Zika Virus: A Tale of Two Lineages
Abstract
1. Introduction
2. Methodology of the Review—Literature Search Strategy
3. Zika Virus Biology
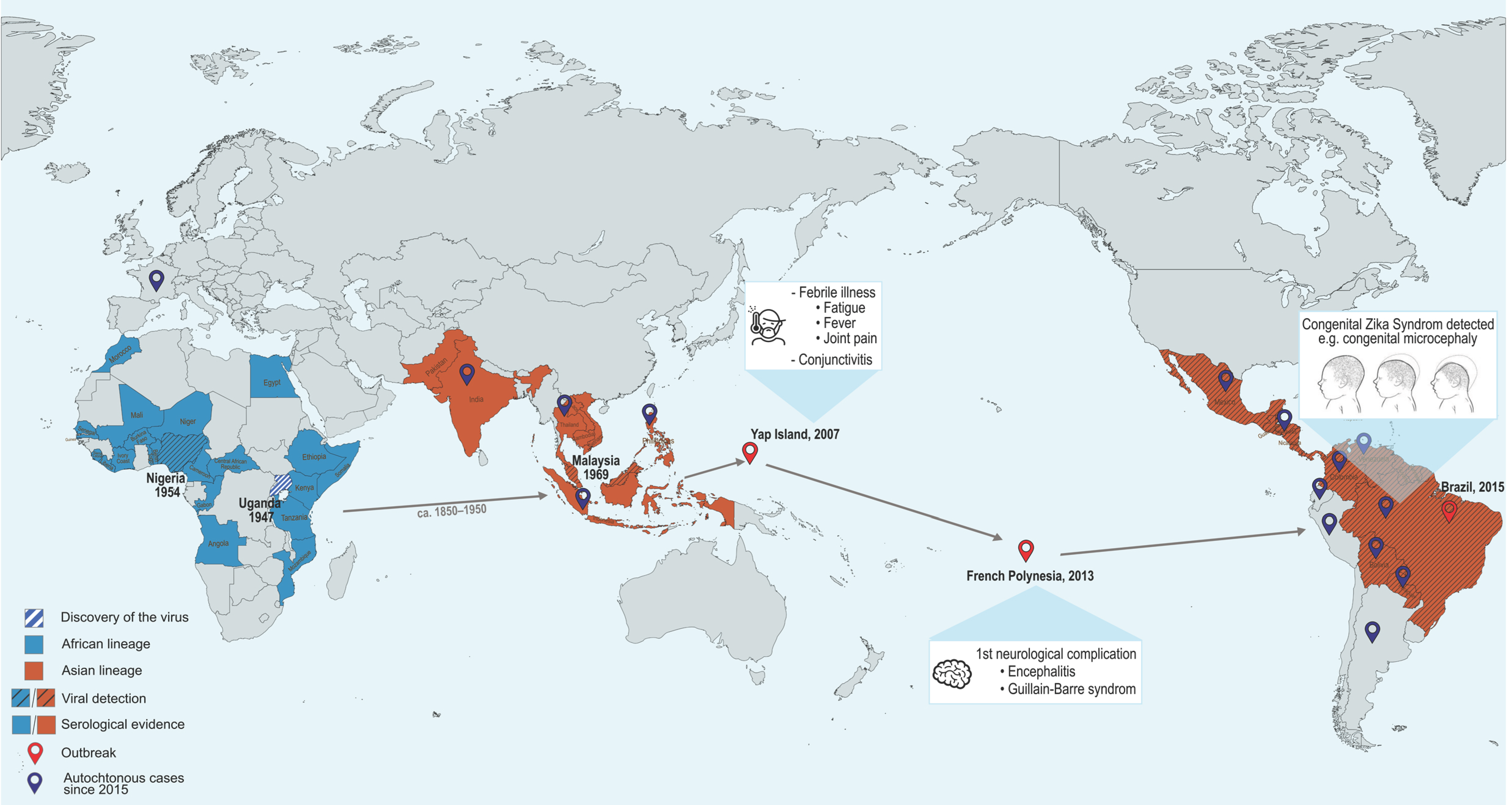
3.1. Epidemiology
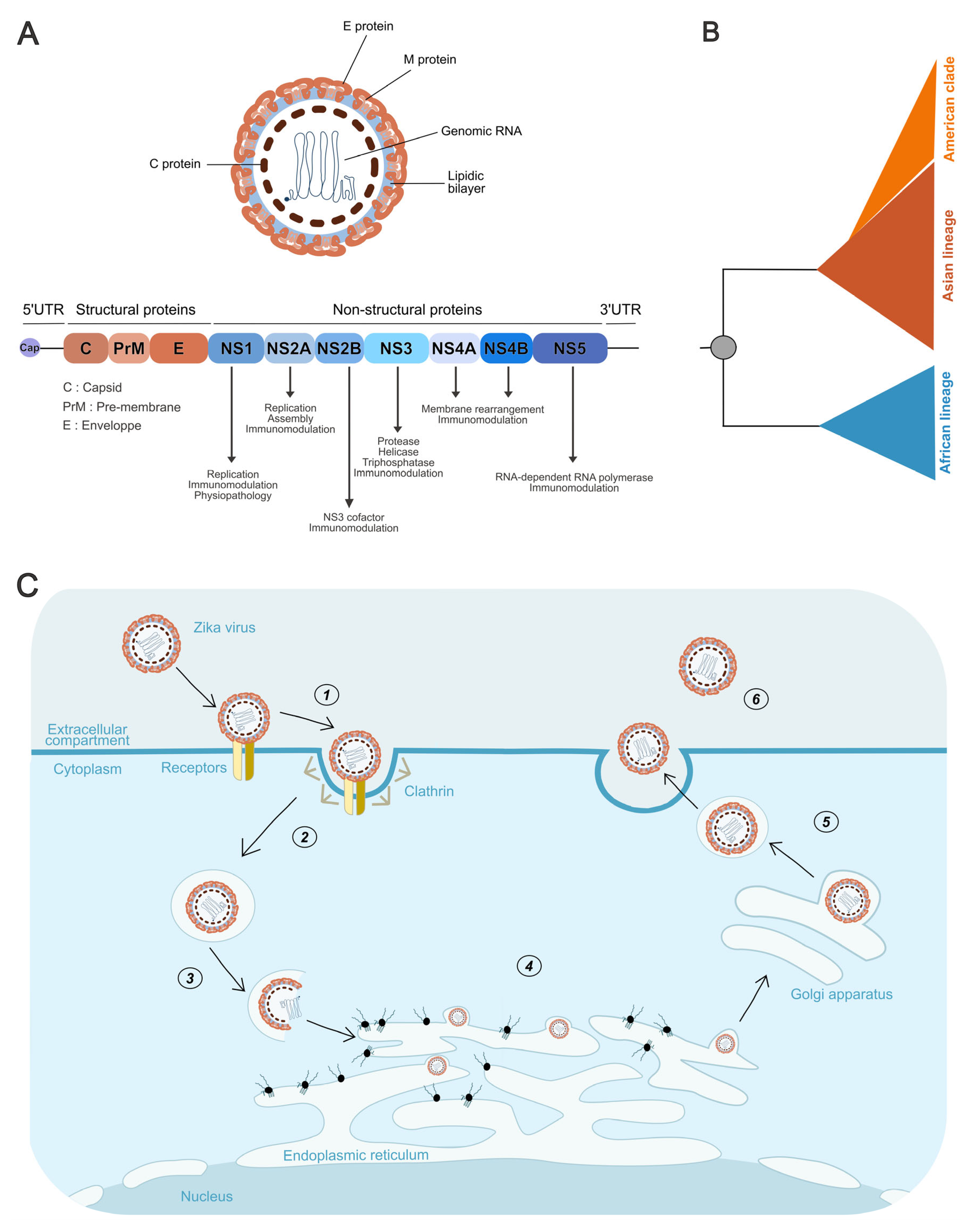
3.2. Virion Structure and Genome Organization
3.3. Phylogeny of Zika Lineages
3.4. Viral Cycle
4. In Vitro Differences Between Lineages
4.1. Viral Replication
4.2. Cytopathogenicity
4.3. Comparison Regarding Immunity
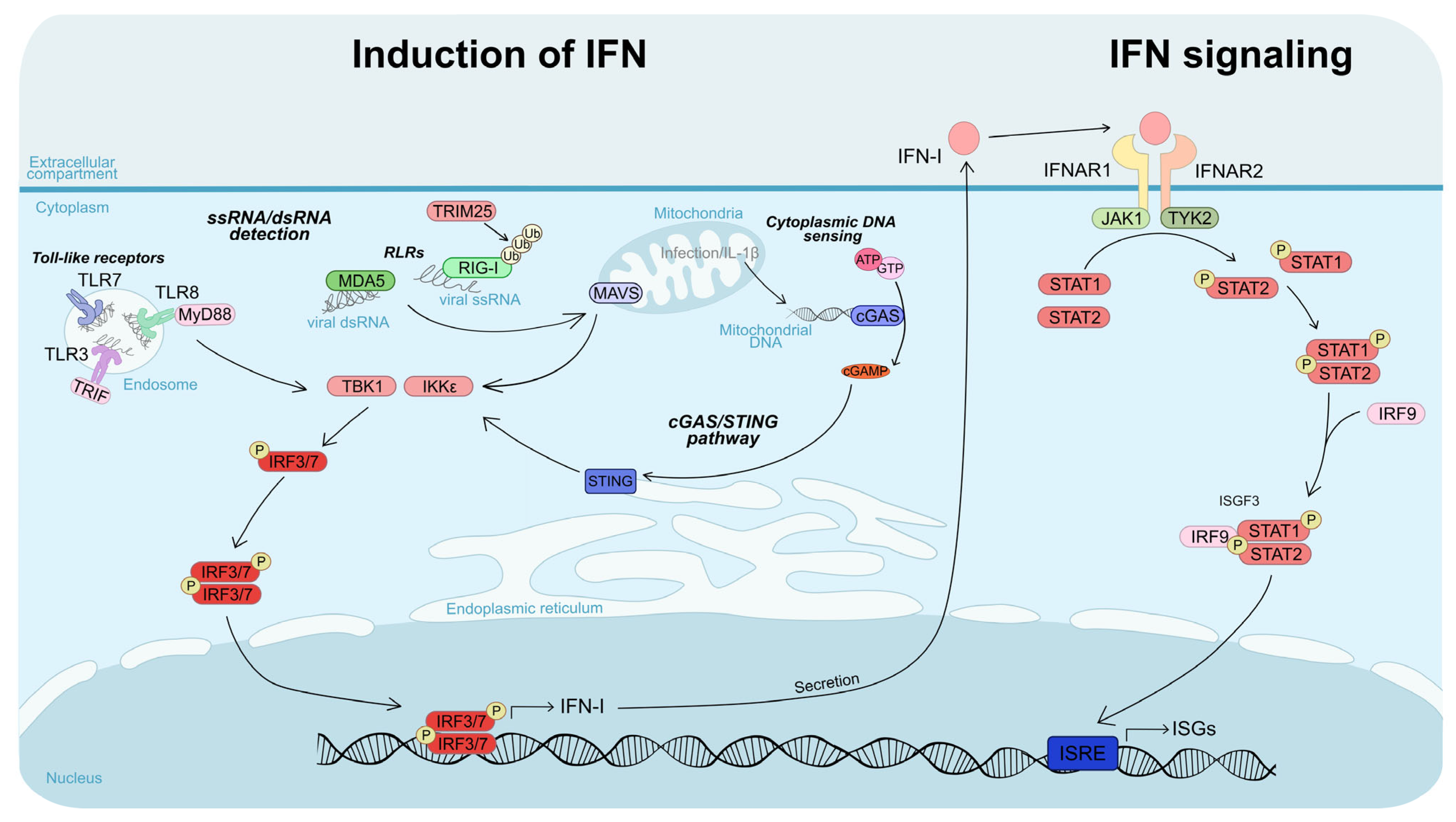
4.3.1. Induction of IFN
4.3.2. Interferon Signaling
4.3.3. Immune Response
| Characteristic | Favored Lineage | Reported Difference | References |
|---|---|---|---|
| Replication in cell lines | African | African strains replicate faster and reach higher viral titers | [89,90,91,92] |
| Replication in primary cells | African | African strains show faster replication kinetics and higher titers | [91,93,94,95,96,97,98,99,100,101,102,103,104] |
| Cytotoxicity | African | African strains induce stronger cytopathic effects and higher levels of cell death | [89,93,95,96,99,100,101,102,103,105,106,107] |
| IFN-I response activation | No consensus (African/Asian) | Results vary depending on the cell type and viral strain tested | [50,94,98,100,106,109,110] |
| IFN-I resistance | African | African strains exhibit enhanced resistance to type I IFN responses | [90,91,93] |
| Monocyte polarization | No consensus (African/Asian) | Variable outcomes depending on strain and conditions | [113,114] |
5. In Vivo Studies
5.1. Murine Model
5.2. Non-Human Primate Models
5.3. Other Models
6. Vector Competence
| Host Model | Characteristic | Favored Lineage | Reported Differences | References |
|---|---|---|---|---|
| Mouse | Physiological alterations and lethality in adults | African | African strains cause greater tissue damage and higher lethality in adult mice | [89,92,97,99,116,117,118,120] |
| Fetal damage | African | African strains induce spontaneous abortion and more severe brain damage in pups | [101,119] | |
| Avian | Lethality | African | African strains increase mortality in chicken embryos | [90] |
| Non-human primates | Infection level | No consensus (African/Asian) | Infection levels vary depending on viral strain and primate species | [123,124,125] |
| Fetal damage | African | African strains result in higher viral loads at the maternal–fetal interface | [121,122,123] | |
| Mosquito vectors | Infection | African | African strains generally exhibit higher vector competence | [51,89,90,92,119,128,129,130,131] |
| Dissemination | African | |||
| Transmission | African |
7. Exceptions to the General Trend
8. Molecular Basis of Lineage-Specific Traits
8.1. Mutations
8.1.1. Structural Proteins
8.1.2. Non-Structural Proteins
8.1.3. UTRs
| Protein | Mutation | Reported Effect | Predominant Lineage | References |
|---|---|---|---|---|
| E | VNDT motif | ↑ Lethality and neuroinvasion | Asian Some African strains | [137] |
| V763M | ↑ Replication, ↑ maternal–fetal transmission, ↑ neurovirulence in mouse pups | Asian (post-2015) | [139] | |
| prM | E21K | Neurovirulence and neuroinvasion factor | African | [143] |
| S139N | Neurovirulence factor (although African strains without this mutation cause greater neuronal damage) | Epidemic Asian strains | [140,141,142] | |
| NS1 | A188V | ↑ Type I IFN resistance, ↑ secretion, ↑ mosquito transmission | African, Asian (post-2012) | [51,110,132] |
| 5′UTR | Unique uORF | ↑ Replication, ↑ translation | African | [34] |
| uORF1 and uORF2 | uORF1 linked to neuronal dissemination | Asian |
8.2. Codon Usage and Its Implications for Lineage-Specific Pathogenicity
9. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADE | antibody-dependent enhancement |
| BBB | blood–brain barrier |
| C | capsid protein |
| CZS | congenital Zika syndrome |
| DCs | dendritic cells |
| DENV | dengue virus |
| E | envelope protein |
| hNPCs | human neural progenitor cells |
| hNSCs | human neural stem cells |
| IFN | interferon |
| ISG | interferon-stimulated gene |
| JEV | Japanese encephalitis virus |
| M | membrane protein |
| MTase | methyltransferase |
| NC | nucleocapsid |
| ORF | open reading frame |
| prM | precursor membrane protein |
| PHEIC | Public Health Emergency of International Concern |
| RdRp | RNA-dependent RNA polymerase |
| sfRNA | subgenomic flaviviral RNA |
| uORF | upstream open reading frame |
| WHO | World Health Organization |
| WNV | West Nile virus |
| YFV | yellow fever virus |
| ZIKV | Zika virus |
References
- Ryan, S.J.; Carlson, C.J.; Mordecai, E.A.; Johnson, L.R. Global Expansion and Redistribution of Aedes-Borne Virus Transmission Risk with Climate Change. PLoS Negl. Trop. Dis. 2019, 13, e0007213. [Google Scholar] [CrossRef]
- Kauffman, E.B.; Kramer, L.D. Zika Virus Mosquito Vectors: Competence, Biology, and Vector Control. J. Infect. Dis. 2017, 216, S976. [Google Scholar] [CrossRef]
- Liang, Y.; Dai, X. The Global Incidence and Trends of Three Common Flavivirus Infections (Dengue, Yellow Fever, and Zika) from 2011 to 2021. Front. Microbiol. 2024, 15, 1458166. [Google Scholar] [CrossRef]
- Hiscox, A.; Jones, R.T.; Dennehy, J.; Dyall, W.; Paris, L.; Spencer, F.I.; Keating, F.; Seelig, F.; Narendran, A.; Das, A.; et al. An Exploration of Current and Future Vector-Borne Disease Threats and Opportunities for Change. Front. Public Health 2025, 13, 1585412. [Google Scholar] [CrossRef] [PubMed]
- Pierson, T.C.; Diamond, M.S. The Continued Threat of Emerging Flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef] [PubMed]
- Dick, G.W.A.; Kitchen, S.F.; Haddow, A.J. Zika Virus (I). Isolations and Serological Specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Gubler, D.J. Zika Virus. Clin. Microbiol. Rev. 2016, 29, 487–524. [Google Scholar] [CrossRef]
- Pettersson, J.H.-O.; Eldholm, V.; Seligman, S.J.; Lundkvist, Å.; Falconar, A.K.; Gaunt, M.W.; Musso, D.; Nougairède, A.; Charrel, R.; Gould, E.A.; et al. How Did Zika Virus Emerge in the Pacific Islands and Latin America? mBio 2016, 7, e01239-16. [Google Scholar] [CrossRef]
- Gubler, D.J.; Vasilakis, N.; Musso, D. History and Emergence of Zika Virus. J. Infect. Dis. 2017, 216, S860–S867. [Google Scholar] [CrossRef]
- Wilder-Smith, A.; Osman, S. Public Health Emergencies of International Concern: A Historic Overview. J. Travel Med. 2020, 27, taaa227. [Google Scholar] [CrossRef]
