Estimating the Optimal COVID-19 Booster Timing Using Surrogate Correlates of Protection: A Longitudinal Antibody Study in Naïve and Previously Infected Individuals
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data and Sample Collection
2.3. Measurement of Anti-SARS-CoV-2 Antibody
2.4. Surrogate Indicator for Correlates of Protection
2.5. Statistical Analyses
3. Results
3.1. Characteristics of Participants
3.2. Dynamics of Vaccine Response Among Naïve and Prior-Infected Group
3.3. Timing of Booster Vaccine Administration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COVID-19 | Coronavirus disease 2019 |
| CoP | Correlate of protection |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| S-RBD | Anti-spike receptor-binding domain |
| N-IgG | Anti-nucleocapsid IgG |
| S-IgG | Anti-spike protein IgG |
| sVNT | Surrogate virus neutralization test |
| GMT | Geometric mean titer |
| cVNT | Conventional virus neutralization test |
| BSL | Biosafety level |
References
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef]
- Lopez Bernal, J.L.; Andrews, N.; Gower, C.; Robertson, C.; Stowe, J.; Tessier, E.; Simmons, R.; Cottrell, S.; Roberts, R.; O’Doherty, M.; et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in England: Test negative case-control study. BMJ 2021, 373, n1088. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Self, W.H.; Gaglani, M.; Ginde, A.A.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; Zepeski, A.; McNeal, T.; et al. Effectiveness of mRNA vaccination in preventing COVID-19-associated invasive mechanical ventilation and death—United States, March 2021–January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 459–465. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning immune humoral response to BNT162b2 COVID-19 vaccine over 6 months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef] [PubMed]
- Naaber, P.; Tserel, L.; Kangro, K.; Sepp, E.; Jürjenson, V.; Adamson, A.; Haljasmägi, L.; Rumm, A.P.; Maruste, R.; Kärner, J.; et al. Dynamics of antibody response to BNT162b2 vaccine after six months: A longitudinal prospective study. Lancet Reg. Health Eur. 2021, 10, 100208. [Google Scholar] [CrossRef] [PubMed]
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C.; Mandelboim, M.; Levin, E.G.; Rubin, C.; Indenbaum, V.; et al. COVID-19 breakthrough infections in vaccinated health care workers. N. Engl. J. Med. 2021, 385, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- Levine-Tiefenbrun, M.; Yelin, I.; Alapi, H.; Katz, R.; Herzel, E.; Kuint, J.; Chodick, G.; Gazit, S.; Patalon, T.; Kishony, R. Viral loads of Delta-variant SARS-CoV-2 breakthrough infections after vaccination and booster with BNT162b2. Nat. Med. 2021, 27, 2108–2110. [Google Scholar] [CrossRef]
- World Health Organization. COVID-19 Vaccines. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines?utm_source=chatgpt.com (accessed on 6 July 2025).
- US Center for Disease Control and Prevention. Staying Up to Date With COVID-19 Vaccines. Available online: https://www.cdc.gov/covid/vaccines/stay-up-to-date.html?utm_source=chatgpt.com (accessed on 6 July 2025).
- Pilz, S.; Theiler-Schwetz, V.; Trummer, C.; Krause, R.; Ioannidis, J.P.A. SARS-CoV-2 reinfections: Overview of efficacy and duration of natural and hybrid immunity. Environ. Res. 2022, 209, 112911. [Google Scholar] [CrossRef]
- Lai, K.Z.H.; Greenstein, S.; Govindasamy, R.; Paranilam, J.; Brown, J.; Kimball-Carroll, S. COVID-19 vaccination recommendations for immunocompromised patient populations: Delphi panel and consensus statement generation in the United States. Infect. Dis. Ther. 2024, 13, 2255–2283. [Google Scholar] [CrossRef]
- US Center for Disease Control and Prevention. COVID-19 Vaccination Guidance for People Who Are Immunocompromised. Available online: https://www.cdc.gov/covid/hcp/vaccine-considerations/immunocompromised.html?utm_source=chatgpt.com (accessed on 6 July 2025).
