Clinical Progression of Cardiac Chronic Trypanosoma cruzi Infection in a Long-Term Prospective Cohort from Rural Colombia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants
2.3. Clinical and Laboratory Procedures and Outcomes
2.4. Statistical Analysis
3. Results
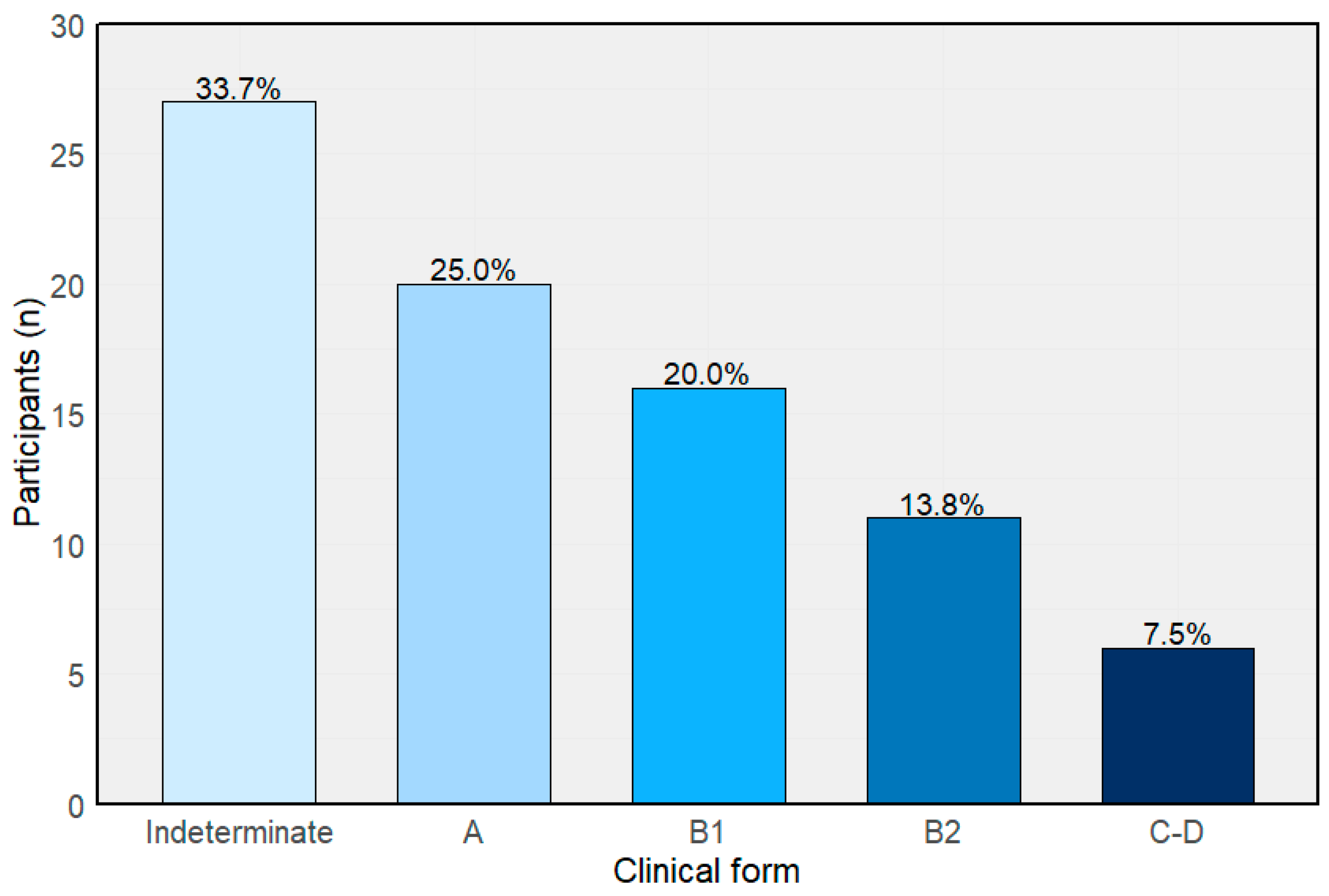
3.1. Baseline Characteristics and Clinical Classification
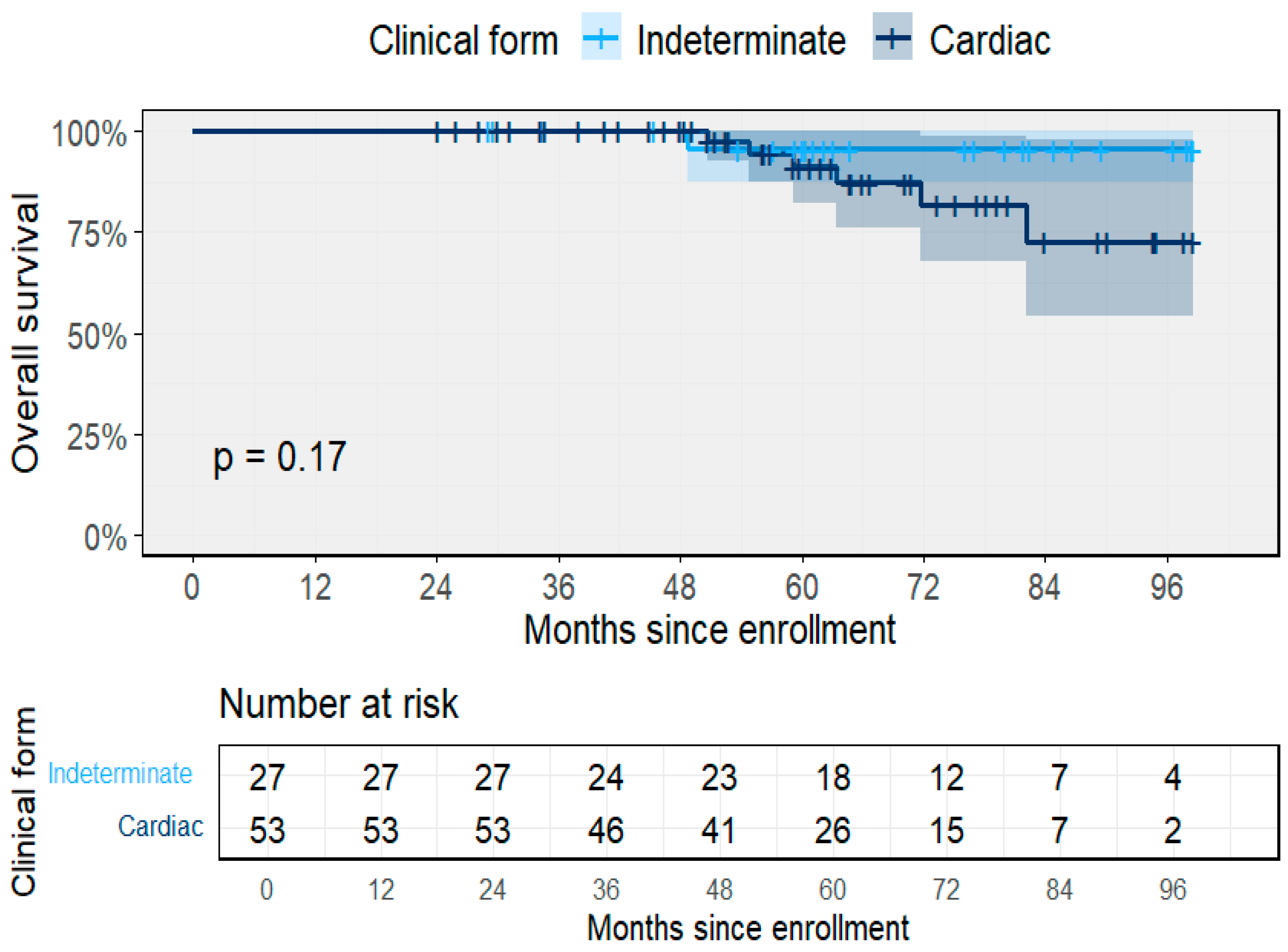
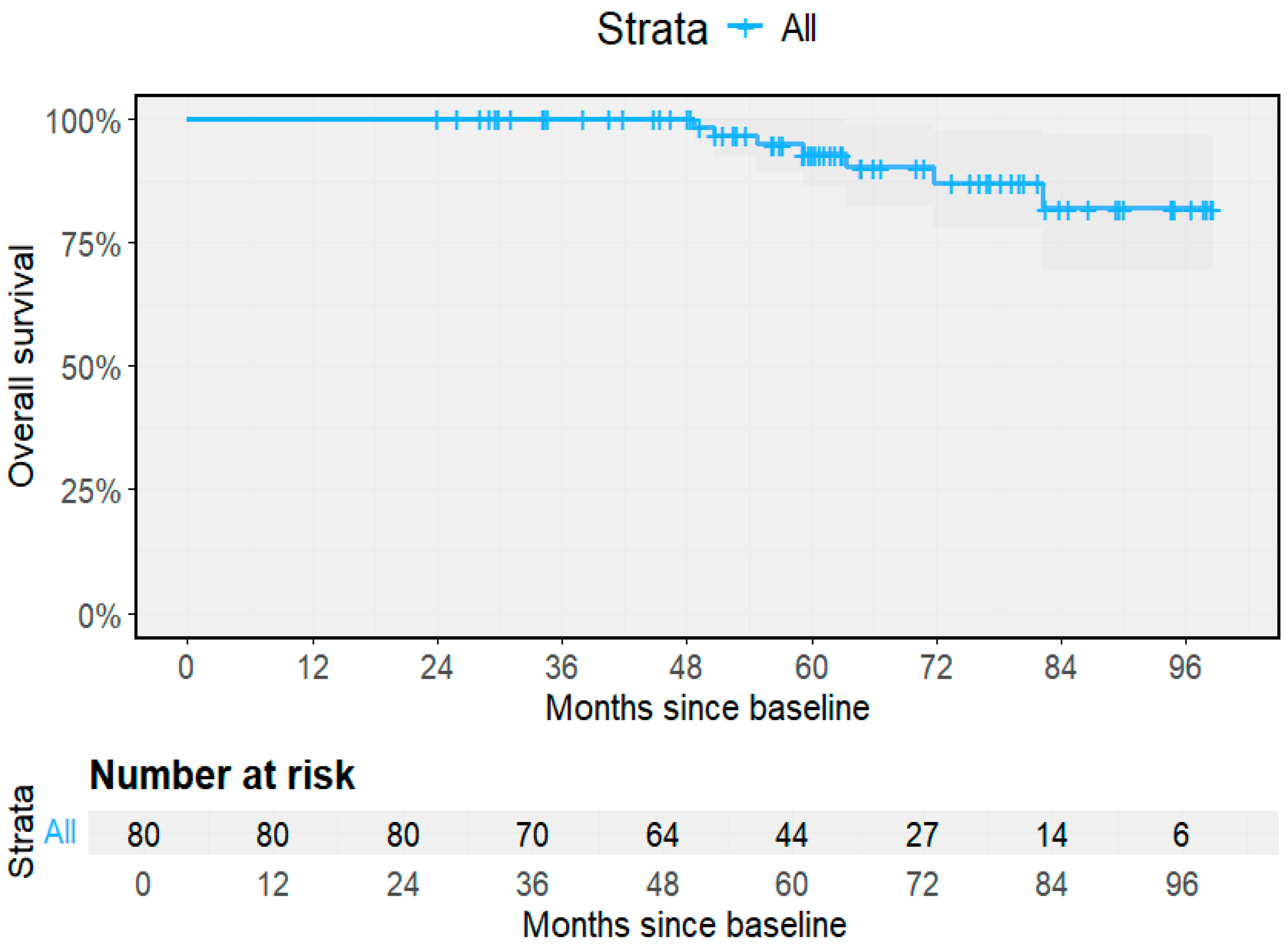
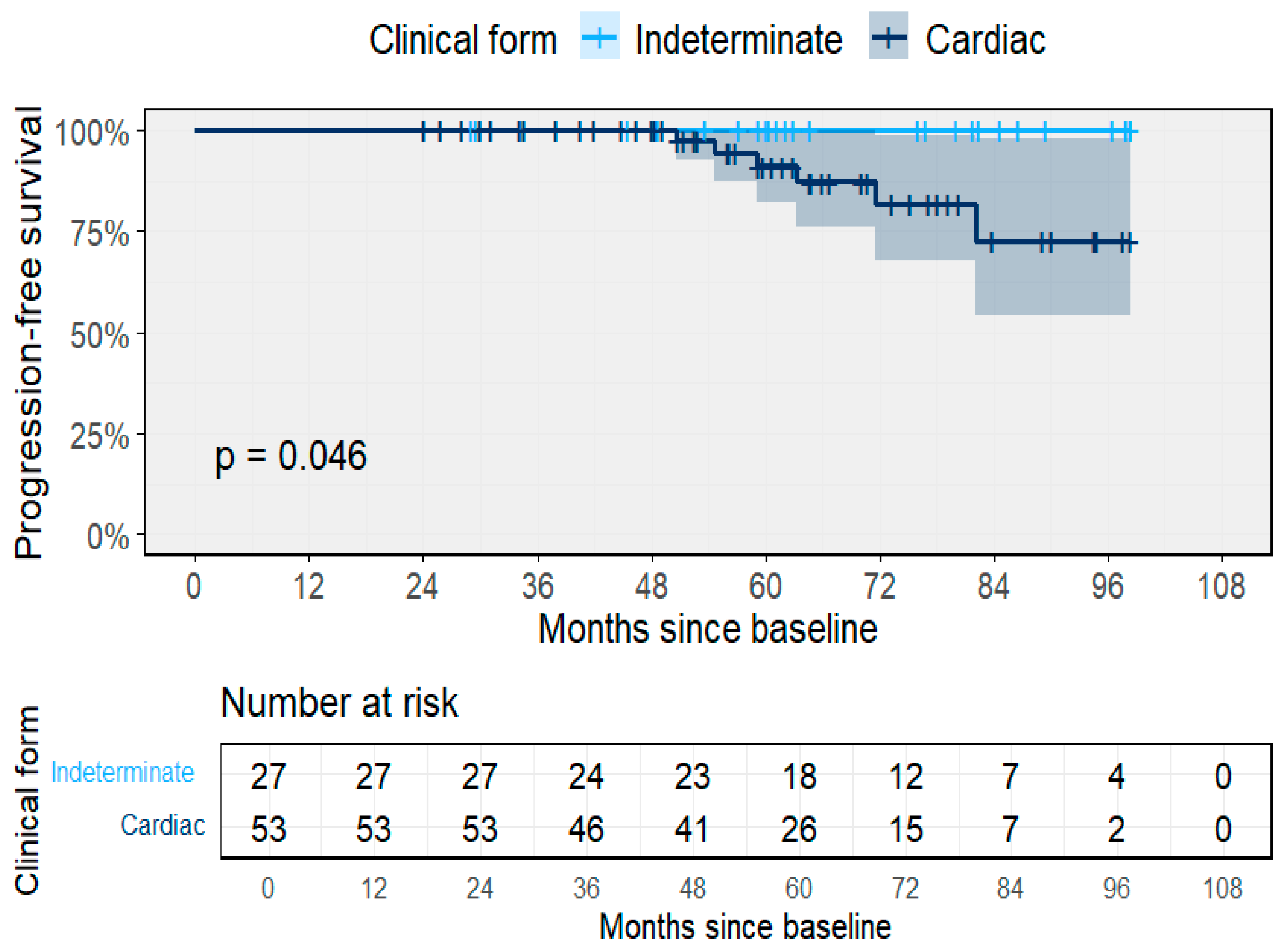
3.2. Clinical Outcomes and Progression
3.3. Predictors of Clinical Progression
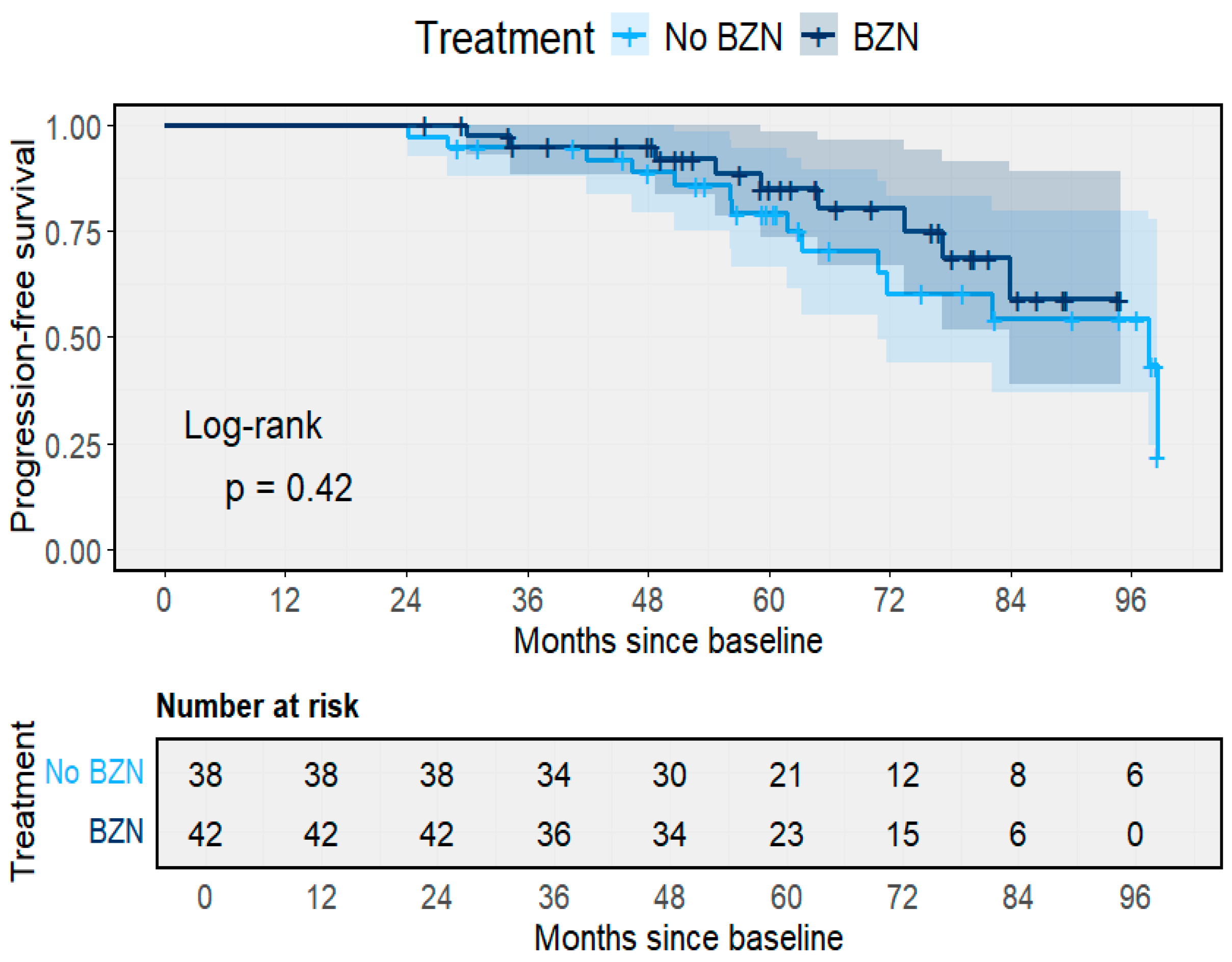
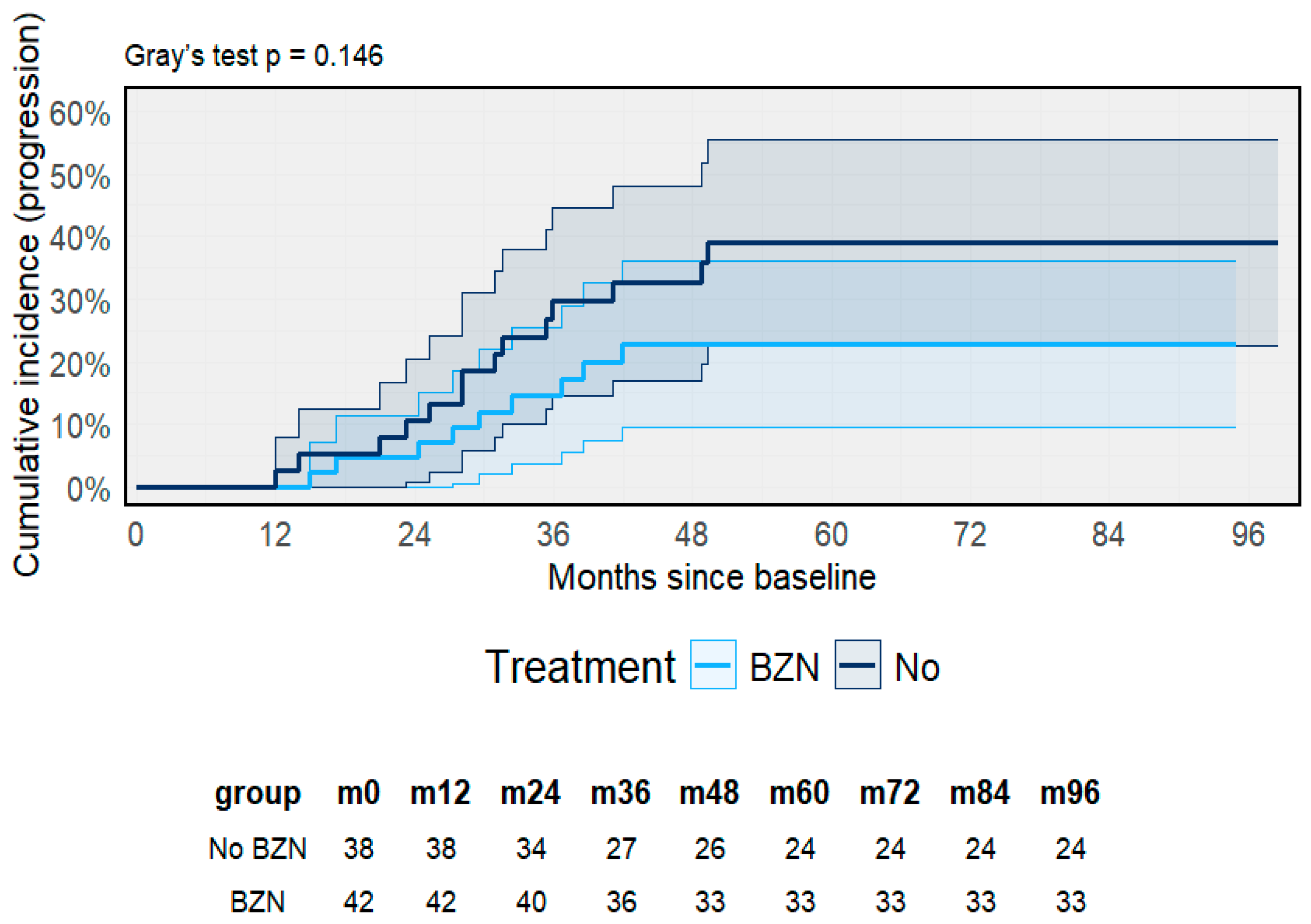
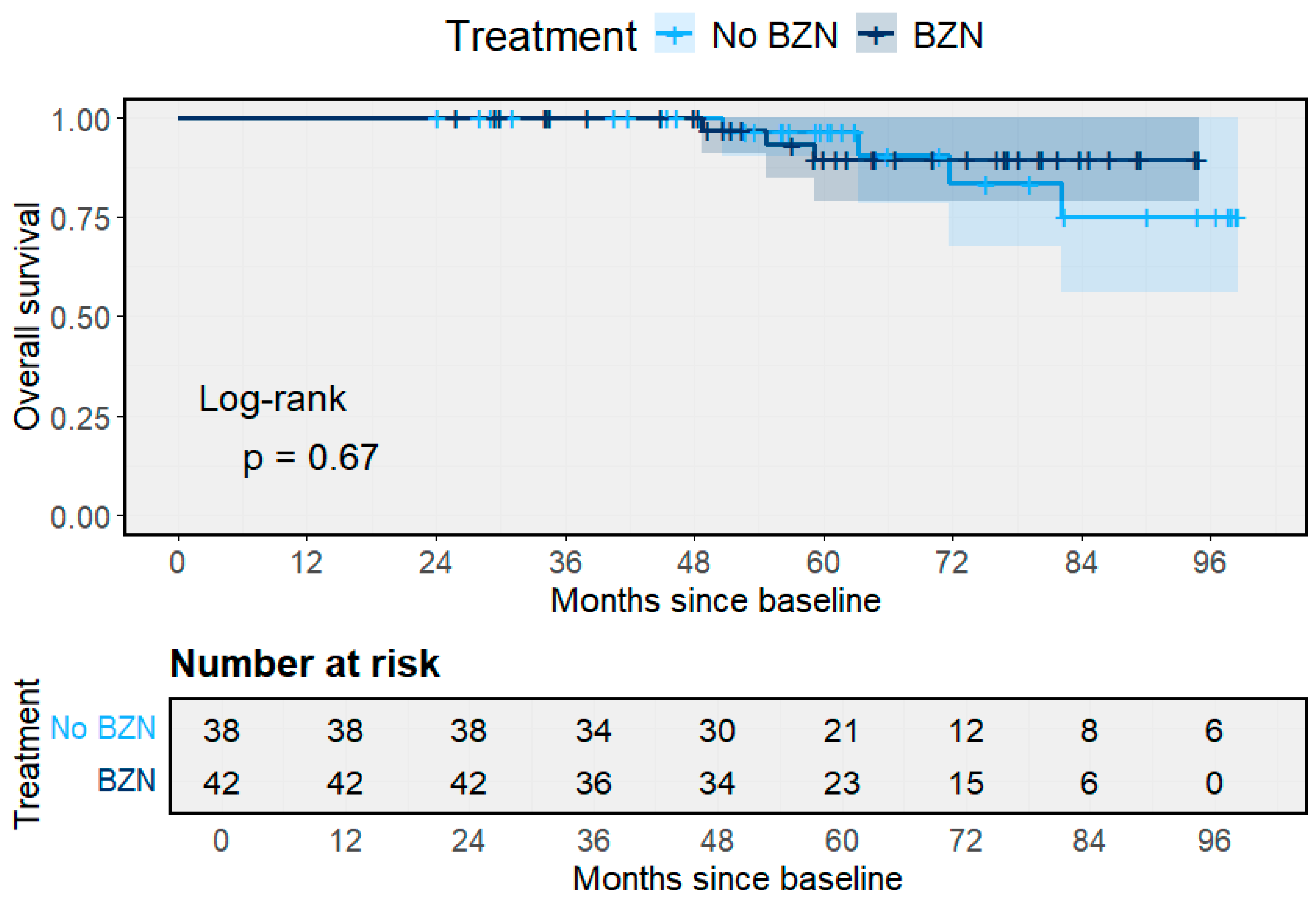
3.4. Treatment and Molecular Follow-Up
3.5. Treatment Outcomes and Clinical Progression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Organización Panamericana de la Salud. Actualización de la Estimación de la Enfermedad de Chagas en los Países Endémicos de las Américas, 2018; PAHO: Washington, DC, USA, 2025. [Google Scholar]
- Cucunubá, Z.M.; Gutiérrez-Romero, S.A.; Ramírez, J.-D.; Velásquez-Ortiz, N.; Ceccarelli, S.; Parra-Henao, G.; Henao-Martínez, A.F.; Rabinovich, J.; Basáñez, M.-G.; Nouvellet, P.; et al. The epidemiology of Chagas disease in the Americas. Lancet Reg. Health Am. 2024, 37, 100881. [Google Scholar] [CrossRef]
- de Sousa, A.S.; Vermeij, D.; Ramos, A.N.; Luquetti, A.O. Chagas disease. Lancet 2024, 403, 203–218. [Google Scholar] [CrossRef]
- Echavarría, N.G.; Echeverría, L.E.; Stewart, M.; Gallego, C.; Saldarriaga, C. Chagas Disease: Chronic Chagas Cardiomyopathy. Curr. Probl. Cardiol. 2021, 46, 100507. [Google Scholar] [CrossRef]
- Olivera, M.J.; Porras-Villamil, J.F.; Villar, J.C.; Herrera, E.V.; Buitrago, G. Chagas disease-related mortality in Colombia from 1979 to 2018: Temporal and spatial trends. Rev. Soc. Bras. Med. Trop. 2021, 54, e07682020. [Google Scholar] [CrossRef] [PubMed]
