Stage-Specific Expression and Subcellular Localization of Calcineurin in Infective Forms of Leishmania amazonensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Bone Marrow-Derived Macrophages Isolation
2.2. Leishmania amazonensis Parasites
2.3. CaN Inhibition and Cell Viability Assay
2.4. Western Blot
2.5. Immunofluorescence
2.6. Prediction of the Subcellular Localization of CaN
2.7. Statistical Analysis
3. Results
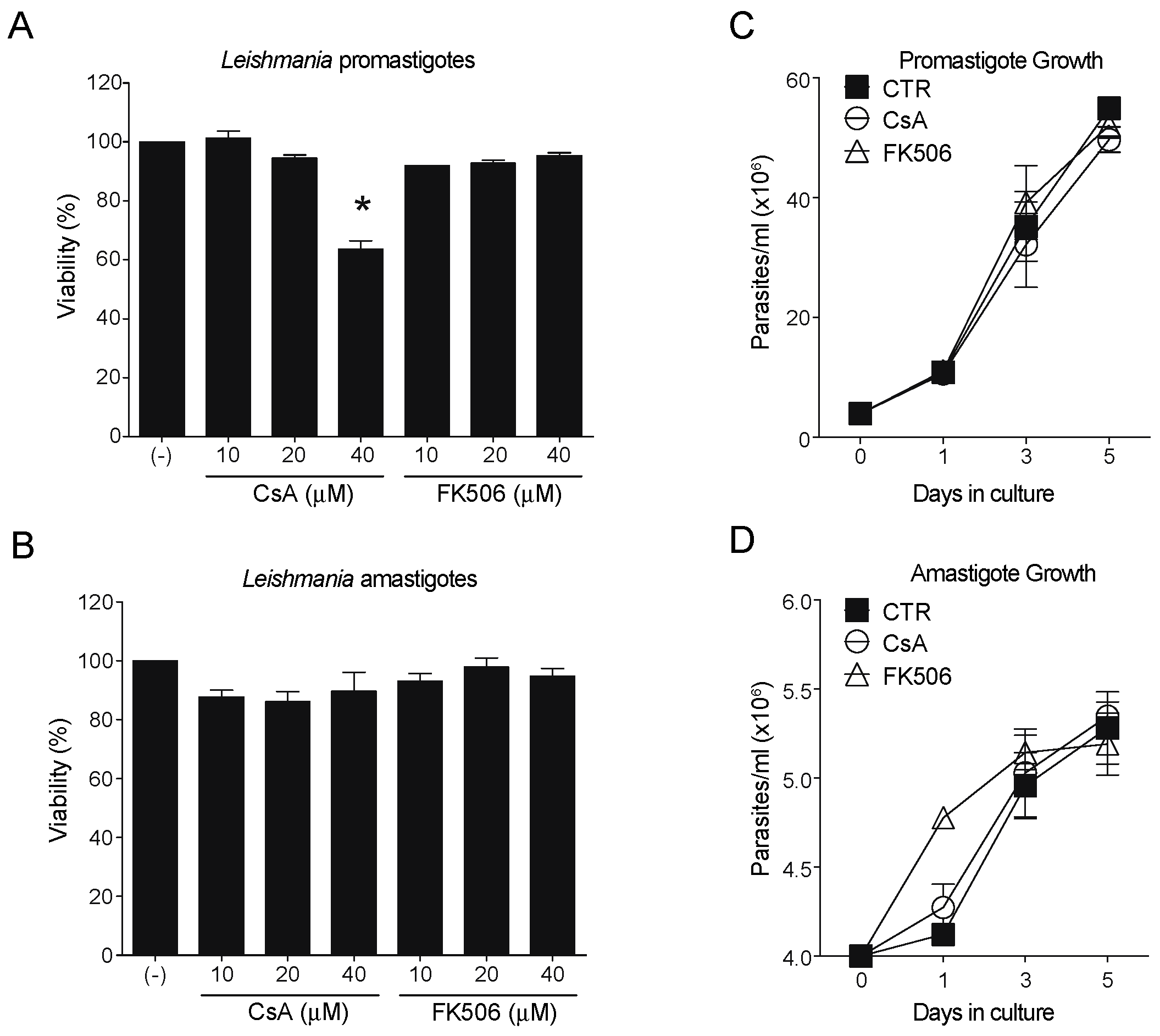
3.1. CaN Inhibitors Differentially Affect Parasite Viability
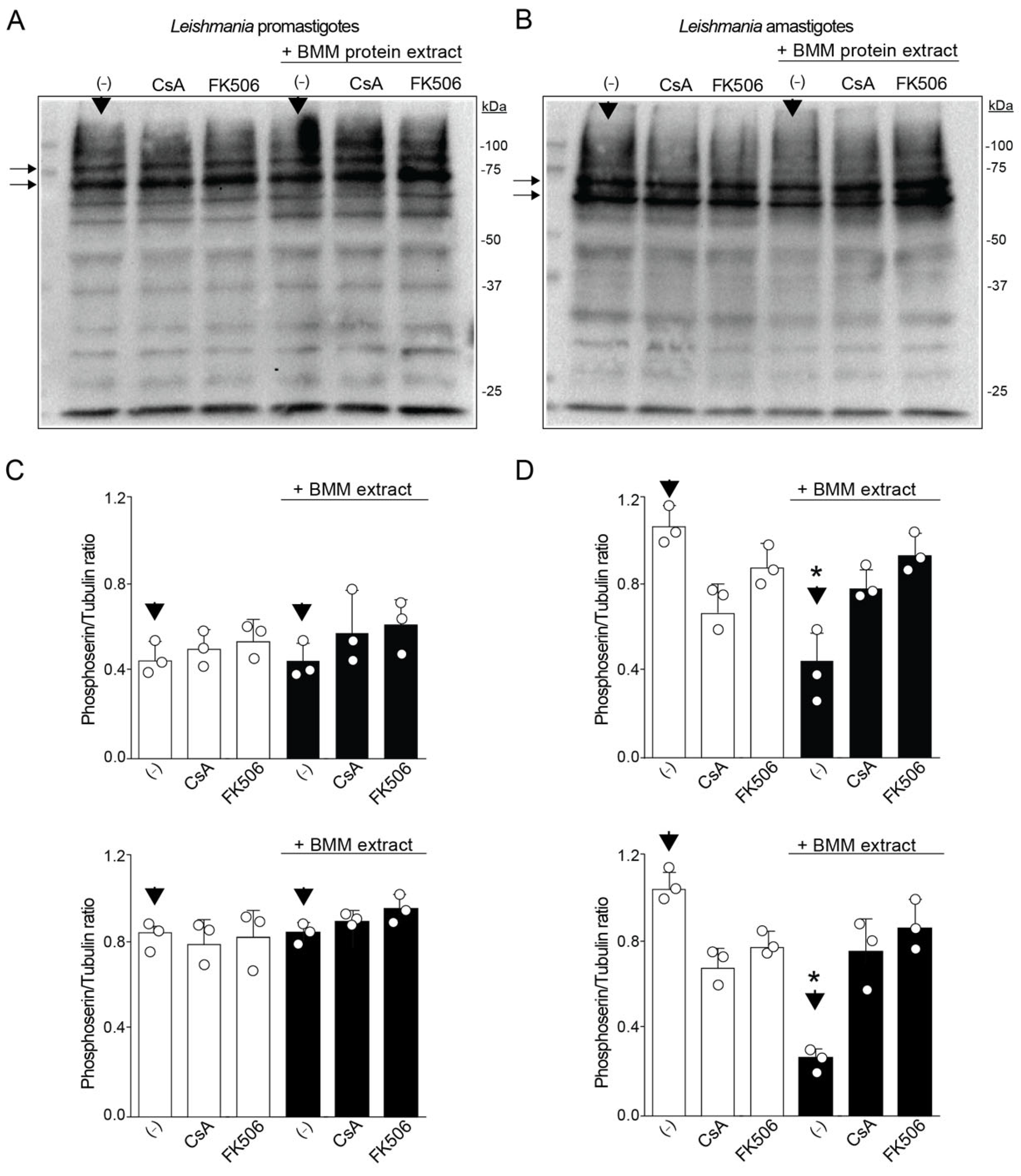
3.2. Phosphorylation Profile Altered by CaN Inhibition Only in Amastigotes
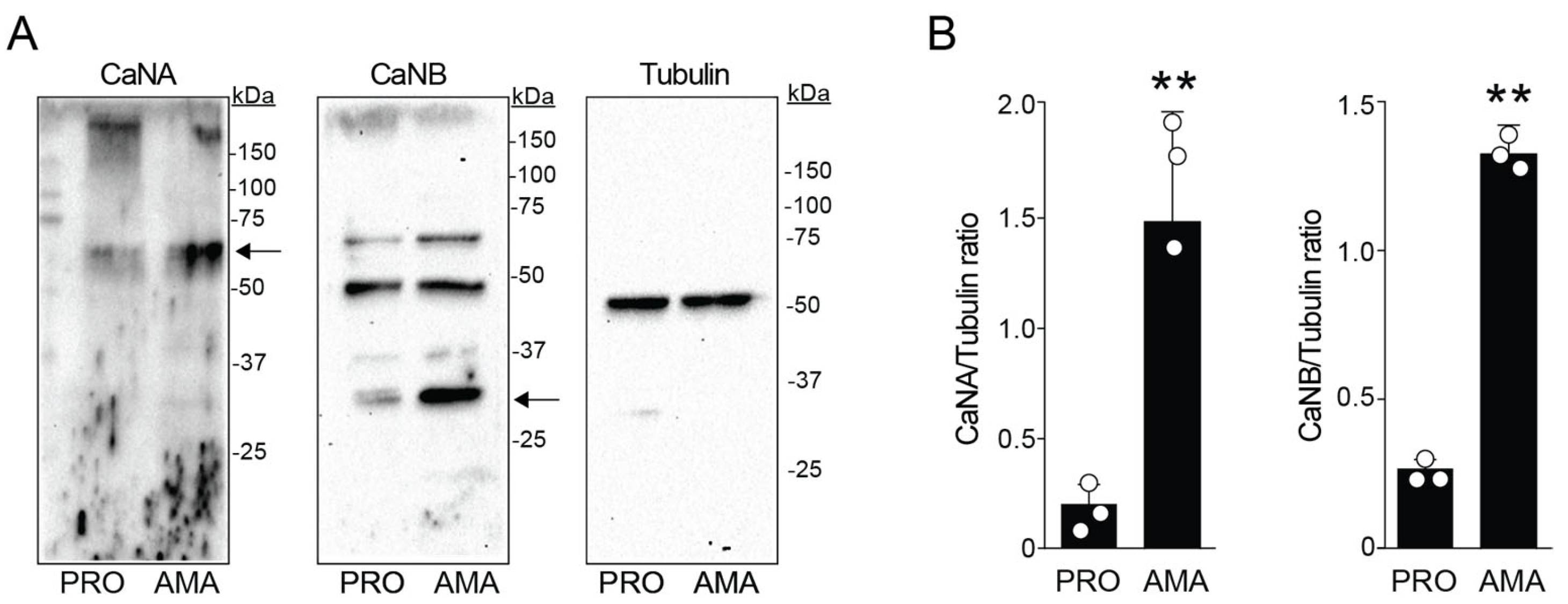
3.3. Differential Expression of CaN Subunits in Leishmania amazonensis
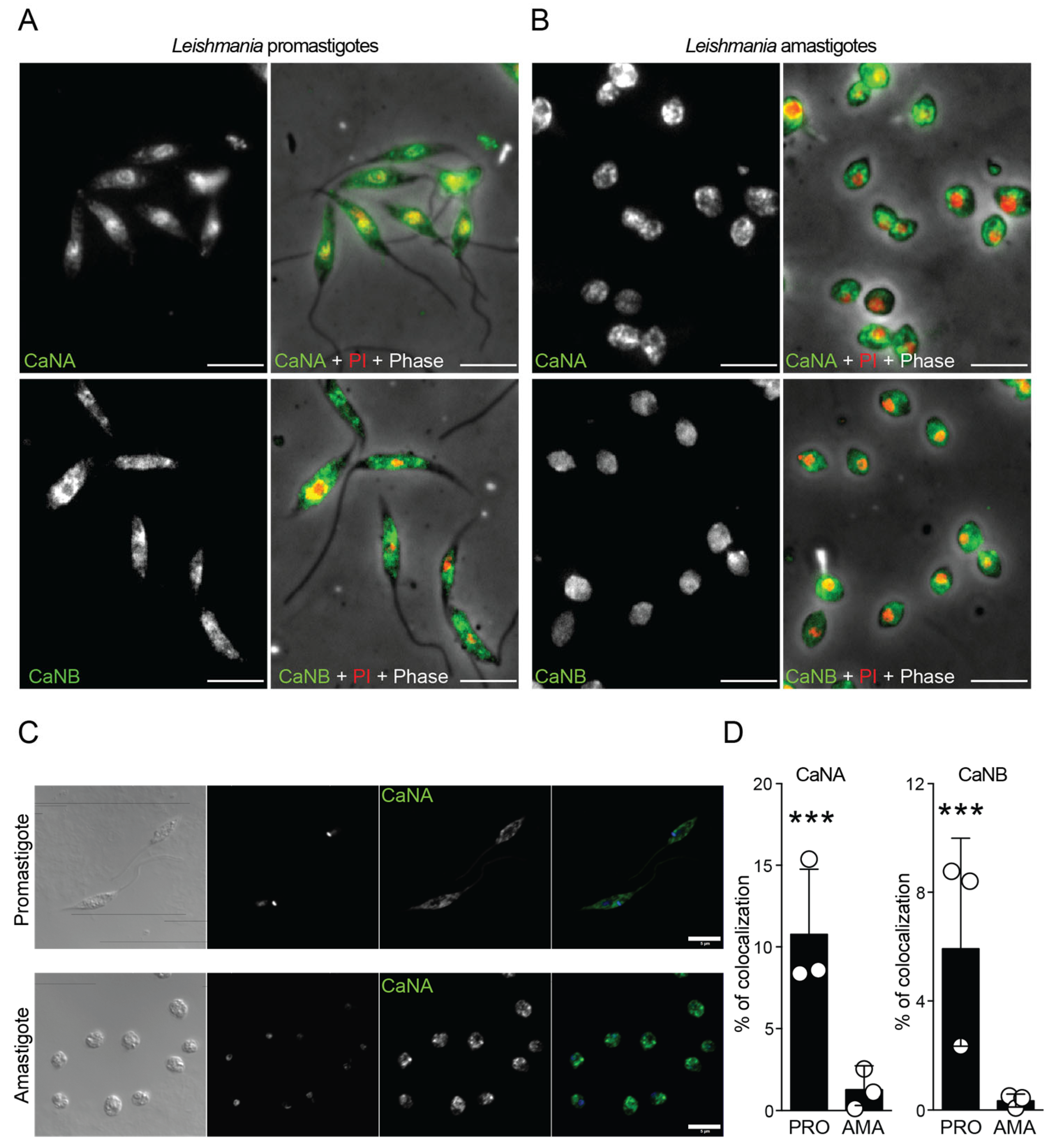
3.4. Subcellular Localization of CaNA and CaNB Differs Between the Infective Forms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CaN | Calcineurin |
| PBS | phosphate-buffered saline |
References
- Soulat, D.; Bogdan, C. Function of Macrophage and Parasite Phosphatases in leishmaniasis. Front. Immunol. 2017, 8, 1838. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Convit, J.; Ulrich, M. Antigen-specific immunodeficiency and its relation to the spectrum of American cutaneous leishmaniasis. Biol. Res. 1993, 26, 159–166. [Google Scholar] [PubMed]
- Reithinger, R.; Dujardin, J.C.; Louzir, H.; Pirmez, C.; Alexander, B.; Brooker, S. Cutaneous leishmaniasis. Lancet Infect. Dis. 2007, 7, 581–596. [Google Scholar] [CrossRef] [PubMed]
- Pacakova, L.; Harant, K.; Volf, P.; Lestinova, T. Three types of Leishmania mexicana amastigotes: Proteome comparison by quantitative proteomic analysis. Front. Cell. Infect. Microbiol. 2022, 12, 1022448. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cortazzo da Silva, L.; Aoki, J.I.; Floeter-Winter, L.M. Finding Correlations Between mRNA and Protein Levels in Leishmania Development: Is There a Discrepancy? Front. Cell. Infect. Microbiol. 