pseudorabies virus, and porcine reproductive and respiratory syndrome virus in clinical specimens. Virus Genes 2009, 38, 392–397. [Google Scholar] [CrossRef]
- Butler, J.E.; Lager, K.M.; Golde, W.; Faaberg, K.S.; Sinkora, M.; Loving, C.; Zhang, Y.I. Porcine reproductive and respiratory syndrome (PRRS): An immune dysregulatory pandemic. Immunol. Res. 2014, 59, 81–108. [Google Scholar] [CrossRef]
- Meng, X.-J. Porcine circovirus type 2 (PCV2): Pathogenesis and interaction with the immune system. Annu. Rev. Anim. Biosci. 2013, 1, 43–64. [Google Scholar] [CrossRef]
- Poonsuk, K.; Zimmerman, J. Historical and contemporary aspects of maternal immunity in swine. Anim. Health Res. Rev. 2018, 19, 31–45. [Google Scholar] [CrossRef]
- Figueras-Gourgues, S.; Fraile, L.; Segalés, J.; Hernández-Caravaca, I.; López-Úbeda, R.; García-Vázquez, F.A.; Gomez-Duran, O.; Grosse-Liesner, B. Effect of Porcine circovirus 2 (PCV-2) maternally derived antibodies on performance and PCV-2 viremia in vaccinated piglets under field conditions. Porcine Health Manag. 2019, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Mészáros, I.; Olasz, F.; Cságola, A.; Tijssen, P.; Zádori, Z. Biology of Porcine Parvovirus (Ungulate parvovirus 1). Viruses 2017, 9, 393. [Google Scholar] [CrossRef] [PubMed]
- Lopez, O.J.; Oliveira, M.F.; Garcia, E.A.; Kwon, B.J.; Doster, A.; Osorio, F.A. Protection against porcine reproductive and respiratory syndrome virus (PRRSV) infection through passive transfer of PRRSV-neutralizing antibodies is dose dependent. Clin. Vaccine Immunol. 2007, 14, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.; McAuley, J.; Jacobson, J.; Nguyen, C.D.; Ullah, M.A.; Sebina, I.; Williamson, V.; Mulholland, E.K.; Wijburg, O.; Phipps, S.; et al. Synergism and Antagonism of Bacterial-Viral Coinfection in the Upper Respiratory Tract. mSphere 2022, 7, e0098421. [Google Scholar] [CrossRef]



| Sows | Insemination | Day 40 | Day 80 | Delivery | |||
|---|---|---|---|---|---|---|---|
| Virus | Antibodies S/P (±), n | Viral Loads Log Copies (±), n | Viral Loads Log Copies (±), n | Viral Loads Log Copies (±), n | Antibodies S/P (±), n | Viral Loads Log Copies (±), n | |
| PCV2 | Primiparous | 0.9 (0.21) 18/18 | 3.9 (0.6) 6/18 | 3 (0.0) 1/18 | 3.8 (0.4) 8/18 | 1.2 (0.32) 18/18 | 3.8 (0.76) 15/18 |
| Multiparous | 0.91 (0.25) 22/22 | 4 (0.67) 8/22 | 4.25 (0.0) 1/22 | 4.2 (0.71) 9/22 | 1.29 (0.41) 20/20 | 3.8 (0.82) 18/20 | |
| PCV3 | Primiparous | NP | 3.8 (1) 7/18 | 4.2 (0.25) 7/18 | 3.6 (0.3) 8/18 | 0.23 (1.4) 6/18 | 4 (0.4) 2/18 |
| Multiparous | NP | 3.8 (0.74) 8/22 | 4 (0.21) 8/22 | 3.65 (0.51) 10/22 | 1.3 (0.98) * 13/20 | 4 (0.15) 3/20 | |
| PPV1 | Primiparous | 0.76 (0.41) 12/18 | 3.7 (0.14) 10/18 | 4 (0.4) 3/18 | 3.7 (0.05) 3/18 | 0.34 (0.3) 8/18 | 3.7 (0.56) 7/18 |
| Multiparous | 0.95 (0.4) 14/22 | 3.8 (0.16) 9/22 | 3.8 (0.17) 4/22 | 4 (0) 1/22 | 0.7 (0.36) * 13/20 | 3.76 (0.23) 6/20 | |
| PRRSV | Primiparous | 0.01 (0.56) 5/18 | 0/18 | 3 (0.2) 4/18 | 3 (0.2) 4/18 | 0.21 (0.64) 7/18 | 3 (0.0) 1/18 |