- Haddow, A.D.; Schuh, A.J.; Yasuda, C.Y.; Kasper, M.R.; Heang, V.; Huy, R.; Guzman, H.; Tesh, R.B.; Weaver, S.C. Genetic Characterization of Zika Virus Strains: Geographic Expansion of the Asian Lineage. PLoS Negl. Trop. Dis. 2012, 6, e1477. [Google Scholar] [CrossRef]
- Marchette, N.J.; Garcia, R.; Rudnick, A. Isolation of Zika Virus from Aedes Aegypti Mosquitoes in Malaysia. Am. J. Trop. Med. Hyg. 1969, 18, 411–415. [Google Scholar] [CrossRef] [PubMed]
- MacNamara, F.N. Zika Virus: A Report on Three Cases of Human Infection during an Epidemic of Jaundice in Nigeria. Trans. R. Soc. Trop. Med. Hyg. 1954, 48, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Villarroel, P.M.S.; Hamel, R.; Gumpangseth, N.; Yainoy, S.; Koomhin, P.; Missé, D.; Wichit, S. Global Seroprevalence of Zika Virus in Asymptomatic Individuals: A Systematic Review. PLoS Negl. Trop. Dis. 2024, 18, e0011842. [Google Scholar] [CrossRef] [PubMed]
- Kindhauser, M.K.; Allen, T.; Frank, V.; Santhana, R.S.; Dye, C. Zika: The Origin and Spread of a Mosquito-Borne Virus. Bull. World Health Organ. 2016, 94, 675–686C. [Google Scholar] [CrossRef]
- Duffy, M.R.; Chen, T.-H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika Virus Outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef]
- Yan, G.; Pang, L.; Cook, A.R.; Ho, H.J.; Win, M.S.; Khoo, A.L.; Wong, J.G.X.; Lee, C.K.; Yan, B.; Jureen, R.; et al. Distinguishing Zika and Dengue Viruses through Simple Clinical Assessment, Singapore. Emerg. Infect. Dis. 2018, 24, 1565–1568. [Google Scholar] [CrossRef]
- Cao-Lormeau, V.-M.; Musso, D. Emerging Arboviruses in the Pacific. Lancet 2014, 384, 1571–1572. [Google Scholar] [CrossRef]
- Musso, D.; Bossin, H.; Mallet, H.P.; Besnard, M.; Broult, J.; Baudouin, L.; Levi, J.E.; Sabino, E.C.; Ghawche, F.; Lanteri, M.C.; et al. Zika Virus in French Polynesia 2013–14: Anatomy of a Completed Outbreak. Lancet Infect. Dis. 2018, 18, e172–e182. [Google Scholar] [CrossRef]
- Cao-Lormeau, V.-M.; Roche, C.; Teissier, A.; Robin, E.; Berry, A.-L.; Mallet, H.-P.; Sall, A.A.; Musso, D. Zika Virus, French Polynesia, South Pacific, 2013. Emerg. Infect. Dis. 2014, 20, 1084–1086. [Google Scholar] [CrossRef]
- Oehler, E.; Watrin, L.; Larre, P.; Leparc-Goffart, I.; Lastere, S.; Valour, F.; Baudouin, L.; Mallet, H.; Musso, D.; Ghawche, F. Zika Virus Infection Complicated by Guillain-Barre Syndrome—Case Report, French Polynesia, December 2013. Euro Surveill. 2014, 19, 20720. [Google Scholar] [CrossRef] [PubMed]
- Cao-Lormeau, V.-M.; Blake, A.; Mons, S.; Lastère, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain-Barré Syndrome Outbreak Associated with Zika Virus Infection in French Polynesia: A Case-Control Study. The Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Heukelbach, J.; Alencar, C.H.; Kelvin, A.A.; de Oliveira, W.K.; Pamplona de Góes Cavalcanti, L. Zika Virus Outbreak in Brazil. J. Infect. Dev. Ctries. 2016, 10, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Filho, D.D.B.; Martelli, C.M.T.; Ximenes, R.A.D.A.; Araújo, T.V.B.; Rocha, M.A.W.; Ramos, R.C.F.; Dhalia, R.; França, R.F.D.O.; Marques Júnior, E.T.D.A.; Rodrigues, L.C. Initial Description of the Presumed Congenital Zika Syndrome. Am. J. Public Health 2016, 106, 598–600. [Google Scholar] [CrossRef]
- Weaver, S.C. Emergence of Epidemic Zika Virus Transmission and Congenital Zika Syndrome: Are Recently Evolved Traits to Blame? mBio 2017, 8, e02063-16. [Google Scholar] [CrossRef]
- Faye, O.; De Lourdes Monteiro, M.; Vrancken, B.; Prot, M.; Lequime, S.; Diarra, M.; Ndiaye, O.; Valdez, T.; Tavarez, S.; Ramos, J.; et al. Genomic Epidemiology of 2015–2016 Zika Virus Outbreak in Cape Verde. Emerg. Infect. Dis. 2020, 26, 1084–1090. [Google Scholar] [CrossRef]
- Rabe, I.B.; Hills, S.L.; Haussig, J.M.; Walker, A.T.; Dos Santos, T.; San Martin, J.L.; Gutierrez, G.; Mendez-Rico, J.; Rodriguez, J.C.; Elizondo-Lopez, D.; et al. A Review of the Recent Epidemiology of Zika Virus Infection. Am. J. Trop. Med. Hyg. 2025, 112, 1026–1035. [Google Scholar] [CrossRef]
- Jupille, H.; Seixas, G.; Mousson, L.; Sousa, C.A.; Failloux, A.-B. Zika Virus, a New Threat for Europe? PLoS Negl. Trop. Dis. 2016, 10, e0004901. [Google Scholar] [CrossRef]
- Charnley, G.E.C.; Alcayna, T.; Almuedo-Riera, A.; Antoniou, C.; Badolo, A.; Bartumeus, F.; Boodram, L.-L.; Bueno-Marí, R.; Codeço, C.; Codeço Coelho, F.; et al. Strengthening Resilience to Emerging Vector-Borne Diseases in Europe: Lessons Learnt from Countries Facing Endemic Transmission. Lancet Reg. Health—Eur. 2025, 53, 101271. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Kuhn, R.J.; Rossmann, M.G. A Structural Perspective of the Flavivirus Life Cycle. Nat. Rev. Microbiol. 2005, 3, 13–22. [Google Scholar] [CrossRef]
- Dong, H.; Fink, K.; Züst, R.; Lim, S.P.; Qin, C.-F.; Shi, P.-Y. Flavivirus RNA Methylation. J. General. Virol. 2014, 95, 763–778. [Google Scholar] [CrossRef]
- Černý, J.; Selinger, M.; Palus, M.; Vavrušková, Z.; Tykalová, H.; Bell-Sakyi, L.; Štěrba, J.; Grubhoffer, L.; Růžek, D. Expression of a Second Open Reading Frame Present in the Genome of Tick-Borne Encephalitis Virus Strain Neudoerfl Is Not Detectable in Infected Cells. Virus Genes 2016, 52, 309–316. [Google Scholar] [CrossRef]
- Singh, K.; Martinez, M.G.; Lin, J.; Gregory, J.; Nguyen, T.U.; Abdelaal, R.; Kang, K.; Brennand, K.; Grünweller, A.; Ouyang, Z.; et al. Transcriptional and Translational Dynamics of Zika and Dengue Virus Infection. Viruses 2022, 14, 1418. [Google Scholar] [CrossRef]
- Lefèvre, C.; Cook, G.M.; Dinan, A.M.; Torii, S.; Stewart, H.; Gibbons, G.; Nicholson, A.S.; Echavarría-Consuegra, L.; Meredith, L.W.; Lulla, V.; et al. Zika Viruses Encode 5′ Upstream Open Reading Frames Affecting Infection of Human Brain Cells. Nat. Commun. 2024, 15, 8822. [Google Scholar] [CrossRef] [PubMed]
- De Falco, L.; Silva, N.M.; Santos, N.C.; Huber, R.G.; Martins, I.C. The Pseudo-Circular Genomes of Flaviviruses: Structures, Mechanisms, and Functions of Circularization. Cells 2021, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- Manzano, M.; Reichert, E.D.; Polo, S.; Falgout, B.; Kasprzak, W.; Shapiro, B.A.; Padmanabhan, R. Identification of Cis-Acting Elements in the 3′-Untranslated Region of the Dengue Virus Type 2 RNA That Modulate Translation and Replication*. J. Biol. Chem. 2011, 286, 22521–22534. [Google Scholar] [CrossRef] [PubMed]