2022, 12, 852902. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rossi, I.V.; Ferreira Nunes, M.A.; Vargas-Otalora, S.; da Silva Ferreira, T.C.; Cortez, M.; Ramirez, M.I. Extracellular Vesicles during TriTryps infection: Complexity and future challenges. Mol. Immunol. 2021, 132, 172–183. [Google Scholar] [CrossRef]
- Clos, J.; Grünebast, J.; Holm, M. Promastigote-to-Amastigote Conversion in Leishmania spp.—A Molecular View. Pathogens 2022, 11, 1052. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mule, S.N.; Saad, J.S.; Sauter, I.P.; Fernandes, L.R.; de Oliveira, G.S.; Quina, D.; Tano, F.T.; Brandt-Almeida, D.; Padrón, G.; Stolf, B.S.; et al. The protein map of the protozoan parasite Leishmania (Leishmania) amazonensis, Leishmania (Viannia) braziliensis and Leishmania (Leishmania) infantum during growth phase transition and temperature stress. J. Proteom. 2024, 295, 105088. [Google Scholar] [CrossRef] [PubMed]
- Freitas-Mesquita, A.L.; Dos-Santos, A.L.A.; Meyer-Fernandes, J.R. Involvement of Leishmania Phosphatases in Parasite Biology and Pathogeny. Front. Cell. Infect. Microbiol. 2021, 11, 633146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ashrafmansouri, M.; Amiri-Dashatan, N.; Ahmadi, N. Identification of protein profile in metacyclic and amastigote-like stages of Leishmania tropica: A proteomic approach. AMB Express 2022, 12, 142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- da Silva, F.T.C.; Cínthia, S.-P.; Orrego, P.R.; Araya, J.E.; Cortez, M. Papel de Calcineurinas na Interação Leishmania-Macrófago. Atualidades em Medicina Tropical no Brasil: Protozoários. Chapter 18. 2020. Available online: https://sseditora.com.br/ebooks/atualidades-em-medicina-tropical-no-brasil-protozoarios/ (accessed on 12 September 2025).
- Orrego, P.R.; Serrano-Rodríguez, M.; Cortez, M.; Araya, J.E. In Silico Characterization of Calcineurin from Pathogenic Obligate Intracellular Trypanosomatids: Potential New Biological Roles. Biomolecules 2021, 11, 1322. [Google Scholar] [CrossRef] [PubMed]
