Dengue Virus and the Host Immune System: A Battle of Immune Modulation, Response and Evasion
Abstract
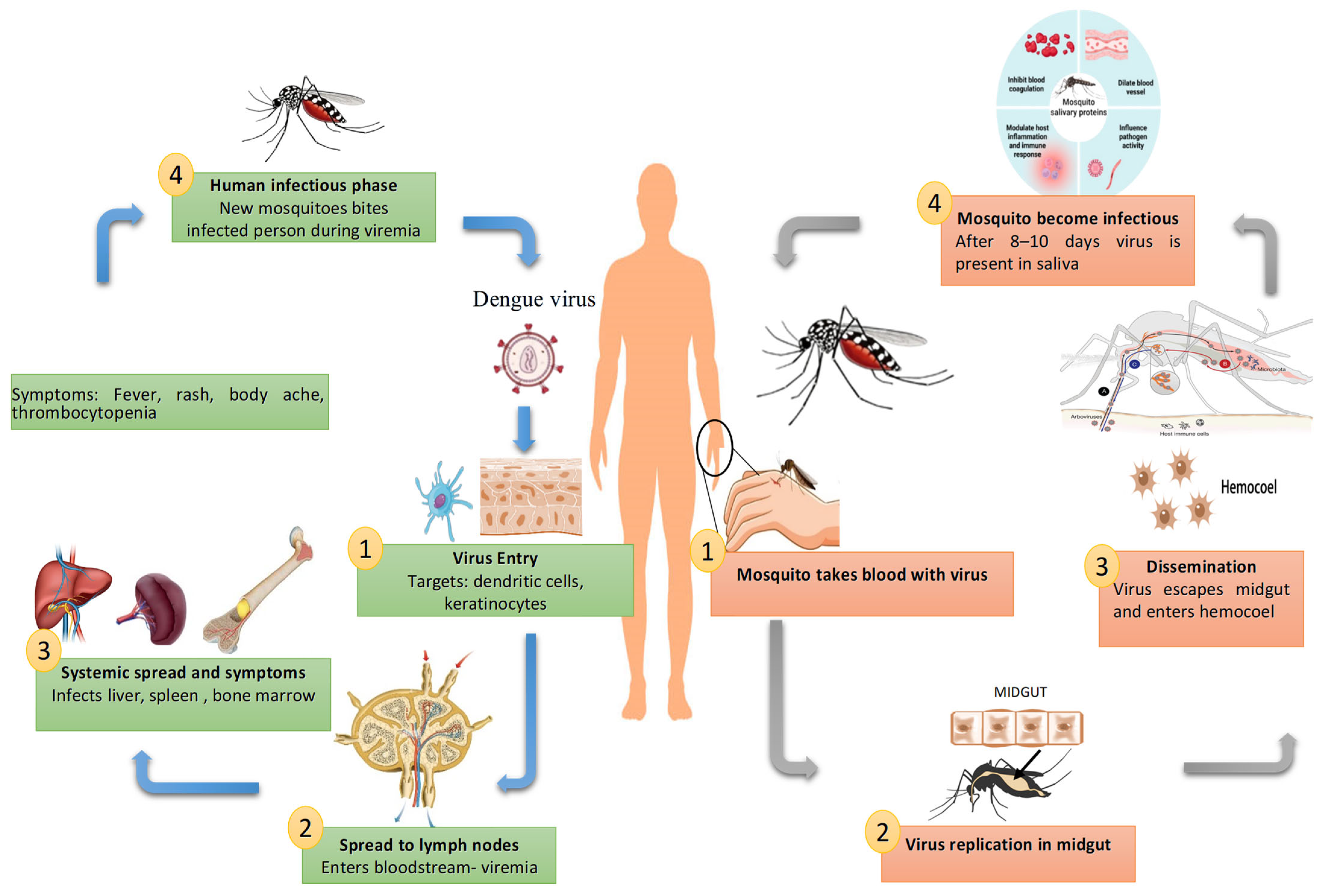
1. Introduction
2. Structure of DENV
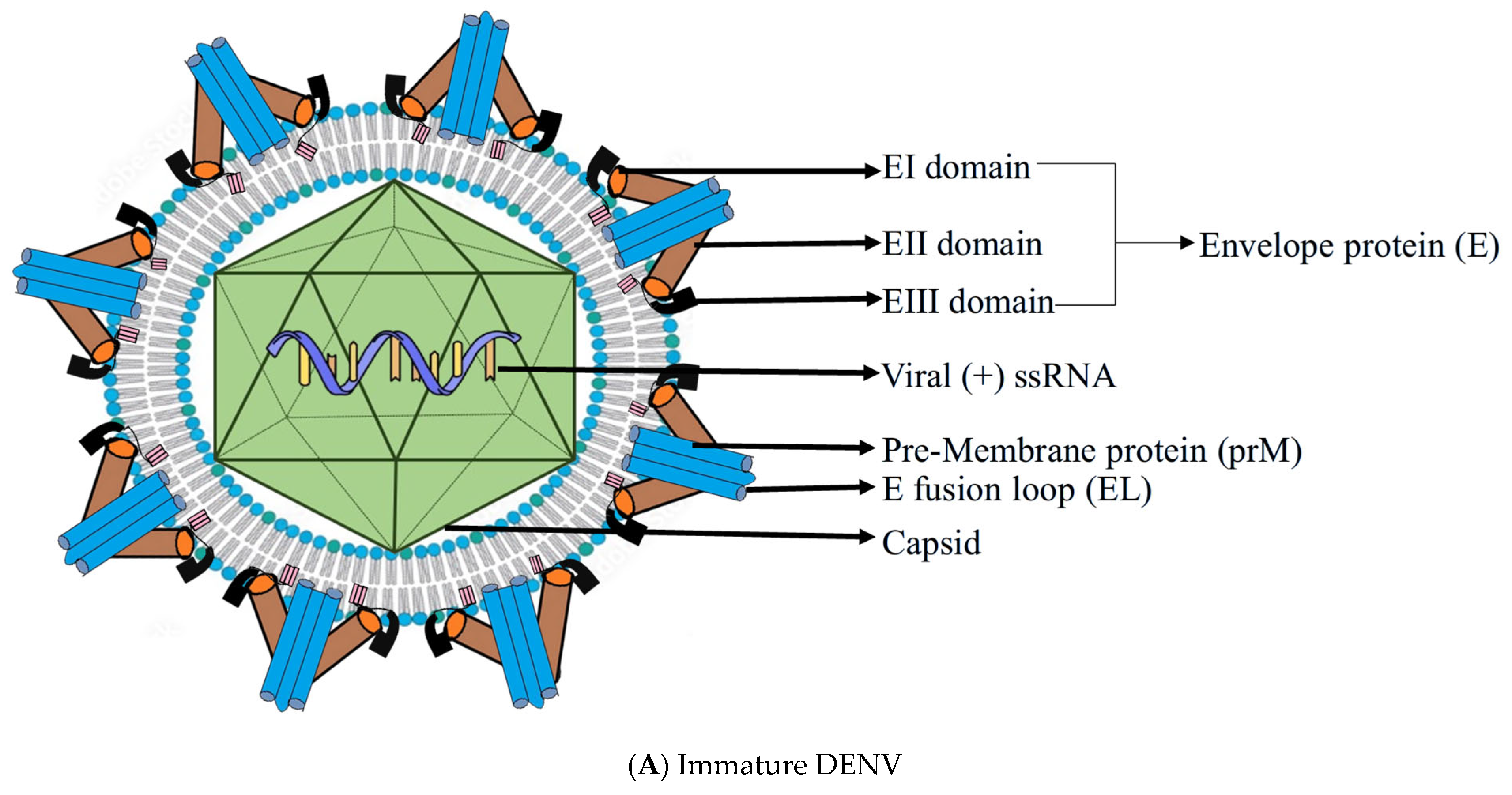
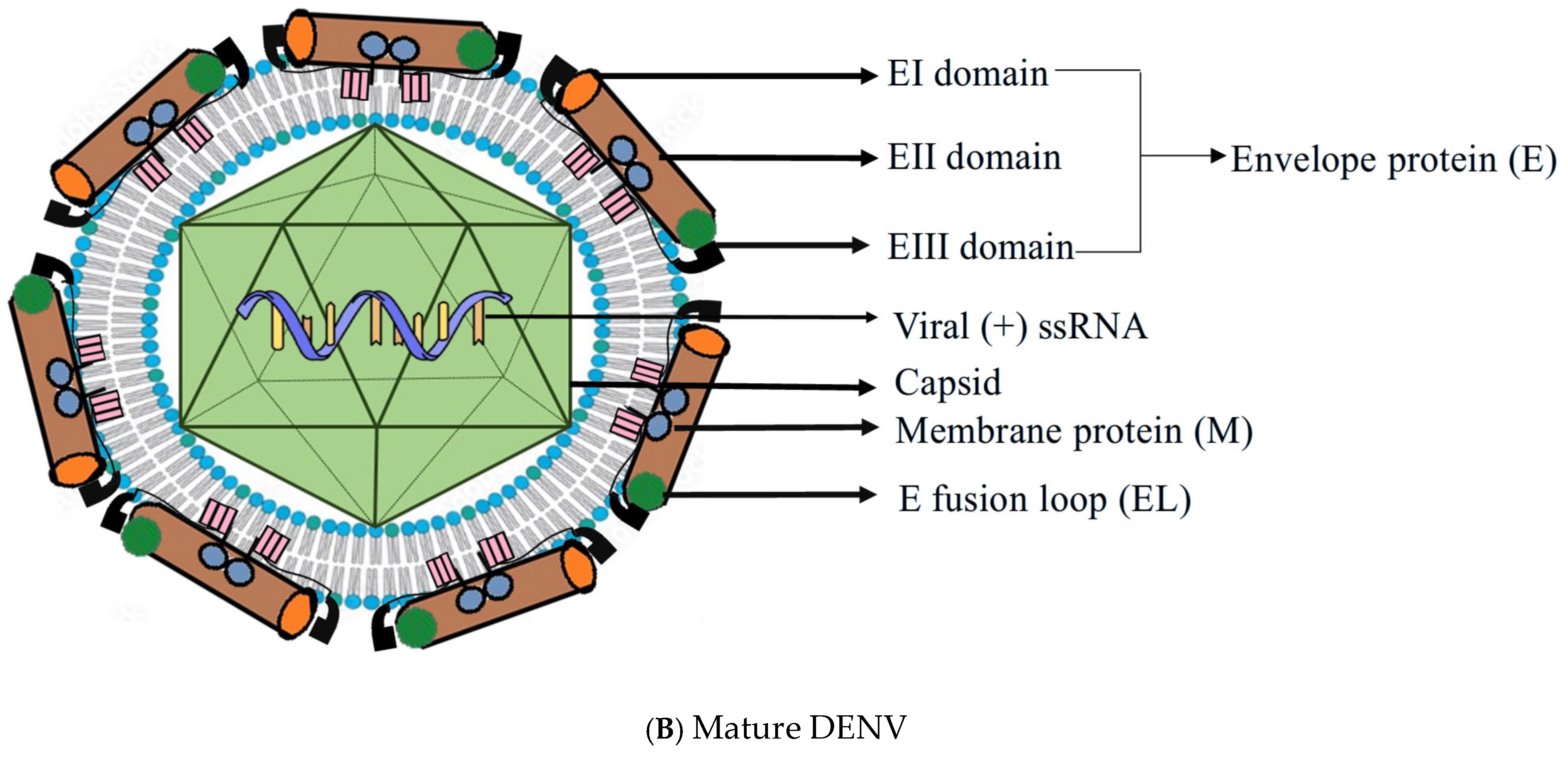
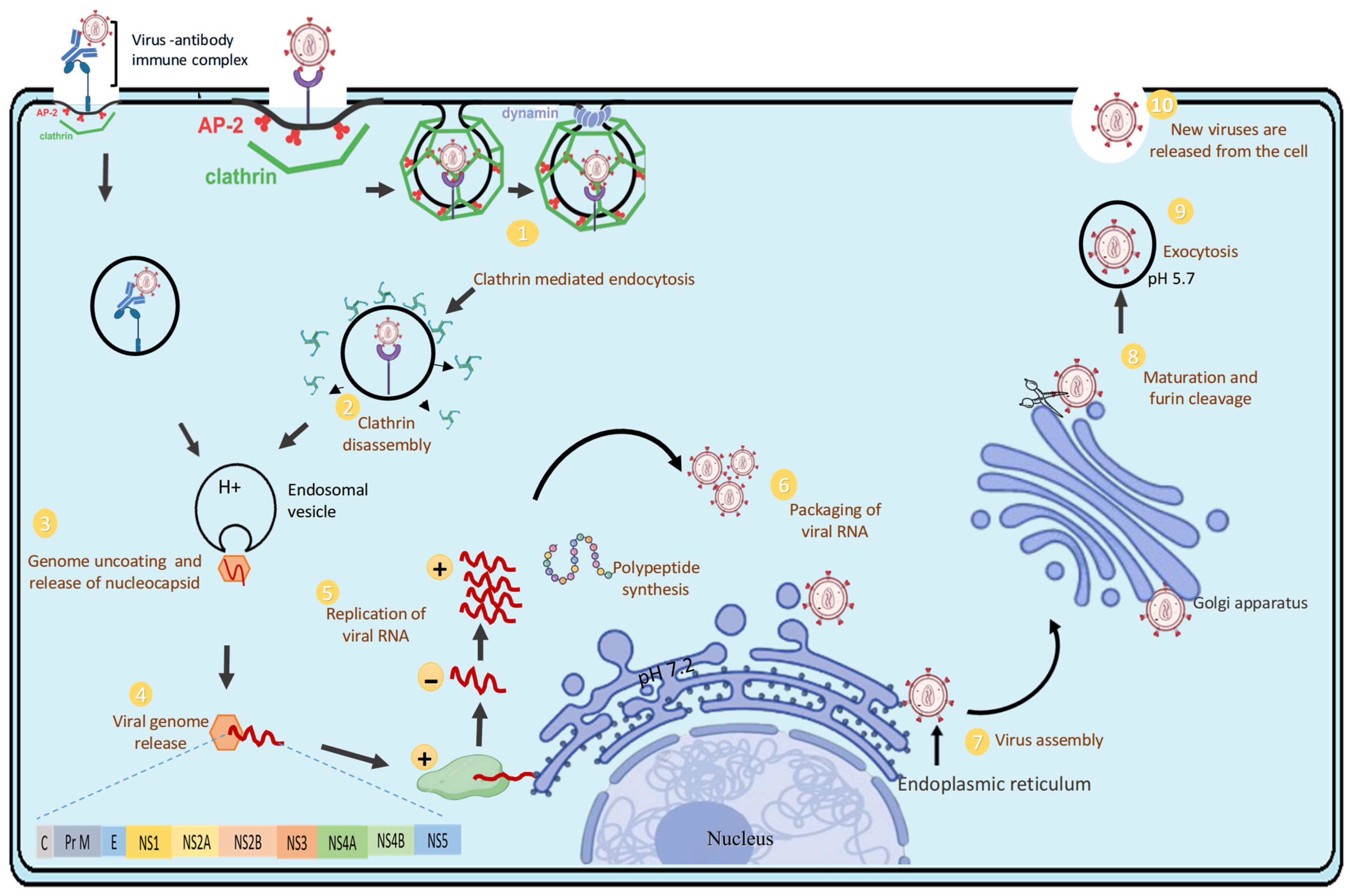
3. Replication Cycle of DENV
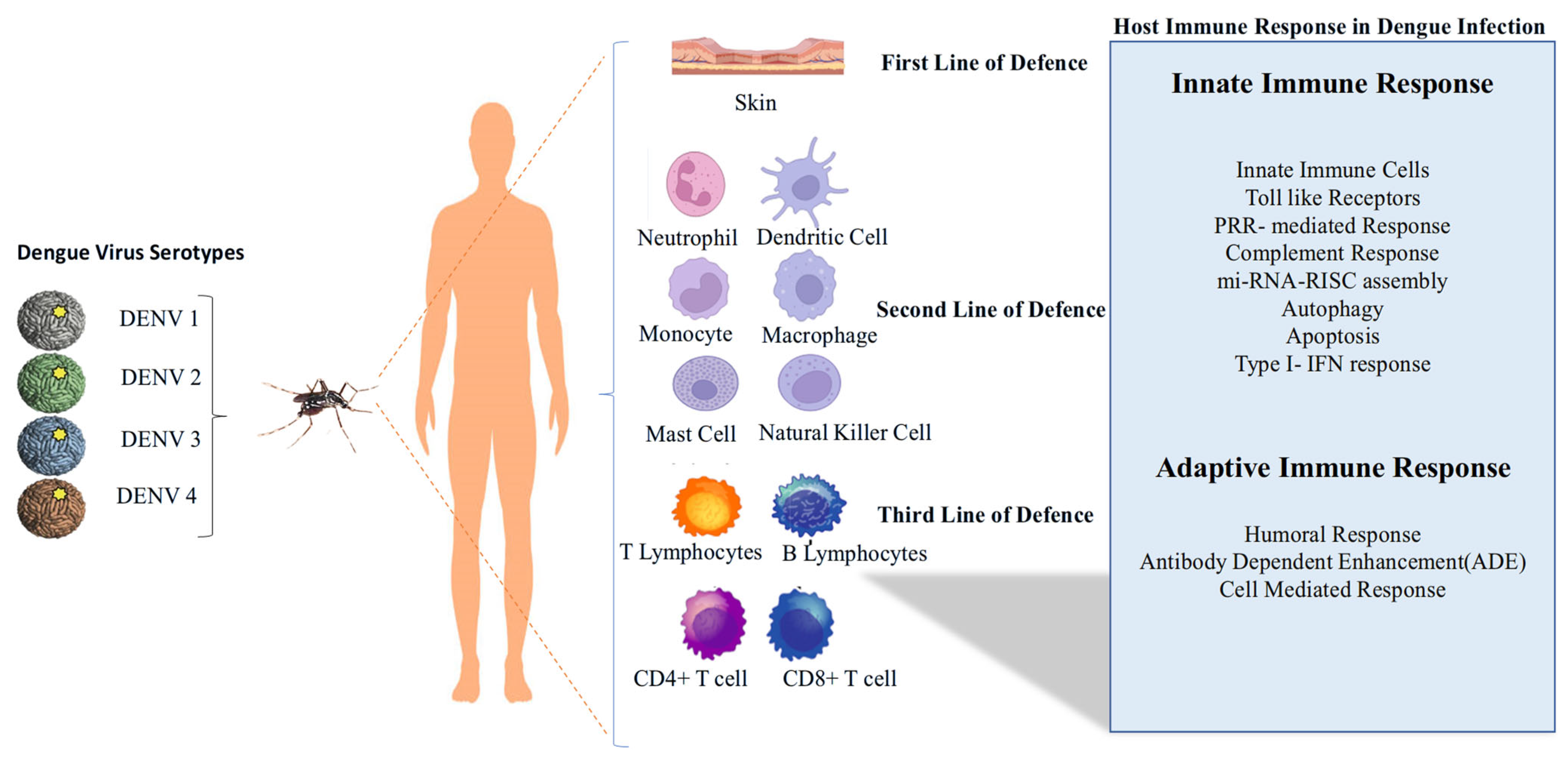
4. Host Immune Response Models and Immune Escape Mechanisms of DENV
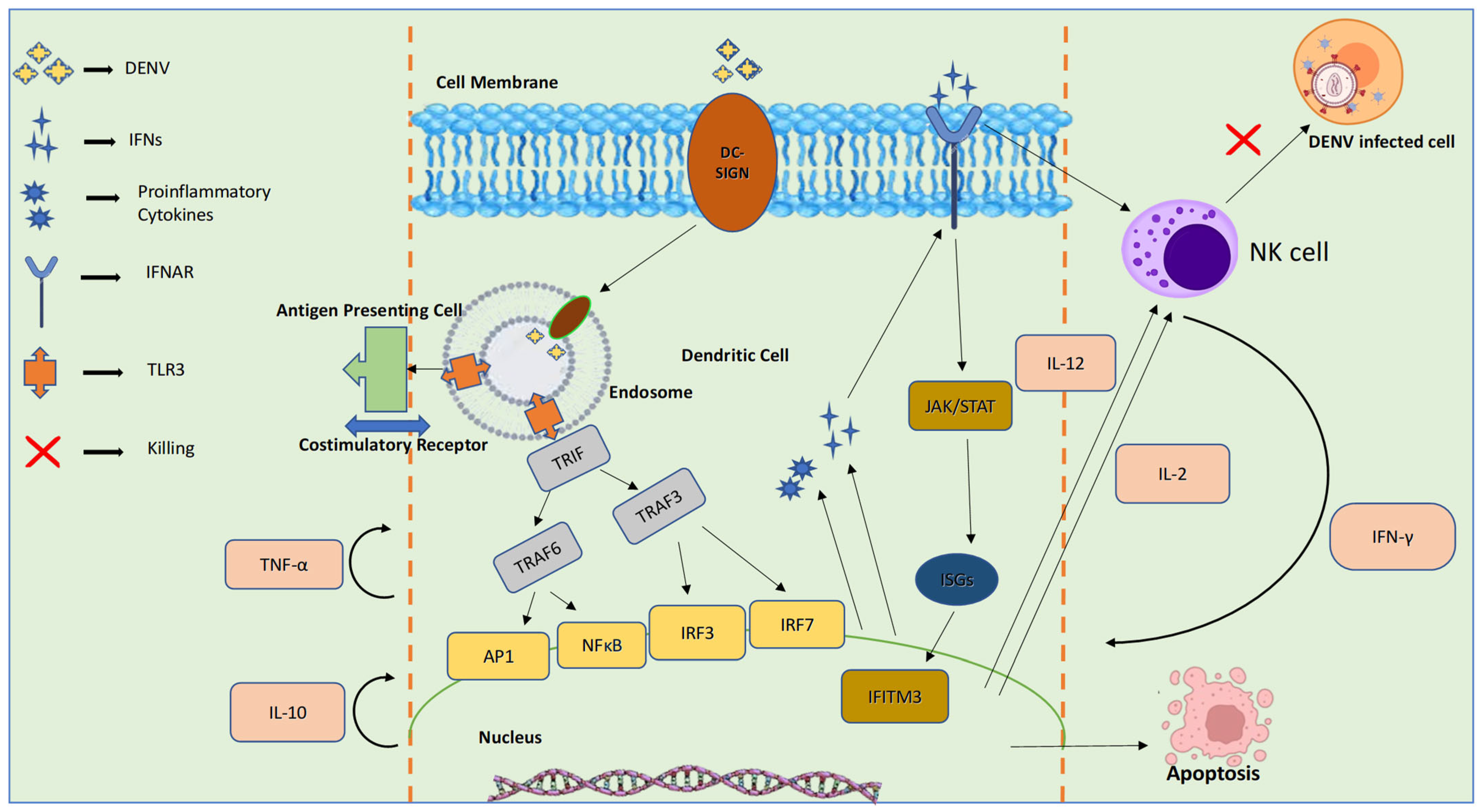
4.1. Innate Immune Response to DENV
4.1.1. Initial Immunological Encounter: Skin Resident IMMUNE Cells and Keratinocytes
4.1.2. Subsequent Wave: Monocyte-Derived Immune Cells Are Recruited and Infected; Concurrent Activation of Plasmacytoid Dendritic Cells
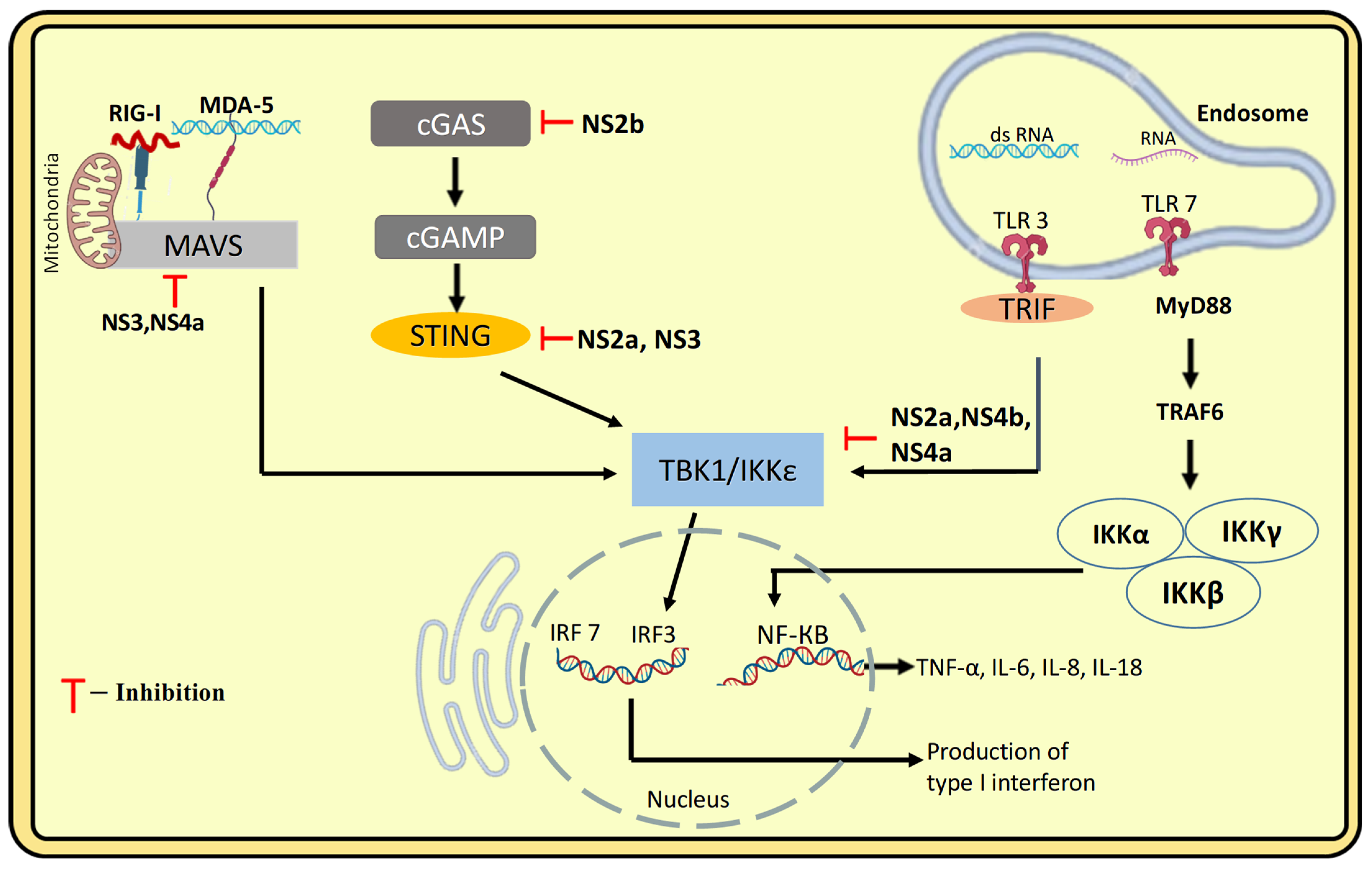
4.1.3. PRR-Mediated Recognition and Response, Type-I IFN Response to DENV Infection
4.1.4. Infection-Driven Activation and Homing of Natural Killer Cells and Neutrophils
4.2. Activation and Regulation of the Complement System in DENV Infection