- de Borba, L.; Villordo, S.M.; Marsico, F.L.; Carballeda, J.M.; Filomatori, C.V.; Gebhard, L.G.; Pallarés, H.M.; Lequime, S.; Lambrechts, L.; Sánchez Vargas, I.; et al. RNA Structure Duplication in the Dengue Virus 3′ UTR: Redundancy or Host Specificity? mBio 2019, 10, e02506-18. [Google Scholar] [CrossRef]
- Zoladek, J.; El Kazzi, P.; Caval, V.; Vivet-Boudou, V.; Cannac, M.; Davies, E.L.; Rossi, S.; Bribes, I.; Rouilly, L.; Simonin, Y.; et al. A Specific Domain within the 3′ Untranslated Region of Usutu Virus Confers Resistance to the Exonuclease ISG20. Nat. Commun. 2024, 15, 8528. [Google Scholar] [CrossRef]
- Villordo, S.M.; Gamarnik, A.V. Differential RNA Sequence Requirement for Dengue Virus Replication in Mosquito and Mammalian Cells. J. Virol. 2013, 87, 9365–9372. [Google Scholar] [CrossRef]
- Pijlman, G.P.; Funk, A.; Kondratieva, N.; Leung, J.; Torres, S.; van der Aa, L.; Liu, W.J.; Palmenberg, A.C.; Shi, P.-Y.; Hall, R.A.; et al. A Highly Structured, Nuclease-Resistant, Noncoding RNA Produced by Flaviviruses Is Required for Pathogenicity. Cell Host Microbe 2008, 4, 579–591. [Google Scholar] [CrossRef]
- Clarke, B.D.; Roby, J.A.; Slonchak, A.; Khromykh, A.A. Functional Non-Coding RNAs Derived from the Flavivirus 3′ Untranslated Region. Virus Res. 2015, 206, 53–61. [Google Scholar] [CrossRef]
- Ivanyi-Nagy, R.; Lavergne, J.-P.; Gabus, C.; Ficheux, D.; Darlix, J.-L. RNA Chaperoning and Intrinsic Disorder in the Core Proteins of Flaviviridae. Nucleic Acids Res. 2008, 36, 712–725. [Google Scholar] [CrossRef]
- Barrows, N.J.; Campos, R.K.; Liao, K.-C.; Prasanth, K.R.; Soto-Acosta, R.; Yeh, S.-C.; Schott-Lerner, G.; Pompon, J.; Sessions, O.M.; Bradrick, S.S.; et al. Biochemistry and Molecular Biology of Flaviviruses. Chem. Rev. 2018, 118, 4448–4482. [Google Scholar] [CrossRef]
- Dussart, P.; Labeau, B.; Lagathu, G.; Louis, P.; Nunes, M.R.T.; Rodrigues, S.G.; Storck-Herrmann, C.; Cesaire, R.; Morvan, J.; Flamand, M.; et al. Evaluation of an Enzyme Immunoassay for Detection of Dengue Virus NS1 Antigen in Human Serum. Clin. Vaccine Immunol. 2006, 13, 1185–1189. [Google Scholar] [CrossRef]
- Muller, D.A.; Young, P.R. The Flavivirus NS1 Protein: Molecular and Structural Biology, Immunology, Role in Pathogenesis and Application as a Diagnostic Biomarker. Antivir. Res. 2013, 98, 192–208. [Google Scholar] [CrossRef]
- Puerta-Guardo, H.; Glasner, D.R.; Espinosa, D.A.; Biering, S.B.; Patana, M.; Ratnasiri, K.; Wang, C.; Beatty, P.R.; Harris, E. Flavivirus NS1 Triggers Tissue-Specific Vascular Endothelial Dysfunction Reflecting Disease Tropism. Cell Rep. 2019, 26, 1598–1613.e8. [Google Scholar] [CrossRef]
- Whiteman, M.C.; Wicker, J.A.; Kinney, R.M.; Huang, C.Y.-H.; Solomon, T.; Barrett, A.D.T. Multiple Amino Acid Changes at the First Glycosylation Motif in NS1 Protein of West Nile Virus Are Necessary for Complete Attenuation for Mouse Neuroinvasiveness. Vaccine 2011, 29, 9702–9710. [Google Scholar] [CrossRef]
- Clé, M.; Constant, O.; Barthelemy, J.; Desmetz, C.; Martin, M.F.; Lapeyre, L.; Cadar, D.; Savini, G.; Teodori, L.; Monaco, F.; et al. Differential Neurovirulence of Usutu Virus Lineages in Mice and Neuronal Cells. J. Neuroinflammation 2021, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Tsetsarkin, K.A.; Kenney, H.; Chen, R.; Liu, G.; Manukyan, H.; Whitehead, S.S.; Laassri, M.; Chumakov, K.; Pletnev, A.G. A Full-Length Infectious cDNA Clone of Zika Virus from the 2015 Epidemic in Brazil as a Genetic Platform for Studies of Virus-Host Interactions and Vaccine Development. mBio 2016, 7, e01114-16. [Google Scholar] [CrossRef] [PubMed]
- Esser-Nobis, K.; Aarreberg, L.D.; Roby, J.A.; Fairgrieve, M.R.; Green, R.; Gale, M. Comparative Analysis of African and Asian Lineage-Derived Zika Virus Strains Reveals Differences in Activation of and Sensitivity to Antiviral Innate Immunity. J. Virol. 2019, 93, e00640-19. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Du, S.; Shan, C.; Nie, K.; Zhang, R.; Li, X.-F.; Zhang, R.; Wang, T.; Qin, C.-F.; et al. Evolutionary Enhancement of Zika Virus Infectivity in Aedes Aegypti Mosquitoes. Nature 2017, 545, 482–486. [Google Scholar] [CrossRef]
- Leung, J.Y.; Pijlman, G.P.; Kondratieva, N.; Hyde, J.; Mackenzie, J.M.; Khromykh, A.A. Role of Nonstructural Protein NS2A in Flavivirus Assembly. J. Virol. 2008, 82, 4731–4741. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, Y.l.; Chen, M. Flavivirus NS2A Orchestrates Reticulophagy to Enhance Viral Pathogenicity. Autophagy 2025, 21, 1167–1168. [Google Scholar] [CrossRef]
- Gopala Reddy, S.B.; Chin, W.-X.; Shivananju, N.S. Dengue Virus NS2 and NS4: Minor Proteins, Mammoth Roles. Biochem. Pharmacol. 2018, 154, 54–63. [Google Scholar] [CrossRef]
- Zoladek, J.; Nisole, S. Mosquito-Borne Flaviviruses and Type I Interferon: Catch Me If You Can! Front. Microbiol. 2023, 14, 1257024. [Google Scholar] [CrossRef]
- Wang, L.; Valderramos, S.G.; Wu, A.; Ouyang, S.; Li, C.; Brasil, P.; Bonaldo, M.; Coates, T.; Nielsen-Saines, K.; Jiang, T.; et al. From Mosquitos to Humans: Genetic Evolution of Zika Virus. Cell Host Microbe 2016, 19, 561–565. [Google Scholar] [CrossRef]
- Faye, O.; Freire, C.C.M.; Iamarino, A.; Faye, O.; De Oliveira, J.V.C.; Diallo, M.; Zanotto, P.M.A.; Sall, A.A. Molecular Evolution of Zika Virus during Its Emergence in the 20th Century. PLoS Negl. Trop. Dis. 2014, 8, e2636. [Google Scholar] [CrossRef] [PubMed]
- Dupont-Rouzeyrol, M.; Diancourt, L.; Calvez, E.; Vandenbogaert, M.; O’Connor, O.; Teissier, A.; Pol, M.; Aubry, M.; Faye, O.; Tou, D.; et al. Zika Virus Evolution on the Edges of the Pacific Ocean. Emerg. Microbes Infect. 2017, 6, e111. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Xu, X.; Han, G.-Z. The Diversification of Zika Virus: Are There Two Distinct Lineages? Genome Biol. Evol. 2017, 9, 2940–2945. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Shi, J.; Wang, J.; Tang, S.; Wang, H.; Hu, Z.; Deng, F. Phylogenetic Analysis Revealed the Central Roles of Two African Countries in the Evolution and Worldwide Spread of Zika Virus. Virol. Sin. 2016, 31, 118–130. [Google Scholar] [CrossRef]
- Kasprzykowski, J.I.; Fukutani, K.F.; Fabio, H.; Fukutani, E.R.; Costa, L.C.; Andrade, B.B.; Queiroz, A.T.L. A Recursive Sub-Typing Screening Surveillance System Detects the Appearance of the ZIKV African Lineage in Brazil: Is There a Risk of a New Epidemic? Int. J. Infect. Dis. 2020, 96, 579–581. [Google Scholar] [CrossRef]