- Sobhani, K.; Cheng, S.; Binder, R.A.; Mantis, N.J.; Crawford, J.M.; Okoye, N.; Braun, J.G.; Joung, S.; Wang, M.; Lozanski, G.; et al. Clinical utility of SARS-CoV-2 serological testing and defining a correlate of protection. Vaccines 2023, 11, 1644. [Google Scholar] [CrossRef]
- Rahmani, A.; Montecucco, A.; Priano, L.; Mandolini, L.; Dini, G.; Durando, P. Serological correlates of protection induced by COVID-19 vaccination in the working age population: A systematic review and meta-analysis. Vaccines 2024, 12, 494. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, R.; Suzuki, E.; Murai, R.; Tanaka, M.; Fujiya, Y.; Takahashi, S. Performance analysis among multiple fully automated anti-SARS-CoV-2 antibody measurement reagents: A potential indicator for the correlation of protection in the antibody titer. J. Infect. Chemother. 2022, 28, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Cromer, D.; Steain, M.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Kent, S.J.; Triccas, J.A.; Khoury, D.S.; Davenport, M.P. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: A meta-analysis. Lancet Microbe 2022, 3, e52–e61. [Google Scholar] [CrossRef]
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.-C.; Tiu, C.; Hu, Z.; Chen, V.C.-W.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction. Nat. Biotechnol. 2020, 38, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Pezzati, L.; Milazzo, L.; Carrozzo, G.; Kullmann, C.; Oreni, L.; Beltrami, M.; Caronni, S.; Lai, A.; Caberlotto, L.; Ottomano, C.; et al. Evaluation of residual humoral immune response against SARS-CoV-2 by a surrogate virus neutralization test (sVNT) 9 months after BNT162b2 primary vaccination. J. Infect. Chemother. 2023, 29, 624–627. [Google Scholar] [CrossRef]
- Gilboa, M.; Regev-Yochay, G.; Mandelboim, M.; Indenbaum, V.; Asraf, K.; Fluss, R.; Amit, S.; Mendelson, E.; Doolman, R.; Afek, A.; et al. Durability of Immune Response after COVID-19 Booster Vaccination and Association with COVID-19 Omicron Infection. JAMA Netw. Open 2022, 5, e2231778. [Google Scholar] [CrossRef]
- Regev-Yochay, G.; Lustig, Y.; Joseph, G.; Gilboa, M.; Barda, N.; Gens, I.; Indenbaum, V.; Halpern, O.; Katz-Likvornik, S.; Levin, T.; et al. Correlates of protection against COVID-19 infection and intensity of symptomatic disease in vaccinated individuals exposed to SARS-CoV-2 in households in Israel (ICoFS): A prospective cohort study. Lancet Microbe 2023, 4, e309–e318. [Google Scholar] [CrossRef]