4.3. Role of miRNA-RISC Assembly in Viral RNA Silencing and Degradation of DENV
4.4. DENV and Autophagy
4.5. DENV and Apoptosis
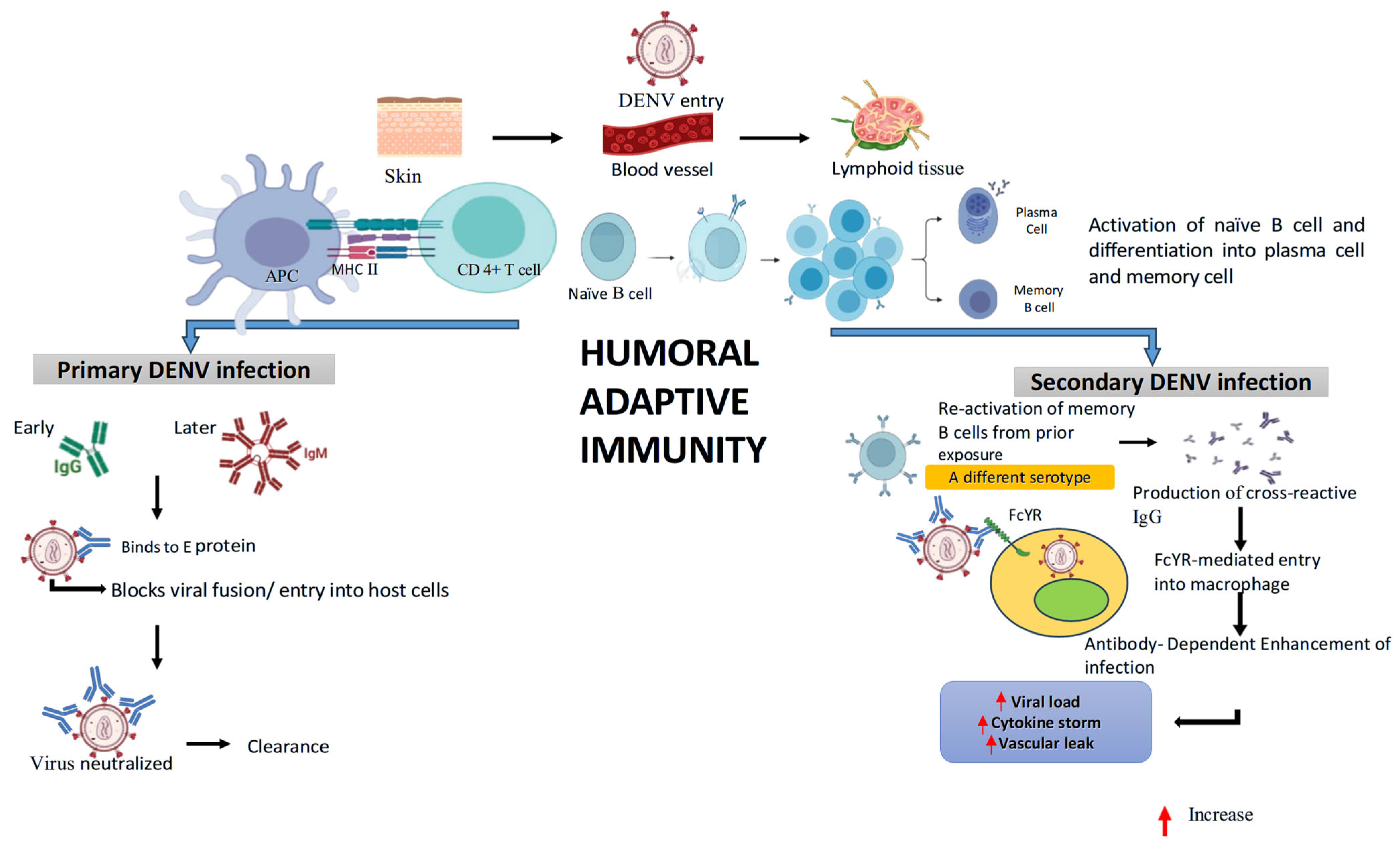
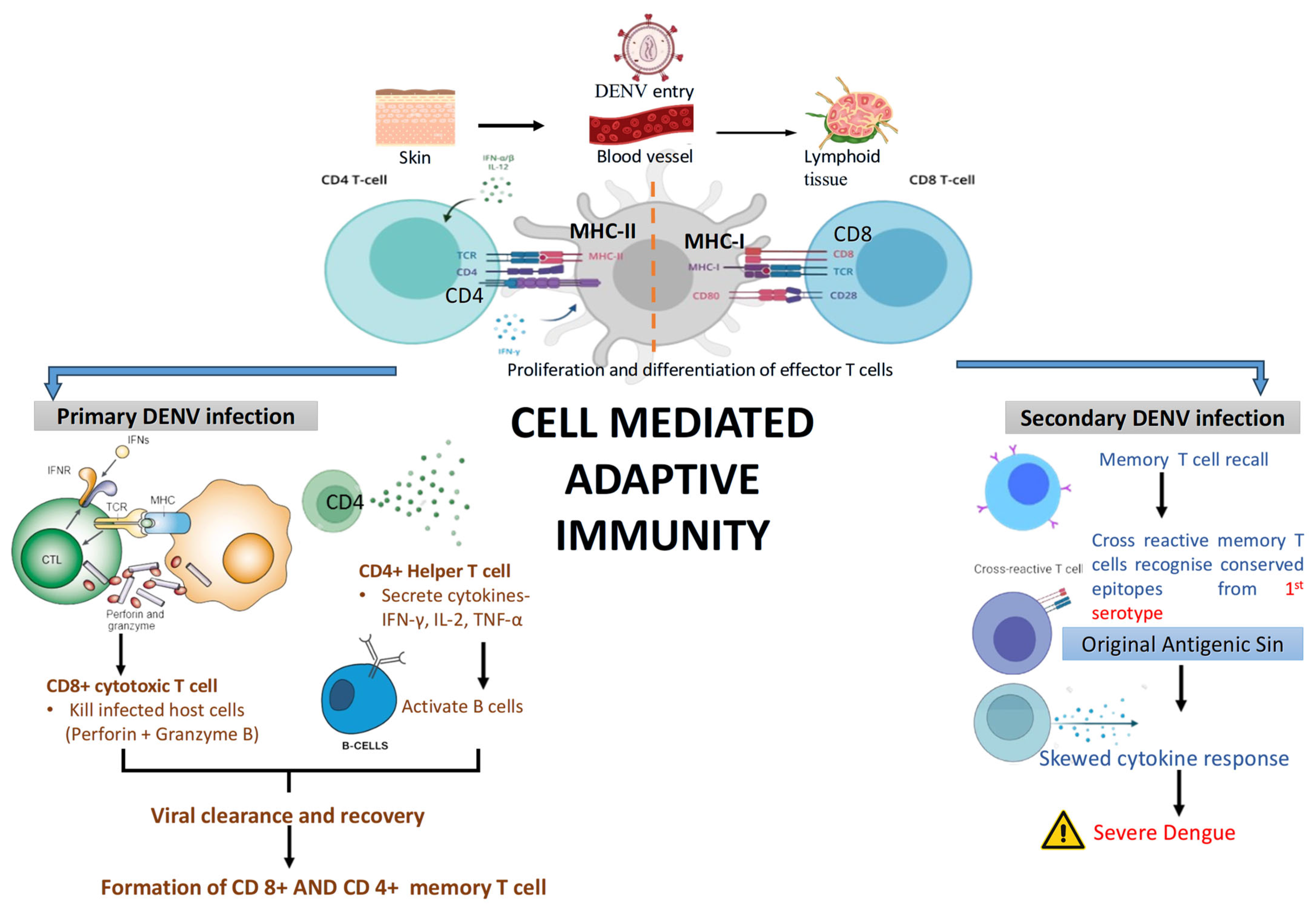
4.6. Adaptive Immune Response
4.6.1. Humoral Immune Response
4.6.2. Cell-Mediated Immune Response
5. Host Immune Evasion Strategies of DENV
5.1. Movement of Immune Cells from Skin to Lymph Nodes: A Key Mechanism for DENV Immune Escape and Viral Spread
5.2. Innate Immune Evasion: Structural Hiding in the Host Cell, Inhibition of IFN-Production and Signalling
5.3. Immune Evasion from Complement Response
5.4. Evasion of Adaptive Immune Response
5.4.1. Antigenic Variation to Evade Recognition by Neutralizing Antibodies
5.4.2. Antibody Dependent Enhancement (ADE) of Infection
5.4.3. Evasion by Antigen Presentation Blockade and T Cell Recognition
6. Influence of Host Immunity and Genetic Factors on DENV Infection and Disease Severity
6.1. Effect of Host Immunity on the Pathogenesis of Dengue Infection