- de Matos, S.M.S.; Hennigen, A.F.; Wachholz, G.E.; Rengel, B.D.; Schuler-Faccini, L.; Roehe, P.M.; Varela, A.P.M.; Fraga, L.R. Possible Emergence of Zika Virus of African Lineage in Brazil and the Risk for New Outbreaks. Front. Cell. Infect. Microbiol. 2021, 11, 680025. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Starmer, W.T. Possible Roles of New Mutations Shared by Asian and American Zika Viruses. Mol. Biol. Evol. 2017, 34, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Metsky, H.C.; Matranga, C.B.; Wohl, S.; Schaffner, S.F.; Freije, C.A.; Winnicki, S.M.; West, K.; Qu, J.; Baniecki, M.L.; Gladden-Young, A.; et al. Zika Virus Evolution and Spread in the Americas. Nature 2017, 546, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.-J.; Huang, S.-W. Contributions of Genetic Evolution to Zika Virus Emergence. Front. Microbiol. 2021, 12, 655065. [Google Scholar] [CrossRef]
- Muñoz, L.S.; Parra, B.; Pardo, C.A. Neurological Implications of Zika Virus Infection in Adults. J. Infect. Dis. 2017, 216, S897–S905. [Google Scholar] [CrossRef]
- Hu, T.; Li, J.; Carr, M.J.; Duchêne, S.; Shi, W. The Asian Lineage of Zika Virus: Transmission and Evolution in Asia and the Americas. Virol. Sin. 2019, 34, 1–8. [Google Scholar] [CrossRef]
- Zhu, Z.; Chan, J.F.-W.; Tee, K.-M.; Choi, G.K.-Y.; Lau, S.K.-P.; Woo, P.C.-Y.; Tse, H.; Yuen, K.-Y. Comparative Genomic Analysis of Pre-Epidemic and Epidemic Zika Virus Strains for Virological Factors Potentially Associated with the Rapidly Expanding Epidemic. Emerg. Microbes Infect. 2016, 5, e22. [Google Scholar] [CrossRef]
- Chen, R.; Vasilakis, N. Dengue—Quo Tu et Quo Vadis? Viruses 2011, 3, 1562–1608. [Google Scholar] [CrossRef]
- Twiddy, S.S.; Holmes, E.C. The Extent of Homologous Recombination in Members of the Genus Flavivirus. J. General. Virol. 2003, 84, 429–440. [Google Scholar] [CrossRef]
- Taucher, C.; Berger, A.; Mandl, C.W. A Trans-Complementing Recombination Trap Demonstrates a Low Propensity of Flaviviruses for Intermolecular Recombination. J. Virol. 2010, 84, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Fukuhara, T.; Uchida, T.; Ono, C.; Mori, H.; Sato, A.; Fauzyah, Y.; Okamoto, T.; Kurosu, T.; Setoh, Y.X.; et al. Characterization of Recombinant Flaviviridae Viruses Possessing a Small Reporter Tag. J. Virol. 2018, 92, e01582-17. [Google Scholar] [CrossRef] [PubMed]
- Sharov, V.; Rezelj, V.V.; Galatenko, V.V.; Titievsky, A.; Panov, J.; Chumakov, K.; Andino, R.; Vignuzzi, M.; Brodsky, L. Intra- and Inter-Cellular Modeling of Dynamic Interaction between Zika Virus and Its Naturally Occurring Defective Viral Genomes. J. Virol. 2021, 95, e00977-21. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.-L.; Lee, P.-L.; Chen, H.-W.; Chen, L.-K.; Kao, C.-L.; King, C.-C. Analysis of the Steps Involved in Dengue Virus Entry into Host Cells. Virology 1999, 257, 156–167. [Google Scholar] [CrossRef]
- Kroschewski, H.; Allison, S.L.; Heinz, F.X.; Mandl, C.W. Role of Heparan Sulfate for Attachment and Entry of Tick-Borne Encephalitis Virus. Virology 2003, 308, 92–100. [Google Scholar] [CrossRef]
- Navarro-Sanchez, E.; Altmeyer, R.; Amara, A.; Schwartz, O.; Fieschi, F.; Virelizier, J.-L.; Arenzana-Seisdedos, F.; Desprès, P. Dendritic-Cell-Specific ICAM3-Grabbing Non-Integrin Is Essential for the Productive Infection of Human Dendritic Cells by Mosquito-Cell-Derived Dengue Viruses. EMBO Rep. 2003, 4, 723–728. [Google Scholar] [CrossRef]
- Lozach, P.-Y.; Amara, A.; Bartosch, B.; Virelizier, J.-L.; Arenzana-Seisdedos, F.; Cosset, F.-L.; Altmeyer, R. C-Type Lectins L-SIGN and DC-SIGN Capture and Transmit Infectious Hepatitis C Virus Pseudotype Particles*. J. Biol. Chem. 2004, 279, 32035–32045. [Google Scholar] [CrossRef]
- Routhu, N.K.; Lehoux, S.D.; Rouse, E.A.; Bidokhti, M.R.M.; Giron, L.B.; Anzurez, A.; Reid, S.P.; Abdel-Mohsen, M.; Cummings, R.D.; Byrareddy, S.N. Glycosylation of Zika Virus Is Important in Host–Virus Interaction and Pathogenic Potential. Int. J. Mol. Sci. 2019, 20, 5206. [Google Scholar] [CrossRef]
- Jindadamrongwech, S.; Thepparit, C.; Smith, D.R. Identification of GRP 78 (BiP) as a Liver Cell Expressed Receptor Element for Dengue Virus Serotype 2. Arch. Virol. 2004, 149, 915–927. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Wang, S.-Y.; King, C.-C. Bacterial Lipopolysaccharide Inhibits Dengue Virus Infection of Primary Human Monocytes/Macrophages by Blockade of Virus Entry via a CD14-Dependent Mechanism. J. Virol. 1999, 73, 2650–2657. [Google Scholar] [CrossRef]
- Meertens, L.; Carnec, X.; Lecoin, M.P.; Ramdasi, R.; Guivel-Benhassine, F.; Lew, E.; Lemke, G.; Schwartz, O.; Amara, A. The TIM and TAM Families of Phosphatidylserine Receptors Mediate Dengue Virus Entry. Cell Host Microbe 2012, 12, 544–557. [Google Scholar] [CrossRef]
- van der Schaar, H.M.; Rust, M.J.; Chen, C.; van der Ende-Metselaar, H.; Wilschut, J.; Zhuang, X.; Smit, J.M. Dissecting the Cell Entry Pathway of Dengue Virus by Single-Particle Tracking in Living Cells. PLoS Pathog. 2008, 4, e1000244. [Google Scholar] [CrossRef]
- Shukla, R.; Ramasamy, V.; Shanmugam, R.K.; Ahuja, R.; Khanna, N. Antibody-Dependent Enhancement: A Challenge for Developing a Safe Dengue Vaccine. Front. Cell. Infect. Microbiol. 2020, 10, 572681. [Google Scholar] [CrossRef] [PubMed]
- Sarker, A.; Dhama, N.; Gupta, R.D. Dengue Virus Neutralizing Antibody: A Review of Targets, Cross-Reactivity, and Antibody-Dependent Enhancement. Front. Immunol. 2023, 14, 1200195. [Google Scholar] [CrossRef] [PubMed]
- Bidet, K.; Garcia-Blanco, M.A. Flaviviral RNAs: Weapons and Targets in the War between Virus and Host. Biochem. J. 2014, 462, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Ci, Y.; Liu, Z.-Y.; Zhang, N.-N.; Niu, Y.; Yang, Y.; Xu, C.; Yang, W.; Qin, C.-F.; Shi, L. Zika NS1–Induced ER Remodeling Is Essential for Viral Replication. J. Cell Biol. 2019, 219, e201903062. [Google Scholar] [CrossRef]
- Smit, J.M.; Moesker, B.; Rodenhuis-Zybert, I.; Wilschut, J. Flavivirus Cell Entry and Membrane Fusion. Viruses 2011, 3, 160–171. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Jumnainsong, A.; Onsirisakul, N.; Fitton, P.; Vasanawathana, S.; Limpitikul, W.; Puttikhunt, C.; Edwards, C.; Duangchinda, T.; Supasa, S.; et al. Cross-Reacting Antibodies Enhance Dengue Virus Infection in Humans. Science 2010, 328, 745–748. [Google Scholar] [CrossRef]