- Creamer, T.P. Calcineurin. Cell Commun. Signal. CCS 2020, 18, 137. [Google Scholar] [CrossRef] [PubMed]
- Rusnak, F.; Mertz, P. Calcineurin: Form and function. Physiol. Rev. 2000, 80, 1483–1521. [Google Scholar] [CrossRef] [PubMed]
- Sikkink, R.; Haddy, A.; MacKelvie, S.; Mertz, P.; Litwiller, R.; Rusnak, F. Calcineurin subunit interactions: Mapping the calcineurin B binding domain on calcineurin A. Biochemistry 1995, 34, 8348–8356. [Google Scholar] [CrossRef]
- Watanabe, Y.; Perrino, B.A.; Chang, B.H.; Soderling, T.R. Identification in the calcineurin A subunit of the domain that binds the regulatory B subunit. J. Biol. Chem. 1995, 270, 456–460. [Google Scholar] [CrossRef]
- Kincaid, R.L.; Nightingale, M.S.; Martin, B.M. Characterization of a cDNA clone encoding the calmodulin-binding domain of mouse brain calcineurin. Proc. Natl. Acad. Sci. USA 1988, 85, 8983–8987. [Google Scholar] [CrossRef]
- Guerini, D.; Montell, C.; Klee, C.B. Molecular cloning and characterization of the genes encoding the two subunits of Drosophila melanogaster calcineurin. J. Biol. Chem. 1992, 267, 22542–22549. [Google Scholar] [CrossRef]
- Li, S.J.; Wang, J.; Ma, L.; Lu, C.; Wang, J.; Wu, J.W.; Wang, Z.X. Cooperative autoinhibition and multi-level activation mechanisms of calcineurin. Cell Res. 2016, 26, 336–349. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Perrino, B.A.; Soderling, T.R. Identification of an autoinhibitory domain in calcineurin. J. Biol. Chem. 1990, 265, 1924–1927. [Google Scholar] [CrossRef]
- Perrino, B.A.; Ng, L.Y.; Soderling, T.R. Calcium regulation of calcineurin phosphatase activity by its B subunit and calmodulin. Role of the autoinhibitory domain. J. Biol. Chem. 1995, 270, 340–346. [Google Scholar] [CrossRef]