6.2. Host Genetics Determinants and Susceptibility
6.2.1. Major Histocompatibility Complex Genes
6.2.2. Non-MHC Genes
7. Recent Advances in Therapeutic Approaches and Vaccine Development
7.1. Symptomatic Management of Dengue
7.2. Role of Therapeutics in Targeting the DENV
7.3. Vaccine Development
8. Current Challenges and Future Directions in Dengue Therapy and Vaccine Development
9. Proteomic and Transcriptomic Insights into Early Host Biomarkers of Severe Dengue
9.1. Proteomics Insights
9.2. Transcriptomics Insights
10. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADE | Antibody-dependent enhancement |
| APRIL | A Proliferation-inducing ligand |
| Argo | Argonaute |
| CMI | Cell-mediated immunity |
| CTLA-4 | Cytotoxic T-lymphocyte antigen 4 |
| CTLs | Cytotoxic T Lymphocytes |
| CXCL8 | C-X-C motif chemokine ligand 8 |
| DAAs | Direct Acting Antivirals |
| DAK | Dihydroxyacetone kinase |
| DAMPs | Damage-associated molecular patterns |
| DC-SIGN | Dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin |
| DENV | Dengue Virus |
| DHF | Dengue haemorrhagic fever |
| DSS | Dengue shock syndrome |
| FGL1 | Fibrinogen-like protein 1 |
| GATA3 | GATA binding protein 3 |
| GLUL | Glutamate-ammonia ligase |
| ICAM1 | Intercellular Adhesion Molecule 1 |
| IFIT3 | Interferon-Induced Protein with Tetratricopeptide Repeats 3. |
| IL-6 | Interleukin-6 |
| IL1R1 | Interleukin-1 Receptor, Type I |
| IL1RN | Interleukin-1 Receptor Antagonist |
| ISGs | Induces interferon-stimulated genes |
| LAMB2 | laminin subunit beta-2 |
| MAC | Membrane attack complex |
| MBL | Mannose-binding lectin pathway |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| MDA-5 | Melanoma Differentiation-Associated gene 5 |
| MFAP4 | Microfibril-associated protein 4 |
| MICB | Metabolic, Circulatory, Immune, and microbial microenvironment |
| moDCs | Monocyte-Derived Dendritic Cells |
| NIAID | National Institute of Allergy and Infectious Diseases |
| NK Cells | Natural Killer Cells |
| OAS | Original antigenic sin |
| PAMPs | Pathogen-associated molecular patterns |
| pDCs | Plasmacytoid dendritic cells |
| PF4 | Platelet Factor 4 |
| PLAT | Tissue-type Plasminogen Activator |
| PRRs | Pattern recognition receptors |
| RIG-I | Retinoic acid-inducible gene I |
| RISC | RNA-induced silencing complex |
| RLRs | RIG-like receptors |
| RSAD2 | Radical S-adenosyl methionine domain containing 2 |
| SARM | Selective androgen-receptor modulator |
| SOCS-3 | Suppressor of cytokine signalling 3 |
| STAT2 | Signal Transducer and Activator of Transcription 2 |
| TBK1 | TANK-binding kinase 1 |
| TLRs | Toll-like Receptors |
| TNF-α | Tumor necrosis factor alpha |
| TNFS10 | Tumor Necrosis Factor (Ligand) Superfamily, Member 10 |
| TRIF | TIR-domain-containing adapter-inducing interferon-β |
| VCAM1 | Vascular Cell Adhesion Molecule 1 |
| VDR | Vitamin D receptor |
| VN | Vitronectin |
| VPs | Vesicle packets |
| WHO | World Health Organization |
References
- Dengue-Global Situation, WHO. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 3 October 2025).