- Lima-Costa, M.F.; Firmo, J.O.A. Uchoa ECohort profile: The Bambui (Brazil) Cohort Study of Ageing. Int. J. Epidemiol. 2011, 40, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Hasslocher-Moreno, A.M.; Xavier, S.S.; Saraiva, R.M.; Sangenis, L.H.C.; De Holanda, M.T.; Veloso, H.H.; da Costa, A.R.; Mendes, F.d.S.N.S.; Brasil, P.E.A.A.D.; da Silva, G.M.S.; et al. Progression Rate from the Indeterminate Form to the Cardiac Form in Patients with Chronic Chagas Disease: Twenty-Two-Year Follow-Up in a Brazilian Urban Cohort. Trop. Med. Infect. Dis. 2020, 5, 76. [Google Scholar] [CrossRef] [PubMed]
- Sabino, E.C.; Ribeiro, A.L.; Salemi, V.M.C.; Di Lorenzo Oliveira, C.; Antunes, A.P.; Menezes, M.M.; Ianni, B.M.; Nastari, L.; Fernandes, F.; Patavino, G.M.; et al. Ten-Year Incidence of Chagas Cardiomyopathy Among Asymptomatic Trypanosoma cruzi–Seropositive Former Blood Donors. Circulation 2013, 127, 1105–1115. [Google Scholar] [CrossRef]
- Basquiera, A.L.; Sembaj, A.; Aguerri, A.M.; Omelianiuk, M.; Guzmán, S.; Moreno Barral, J.; Madoery, R.J.; Salomone, O.A. Risk progression to chronic Chagas cardiomyopathy: Influence of male sex and of parasitaemia detected by polymerase chain reaction. Heart 2003, 89, 1186–1190. [Google Scholar] [CrossRef]
- Cardoso, C.S.; Sabino, E.C.; Di Lorenzo Oliveira, C.; De Oliveira, L.C.; Ferreira, A.M.; Cunha-Neto, E.; Bierrenbach, A.L.; Ferreira, J.E.; Haikal, D.S.; Reingold, A.L.; et al. Longitudinal study of patients with chronic Chagas cardiomyopathy in Brazil (SaMi-Trop project): A cohort profile. BMJ Open 2016, 6, e011181. [Google Scholar] [CrossRef]
- Chadalawada, S.; Sillau, S.; Archuleta, S.; Mundo, W.; Bandali, M.; Parra-Henao, G.; Rodriguez-Morales, A.J.; Villamil-Gomez, W.E.; Suárez, J.A.; Shapiro, L.; et al. Risk of Chronic Cardiomyopathy Among Patients With the Acute Phase or Indeterminate Form of Chagas Disease: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e2015072. [Google Scholar] [CrossRef]
- Zingales, B. Trypanosoma cruzi genetic diversity: Something new for something known about Chagas disease manifestations, serodiagnosis and drug sensitivity. Acta Trop. 2018, 184, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Salud y Protección Social. Plan de Interrupción de la Transmisión de la Enfermedad de Chagas por Vectores Intradomiciliados en Colombia; Ministerio de Salud y Protección Social: Bogotá, Colombia, 2019. [Google Scholar]