- Aitken, A.; Klee, C.B.; Cohen, P. The structure of the B subunit of calcineurin. Eur. J. Biochem. 1984, 139, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Juvvadi, P.R.; Lee, S.C.; Heitman, J.; Steinbach, W.J. Calcineurin in fungal virulence and drug resistance: Prospects for harnessing targeted inhibition of calcineurin for an antifungal therapeutic approach. Virulence 2017, 8, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Lee, S.C.; Cardenas, M.E.; Heitman, J. Calcium-Calmodulin-Calcineurin Signaling: A Globally Conserved Virulence Cascade in Eukaryotic Microbial Pathogens. Cell Host Microbe 2019, 26, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Thewes, S. Calcineurin-Crz1 signaling in lower eukaryotes. Eukaryot. Cell 2014, 13, 694–705. [Google Scholar] [CrossRef]
- Chow, E.W.; Clancey, S.A.; Billmyre, R.B.; Averette, A.F.; Granek, J.A.; Mieczkowski, P.; Cardenas, M.E.; Heitman, J. Elucidation of the calcineurin-Crz1 stress response transcriptional network in the human fungal pathogen Cryptococcus neoformans. PLoS Genet. 2017, 13, e1006667. [Google Scholar] [CrossRef]
- Jiang, L.; Gu, Y.; Jiang, Y.; Wei, L.; Meng, L.; Guan, N. The calcineurin-responsive transcription factor Crz1 regulates the expression of CMK2 via a sole CDRE site in its promoter in budding yeast. Sci. Rep. 2025, 15, 7046. [Google Scholar] [CrossRef]
- Ulengin-Talkish, I.; Cyert, M.S. A cellular atlas of calcineurin signaling. Biochim. Biophys. Acta Mol. Cell Res. 2023, 1870, 119366. [Google Scholar] [CrossRef]
- Naderer, T.; Dandash, O.; McConville, M.J. Calcineurin is required for Leishmania major stress response pathways and for virulence in the mammalian host. Mol. Microbiol. 2011, 80, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Bagher Khadem Erfan, M.; Mohebali, M.; Kazemi-Rad, E.; Hajjaran, H.; Edrissian, G.; Mamishi, S.; Saffari, M.; Raoofian, R.; Heidari, M. Downregulation of Calcineurin Gene Is Associated with Glucantime® Resiatance in Leishmania infantum. Iran. J. Parasitol. 2013, 8, 359–366. [Google Scholar]