- Perry, J.; Osman, S.; Wright, J.; Richard-Greenblatt, M.; Buchan, S.A.; Sadarangani, M.; Bolotin, S. Does a humoral correlate of protection exist for SARS-CoV-2? A systematic review. PLoS ONE 2022, 17, e0266852. [Google Scholar] [CrossRef]
- Kato, H.; Miyakawa, K.; Ohtake, N.; Yamaoka, Y.; Yajima, S.; Yamazaki, E.; Shimada, T.; Goto, A.; Nakajima, H.; Ryo, A. Vaccine-induced humoral response against SARS-CoV-2 dramatically declined but cellular immunity possibly remained at 6 months post BNT162b2 vaccination. Vaccine 2022, 40, 2652–2655. [Google Scholar] [CrossRef]
- Speletas, M.; Voulgaridi, I.; Bogogiannidou, Z.; Sarrou, S.; Kyritsi, M.A.; Theodoridou, A.; Dadouli, K.; Matziri, A.; Vontas, A.; Pappa, D.; et al. Dynamics of Anti-SARS-CoV-2 IgA and IgG Responses and Their Protective Effect against Fatal Disease after Booster COVID-19 Vaccination. Vaccines 2023, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.R.; Apostolidis, S.A.; Painter, M.M.; Mathew, D.; Pattekar, A.; Kuthuru, O.; Gouma, S.; Hicks, P.; Meng, W.; Rosenfeld, A.M.; et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci. Immunol. 2021, 6, eabi6950. [Google Scholar] [CrossRef] [PubMed]
- Desmecht, S.; Tashkeev, A.; El Moussaoui, M.; Marechal, N.; Perée, H.; Tokunaga, Y.; Fombellida-Lopez, C.; Polese, B.; Legrand, C.; Wéry, M.; et al. Kinetics and persistence of the cellular and humoral immune responses to BNT162b2 mRNA vaccine in SARS-CoV-2-Naive and—Experienced subjects: Impact of booster dose and breakthrough infections. Front. Immunol. 2022, 13, 863554. [Google Scholar] [CrossRef] [PubMed]
- Mak, W.A.; Koeleman, J.G.M.; van der Vliet, M.; Keuren, F.; Ong, D.S.Y. SARS-CoV-2 antibody and T cell responses one year after COVID-19 and the booster effect of vaccination: A prospective cohort study. J. Infect. 2022, 84, 171–178. [Google Scholar] [CrossRef]
- Noh, J.Y.; Cheong, H.J.; Kim, W.J.; Choi, J.Y.; Lee, H.W.; Kim, S.S.; Kim, B.; Song, J.Y. Robust neutralizing antibody responses after single-dose BNT162b2 vaccination at long intervals from prior SARS-CoV-2 infection and ceiling effect with repeated vaccination. J. Infect. 2022, 85, 573–607. [Google Scholar] [CrossRef]
- Bates, T.A.; Leier, H.C.; McBride, S.K.; Schoen, D.; Lyski, Z.L.; Lee, D.X.; Messer, W.B.; Curlin, M.E.; Tafesse, F.G. An extended interval between vaccination and infection enhances hybrid immunity against SARS-CoV-2 variants. JCI Insight 2023, 8, e165265. [Google Scholar] [CrossRef]
- Buckner, C.M.; Kardava, L.; El Merhebi, O.; Narpala, S.R.; Serebryannyy, L.; Lin, B.C.; Wang, W.; Zhang, X.; Lopes de Assis, F.; Kelly, S.E.M.; et al. Interval between prior SARS-CoV-2 infection and booster vaccination impacts magnitude and quality of antibody and B cell responses. Cell 2022, 185, 4333–4346.e14. [Google Scholar] [CrossRef]