- Ramírez, J.D.; Montilla, M.; Cucunubá, Z.M.; Floréz, A.C.; Zambrano, P.; Guhl, F. Molecular Epidemiology of Human Oral Chagas Disease Outbreaks in Colombia. PLoS Neglected Trop. Dis. 2013, 7, e2041. [Google Scholar] [CrossRef] [PubMed]
- Zafra, G.; Mantilla, J.C.; Jácome, J.; MacEdo, A.M.; González, C.I. Direct analysis of genetic variability in Trypanosoma cruzi populations from tissues of Colombian chagasic patients. Hum. Pathol. 2011, 42, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Olivera, M.J.; Fory, J.A.; Porras, J.F.; Buitrago, G. Prevalence of Chagas disease in Colombia: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0210156. [Google Scholar] [CrossRef] [PubMed]
- Olivera, M.J.; Porras, J.; Toquica, C.; Rodríguez, J. Barriers to Diagnosis Access for Chagas Disease in Colombia. J. Parasitol. Res. 2018, 2018, 4940796. [Google Scholar] [CrossRef]
- Segura-Alba, M.L.; Hernandez, C.; Guerra, A.P.; Luna, N.; Cortes, L.J.; Acevedo, C.R.; Ballesteros, N.; Ayala, M.S.; Vera, M.J.; Diaz, R.A.C.; et al. Acute Chagas disease outbreaks in Colombia in 2019. IJID Regions 2024, 12, 100410. [Google Scholar] [CrossRef]
- Guhl, F.; Ramírez, J.D. Poverty, Migration, and Chagas Disease. Curr. Trop. Med. Rep. 2021, 8, 52–58. [Google Scholar] [CrossRef]
- Hernández, C.; Cucunubá, Z.; Flórez, C.; Olivera, M.; Valencia, C.; Zambrano, P.; León, C.; Ramírez, J.D. Molecular Diagnosis of Chagas Disease in Colombia: Parasitic Loads and Discrete Typing Units in Patients from Acute and Chronic Phases. PLoS Neglected Trop. Dis. 2016, 10, e0004997. [Google Scholar] [CrossRef]
- Burgos, J.M.; Diez, M.; Vigliano, C.; Bisio, M.; Risso, M.; Duffy, T.; Cura, C.; Brusses, B.; Favaloro, L.; Leguizamon, M.S.; et al. Molecular identification of Trypanosoma cruzi discrete typing units in end-stage chronic Chagas heart disease and reactivation after heart transplantation. Clin. Infect. Dis. 2010, 51, 485–495. [Google Scholar] [CrossRef]