- Serrano-Rodríguez, M.; Araya, J.E.; Cortez, M.; Orrego, P.R. Cytotoxic Effect of Trypanosoma cruzi Calcineurin B Against Melanoma and Adenocarcinoma Cells In Vitro. Adv. Pharmacol. Pharm. Sci. 2024, 2024, 5394494. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Balaji, S.; Babu, M.M.; Iyer, L.M.; Aravind, L. Discovery of the prin- cipal specific transcription factors of Apicomplexa and their implication for the evolution of the AP2-integrase DNA binding domains. Nucleic Acids Res. 2005, 33, 3994–4006. [Google Scholar] [CrossRef]
- Sauter, I.P.; Madrid, K.G.; de Assis, J.B.; Sá-Nunes, A.; Torrecilhas, A.C.; Staquicini, D.I.; Pasqualini, R.; Arap, W.; Cortez, M. TLR9/MyD88/TRIF signaling activates host immune inhibitory CD200 in Leishmania infection. JCI Insight 2019, 4, e126207. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferreira, T.C.S.; Sauter, I.P.; Borda-Samper, L.; Bentivoglio, E.; DaMata, J.P.; Taniwaki, N.N.; Orrego, P.R.; Araya, J.E.; Lincopan, N.; Cortez, M. Effect of DODAB Nano-Sized Cationic Bilayer Fragments Against Leishmania amazonensis. Molecules 2020, 25, 5741. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luczywo, A.; Sauter, I.P.; da Silva Ferreira, T.C.; Cortez, M.; Romanelli, G.P.; Sathicq, G.; Asis, S.E. Microwave-assisted synthesis of 2-styrylquinoline-4-carboxylic acid derivatives to improve the toxic effect against Leishmania (Leishmania) amazonensis. J. Heterocycl. Chem. 2021, 58, 822–832. [Google Scholar] [CrossRef]
- Orrego, P.R.; Olivares, H.; Cordero, E.M.; Bressan, A.; Cortez, M.; Sagua, H.; Neira, I.; González, J.; da Silveira, J.F.; Yoshida, N.; et al. A cytoplasmic new catalytic subunit of calcineurin in Trypanosoma cruzi and its molecular and functional characterization. PLoS Negl. Trop. Dis. 2014, 8, e2676. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dijkers, P.F.; O’Farrell, P.H. Drosophila calcineurin promotes induction of innate immune responses. Curr. Biol. 2007, 17, 2087–2093. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Araya, J.E.; Cornejo, A.; Orrego, P.R.; Cordero, E.M.; Cortéz, M.; Olivares, H.; Neira, I.; Sagua, H.; da Silveira, J.F.; Yoshida, N.; et al. Calcineurin B of the human protozoan parasite Trypanosoma cruzi is involved in cell invasion. Microbes Infect. 2008, 10, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.C.; Dillon, L.A.L.; Belew, A.T.; Bravo, H.C.; Mosser, D.M.; El-Sayed, N.M. Dual Transcriptome Profiling of Leishmania-Infected Human Macrophages Reveals Distinct Reprogramming Signatures. mBio 2016, 7, e00027-16. [Google Scholar] [CrossRef]
- Amos, B.; Aurrecoechea, C.; Barba, M.; Barreto, A.; Basenko, E.Y.; Bażant, W.; Belnap, R.; Blevins, A.S.; Böhme, U.; Brestelli, J.; et al. VEuPathDB: The eukaryotic pathogen, vector and host bioinformatics resource center. Nucleic Acids Res. 2022, 50, D898–D911. [Google Scholar] [CrossRef]
- Shanmugasundram, A.; Starns, D.; Böhme, U.; Amos, B.; Wilkinson, P.A.; Harb, O.S.; Warrenfeltz, S.; Kissinger, J.C.; McDowell, M.A.; Roos, D.S.; et al. TriTrypDB: An integrated functional genomics resource for kinetoplastida. PLoS Neglected Trop. Dis. 2023, 17, e0011058. [Google Scholar] [CrossRef]
- Banerjee, C.; Sarkar, D.; Bhaduri, A. Ca2+ and calmodulin-dependent protein phosphatase from Leishmania donovani. Parasitology 1999, 118 Pt 6, 567–573. [Google Scholar] [CrossRef]
- Ho, S.; Clipstone, N.; Timmermann, L.; Northrop, J.; Graef, I.; Fiorentino, D.; Nourse, J.; Crabtree, G.R. The mechanism of action of cyclosporin A and FK506. Clin. Immunol. Immunopathol. 1996, 80, S40–S45. [Google Scholar] [CrossRef]
- Rascher, C.; Pahl, A.; Pecht, A.; Brune, K.; Solbach, W.; Bang, H. Leishmania major parasites express cyclophilin isoforms with an unusual interaction with calcineurin. Biochem. J. 1998, 334 Pt 3, 659–667. [Google Scholar] [CrossRef]
- Hoerauf, A.; Rascher, C.; Bang, R.; Pahl, A.; Solbach, W.; Brune, K.; Rollinghoff, M.; Bang, H. Host-cell cyclophilin is important for the intracellular replication of Leishmania major. Mol. Microbiol. 1997, 24, 421–429. [Google Scholar] [CrossRef]