- Smith, D.R.; Sprague, T.R.; Hollidge, B.S.; Valdez, S.M.; Padilla, S.L.; Bellanca, S.A.; Golden, J.W.; Coyne, S.R.; Kulesh, D.A.; Miller, L.J.; et al. African and Asian Zika Virus Isolates Display Phenotypic Differences Both In Vitro and In Vivo. Am. J. Trop. Med. Hyg. 2018, 98, 432–444. [Google Scholar] [CrossRef]
- Willard, K.; Demakovsky, L.; Tesla, B.; Goodfellow, F.; Stice, S.; Murdock, C.; Brindley, M. Zika Virus Exhibits Lineage-Specific Phenotypes in Cell Culture, in Aedes Aegypti Mosquitoes, and in an Embryo Model. Viruses 2017, 9, 383. [Google Scholar] [CrossRef]
- Bribes, I.; Zoladek, J.; Cannac, M.; Salinas, S.; Wilson, S.J.; Nisole, S. African Strains of Zika Virus Resist ISG-Mediated Restriction. PLoS Negl. Trop. Dis. 2025, 19, e0013326. [Google Scholar] [CrossRef]
- Ou, T.P.; Auerswald, H.; In, S.; Peng, B.; Pang, S.; Boyer, S.; Choeung, R.; Dupont-Rouzeyrol, M.; Dussart, P.; Duong, V. Replication Variance of African and Asian Lineage Zika Virus Strains in Different Cell Lines, Mosquitoes and Mice. Microorganisms 2021, 9, 1250. [Google Scholar] [CrossRef]
- Bowen, J.R.; Quicke, K.M.; Maddur, M.S.; O’Neal, J.T.; McDonald, C.E.; Fedorova, N.B.; Puri, V.; Shabman, R.S.; Pulendran, B.; Suthar, M.S. Zika Virus Antagonizes Type I Interferon Responses during Infection of Human Dendritic Cells. PLOS Pathog. 2017, 13, e1006164. [Google Scholar] [CrossRef]
- Österlund, P.; Jiang, M.; Westenius, V.; Kuivanen, S.; Järvi, R.; Kakkola, L.; Lundberg, R.; Melén, K.; Korva, M.; Avšič-Županc, T.; et al. Asian and African Lineage Zika Viruses Show Differential Replication and Innate Immune Responses in Human Dendritic Cells and Macrophages. Sci. Rep. 2019, 9, 15710. [Google Scholar] [CrossRef] [PubMed]
- Anfasa, F.; Siegers, J.Y.; van der Kroeg, M.; Mumtaz, N.; Stalin Raj, V.; de Vrij, F.M.S.; Widagdo, W.; Gabriel, G.; Salinas, S.; Simonin, Y.; et al. Phenotypic Differences between Asian and African Lineage Zika Viruses in Human Neural Progenitor Cells. mSphere 2017, 2, e00292-17. [Google Scholar] [CrossRef] [PubMed]
- Simonin, Y.; Loustalot, F.; Desmetz, C.; Foulongne, V.; Constant, O.; Fournier-Wirth, C.; Leon, F.; Molès, J.-P.; Goubaud, A.; Lemaitre, J.-M.; et al. Zika Virus Strains Potentially Display Different Infectious Profiles in Human Neural Cells. EBioMedicine 2016, 12, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Herrlinger, S.; Zhu, Y.-N.; Yang, M.; Goodfellow, F.; Stice, S.L.; Qi, X.-P.; Brindley, M.A.; Chen, J.-F. The African Zika Virus MR-766 Is More Virulent and Causes More Severe Brain Damage than Current Asian Lineage and Dengue Virus. Development 2017, 144, 4114–4124. [Google Scholar] [CrossRef]
- Hamel, R.; Ferraris, P.; Wichit, S.; Diop, F.; Talignani, L.; Pompon, J.; Garcia, D.; Liégeois, F.; Sall, A.A.; Yssel, H.; et al. African and Asian Zika Virus Strains Differentially Induce Early Antiviral Responses in Primary Human Astrocytes. Infect. Genet. Evol. 2017, 49, 134–137. [Google Scholar] [CrossRef]
- Simonin, Y.; Erkilic, N.; Damodar, K.; Clé, M.; Desmetz, C.; Bolloré, K.; Taleb, M.; Torriano, S.; Barthelemy, J.; Dubois, G.; et al. Zika Virus Induces Strong Inflammatory Responses and Impairs Homeostasis and Function of the Human Retinal Pigment Epithelium. eBioMedicine 2019, 39, 315–331. [Google Scholar] [CrossRef]
- Clé, M.; Desmetz, C.; Barthelemy, J.; Martin, M.-F.; Constant, O.; Maarifi, G.; Foulongne, V.; Bolloré, K.; Glasson, Y.; De Bock, F.; et al. Zika Virus Infection Promotes Local Inflammation, Cell Adhesion Molecule Upregulation, and Leukocyte Recruitment at the Blood-Brain Barrier. mBio 2020, 11, e01183-20. [Google Scholar] [CrossRef]
- Chen, W.; Foo, S.-S.; Hong, E.; Wu, C.; Lee, W.-S.; Lee, S.-A.; Evseenko, D.; Lopes Moreira, M.E.; García-Sastre, A.; Cheng, G.; et al. Zika Virus NS3 Protease Induces Bone Morphogenetic Protein-Dependent Brain Calcification in Human Fetuses. Nat. Microbiol. 2021, 6, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, M.A.; Yunusov, D.; Balaraman, V.; Alexenko, A.P.; Yabe, S.; Verjovski-Almeida, S.; Schust, D.J.; Franz, A.W.; Sadovsky, Y.; Ezashi, T.; et al. Vulnerability of Primitive Human Placental Trophoblast to Zika Virus. Proc. Natl. Acad. Sci. USA 2017, 114, E1587–E1596. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, M.A.; Balaraman, V.; Schust, D.J.; Ezashi, T.; Roberts, R.M.; Franz, A.W.E. African and Asian Strains of Zika Virus Differ in Their Ability to Infect and Lyse Primitive Human Placental Trophoblast. PLoS ONE 2018, 13, e0200086. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, A.B.; Doobin, D.J.; Warren, A.L.; Racaniello, V.R.; Vallee, R.B. Replication of Early and Recent Zika Virus Isolates throughout Mouse Brain Development. Proc. Natl. Acad. Sci. USA 2017, 114, 12273–12278. [Google Scholar] [CrossRef]
- Vielle, N.J.; Zumkehr, B.; García-Nicolás, O.; Blank, F.; Stojanov, M.; Musso, D.; Baud, D.; Summerfield, A.; Alves, M.P. Silent Infection of Human Dendritic Cells by African and Asian Strains of Zika Virus. Sci. Rep. 2018, 8, 5440. [Google Scholar] [CrossRef]
- Zhang, F.; Hammack, C.; Ogden, S.C.; Cheng, Y.; Lee, E.M.; Wen, Z.; Qian, X.; Nguyen, H.N.; Li, Y.; Yao, B.; et al. Molecular Signatures Associated with ZIKV Exposure in Human Cortical Neural Progenitors. Nucleic Acids Res. 2016, 44, 8610–8620. [Google Scholar] [CrossRef]
- Gabriel, E.; Ramani, A.; Karow, U.; Gottardo, M.; Natarajan, K.; Gooi, L.M.; Goranci-Buzhala, G.; Krut, O.; Peters, F.; Nikolic, M.; et al. Recent Zika Virus Isolates Induce Premature Differentiation of Neural Progenitors in Human Brain Organoids. Cell Stem Cell 2017, 20, 397–406.e5. [Google Scholar] [CrossRef]
- Schoggins, J.W. Interferon-Stimulated Genes: What Do They All Do? Annu. Rev. Virol. 2019, 6, 567–584. [Google Scholar] [CrossRef]
- McGrath, E.L.; Rossi, S.L.; Gao, J.; Widen, S.G.; Grant, A.C.; Dunn, T.J.; Azar, S.R.; Roundy, C.M.; Xiong, Y.; Prusak, D.J.; et al. Differential Responses of Human Fetal Brain Neural Stem Cells to Zika Virus Infection. Stem Cell Rep. 2017, 8, 715–727. [Google Scholar] [CrossRef]
- Xia, H.; Luo, H.; Shan, C.; Muruato, A.E.; Nunes, B.T.D.; Medeiros, D.B.A.; Zou, J.; Xie, X.; Giraldo, M.I.; Vasconcelos, P.F.C.; et al. An Evolutionary NS1 Mutation Enhances Zika Virus Evasion of Host Interferon Induction. Nat. Commun. 2018, 9, 414. [Google Scholar] [CrossRef]