- Dias, J.C.P.; Ramos, A.N.; Gontijo, E.D.; Luquetti, A.; Shikanai-Yasuda, M.A.; Coura, J.R.; Torres, R.M.; Melo, J.R.D.C.; De Almeida, E.A.; de Oliveira, W., Jr.; et al. 2nd Brazilian Consensus on Chagas Disease, 2015. Rev. Soc. Bras. Med. Trop. 2016, 49 (Suppl. S1), 3–60. [Google Scholar] [CrossRef]
- Murillo-Godínez, G. Chagas disease (American trypanosomiasis). Med. Int. Méx. 2018, 34, 959–970. [Google Scholar] [CrossRef]
- Nunes, M.C.P.; Bern, C.; Clark, E.H.; Teixeira, A.L.; Molina, I. Clinical features of Chagas disease progression and severity. Lancet Reg. Health 2024, 37, 100832. [Google Scholar] [CrossRef]
- Sosa-Estani, S.; Segura, E.L. Integrated control of Chagas disease for its elimination as public health problem—A Review. Mem. Inst. Oswaldo Cruz 2015, 110, 289–298. [Google Scholar] [CrossRef]
- Apt, W.; Llancaqueo, M.; Zulantay, I.; Canals, M.; Kara, S.; Arribada, A.; Muñoz, G.; Martínez, G. Clinical, electrocardiographic and echocardiographic evolution of chronic Chagas disease treated with nifurtimox on prolonged follow-up in Chile: Observational study. J. Glob. Antimicrob. Resist. 2021, 27, 160–166. [Google Scholar] [CrossRef]
- Fabbro, D.L.; Streiger, M.L.; Arias, E.D.; Bizai, M.L.; del Barco, M.; Amicone, N.A. Trypanocide treatment among adults with chronic Chagas disease living in Santa Fe city (Argentina), over a mean follow-up of 21 years: Parasitological, serological and clinical evolution. Rev. Soc. Bras. Med. Trop. 2007, 40, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.R.L.; Hecht, M.M.; Guimaro, M.C.; Sousa, A.O.; Nitz, N. Pathogenesis of Chagas’ Disease: Parasite Persistence and Autoimmunity. Clin. Microbiol. Rev. 2011, 24, 592–630. [Google Scholar] [CrossRef] [PubMed]
- Bonney, K.M.; Luthringer, D.J.; Kim, S.A.; Garg, N.J.; Engman, D.M. Pathology and Pathogenesis of Chagas Heart Disease. Annu. Rev. Pathol. 2018, 14, 421–447. [Google Scholar] [CrossRef] [PubMed]
- Cutshaw, M.K.; Sciaudone, M.; Bowman, N.M. Risk Factors for Progression to Chronic Chagas Cardiomyopathy: A Systematic Review and Meta-Analysis. Am. J. Trop. Med. Hyg. 2023, 108, 791–800. [Google Scholar] [CrossRef]