- Yau, W.L.; Blisnick, T.; Taly, J.F.; Helmer-Citterich, M.; Schiene-Fischer, C.; Leclercq, O.; Li, J.; Schmidt-Arras, D.; Morales, M.A.; Notredame, C.; et al. Cyclosporin A treatment of Leishmania donovani reveals stage-specific functions of cyclophilins in parasite proliferation and viability. PLoS Neglected Trop. Dis. 2010, 4, e729. [Google Scholar] [CrossRef] [PubMed]
- Yau, W.L.; Pescher, P.; MacDonald, A.; Hem, S.; Zander, D.; Retzlaff, S.; Blisnick, T.; Rotureau, B.; Rosenqvist, H.; Wiese, M.; et al. The Leishmania donovani chaperone cyclophilin 40 is essential for intracellular infection independent of its stage-specific phosphorylation status. Mol. Microbiol. 2014, 93, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Ivens, A.C.; Peacock, C.S.; Worthey, E.A.; Murphy, L.; Aggarwal, G.; Berriman, M.; Sisk, E.; Rajandream, M.-A.; Adlem, E.; Aert, R.; et al. The genome of the kinetoplastid parasite, Leishmania major. Science 2005, 309, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Real, F.; Vidal, R.O.; Carazzolle, M.F.; Mondego, J.M.; Costa, G.G.; Herai, R.H.; Wurtele, M.; de Carvalho, L.M.; Carmona e Ferreira, R.; Mortara, R.A.; et al. The genome sequence of Leishmania (Leishmania) amazonensis: Functional annotation and extended analysis of gene models. DNA Res. 2013, 20, 567–581. [Google Scholar] [CrossRef]
- Aslett, M.; Aurrecoechea, C.; Berriman, M.; Brestelli, J.; Brunk, B.P.; Carrington, M.; Depledge, D.P.; Fischer, S.; Gajria, B.; Gao, X.; et al. TriTrypDB: A functional genomic resource for the Trypanosomatidae. Nucleic Acids Res. 2010, 38, D457–D462. [Google Scholar] [CrossRef]
- Moreno, V.R.; Aguero, F.; Tekiel, V.; Sanchez, D.O. The Calcineurin A homologue from Trypanosoma cruzi lacks two important regulatory domains. Acta Trop. 2007, 101, 80–89. [Google Scholar] [CrossRef]