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Sinha, S.; Singh, K.; Kumar, Y.S.R.; Roy, R.; Phadnis, S.; Meena, V. Dengue virus pathogenesis and host molecular machineries. J. Biomed. Sci. 2024, 31, 43. [Google Scholar] [CrossRef]
- Scott, B. Pathogenesis of Dengue: Challenges to Molecular Biology. Science 1893, 239, 476–481. [Google Scholar]
- Halstead, S.B. Antibody, Macrophages, Dengue Virus Infection, Shock, and Hemorrhage: A Pathogenetic Cascade. Rev. Infect. Dis. 1989, 11 (Suppl. S4), S830–S839. [Google Scholar] [CrossRef]
- Glasner, D.R.; Puerta-Guardo, H.; Beatty, P.R.; Harris, E. The good, the bad, and the shocking: The multiple roles of dengue virus nonstructural protein 1 in protection and pathogenesis. Annu. Rev. Virol. 2018, 5, 227–253. [Google Scholar] [CrossRef] [PubMed]
- Puerta-Guardo, H.; Biering, S.B.; Castillo-Rojas, B.; DiBiasio-White, M.J.; Lo, N.T.; Espinosa, D.A.; Warnes, C.M.; Wang, C.; Cao, T.; Glasner, D.R.; et al. Flavivirus NS1-triggered endothelial dysfunction promotes virus dissemination. bioRxiv 2024. [Google Scholar] [CrossRef]
- Ashour, J.; Laurent-rolle, M.; Shi, P.; García-sastre, A. NS5 of Dengue Virus Mediates STAT2 Binding and Degradation. J. Virol. 2009, 83, 5408–5418. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-santos, C. Innate Immune Response to Dengue Virus: Toll-like Receptors and Antiviral Response. Viruses 2022, 14, 992. [Google Scholar] [CrossRef]
- Dash, M.K.; Samal, S.; Rout, S.; Behera, C.K.; Sahu, M.C. Immunomodulation in dengue: Towards deciphering dengue severity markers. Cell Commun. Signal. 2024, 22, 451. [Google Scholar] [CrossRef]
- Weiskopf, D.; Angelo, M.A.; de Azeredo, E.L.; Sidney, J.; Greenbaum, J.A.; Fernando, A.N.; Broadwater, A.; Kolla, R.V.; De Silva, A.D.; de Silva, A.M.; et al. Comprehensive analysis of dengue virus-speci fi c responses supports an HLA-linked protective role for CD8 + T cells. Proc. Natl. Acad. Sci. USA 2013, 110, E2046–E2053. [Google Scholar] [CrossRef]
- Mongkolsapaya, J.; Dejnirattisai, W.; Xu, X.N.; Vasanawathana, S.; Tangthawornchaikul, N.; Chairunsri, A.; Sawasdivorn, S.; Duangchinda, T.; Dong, T.; Rowland-Jones, S.; et al. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat. Med. 2003, 9, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Stephens, H.A.F. HLA and Other Gene Associations with Dengue Disease Severity. Dengue Viru 2009, 338, 99–114. [Google Scholar] [CrossRef]
- Chuansumrit, A.; Turbpaiboon, C.; Casadémont, I.; Chuansumrit, A.; Lowhnoo, T.; Kajaste-Rudnitski, A.; Kalayanarooj, S.M.; Tangnararatchakit, K.; Tangthawornchaikul, N.; Vasanawathana, S.; et al. A variant in the CD209 promoter is associated with severity of dengue disease. Nat. Genet. 2005, 37, 507–513. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, L.; Rossmann, M.G.; Lafayette, W. Temperature dependent conformational change of dengue virus. Curr. Opin. Virol. 2016, 765, 109–112. [Google Scholar] [CrossRef]
- Anumanthan, G.; Sahay, B. Current Dengue Virus Vaccine Developments and Future Directions. Viruses 2025, 17, 212. [Google Scholar] [CrossRef]
- Thomas, A.; Thiono, D.J.; Kudlacek, S.T.; Forsberg, J.; Premkumar, L.; Tian, S.; Kuhlman, B.; de Silva, A.M.; Metz, S.W. Dimerization of Dengue Virus E Subunits Impacts Antibody Function and Domain Focus. J. Virol. 2020, 94, e00745-20. [Google Scholar] [CrossRef]
- Asarnow, D.; Becker, V.A.; Bobe, D.; Dubbledam, C.; Johnston, J.D.; Kopylov, M.; Lavoie, N.R.; Li, Q.; Mattingly, J.M.; Mendez, J.H.; et al. Recent advances in infectious disease research using cryo-electron tomography. Front. Mol. Biosci. 2024, 10, 1296941. [Google Scholar] [CrossRef]
- Khetarpal, N.; Khanna, I. Dengue Fever: Causes, Complications, and Vaccine Strategies. J. Immunol. Res. 2016, 2016, 6803098. [Google Scholar] [CrossRef]
- Naveed, M.; Akhtar, R.; Abid, M.; Khan, S.A.; Rehman, Z.U.; Tayyub, M.; Malik, M.I.; Shahzad, M.K.; Mubeen, H.; Qadir, M.S.; et al. The interactions of flaviviruses with cellular receptors: Implications for virus entry. Virology 2022, 568, 77–85. [Google Scholar] [CrossRef]
- Klema, V.J.; Padmanabhan, R.; Choi, K.H. Flaviviral Replication Complex: Coordination between RNA Synthesis and 5′-RNA Capping. Viruses 2015, 7, 4640–4656. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Sun, Y.; Qiu, X. Hijacking autophagy for infection by flaviviruses. Virus Res. 2024, 347, 199422. [Google Scholar] [CrossRef]
- Xie, Y.; Sun, Q. Dengue virus and lipid metabolism: Unravelling the interplay for future therapeutic approaches. Emerg. Microbes Infect. 2025, 14, 2477647. [Google Scholar] [CrossRef]
- Jablunovsky, A.; Jose, J. The Dynamic Landscape of Capsid Proteins and Viral RNA Interactions in Flavivirus Genome Packaging and Virus Assembly. Pathogens 2024, 13, 120. [Google Scholar] [CrossRef]
- Tognarelli, E.I.; Reyes, A.; Carreño, L.J.; Bueno, S.M.; Kalergis, A.M.; Gonz, P.A. Modulation of Endosome Function, Vesicle Trafficking and Autophagy by Human Herpesviruses. Cells 2021, 10, 542. [Google Scholar] [CrossRef]
- Dhole, P.; Zaidi, A.; Nariya, H.K.; Sinha, S.; Jinesh, S.; Srivastava, S. Host Immune Response to Dengue Virus Infection: Friend or Foe? Immuno 2024, 4, 549–577. [Google Scholar] [CrossRef]
- King, C.A.; Wegman, A.D.; Endy, T.P. Mobilization and Activation of the Innate Immune Response to Dengue Virus. Front. Cell. Infect. Microbiol. 2020, 10, 574417. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.A.; Harris, E. Monocyte Recruitment to the Dermis and Differentiation to Dendritic Cells Increases the Targets for Dengue Virus Replication. PLoS Pathog. 2014, 10, e1004541. [Google Scholar] [CrossRef] [PubMed]
- Duangkhae, P.; Erdos, G.; Ryman, K.D.; Watkins, S.C.; Falo, L.D., Jr.; Marques, E.T., Jr.; Barratt-Boyes, S.M. Interplay between Keratinocytes and Myeloid Cells Drives Dengue Virus Spread in Human Skin. J. Investig. Dermatol. 2018, 138, 618–626. [Google Scholar] [CrossRef]
- Brown, R.A.M.G.; Hermann, L.L.; Issekutz, A.C.; Marshall, J.S.; Rowter, D.; Al-Afif, A. Dengue Virus Infection of Mast Cells Triggers Endothelial Cell Activation. J. Virol. 2011, 85, 1145–1150. [Google Scholar] [CrossRef]