- Gobillot, T.A.; Humes, D.; Sharma, A.; Kikawa, C.; Overbaugh, J. The Robust Restriction of Zika Virus by Type-I Interferon in A549 Cells Varies by Viral Lineage and Is Not Determined by IFITM3. Viruses 2020, 12, 503. [Google Scholar] [CrossRef]
- Grant, A.; Ponia, S.S.; Tripathi, S.; Balasubramaniam, V.; Miorin, L.; Sourisseau, M.; Schwarz, M.C.; Sánchez-Seco, M.P.; Evans, M.J.; Best, S.M.; et al. Zika Virus Targets Human STAT2 to Inhibit Type I Interferon Signaling. Cell Host Microbe 2016, 19, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Foo, S.-S.; Chen, W.; Chan, Y.; Bowman, J.W.; Chang, L.-C.; Choi, Y.; Yoo, J.S.; Ge, J.; Cheng, G.; Bonnin, A.; et al. Asian Zika Virus Strains Target CD14+ Blood Monocytes and Induce M2-Skewed Immunosuppression during Pregnancy. Nat. Microbiol. 2017, 2, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Sarmiento, L.J.; Valdés-López, J.F.; Urcuqui-Inchima, S. American-Asian- and African Lineages of Zika Virus Induce Differential pro-Inflammatory and Interleukin 27-Dependent Antiviral Responses in Human Monocytes. Virus Res. 2023, 325, 199040. [Google Scholar] [CrossRef] [PubMed]
- Beaver, J.T.; Lelutiu, N.; Habib, R.; Skountzou, I. Evolution of Two Major Zika Virus Lineages: Implications for Pathology, Immune Response, and Vaccine Development. Front. Immunol. 2018, 9, 1640. [Google Scholar] [CrossRef]
- Dowall, S.D.; Graham, V.A.; Rayner, E.; Hunter, L.; Atkinson, B.; Pearson, G.; Dennis, M.; Hewson, R. Lineage-Dependent Differences in the Disease Progression of Zika Virus Infection in Type-I Interferon Receptor Knockout (A129) Mice. PLoS Negl. Trop. Dis. 2017, 11, e0005704. [Google Scholar] [CrossRef]
- Tripathi, S.; Balasubramaniam, V.R.M.T.; Brown, J.A.; Mena, I.; Grant, A.; Bardina, S.V.; Maringer, K.; Schwarz, M.C.; Maestre, A.M.; Sourisseau, M.; et al. A Novel Zika Virus Mouse Model Reveals Strain Specific Differences in Virus Pathogenesis and Host Inflammatory Immune Responses. PLoS Pathog. 2017, 13, e1006258. [Google Scholar] [CrossRef]
- Ngono, A.E.; Vizcarra, E.A.; Tang, W.W.; Sheets, N.; Joo, Y.; Kim, K.; Gorman, M.J.; Diamond, M.S.; Shresta, S. Mapping and Role of the CD8+ T Cell Response During Primary Zika Virus Infection in Mice. Cell Host Microbe 2017, 21, 35–46. [Google Scholar] [CrossRef]
- Aubry, F.; Jacobs, S.; Darmuzey, M.; Lequime, S.; Delang, L.; Fontaine, A.; Jupatanakul, N.; Miot, E.F.; Dabo, S.; Manet, C.; et al. Recent African Strains of Zika Virus Display Higher Transmissibility and Fetal Pathogenicity than Asian Strains. Nat. Commun. 2021, 12, 916. [Google Scholar] [CrossRef]
- Duggal, N.K.; Ritter, J.M.; McDonald, E.M.; Romo, H.; Guirakhoo, F.; Davis, B.S.; Chang, G.-J.J.; Brault, A.C. Differential Neurovirulence of African and Asian Genotype Zika Virus Isolates in Outbred Immunocompetent Mice. Am. J. Trop. Med. Hyg. 2017, 97, 1410–1417. [Google Scholar] [CrossRef]
- Koenig, M.R.; Mitzey, A.M.; Morgan, T.K.; Zeng, X.; Simmons, H.A.; Mejia, A.; Leyva Jaimes, F.; Keding, L.T.; Crooks, C.M.; Weiler, A.M.; et al. Infection of the Maternal-Fetal Interface and Vertical Transmission Following Low-Dose Inoculation of Pregnant Rhesus Macaques (Macaca Mulatta) with an African-Lineage Zika Virus. PLoS ONE 2023, 18, e0284964. [Google Scholar] [CrossRef]
- Raasch, L.E.; Yamamoto, K.; Newman, C.M.; Rosinski, J.R.; Shepherd, P.M.; Razo, E.; Crooks, C.M.; Bliss, M.I.; Breitbach, M.E.; Sneed, E.L.; et al. Fetal Loss in Pregnant Rhesus Macaques Infected with High-Dose African-Lineage Zika Virus. PLoS Negl. Trop. Dis. 2022, 16, e0010623. [Google Scholar] [CrossRef]
- Crooks, C.M.; Weiler, A.M.; Rybarczyk, S.L.; Bliss, M.; Jaeger, A.S.; Murphy, M.E.; Simmons, H.A.; Mejia, A.; Fritsch, M.K.; Hayes, J.M.; et al. African-Lineage Zika Virus Replication Dynamics and Maternal-Fetal Interface Infection in Pregnant Rhesus Macaques. J. Virol. 2021, 95, e02220-20. [Google Scholar] [CrossRef]
- Koide, F.; Goebel, S.; Snyder, B.; Walters, K.B.; Gast, A.; Hagelin, K.; Kalkeri, R.; Rayner, J. Development of a Zika Virus Infection Model in Cynomolgus Macaques. Front. Microbiol. 2016, 7, 2028. [Google Scholar] [CrossRef]
- Rayner, J.O.; Kalkeri, R.; Goebel, S.; Cai, Z.; Green, B.; Lin, S.; Snyder, B.; Hagelin, K.; Walters, K.B.; Koide, F. Comparative Pathogenesis of Asian and African-Lineage Zika Virus in Indian Rhesus Macaque’s and Development of a Non-Human Primate Model Suitable for the Evaluation of New Drugs and Vaccines. Viruses 2018, 10, 229. [Google Scholar] [CrossRef] [PubMed]
- Sow, A.A.; Jamadagni, P.; Scaturro, P.; Patten, S.A.; Chatel-Chaix, L. A Zebrafish-Based in Vivo Model of Zika Virus Infection Unveils Alterations of the Glutamatergic Neuronal Development and NS4A as a Key Viral Determinant of Neuropathogenesis. PLoS Pathog. 2024, 20, e1012756. [Google Scholar] [CrossRef] [PubMed]
- Maleski, A.L.A.; Rosa, J.G.S.; Bernardo, J.T.G.; Astray, R.M.; Walker, C.I.B.; Lopes-Ferreira, M.; Lima, C. Recapitulation of Retinal Damage in Zebrafish Larvae Infected with Zika Virus. Cells 2022, 11, 1457. [Google Scholar] [CrossRef] [PubMed]
- Calvez, E.; O’Connor, O.; Pol, M.; Rousset, D.; Faye, O.; Richard, V.; Tarantola, A.; Dupont-Rouzeyrol, M. Differential Transmission of Asian and African Zika Virus Lineages by Aedes Aegypti from New Caledonia. Emerg. Microbes Infect. 2018, 7, 159. [Google Scholar] [CrossRef]
- Fernandes, R.S.; O’Connor, O.; Bersot, M.I.L.; Girault, D.; Dokunengo, M.R.; Pocquet, N.; Dupont-Rouzeyrol, M.; Lourenço-de-Oliveira, R. Vector Competence of Aedes Aegypti, Aedes Albopictus and Culex Quinquefasciatus from Brazil and New Caledonia for Three Zika Virus Lineages. Pathogens 2020, 9, 575. [Google Scholar] [CrossRef]
- Weger-Lucarelli, J.; Rückert, C.; Chotiwan, N.; Nguyen, C.; Garcia Luna, S.M.; Fauver, J.R.; Foy, B.D.; Perera, R.; Black, W.C.; Kading, R.C.; et al. Vector Competence of American Mosquitoes for Three Strains of Zika Virus. PLoS Negl. Trop. Dis. 2016, 10, e0005101. [Google Scholar] [CrossRef]
- Roundy, C.M.; Azar, S.R.; Rossi, S.L.; Huang, J.H.; Leal, G.; Yun, R.; Fernandez-Salas, I.; Vitek, C.J.; Paploski, I.A.D.; Kitron, U.; et al. Variation in Aedes Aegypti Mosquito Competence for Zika Virus Transmission. Emerg. Infect. Dis. 2017, 23, 625–632. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Shan, C.; Nunes, B.T.D.; Yun, R.; Haller, S.L.; Rafael, G.H.; Azar, S.R.; Andersen, C.R.; Plante, K.; et al. Role of Mutational Reversions and Fitness Restoration in Zika Virus Spread to the Americas. Nat. Commun. 2021, 12, 595. [Google Scholar] [CrossRef]