- Deschacht, M.; Van Assche, T.; Hendrickx, S.; Bult, H.; Maes, L.; Cos, P. Role of oxidative stress and apoptosis in the cellular response of murine macrophages upon Leishmania infection. Parasitology 2012, 139, 1429–1437. [Google Scholar] [CrossRef]
- Teixeira, P.C.; Velasquez, L.G.; Lepique, A.P.; de Rezende, E.; Bonatto, J.M.; Barcinski, M.A.; Cunha-Neto, E.; Stolf, B.S. Regulation of Leishmania (L.) amazonensis protein expression by host T cell dependent responses: Differential expression of oligopeptidase B, tryparedoxin peroxidase and HSP70 isoforms in amastigotes isolated from BALB/c and BALB/c nude mice. PLoS Neglected Trop. Dis. 2015, 9, e0003411. [Google Scholar] [CrossRef]
- Inbar, E.; Hughitt, V.K.; Dillon, L.A.; Ghosh, K.; El-Sayed, N.M.; Sacks, D.L. The Transcriptome of Leishmania major Developmental Stages in Their Natural Sand Fly Vector. mBio 2017, 8, e00029-17. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandt-Almeida, D.; Sauter, I.P.; Cruz, M.C.; Cortez, C.; Orrego, P.R.; Cortez, M. Stage-Specific Expression and Subcellular Localization of Calcineurin in Infective Forms of Leishmania amazonensis. Pathogens 2025, 14, 1139. https://doi.org/10.3390/pathogens14111139
Brandt-Almeida D, Sauter IP, Cruz MC, Cortez C, Orrego PR, Cortez M. Stage-Specific Expression and Subcellular Localization of Calcineurin in Infective Forms of Leishmania amazonensis. Pathogens. 2025; 14(11):1139. https://doi.org/10.3390/pathogens14111139
Chicago/Turabian StyleBrandt-Almeida, Deborah, Ismael Pretto Sauter, Mario Costa Cruz, Cristian Cortez, Patricio Reyes Orrego, and Mauro Cortez. 2025. "Stage-Specific Expression and Subcellular Localization of Calcineurin in Infective Forms of Leishmania amazonensis" Pathogens 14, no. 11: 1139. https://doi.org/10.3390/pathogens14111139
APA StyleBrandt-Almeida, D., Sauter, I. P., Cruz, M. C., Cortez, C., Orrego, P. R., & Cortez, M. (2025). Stage-Specific Expression and Subcellular Localization of Calcineurin in Infective Forms of Leishmania amazonensis. Pathogens, 14(11), 1139. https://doi.org/10.3390/pathogens14111139