- Thomas, R.; Zaqout, A.; Meqbel, B.; Jafar, U.; Vaikath, N.N.; Aldushain, A.; Naik, A.; Shaath, H.; Al-Akl, N.S.; Adam, A.; et al. Longitudinal cellular and humoral immune responses following COVID-19 BNT162b2-mRNA-based booster vaccination of craft and manual workers in Qatar. Front. Immunol. 2025, 16, 1557426. [Google Scholar] [CrossRef]




| Total (n = 177) | Naïve (n = 169) | Prior-Infected ≤ 6 Months (n = 6) | Prior-Infected > 6 Months (n = 2) | |||
|---|---|---|---|---|---|---|
| Characteristics, n (%) | ||||||
| Age, years | ||||||
| Median (range) | 41 (23–64) | 41 (23–64) | 46 (28–57) | 44.5 (43–46) | ||
| 20–29 | 23 (13) | 22 (13) | 1 (17) | 0 (0) | ||
| 30–39 | 58 (33) | 57 (34) | 1 (17) | 0 (0) | ||
| 40–49 | 68 (38) | 64 (38) | 2 (33) | 2 (100) | ||
| 50–59 | 25 (14) | 23 (14) | 2 (33) | 0 (0) | ||
| ≥60 | 3 (2) | 3 (2) | 0 (0) | 0 (0) | ||
| Sex | ||||||
| Male | 98 (55) | 96 (57) | 1 (17) | 1 (50) | ||
| Female | 79 (45) | 73 (43) | 5 (83) | 1 (50) | ||
| Occupation | ||||||
| Doctor | 58 (33) | 57 (34) | 0 (0) | 1 (50) | ||
| Nurse | 46 (26) | 41 (24) | 5 (83) | 0 (0) | ||
| Laboratory technician | 44 (25) | 43 (25) | 0 (0) | 1 (50) | ||
| Radiology technician | 14 (8) | 14 (8) | 0 (0) | 0 (0) | ||
| Clinical engineer | 7 (4) | 7 (4) | 0 (0) | 0 (0) | ||
| Pharmacist | 5 (3) | 4 (2) | 1 (16) | 0 (0) | ||
| Others | 3 (2) | 3 (2) | 0 (0) | 0 (0) | ||
| Involved in the treatment of COVID-19 patients | 111 (63) | 104 (62) | 6 (100) | 1 (50) | ||
| Comorbidities, n (%) | ||||||
| Allergic rhinitis or hay fever | 13 (7) | 13 (8) | 0 (0) | 0 (0) | ||
| Hypertension | 7 (4) | 7 (4) | 0 (0) | 0 (0) | ||
| Athma | 7 (4) | 7 (4) | 0 (0) | 0 (0) | ||
| Dyslipidemia | 4 (2) | 4 (2) | 0 (0) | 0 (0) | ||
| Hyperuricemia | 4 (2) | 4 (2) | 0 (0) | 0 (0) | ||
| Chronic kidney disease | 2 (1) | 2 (1) | 0 (0) | 0 (0) | ||
| Inflammatory bowel disease | 2 (1) | 2 (1) | 0 (0) | 0 (0) | ||
| Cancer | 2 (1) | 2 (1) | 0 (0) | 0 (0) | ||
| Others | 22 (12) | 22 (13) | 0 (0) | 0 (0) | ||
| Unknown | 20 (11) | 20 (12) | 0 (0) | 0 (0) | ||
| Infected after vaccination, n (%) | 6 (3) | 6 (4) | - | - | ||
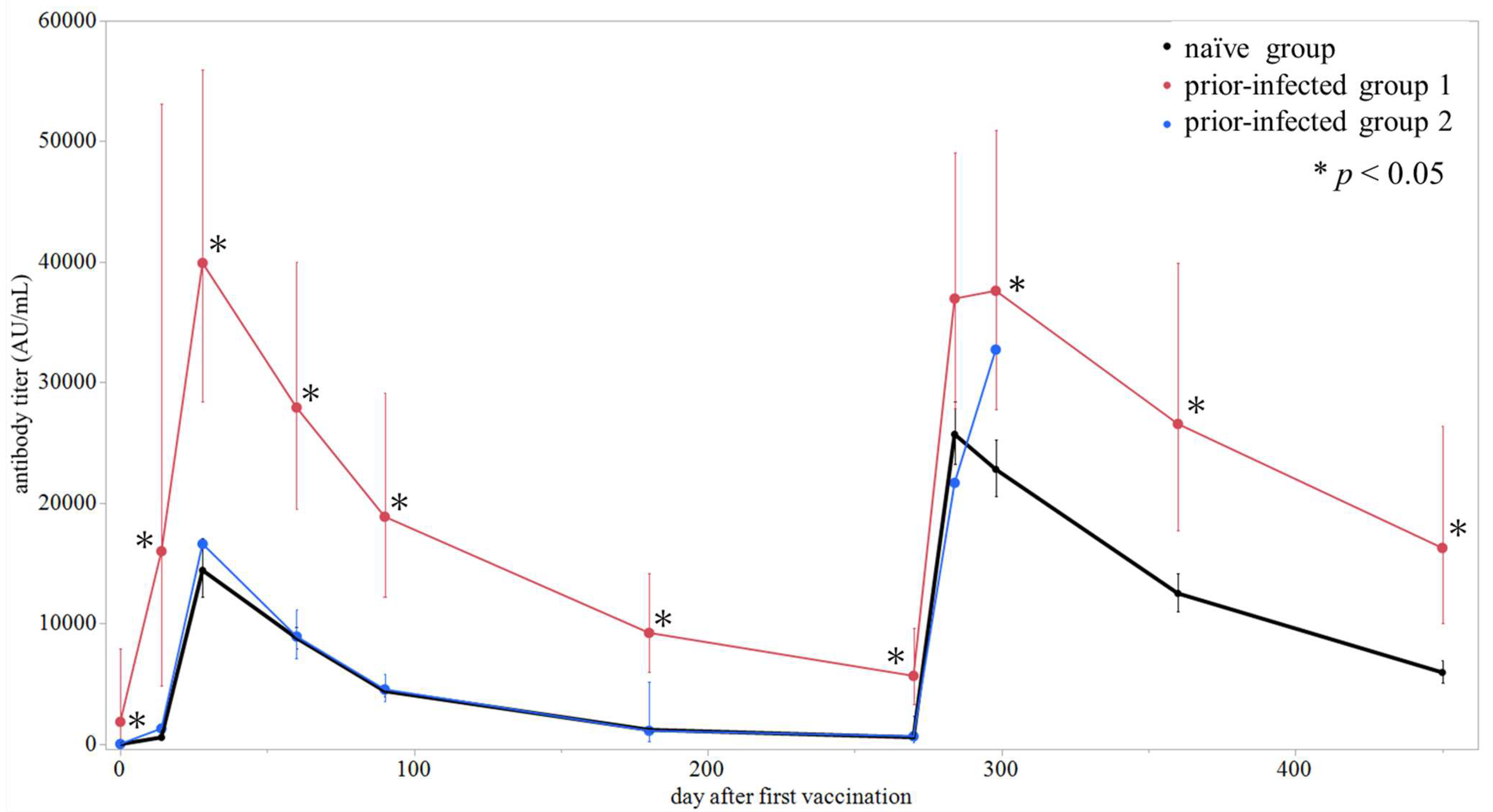
| Prior COVID-19 Status | Day 0 | Day 14 | Day 28 | Day 60 | Day 90 | Day 180 | Day 270 | Day 284 | Day 298 | Day 360 | Day 450 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | GMT (95% CI) | n | GMT (95% CI) | n | GMT (95% CI) | n | GMT (95% CI) | n | GMT (95% CI) | n | GMT (95% CI) | n | GMT (95% CI) | n | GMT (95% CI) | n | GMT (95% CI) | n | GMT (95% CI) | n | GMT (95% CI) | |