| Multiparous | 0.02 (0.7) 5/22 | 0/22 | 3 (0.2) 4/22 | 3 (0.0) 3/22 | 0.07 (0.39) 6/20 | 3 (0.11) 3/20 | |
| Virus | Sows | Placenta Viral Load Log 10 Copies (±), n | Umbilical Cord Viral Load Log 10 Copies (±), n | Colostrum Viral Load Log10 Copies (±), n |
|---|---|---|---|---|
| PCV2 | Primiparous | 4 (0.23), 12/18 | 4 (0.3), 14/18 | 3.9 (0.5), 12/18 |
| Multiparous | 4.2 (0.57), 15/19 | 4.2 (0.6), 18/19 | 4.1 (0.43), 15/19 | |
| PCV3 | Primiparous | 4 (0.26), 4/18 | 3.7 (0.23), 4/18 | 4 (0.6), 6/18 |
| Multiparous | 3.7 (0.01), 4/19 | 3.7 (0.1), 5/19 | 3.7 (0.16), 5/19 | |
| PPV1 | Primiparous | 3.9 (0.37), 8/18 | 4 (0.59), 9/18 | 3.8 (0.64), 6/18 |
| Multiparous | 3.8 (0.32), 8/19 | 3.9 (0.39), 6/19 | 3.95 (0.44), 7/19 | |
| PRRSV | Primiparous | 3 (0.0), 1/18 | 3 (0.0), 1/18 | 3 (0.0), 1/18 |
| Multiparous | 3 (0.75), 4/19 | 3 (0.46), 3/19 | 3.4 (0.56), 2/19 |
| Pre-Suckling | Week 1 | Week 3 | |||||
|---|---|---|---|---|---|---|---|
| Virus | Sows | Antibodies S/P (±), n | Viral Load Log Copies (±), n | Antibodies S/P (±), n | Viral Load Log Copies (±), n | Antibodies S/P (±), n | Viral Load Log Copies (±), n |
| PCV2 | Primiparous | 0.39 (0.28), 12/17 | 4 (0.48), 14/17 | 1.2 (0.35), 17/17 | 4 (0.32), 8/17 | 1.16 (0.33), 17/17 | 3.7 (0.34), 6/17 |
| Multiparous | 0.3 (0.35), 10/19 | 4 (0.4), 18/19 | 1.3 (0.38), 19/19 | 3.7 (0.52), 8/19 | 1.14 (0.37), 19/19 | 3.4 (0.35), 7/19 | |
| PCV3 | Primiparous | 0.05(0.28), 2/17 | 3.4 (0.0), 1/17 | NP | 3.5 (0.21), 2/17 | NP | 3.7 (0.8), 10/17 |
| Multiparous | 0.04 (0.27), 1/19 | 3.8 (0.57), 3/19 | NP | 3.55 (0.21), 2/19 | NP | 3.4 (0.34), 5/19 | |
| PPV1 | Primiparous | 0.09 (0.09), 4/17 | 3.8 (0.2), 5/17 | 0.58 (0.38) *, 11/17 | 4.4 (0.23), 4/17 | 0.25 (0.38) *, 8/17 | 4 (0.1), 4/17 |
| Multiparous | 0.06 (0.06), 1/19 | 3.8 (0.13), 5/19 | 1.01 (0.4) 15/19 | 4.3 (0.0), 1/19 | 0.93 (0.43), 12/19 | 4 (0.5), 5/19 | |
| PRRSV | Primiparous | 0.02 (0.01), 0/17 | 5 (0.0), 1/17 | 0.05 (0.45), 6/17 | 3.7 (0.0), 1/17 | 0.03 (0.3), 6/17 | 3 (0.0), 1/17 |
| Multiparous | 0.03 (0.0), 0/19 | 3.7 (0.25), 3/19 | 0.03 (0.56), 6/19 | 4.05 (0.21), 2/19 | 0.1 (0.41), 6/19 | 3 (0.0), 1/19 | |
| Virus | Reproductive Disease | Reproductive Subclinical Infection |
|---|---|---|
| PCV2 | Reproductive failure or SMEDI, return to estrus Fibrous to necrotizing myocarditis of fetuses Moderate (>5 log10 copies) to high (>7 log10 copies) viral load of PCV2 in tissues samples of mummies, stillborns and newborn piglets Seroconversion following return to estrus PCR or qPCR positivity detected during irregular return to estrus [39] | Lack of overt reproductive clinical signs or SMEDI No or minimal histopathological lesions in fetal tissues Low PCV2 viral loads (<4 log10 copies) by qPCR or ISH in fetal and maternal tissues (fetal hearth and placenta) Low PCV2 viral loads (<4 log10 copies) by qPCR in sows during pregnancy and farrowing Detection of pre-suckling PCV2-Abs and low PCV2 viral load (<4 log10 copies) |