- Clark, E.H.; Samuels, A.M.; Bozo-Gutierrez, R.W.; Martin, D.L.; Gilman, R.H.; Fernandez, A.B.; Hidron, A.I.; Marks, M.A.; Bern, C.; Galdos-Cardenas, G.; et al. Circulating Serum Markers and QRS Scar Score in Chagas Cardiomyopathy. Am. J. Trop. Med. Hyg. 2015, 92, 39–44. [Google Scholar] [CrossRef]
- Torres, R.M.; Correia, D.; Nunes, M.D.C.P.; ODutra, W.; Talvani, A.; Sousa, A.S.; Mendes, F.d.S.N.S.; Scanavacca, M.I.; Pisani, C.; Moreira, M.d.C.V.; et al. Prognosis of chronic Chagas heart disease and other pending clinical challenges. Mem. Inst. Oswaldo Cruz 2022, 117, e210172. [Google Scholar] [CrossRef]
- Simões, M.V.; Romano, M.M.D.; Schmidt, A.; Martins, K.S.M.; Marin-Neto, J.A. Chagas Disease Cardiomyopathy. Int. J. Cardiovasc. Sci. 2018, 31, 173–189. [Google Scholar] [CrossRef]
- Mansur, A.P.; Pereira-Barretto, A.C.; del Carlo, C.H.; Ianni, B.M.; Avakian, S.D.; Gonçalinho, G.H.F.; Nakagawa, N.K.; César, L.A.; Bocchi, E.A. Sex Differences in Prognosis of Heart Failure Due to Chronic Chagas Cardiomyopathy. JACC Heart Fail. 2023, 11, 1284–1286. [Google Scholar] [CrossRef]
- Lima-Costa, M.F.; Peixoto, S.V.; Matos, D.L.; Firmo, J.O.A.; Uchôa, E. Predictors of 10-year mortality in a population of community-dwelling Brazilian elderly: The Bambuí Cohort Study of Aging. Cad. Saude Publica 2011, 27 (Suppl. S3), s360–s369. [Google Scholar] [CrossRef]
- Brito, B.O.F.; Lima, E.M.; Soliman, E.Z.; Silva, E.F.; Lima-Costa, M.F.; Ribeiro, A.L.P. The evolution of electrocardiographic abnormalities in the elderly with Chagas disease during 14 years of follow-up: The Bambui Cohort Study of Aging. PLoS Neglected Trop. Dis. 2023, 17, e0011419. [Google Scholar] [CrossRef]
- De Alba-Alvarado, M.C.; Torres-Gutiérrez, E.; Reynoso-Ducoing, O.A.; Zenteno-Galindo, E.; Cabrera-Bravo, M.; Guevara-Gómez, Y.; Salazar-Schettino, P.M.; Rivera-Fernández, N.; Bucio-Torres, M.I. Immunopathological Mechanisms Underlying Cardiac Damage in Chagas Disease. Pathogens 2023, 12, 335. [Google Scholar] [CrossRef]