- Liu, S.; DeLalio, L.J.; Isakson, B.E.; Wang, T.T. AXL-Mediated Productive Infection of Human Endothelial Cells by Zika Virus. Circ. Res. 2016, 119, 1183–1189. [Google Scholar] [CrossRef]
- Qian, X.; Nguyen, H.N.; Song, M.M.; Hadiono, C.; Ogden, S.C.; Hammack, C.; Yao, B.; Hamersky, G.R.; Jacob, F.; Zhong, C.; et al. Brain-Region-Specific Organoids Using Mini-Bioreactors for Modeling ZIKV Exposure. Cell 2016, 165, 1238–1254. [Google Scholar] [CrossRef]
- Cugola, F.R.; Fernandes, I.R.; Russo, F.B.; Freitas, B.C.; Dias, J.L.M.; Guimarães, K.P.; Benazzato, C.; Almeida, N.; Pignatari, G.C.; Romero, S.; et al. The Brazilian Zika Virus Strain Causes Birth Defects in Experimental Models. Nature 2016, 534, 267–271. [Google Scholar] [CrossRef]
- Lazear, H.M.; Govero, J.; Smith, A.M.; Platt, D.J.; Fernandez, E.; Miner, J.J.; Diamond, M.S. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe 2016, 19, 720–730. [Google Scholar] [CrossRef]
- Annamalai, A.S.; Pattnaik, A.; Sahoo, B.R.; Muthukrishnan, E.; Natarajan, S.K.; Steffen, D.; Vu, H.L.X.; Delhon, G.; Osorio, F.A.; Petro, T.M.; et al. Zika Virus Encoding Nonglycosylated Envelope Protein Is Attenuated and Defective in Neuroinvasion. J. Virol. 2017, 91, e01348-17. [Google Scholar] [CrossRef]
- Roozitalab, A.; Zhang, J.; Zhang, C.; Tang, Q.; Zhao, R.Y. The Evolving Role of Zika Virus Envelope Protein in Viral Entry and Pathogenesis. Viruses 2025, 17, 817. [Google Scholar] [CrossRef]
- Shan, C.; Xia, H.; Haller, S.L.; Azar, S.R.; Liu, Y.; Liu, J.; Muruato, A.E.; Chen, R.; Rossi, S.L.; Wakamiya, M.; et al. A Zika Virus Envelope Mutation Preceding the 2015 Epidemic Enhances Virulence and Fitness for Transmission. Proc. Natl. Acad. Sci. USA 2020, 117, 20190–20197. [Google Scholar] [CrossRef]
- Yuan, L.; Huang, X.-Y.; Liu, Z.-Y.; Zhang, F.; Zhu, X.-L.; Yu, J.-Y.; Ji, X.; Xu, Y.-P.; Li, G.; Li, C.; et al. A Single Mutation in the prM Protein of Zika Virus Contributes to Fetal Microcephaly. Science 2017, 358, 933–936. [Google Scholar] [CrossRef]
- Jaeger, A.S.; Murrieta, R.A.; Goren, L.R.; Crooks, C.M.; Moriarty, R.V.; Weiler, A.M.; Rybarczyk, S.; Semler, M.R.; Huffman, C.; Mejia, A.; et al. Zika Viruses of African and Asian Lineages Cause Fetal Harm in a Mouse Model of Vertical Transmission. PLoS Negl. Trop. Dis. 2019, 13, e0007343. [Google Scholar] [CrossRef]
- Shang, J.; Zhou, C.; He, M.; Huang, X.-Y.; Qin, C.; Wu, A. Mutation S139N on Zika Virus prM Protein Shifts Immune Response from Asian to Contemporary Strain. Brain Behav. Immun. 2025, 126, 247–259. [Google Scholar] [CrossRef]
- He, M.-J.; Wang, H.-J.; Yan, X.-L.; Lou, Y.-N.; Song, G.-Y.; Li, R.-T.; Zhu, Z.; Zhang, R.-R.; Qin, C.-F.; Li, X.-F. Key Residue in the Precursor Region of M Protein Contributes to the Neurovirulence and Neuroinvasiveness of the African Lineage of Zika Virus. J. Virol. 2023, 97, e01801-22. [Google Scholar] [CrossRef]
- Li, P.; Wei, Y.; Mei, M.; Tang, L.; Sun, L.; Huang, W.; Zhou, J.; Zou, C.; Zhang, S.; Qin, C.-F.; et al. Integrative Analysis of Zika Virus Genome RNA Structure Reveals Critical Determinants of Viral Infectivity. Cell Host Microbe 2018, 24, 875–886.e5. [Google Scholar] [CrossRef]
- Butt, A.M.; Nasrullah, I.; Qamar, R.; Tong, Y. Evolution of Codon Usage in Zika Virus Genomes Is Host and Vector Specific. Emerg. Microbes Infect. 2016, 5, e107. [Google Scholar] [CrossRef]
- Freire, C.C.d.M.; Palmisano, G.; Braconi, C.T.; Cugola, F.R.; Russo, F.B.; Beltrão-Braga, P.C.; Iamarino, A.; de Lima, D.F.; Sall, A.A.; Rosa-Fernandes, L.; et al. NS1 Codon Usage Adaptation to Humans in Pandemic Zika Virus. Mem. Inst. Oswaldo Cruz 2018, 113, e170385. [Google Scholar] [CrossRef]
- Goodfellow, F.T.; Willard, K.A.; Wu, X.; Scoville, S.; Stice, S.L.; Brindley, M.A. Strain-Dependent Consequences of Zika Virus Infection and Differential Impact on Neural Development. Viruses 2018, 10, 550. [Google Scholar] [CrossRef]
- Calvez, E.; Mousson, L.; Vazeille, M.; O’Connor, O.; Cao-Lormeau, V.-M.; Mathieu-Daudé, F.; Pocquet, N.; Failloux, A.-B.; Dupont-Rouzeyrol, M. Zika Virus Outbreak in the Pacific: Vector Competence of Regional Vectors. PLoS Negl. Trop. Dis. 2018, 12, e0006637. [Google Scholar] [CrossRef]
- Kisuya, B.; Masika, M.M.; Bahizire, E.; Oyugi, J.O. Seroprevalence of Zika Virus in Selected Regions in Kenya. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 735–739. [Google Scholar] [CrossRef]
- Mathé, P.; Egah, D.Z.; Müller, J.A.; Shehu, N.Y.; Obishakin, E.T.; Shwe, D.D.; Pam, V.C.; Okolo, M.O.; Yilgwan, C.; Gomerep, S.S.; et al. Low Zika Virus Seroprevalence among Pregnant Women in North Central Nigeria, 2016. J. Clin. Virol. 2018, 105, 35–40. [Google Scholar] [CrossRef]
- Nurtop, E.; Moyen, N.; Dzia-Lepfoundzou, A.; Dimi, Y.; Ninove, L.; Drexler, J.F.; Gallian, P.; de Lamballerie, X.; Priet, S. A Report of Zika Virus Seroprevalence in Republic of the Congo. Vector Borne Zoonotic Dis. 2020, 20, 40–42. [Google Scholar] [CrossRef]
- Alizon, S.; Hurford, A.; Mideo, N.; Van Baalen, M. Virulence Evolution and the Trade-off Hypothesis: History, Current State of Affairs and the Future. J. Evol. Biol. 2009, 22, 245–259. [Google Scholar] [CrossRef]
- Berngruber, T.W.; Froissart, R.; Choisy, M.; Gandon, S. Evolution of Virulence in Emerging Epidemics. PLoS Pathog. 2013, 9, e1003209. [Google Scholar] [CrossRef]
- Acevedo, M.A.; Dillemuth, F.P.; Flick, A.J.; Faldyn, M.J.; Elderd, B.D. Virulence-Driven Trade-Offs in Disease Transmission: A Meta-Analysis. Evolution 2019, 73, 636–647. [Google Scholar] [CrossRef]
- Ewald, P.W. Evolution of Virulence. Infect. Dis. Clin. North. Am. 2004, 18, 1–15. [Google Scholar] [CrossRef]
- Schneider, D.S. Battling the Bite: Tradeoffs in Immunity to Insect-Borne Pathogens. Immunity 2016, 44, 1251–1252. [Google Scholar] [CrossRef]
- Gandon, S.; Hochberg, M.E.; Holt, R.D.; Day, T. What Limits the Evolutionary Emergence of Pathogens? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20120086. [Google Scholar] [CrossRef]
- Osuna, C.E.; Lim, S.-Y.; Deleage, C.; Griffin, B.D.; Stein, D.; Schroeder, L.T.; Omange, R.; Best, K.; Luo, M.; Hraber, P.T.; et al. Zika Viral Dynamics and Shedding in Rhesus and Cynomolgus Macaques. Nat. Med. 2016, 22, 1448–1455. [Google Scholar] [CrossRef]