| Naïve | 169 | 1.9 (1.7–2.3) | 166 | 563 (490–697) | 164 | 14,424 (12,207–17,044) | 162 | 8800 (7962–9727) | 152 | 4400 (3941–4913) | 153 | 1195 (1077–1327) | 139 | 577 (520–640) | 133 | 25,703 (23,243–28,423) | 122 | 22,799 (20,565–25,277) | 102 | 12,508 (11,020–14,198) | 94 | 5952 (5111–6932) |
| Prior-infected ≤ 6 months | 6 | 1857 (436–7902) | 6 | 16,008 (4827–53,084) | 6 | 39,915 (28,457–55,987) | 6 | 27,926 (19,482–40,029) | 6 | 18,873 (12,229–29,128) | 6 | 9237 (6017–14,180) | 6 | 5671 (3335–9644) | 6 | 36,963 (27,842–49,074) | 6 | 37,610 (27,775–50,929) | 6 | 26,564 (17,688–39,894) | 6 | 16280 (10,051–26,370) |
| Prior-infected > 6 months * | 2 | 3.6, 114.6 | 2 | 262, 6437 | 2 | 12,528, 22,041 | 2 | 8781, 9097 | 2 | 4455, 4636 | 2 | 992, 1263 | 2 | 597, 728 | 2 | 13,256, 35,454 | 1 | 32,729 | 0 | NA | 0 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujiya, Y.; Kobayashi, R.; Tanaka, M.; Suzuki, E.; Hinotsu, S.; Nakae, M.; Sato, Y.; Katayama, Y.; Saeki, M.; Yakuwa, Y.; et al. Estimating the Optimal COVID-19 Booster Timing Using Surrogate Correlates of Protection: A Longitudinal Antibody Study in Naïve and Previously Infected Individuals. Pathogens 2025, 14, 1138. https://doi.org/10.3390/pathogens14111138
Fujiya Y, Kobayashi R, Tanaka M, Suzuki E, Hinotsu S, Nakae M, Sato Y, Katayama Y, Saeki M, Yakuwa Y, et al. Estimating the Optimal COVID-19 Booster Timing Using Surrogate Correlates of Protection: A Longitudinal Antibody Study in Naïve and Previously Infected Individuals. Pathogens. 2025; 14(11):1138. https://doi.org/10.3390/pathogens14111138
Chicago/Turabian StyleFujiya, Yoshihiro, Ryo Kobayashi, Makito Tanaka, Ema Suzuki, Shiro Hinotsu, Mami Nakae, Yuki Sato, Yuki Katayama, Masachika Saeki, Yuki Yakuwa, and et al. 2025. "Estimating the Optimal COVID-19 Booster Timing Using Surrogate Correlates of Protection: A Longitudinal Antibody Study in Naïve and Previously Infected Individuals" Pathogens 14, no. 11: 1138. https://doi.org/10.3390/pathogens14111138
APA StyleFujiya, Y., Kobayashi, R., Tanaka, M., Suzuki, E., Hinotsu, S., Nakae, M., Sato, Y., Katayama, Y., Saeki, M., Yakuwa, Y., Nirasawa, S., Endoh, A., Kuronuma, K., & Takahashi, S. (2025). Estimating the Optimal COVID-19 Booster Timing Using Surrogate Correlates of Protection: A Longitudinal Antibody Study in Naïve and Previously Infected Individuals. Pathogens, 14(11), 1138. https://doi.org/10.3390/pathogens14111138