- Silvestrini, M.M.A.; Alessio, G.D.; Frias, B.E.D.; Sales Júnior, P.A.; Araújo, M.S.S.; Silvestrini, C.M.A.; de Melo, G.E.B.A.; Martins-Filho, O.A.; Teixeira-Carvalho, A.; Martins, H.R. New insights into Trypanosoma cruzi genetic diversity, and its influence on parasite biology and clinical outcomes. Front. Immunol. 2024, 15, 1342431. [Google Scholar] [CrossRef]
- Dumonteil, E.; Desale, H.; Tu, W.; Hernandez-Cuevas, N.; Shroyer, M.; Goff, K.; Marx, P.A.; Herrera, C. Intra-host Trypanosoma cruzi strain dynamics shape disease progression: The missing link in Chagas disease pathogenesis. Microbiol. Spectr. 2023, 11, e0423622. [Google Scholar] [CrossRef]
- Morillo, C.A.; Marin-Neto, J.A.; Avezum, A.; Sosa-Estani, S.; Rassi, A., Jr.; Rosas, F.; Villena, E.; Quiroz, R.; Bonilla, R.; Britto, C.; et al. Randomized Trial of Benznidazole for Chronic Chagas’ Cardiomyopathy. N. Engl. J. Med. 2015, 373, 1295–1306. [Google Scholar] [CrossRef]
- Cardoso, C.S.; Ribeiro, A.L.P.; Oliveira, C.D.L.; Oliveira, L.C.; Ferreira, A.M.; Bierrenbach, A.L.; Silva, J.L.P.; Colosimo, E.A.; Ferreira, J.E.; Lee, T.-H.; et al. Beneficial effects of benznidazole in Chagas disease: NIH SaMi-Trop cohort study. PLoS Neglected Trop. Dis. 2018, 12, e0006814. [Google Scholar] [CrossRef]
- Oliveira, C.D.L.; Cardoso, C.S.; Baldoni, N.R.; Natany, L.; Ferreira, A.M.; de Oliveira, L.C.; Nunes, M.D.C.P.; Quintino, N.D.; Bierrenbach, A.L.; Buss, L.F.; et al. Cohort profile update: The main and new findings from the SaMi-Trop Chagas cohort. Rev. Inst. Med. Trop. Sao Paulo 2021, 63, e75. [Google Scholar] [CrossRef]
- Marin-Neto, J.A.; Rassi, A.; Oliveira, G.M.M.; Correia, L.C.L.; Ramos, A.N.; Luquetti, A.O.; Hasslocher-Moreno, A.M.; de Sousa, A.S.; de Paola, A.A.V.; Sousa, A.C.S.; et al. SBC Guideline on the Diagnosis and Treatment of Patients with Cardiomyopathy of Chagas Disease—2023. Arq. Bras. Cardiol. 2023, 120, e20230269. [Google Scholar] [CrossRef]