- Mishra, A.K.; George, A.A.; Abhilash, K.P.P. The relationship between skin rash and outcome in dengue. J. Vector Borne Dis. 2018, 2012, 310–314. [Google Scholar] [CrossRef]
- Shi, C.; Pamer, E.G. Monocyte recruitment during infection and inflammation Chao. Nat. Rev. Immunol. 2014, 11, 762–774. [Google Scholar] [CrossRef]
- Costa, J.C.V.V.; Ye, W.; Chen, Q.; Preiser, M.M.T.P.; Ooi, E.E. Dengue Virus-Infected Dendritic Cells, but Not Monocytes, Activate Natural Killer Cells through a Contact-Dependent Mechanism Involving Adhesion Molecules. MBio 2017, 8, e00741-17. [Google Scholar] [CrossRef]
- Gandini, M.; Gras, C.; Azeredo, E.L.; Pinto, L.M.D.O.; Smith, N.; Despres, P.; da Cunha, R.V.; de Souza, L.J.; Kubelka, C.F.; Herbeuval, J.-P. Dengue Virus Activates Membrane TRAIL Relocalization and IFN- a Production by Human Plasmacytoid Dendritic Cells In Vitro and In Vivo. PLoS Neglected Trop. Dis. 2013, 7, e2257. [Google Scholar] [CrossRef]
- Nasirudeen, A.M.A.; Wong, H.H.; Thien, P.; Xu, S.; Lam, K.; Xiang, D. RIG-I, MDA5 and TLR3 Synergistically Play an Important Role in Restriction of Dengue Virus Infection. PLoS Neglected Trop. Dis. 2011, 5, e926. [Google Scholar] [CrossRef] [PubMed]
- Uno, N.; Ross, T.M. Dengue virus and the host innate immune response. Emerg. Microbes Infect. 2018, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Thiemmeca, S.; Tamdet, C.; Punyadee, N.; Prommool, T.; Songjaeng, A.; Noisakran, S.; Puttikhunt, C.; Atkinson, J.P.; Diamond, M.S.; Ponlawat, A.; et al. Secreted NS1 protects dengue virus from mannose binding lectin-mediated neutralization. J. Immunol. 2017, 197, 4053–4065. [Google Scholar] [CrossRef] [PubMed]
- Morrison, B.E.; Park, S.J.; Mooney, J.M.; Mehrad, B. Chemokine-mediated recruitment of NK cells is a critical host defense mechanism in invasive aspergillosis. J. Clin. Investig. 2003, 112, 1862–1870. [Google Scholar] [CrossRef]
- Mathew, A. Defining the role of NK cells during dengue virus infection. Immunology 2018, 154, 557–562. [Google Scholar] [CrossRef]
- Gandini, M.; Petitinga-Paiva, F.; Marinho, C.F.; Correa, G.; De Oliveira-Pinto, L.M.; Souza, L.J.D.; Cunha, R.V.; Kubelka, C.F.; de Azeredo, E.L. Dengue Virus Induces NK Cell Activation through TRAIL Expression during Infection. Mediat. Inflamm. 2017, 2017, 5649214. [Google Scholar] [CrossRef]
- Tsioumpekou, M.; Leusen, J.H.W.; Olofsen, P.A. Homing, Function and Plasticity in Health and Disease. Cells 2023, 12, 1981. [Google Scholar] [CrossRef]
- Kunder, M.; Moideen, V.L.A.V. Plasma Neutrophil Elastase, a 1 -Antitrypsin, a 2 -Macroglobulin and Neutrophil Elastase—A 1-Antitrypsin Complex Levels in patients with Dengue Fever. Indian J. Clin. Biochem. 2018, 33, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Jeang, K. RNAi in the regulation of mammalian viral infections. BMC Biol. 2012, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Kakumani, P.K.; Ponia, S.; Rajgokul, K.S.; Sood, V.; Chinnappan, M.; Banerjea, A.C. Role of RNA Interference (RNAi) in Dengue Virus Replication and Identification of NS4B as an RNAi Suppressor. J. Virol. 2013, 87, 8870–8883. [Google Scholar] [CrossRef] [PubMed]
- Urcuqui-inchima, S.; Cabrera, J.; Haenni, A. Interplay between dengue virus and Toll-like receptors, RIG-I/MDA5 and microRNAs: Implications for pathogenesis. Antiviral Res. 2017, 147, 47–57. [Google Scholar] [CrossRef]
- Lennemann, N.J.; Coyne, C.B. Dengue and Zika viruses subvert reticulophagy by NS2B3-mediated cleavage of FAM134B. Autophagy 2017, 13, 322–332. [Google Scholar] [CrossRef]
- Limonta, D.; Cap, V.; Torres, G.; Ana, B.P.; Guzm, G. Apoptosis in tissues from fatal dengue shock syndrome. J. Virol. 2007, 40, 50–54. [Google Scholar] [CrossRef]
- Chen, H.; Chen, C.; Lin, Y.; Lin, C.; Chen, C.; Chang, C. AR-12 suppresses dengue virus replication by down-regulation of PI3K/AKT and GRP78. Antiviral Res. 2017, 142, 158–168. [Google Scholar] [CrossRef]
- Ding, X.; Zhao, F.; Liu, Z.; Yao, J.; Yu, H.; Zhang, X. Biomedicine & Pharmacotherapy Original antigenic sin: A potential double-edged effect for vaccine improvement. Biomed. Pharmacother. 2024, 178, 117187. [Google Scholar] [CrossRef]
- Id, A.T.; Tan, H.D.; Loy, T.; Chia, P.Y.; Lin, C.; Chua, L. Understanding antibody-dependent enhancement in dengue: Are afucosylated IgG1s a concern ? PLoS Pathog. 2023, 19, e1011223. [Google Scholar] [CrossRef]
- Thomas, S.; Smatti, M.K.; Ouhtit, A.; Cyprian, F.S. Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID-19. The COVID-19 resource centre is hosted on Elsevier Connect, the company’ s public news and information. J. Electrocardiol. 2020, 59, 64. [Google Scholar]
- Watanabe, S.; Chan, K.W.K.; Wang, J.; Rivino, L.; Lok, S.M.; Vasudevan, S.G. Dengue Virus Infection with Highly Neutralizing Levels of Cross- Reactive Antibodies Causes Acute Lethal Small Intestinal Pathology without a High Level of Viremia in Mice. J. Virol. 2015, 89, 5847–5861. [Google Scholar] [CrossRef] [PubMed]
- Yauch, L.E.; Zellweger, R.M.; Kotturi, M.F.; Qutubuddin, A.; Sidney, J.; Peters, B.; Prestwood, T.R.; Sette, A.; Shresta, S. A Protective Role for Dengue Virus-Specific CD8+ T Cells. J. Immunol. 2010, 182, 4865–4873. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.P.S.; John, A.L.S. Immune responses to dengue virus in the skin. Open Biol. 2018, 88, 180087. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, M.; Morita, E. Flavivirus Replication Organelle Biogenesis in the Endoplasmic Reticulum: Comparison with Other Single-Stranded Positive-Sense RNA Viruses. Int. J. Mol. Sci. 2019, 20, 2336. [Google Scholar] [CrossRef]
- Kulkarni, R. Antibody-Dependent Enhancement of Viral Infections; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Avirutnan, J.P.A.P.; Hauhart, R.E.; Somnuke, P.; Blom, A.M.; Diamond, M.S. Binding of Flavivirus Non-structural Protein NS1 to C4b Binding Protein Modulates Complement Activation. J. Immunol. 2012, 187, 424–433. [Google Scholar] [CrossRef]
- De Medeiros, L.N.; Menezes, L.; Barbosa, S.; Mohana-borges, R. Inhibition of the Membrane Attack Complex by Dengue Virus NS1 through Interaction with Vitronectin and Terminal Complement Proteins. J. Virol. 2016, 90, 9570–9581. [Google Scholar] [CrossRef]