| PCV3 | Late reproductive signs (abortion, malformations, mummified fetuses, stillborn fetuses, weak-born piglets) and higher perinatal mortality Multisystemic lymphoplasmacytic to lymphohistiocytic perivascular inflammation Focal myocardial necrosis with mononuclear infiltration in stillborn and mummies Moderate to high (>105) PCV3 viral loads in damaged tissues [36] | Lack of overt reproductive clinical signs or SMEDI No or minimal lesions in fetal tissues Low PCV3 viral loads (<4 log10 copies) in fetal and maternal tissues Seropositivity in sows during farrowing and low PCV3 viral loads (<4 log10 copies) in sows during pregnancy by qPCR Detection of pre-suckling PCV3-Abs and low PCV3 viral loads (<4 log10 copies) |
| PPV1 | Reproductive failure or SMEDI PPV1 Ag in tissues [4] High (>5 log10 copies) viral load of PPV1 in fetuses | Lack of overt reproductive clinical signs or SMEDI No or minimal lesions in fetal tissues Low PPV1 viral loads (<4 log10 copies) in fetal and maternal tissues Detection of pre-suckling PPV1-Abs and low PPV1 viral loads (<4 log10 copies) Low PPV1 viral loads (<4 log10 copies) in sows during pregnancy and farrowing by qPCR |
| PRRSV | Late Reproductive failure or SMEDI High PRRSV viral loads (>4 log10 copies) in reproductive lymph nodes of sows Moderate to high PRRSV viral loads (>4 log10 copies) in meconium-stained fetuses [38] | Lack of overt reproductive clinical signs or SMEDI Low PRRSV viral loads (<3.5 log10 copies) in sows during pregnancy and farrowing by qPCR PRRSV resilient litters: low percentage of fetuses dead and low (<3.5 log10 copies) or negative PRRSV-RNA levels [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Bermudez, D.S.; Polo, G.; Mogollon, J.D.; Jaime, J. Longitudinal Monitoring of Mono- and Coinfections Involving Primary Porcine Reproductive Viruses (PCV2, PPV1, and PRRSV) as Well as Emerging Viruses (PCV3, PCV4, and nPPVs) in Primiparous and Multiparous Sows and Their Litters. Pathogens 2025, 14, 573. https://doi.org/10.3390/pathogens14060573
Vargas-Bermudez DS, Polo G, Mogollon JD, Jaime J. Longitudinal Monitoring of Mono- and Coinfections Involving Primary Porcine Reproductive Viruses (PCV2, PPV1, and PRRSV) as Well as Emerging Viruses (PCV3, PCV4, and nPPVs) in Primiparous and Multiparous Sows and Their Litters. Pathogens. 2025; 14(6):573. https://doi.org/10.3390/pathogens14060573
Chicago/Turabian StyleVargas-Bermudez, Diana S., Gina Polo, Jose Dario Mogollon, and Jairo Jaime. 2025. "Longitudinal Monitoring of Mono- and Coinfections Involving Primary Porcine Reproductive Viruses (PCV2, PPV1, and PRRSV) as Well as Emerging Viruses (PCV3, PCV4, and nPPVs) in Primiparous and Multiparous Sows and Their Litters" Pathogens 14, no. 6: 573. https://doi.org/10.3390/pathogens14060573
APA StyleVargas-Bermudez, D. S., Polo, G., Mogollon, J. D., & Jaime, J. (2025). Longitudinal Monitoring of Mono- and Coinfections Involving Primary Porcine Reproductive Viruses (PCV2, PPV1, and PRRSV) as Well as Emerging Viruses (PCV3, PCV4, and nPPVs) in Primiparous and Multiparous Sows and Their Litters. Pathogens, 14(6), 573. https://doi.org/10.3390/pathogens14060573