- Vallejo, M.; Reyes, P.P.A.; Martinez Garcia, M.; Gonzalez Garay, A.G. Trypanocidal drugs for late-stage, symptomatic Chagas disease (Trypanosoma cruzi infection). Cochrane Database Syst. Rev. 2020, 2020, CD004102. [Google Scholar] [CrossRef]
- Velásquez-Ortiz, N.; Herrera, G.; Hernández, C.; Muñoz, M.; Ramírez, J.D. Discrete typing units of Trypanosoma cruzi: Geographical and biological distribution in the Americas. Sci. Data 2022, 9, 360. [Google Scholar] [CrossRef]
- Nielebock, M.A.P.; Moreira, O.C.; Xavier, S.C.D.C.; de Freitas Campos Miranda, L.; de Lima, A.C.B.; de Jesus Sales Pereira, T.O.; Hasslocher-Moreno, A.M.; Britto, C.; Sangenis, L.H.C.; Saraiva, R.M. Association between Trypanosoma cruzi DTU TcII and chronic Chagas disease clinical presentation and outcome in an urban cohort in Brazil. PLoS ONE 2020, 15, e0243008. [Google Scholar] [CrossRef]
- Cordovez, J.M.; Guhl, F. The impact of landscape transformation on the reinfestation rates of Rhodnius prolixus in the Orinoco Region, Colombia. Acta Trop. 2015, 151, 73–79. [Google Scholar] [CrossRef]
- Méndez-Cardona, S.; Ortiz, M.I.; Carrasquilla, M.C.; Fuya, P.; Guhl, F.; González, C. Altitudinal distribution and species richness of triatomines (Hemiptera:Reduviidae) in Colombia. Parasites Vectors 2022, 15, 450. [Google Scholar] [CrossRef]
- Parra-Henao, G.; Suárez-Escudero, L.C.; González-Caro, S. Potential Distribution of Chagas Disease Vectors (Hemiptera, Reduviidae, Triatominae) in Colombia, Based on Ecological Niche Modeling. J. Trop. Med. 2016, 2016, 1439090. [Google Scholar] [CrossRef]
- Cantillo-Barraza, O.; Gual-González, L.; Velásquez-Ortiz, N.; Medina Camargo, M.A.; González, P.; Cruz-Saavedra, L.; Castillo, A.; Zuluaga, S.; Herrera, G.; Cowan, H.; et al. Triatoma venosa and Panstrongylus geniculatus challenge the certification of interruption of vectorial Trypanosoma cruzi transmission by Rhodnius prolixus in eastern Colombia. PLoS Neglected Trop. Dis. 2025, 19, e0012822. [Google Scholar] [CrossRef]
- Genetic Variability of Trypanosoma cruzi TcI Isolates from Rural and Urban Areas of Venezuela—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/25815863/ (accessed on 14 October 2025).