- Gao, D.; Lou, Y.; He, D.; Porco, T.C.; Kuang, Y.; Chowell, G.; Ruan, S. Prevention and Control of Zika as a Mosquito-Borne and Sexually Transmitted Disease: A Mathematical Modeling Analysis. Sci. Rep. 2016, 6, 28070. [Google Scholar] [CrossRef]
- Haddow, A.D.; Nalca, A.; Rossi, F.D.; Miller, L.J.; Wiley, M.R.; Perez-Sautu, U.; Washington, S.C.; Norris, S.L.; Wollen-Roberts, S.E.; Shamblin, J.D.; et al. High Infection Rates for Adult Macaques after Intravaginal or Intrarectal Inoculation with Zika Virus. Emerg. Infect. Dis. 2017, 23, 1274–1281. [Google Scholar] [CrossRef]
- Fritzell, C.; Rousset, D.; Adde, A.; Kazanji, M.; Van Kerkhove, M.D.; Flamand, C. Current Challenges and Implications for Dengue, Chikungunya and Zika Seroprevalence Studies Worldwide: A Scoping Review. PLoS Negl. Trop. Dis. 2018, 12, e0006533. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Bos, S.; Harris, E. Protective and Enhancing Interactions among Dengue Viruses 1-4 and Zika Virus. Curr. Opin. Virol. 2020, 43, 59–70. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Supasa, P.; Wongwiwat, W.; Rouvinski, A.; Barba-Spaeth, G.; Duangchinda, T.; Sakuntabhai, A.; Cao-Lormeau, V.-M.; Malasit, P.; Rey, F.A.; et al. Dengue Virus Sero-Cross-Reactivity Drives Antibody-Dependent Enhancement of Infection with Zika Virus. Nat. Immunol. 2016, 17, 1102–1108. [Google Scholar] [CrossRef]
- Caires-Júnior, L.C.; Goulart, E.; Melo, U.S.; Araujo, B.H.S.; Alvizi, L.; Soares-Schanoski, A.; de Oliveira, D.F.; Kobayashi, G.S.; Griesi-Oliveira, K.; Musso, C.M.; et al. Discordant Congenital Zika Syndrome Twins Show Differential in Vitro Viral Susceptibility of Neural Progenitor Cells. Nat. Commun. 2018, 9, 475. [Google Scholar] [CrossRef]
- Prokunina-Olsson, L.; Muchmore, B.; Tang, W.; Pfeiffer, R.M.; Park, H.; Dickensheets, H.; Hergott, D.; Porter-Gill, P.; Mumy, A.; Kohaar, I.; et al. A Variant Upstream of IFNL3 (IL28B) Creating a Novel Interferon Gene IFNL4 Is Associated with Impaired Clearance of Hepatitis C Virus. Nat. Genet. 2013, 45, 164–171. [Google Scholar] [CrossRef]
- Fang, M.Z.; Jackson, S.S.; O’Brien, T.R. IFNL4: Notable Variants and Associated Phenotypes. Gene 2020, 730, 144289. [Google Scholar] [CrossRef]
- Carmona, P.; Gandon, S. Winter Is Coming: Pathogen Emergence in Seasonal Environments. PLoS Comput. Biol. 2020, 16, e1007954. [Google Scholar] [CrossRef]
- Brady, O.J.; Johansson, M.A.; Guerra, C.A.; Bhatt, S.; Golding, N.; Pigott, D.M.; Delatte, H.; Grech, M.G.; Leisnham, P.T.; Maciel-de-Freitas, R.; et al. Modelling Adult Aedes Aegypti and Aedes Albopictus Survival at Different Temperatures in Laboratory and Field Settings. Parasites Vectors 2013, 6, 351. [Google Scholar] [CrossRef]
- Reinhold, J.M.; Lazzari, C.R.; Lahondère, C. Effects of the Environmental Temperature on Aedes Aegypti and Aedes Albopictus Mosquitoes: A Review. Insects 2018, 9, 158. [Google Scholar] [CrossRef]
- Caminade, C.; Turner, J.; Metelmann, S.; Hesson, J.C.; Blagrove, M.S.C.; Solomon, T.; Morse, A.P.; Baylis, M. Global Risk Model for Vector-Borne Transmission of Zika Virus Reveals the Role of El Niño 2015. Proc. Natl. Acad. Sci. USA 2017, 114, 119–124. [Google Scholar] [CrossRef]
- Wegner, G.I.; Murray, K.A.; Springmann, M.; Muller, A.; Sokolow, S.H.; Saylors, K.; Morens, D.M. Averting Wildlife-Borne Infectious Disease Epidemics Requires a Focus on Socio-Ecological Drivers and a Redesign of the Global Food System. eClinicalMedicine 2022, 47, 101386. [Google Scholar] [CrossRef]
- Morand, S.; Lajaunie, C. Outbreaks of Vector-Borne and Zoonotic Diseases Are Associated with Changes in Forest Cover and Oil Palm Expansion at Global Scale. Front. Vet. Sci. 2021, 8, 661063. [Google Scholar] [CrossRef]
- Gubler, D.J. Dengue, Urbanization and Globalization: The Unholy Trinity of the 21st Century. Trop. Med. Health 2011, 39, 3–11. [Google Scholar] [CrossRef]
- Li, R.; Richmond, P.; Roehner, B.M. Effect of Population Density on Epidemics. Phys. A Stat. Mech. Its Appl. 2018, 510, 713–724. [Google Scholar] [CrossRef]
- Hernandez, A.; Lee, J.; Kang, H. Navigating the Interconnected Web of Health: A Comprehensive Review of the One Health Paradigm and Its Implications for Disease Management. Yonsei Med. J. 2025, 66, 203. [Google Scholar] [CrossRef] [PubMed]
- Chaves, B.A.; Orfano, A.S.; Nogueira, P.M.; Rodrigues, N.B.; Campolina, T.B.; Nacif-Pimenta, R.; Pires, A.C.A.M.; Júnior, A.B.V.; Paz, A.d.C.; Vaz, E.B.d.C.; et al. Coinfection with Zika Virus (ZIKV) and Dengue Virus Results in Preferential ZIKV Transmission by Vector Bite to Vertebrate Host. J. Infect. Dis. 2018, 218, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Göertz, G.P.; Vogels, C.B.F.; Geertsema, C.; Koenraadt, C.J.M.; Pijlman, G.P. Mosquito Co-Infection with Zika and Chikungunya Virus Allows Simultaneous Transmission without Affecting Vector Competence of Aedes Aegypti. PLoS Negl. Trop. Dis. 2017, 11, e0005654. [Google Scholar] [CrossRef]
- Brady, O.J.; Bastos, L.S.; Caldwell, J.M.; Cauchemez, S.; Clapham, H.E.; Dorigatti, I.; Gaythorpe, K.A.M.; Hu, W.; Hussain-Alkhateeb, L.; Johansson, M.A.; et al. Why the Growth of Arboviral Diseases Necessitates a New Generation of Global Risk Maps and Future Projections. PLoS Comput. Biol. 2025, 21, e1012771. [Google Scholar] [CrossRef]
| Protein | Codon Bias in Humans | Codon Bias in A. aegypti |
|---|---|---|
| C | None | None |
| PrM | Asian | African |
| E | African | African |
| NS1 | Asian | Asian |
| NS2A | None | Asian |
| NS2B | African | Asian |
| NS3 | African | African |
| NS4A | None | Asian |
| NS4B | African | None |
| NS5 | African | African |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bribes, I.; Nisole, S. Zika Virus: A Tale of Two Lineages. Pathogens 2025, 14, 1151. https://doi.org/10.3390/pathogens14111151
Bribes I, Nisole S. Zika Virus: A Tale of Two Lineages. Pathogens. 2025; 14(11):1151. https://doi.org/10.3390/pathogens14111151
Chicago/Turabian StyleBribes, Inès, and Sébastien Nisole. 2025. "Zika Virus: A Tale of Two Lineages" Pathogens 14, no. 11: 1151. https://doi.org/10.3390/pathogens14111151
APA StyleBribes, I., & Nisole, S. (2025). Zika Virus: A Tale of Two Lineages. Pathogens, 14(11), 1151. https://doi.org/10.3390/pathogens14111151