- Muñoz-Calderón, A.; Díaz-Bello, Z.; Alarcón de Noya, B.; Noya-González, O.O.; Schijman, A.G. Characterization and Follow-Up of Trypanosoma cruzi Natural Populations Refractory to Etiological Chemotherapy in Oral Chagas Disease Patients. Front. Cell. Infect. Microbiol. 2021, 11, 665063. [Google Scholar] [CrossRef]
- Ramirez, J.D.; Guhl, F.; Rendon, L.M.; Rosas, F.; Marin-Neto, J.A.; Morillo, C.A. Chagas cardiomyopathy manifestations and Trypanosoma cruzi genotypes circulating in chronic Chagasic patients. PLoS Neglected Trop. Dis. 2010, 4, e899. [Google Scholar] [CrossRef]
- Hodo, C.L.; Auckland, L.; Curtis-Robles, R.; Lewis, B.C.; Hamer, S. Trypanosoma cruzi Dtu Tci Disproportionately Associated with Fatal T. cruzi Myocarditis in Dogs in Texas, USA. Vet. Parasitol. Reg. Stud. Rep. 2025, 63, 101293. [Google Scholar] [CrossRef] [PubMed]
- Olivo Freites, C.; Sy, H.; Gharamti, A.; Higuita, N.I.A.; Franco-Paredes, C.; Suárez, J.A.; Henao-Martínez, A.F. Chronic Chagas Disease-the Potential Role of Reinfections in Cardiomyopathy Pathogenesis. Curr. Heart Fail. Rep. 2022, 19, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, J.M.; Novarese, M.; Rivarola, H.W.; Lo Presti, M.S.; Fernández, A.R.; Enders, J.E.; Fretes, R.; Paglini-Oliva, P.A. Reinfections and Trypanosoma cruzi strains can determine the prognosis of the chronic chagasic cardiopathy in mice. Parasitol. Res. 2007, 100, 1407–1410. [Google Scholar] [CrossRef] [PubMed]
- Olivera, M.J.; Rincón Acevedo, C.Y.; Olivera, A.J.; Mendez-Cardona, S.; Vera Soto, M.J. Addressing Chagas disease from a One Health perspective: Risk factors, lessons learned and prevention of oral transmission outbreaks in Colombia. Sci. One Health 2024, 3, 100066. [Google Scholar] [CrossRef]
- Ramirez, J.C.; Moreira, O.C. Quantitative Real-Time PCR for Trypanosoma cruzi Infection Diagnosis and Treatment Response Monitoring of Patients with Chagas Disease. Methods Mol. Biol. 2026, 2982, 163–176. [Google Scholar] [CrossRef]
- Hagström, L.; Marques, A.L.P.; Nitz, N.; Hecht, M.M. The use of qPCR in human Chagas disease: A systematic review. Expert Rev. Mol. Diagn. 2019, 19, 875–894. [Google Scholar] [CrossRef]
- Ministerio de Salud y Protección Social. Protocolo de Vigilancia y Control de Chagas; Ministerio de Salud y Protección Social: Bogotá, Colombia, 2015. [Google Scholar]
- Olivera, M.J.; Fory, J.A. Awareness and Knowledge of Statutory Health Law 1751 of 2015 among Patients in Colombia. Iran. J. Public Health 2022, 51, 2138–2140. [Google Scholar] [CrossRef]
- D’Ávila, D.A.; Galvão, L.M.C.; Sousa, G.R.; Britto, C.; Moreira, O.C.; Chiari, E. Monitoring the parasite load in chronic Chagas disease patients: Comparison between blood culture and quantitative real time PCR. PLoS ONE 2018, 13, e0208133. [Google Scholar] [CrossRef]
| Characteristic | T. cruzi Infection Status |
|---|---|
| Chronic Phase (n = 80) | |
| Age (y), mean ± SD | 54.7 ± 15.7 |
| Female sex, n (%) | 54 (67.5) |
| Systolic BP (mmHg), mean ± SD | 125.9 ± 9.2 |
| Heart rate (beats/min), mean ± SD | 64.8 ± 8.5 |
| BMI (kg/m2), mean ± SD | 27.6 ± 5.3 |
| T. cruzi qPCR-positive, n (%) | 37 (46.2) |
| Median parasitic load, (parasite equivalents/mL) [IQR] | 1.34 [1–4] |
| TcISYL, n (%) | 8 (21.6) |
| TcIDOM, n (%) | 13 (35.1) |
| Diabetes mellitus, n (%) | 15 (18.5) |
| Systemic arterial hypertension, n (%) | 30 (37.5) |
| Dyslipidemia, n (%) | 12 (15.0) |
| Associated heart disease, n (%) | 21 (26.5) |
| Family history of Chagas disease n (%) | 35 (43.8%) |
| ECG abnormalities, n (%) | 43 (53.7%) |
| ECHO abnormalities, n (%) | 17 (21.2) |
| Educational attainment (years) > 12, n (%) | 21 (26.5) |
| Variable | Bivariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Unadjusted HR (95% CI) | p-Value | Adjusted HR (95% CI) | p-Value | |
| Clinical form | ||||
| Indeterminate | Ref | |||
| Stage A | 4.55 (0.47–43.72) | 0.19 | 20.38 (0.98–422.45) | 0.052 |
| Stage B1 | 1.72 (0.11–27.46) | 0.702 | 7.63 (0.27–212.88) | 0.221 |
| Stage B2 | 24.75 (3.08–198.71) | 0.003 | 90.50 (3.70–2210.98) | 0.005 |
| Stage C–D | 7.73 (0.48–124.56) | 0.149 | 35.49 (0.58–2154.75) | 0.09 |
| Sex | ||||
| Female | Ref | |||
| Male | 3.08 (1.07–8.87) | 0.038 | 7.41 (1.32–41.64) | 0.023 |
| ECG altered | ||||
| No | Ref | |||
| Yes | 0.61 (0.21–1.76) | 0.361 | 0.15 (0.02–1.13) | 0.067 |
| ECHO altered | ||||
| No | Ref | |||
| Yes | 6.59 (2.27–19.13) | <0.001 | 0.74 (0.03–18.28) | 0.85 |
| Baseline qPCR positive | ||||
| No | Ref | |||
| Yes | 1.57 (0.54–4.52) | 0.404 | 1.89 (0.47–7.54) | 0.365 |
| Age (per 10 years) | 1.40 (1.02–1.92) | 0.037 | 1.39 (1.07–2.52) | 0.031 |
| LVEF (per 5% absolute) | 0.84 (0.66–1.06) | 0.139 | 1.62 (0.70–3.73) | 0.255 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivera, M.J.; Porras-Villamil, J.F.; Toquica-Gahona, C.; Fuentes, M.V. Clinical Progression of Cardiac Chronic Trypanosoma cruzi Infection in a Long-Term Prospective Cohort from Rural Colombia. Pathogens 2025, 14, 1198. https://doi.org/10.3390/pathogens14121198
Olivera MJ, Porras-Villamil JF, Toquica-Gahona C, Fuentes MV. Clinical Progression of Cardiac Chronic Trypanosoma cruzi Infection in a Long-Term Prospective Cohort from Rural Colombia. Pathogens. 2025; 14(12):1198. https://doi.org/10.3390/pathogens14121198
Chicago/Turabian StyleOlivera, Mario J., Julián F. Porras-Villamil, Christian Toquica-Gahona, and Màrius V. Fuentes. 2025. "Clinical Progression of Cardiac Chronic Trypanosoma cruzi Infection in a Long-Term Prospective Cohort from Rural Colombia" Pathogens 14, no. 12: 1198. https://doi.org/10.3390/pathogens14121198
APA StyleOlivera, M. J., Porras-Villamil, J. F., Toquica-Gahona, C., & Fuentes, M. V. (2025). Clinical Progression of Cardiac Chronic Trypanosoma cruzi Infection in a Long-Term Prospective Cohort from Rural Colombia. Pathogens, 14(12), 1198. https://doi.org/10.3390/pathogens14121198