- Lee, M.F.; Voon, G.Z.; Lim, H.X. Innate and adaptive immune evasion by dengue virus. Front. Cell. Infect. Microbiol. 2022, 12, 1004608. [Google Scholar] [CrossRef]
- Worte, I.; van der Meer, L.T.; Kilberg, M.S.; van Leeuwen, F.N. Antibody Avidity Following Secondary Dengue Virus Type 2 Infection Across a Range of Disease Severity. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef]
- Nguyen, T.; Chau, B.; Hieu, N.T.; Anders, K.L.; Wolbers, M. Dengue Virus Infections and Maternal Antibody Decay in a Prospective Birth Cohort Study of Vietnamese Infants. J. Infect. Dis. 2015, 200, 1893–1900. [Google Scholar] [CrossRef]
- Hieu, T.; Quyen, N.T.H.; Thuy, T.T.; Tuan, N.M.; Hoang, D.M.; Dung, N.T.P.; Lien, L.B.; Quy, N.T.; Hieu, N.T.; Minh Hieu, L.T.; et al. Dengue in Vietnamese Infants—Results of Infection- Enhancement Assays Correlate with Age-Related Disease Epidemiology, and Cellular Immune Responses Correlate with Disease Severity. J. Infect. Dis. 2008, 198, 516–524. [Google Scholar] [CrossRef]
- Castanha, P.M.S.; Braga, C.; Cordeiro, M.T.; Souza, A.I.; Silva, C.D., Jr.; Martelli, C.M.; van Panhuis, W.A.; Nascimento, E.J.M.; Marques, E.T.A. Placental Transfer of Dengue Virus (DENV)-Specific Antibodies and Kinetics of DENV Infection-Enhancing Activity in Brazilian Infants. J. Infect. Dis. 2016, 214, 265–272. [Google Scholar] [CrossRef]
- Balsitis, S.J.; Williams, K.L.; Lachica, R.; Flores, D.; Kyle, J.L.; Mehlhop, E.; Johnson, S.; Diamond, M.S.; Beatty, P.R.; Harris, E. Lethal antibody enhancement of dengue disease in mice is prevented by Fc modification. PLoS Pathog. 2010, 6, e1000790. [Google Scholar] [CrossRef]
- Luo, Y.; Feng, J.J.; Zhou, J.M.; Yu, Z.Z.; Fang, D.Y.; Yan, H.J.; Zeng, G.C.; Jiang, L.F. Identification of a novel infection-enhancing epitope on dengue prM using a dengue cross-reacting monoclonal antibody. BMC Microbiol. 2013, 13, 194. [Google Scholar] [CrossRef]
- Kübler, L.; Bittmann, I.; Kuipers, J.G. Macrophage activation syndrome triggered by active systemic lupus erythematosus: Successful treatment by interleukin-1 inhibition (anakinra). Z. Rheumatol. 2020, 79, 1040–1045. [Google Scholar] [CrossRef]
- Yeh, T.; Chiu, S.C.; Hsiao, Y.L.; Wan, S.W.; Lei, H.Y.; Shiau, A.L.; Liu, H.S.; Yeh, T.M.; Chen, S.H.; Liu, C.C.; et al. Expression of Cytokine, Chemokine, and Adhesion Molecules during Endothelial Cell Activation Induced by Antibodies against Dengue Virus Nonstructural Protein 1. J. Immunol. 2005, 174, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Thi, N.; Lan, P.; Hirayama, K. Host genetic susceptibility to severe dengue infection. Trop. Med. Health 2011, 39, 73–81. [Google Scholar] [CrossRef]
- Ong, S.P.; Jang, J.; Chu, H. An Update on the Host Factors Contributing to Vascular Leakage During Dengue Virus Infection. Future Virol. 2011, 6, 135–138. [Google Scholar] [CrossRef]
- De Vos, T.W.; Porcelijn, L.; Hofstede-Van Egmond, S.; Pajkrt, E.; Oepkes, D.; Lopriore, E.; van der Schoot, C.E.; Winkelhorst, D.; de Haas, M. Clinical characteristics of human platelet antigen (HPA)-1a and HPA-5b alloimmunised pregnancies and the association between platelet HPA-5b antibodies and symptomatic fetal neonatal alloimmune thrombocytopenia. Br. J. Haematol. 2021, 195, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Elshazli, R.; Settin, A.; Salama, A. Cytotoxic T lymphocyte associated antigen-4 (CTLA-4) +49 A>G gene polymorphism in Egyptian cases with rheumatoid arthritis. Gene 2015, 558, 103–107. [Google Scholar] [CrossRef]
- Bhatt, P.; Pillai, S.; Muralidhar, S.; Govindakarnavar, V. Current Understanding of the Pathogenesis of Dengue Virus Infection. Curr. Microbiol. 2021, 78, 17–32. [Google Scholar] [CrossRef]
- Coffey, L.L.; Mertens, E.; Brehin, A.; Fernandez-garcia, M.D.; Amara, A.; Despre, P. Human genetic determinants of dengue virus susceptibility. Microbes Infect. 2009, 11, 143–156. [Google Scholar] [CrossRef]
- World Health Organization. Dengue Guidelines for Diagnosis, Treatment, Prevention and Control; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Trung, D.T.; Thao, L.T.T.; Hien, T.T.; Hung, N.T.; Vinh, N.N.; Hien, P.T.D.; Chinh, N.T.; Simmons, C.; Wills, B. Liver Involvement Associated with Dengue Infection in Adults in Vietnam. Am. J. Trop. Med. Hyg. 2010, 83, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Kala, M.P. Dengue: Update on Clinically Relevant Therapeutic Strategies and Vaccines. Curr. Treat. Options Infect. Dis. 2023, 15, 27–52. [Google Scholar] [CrossRef]
- Rosales-Rosas, A.L.; Goossens, S.; Chiu, W.; Majumder, A.; Soto, A.; Masyn, S.; Stoops, B.; Wang, L.; Kaptein, S.J.F.; Goethals, O.; et al. The antiviral JNJ-A07 significantly reduces dengue virus transmission by Aedes aegypti mosquitoes when delivered via blood-feeding. Sci. Adv. 2024, 10, eadr8338. [Google Scholar] [CrossRef] [PubMed]
- Fredeking, T.; Zavala-Castro, J.; Gonzalez-Martinez, P.; Moguel-Rodríguez, W.; Sanchez, E.; Foster, M.; Diaz-Quijano, F. Dengue Patients Treated with Doxycycline Showed Lower Mortality Associated to a Reduction in IL-6 and TNF Levels. Recent Pat. Anti-Infect. Drug Discov. 2015, 10, 51–58. [Google Scholar] [CrossRef]
- Freund, B.; Gravenstein, S.; Elliott, M.; Miller, I. Zanamivir A Review of Clinical Safety. Drug Saf. 1999, 21, 267–281. [Google Scholar] [CrossRef]
- McArthur, M.A.; Sztein, M.B.; Edelman, R. Dengue vaccines: Recent developments, ongoing challenges and current candidates. Expert Rev. Vaccines 2013, 12, 933–953. [Google Scholar] [CrossRef]
- Hou, J.; Ye, W.; Chen, J. Current Development and Challenges of Tetravalent Live-Attenuated Dengue Vaccines. Front. Immunol. 2022, 13, 840104. [Google Scholar] [CrossRef]
- Torres, J.M.; Arturo, F.; Sandoval, R.; Isabel, M. Dengue Vaccines: An Update. BioDrugs 2022, 36, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, S.S. Development of TV003/TV005, a single dose, highly immunogenic live attenuated dengue vaccine; what makes this vaccine different from the Sanofi-Pasteur CYDTM vaccine? Expert Rev. Vaccines 2017, 15, 509–517. [Google Scholar] [CrossRef]
- Hung, N.T. Fluid management for dengue in children. Ann. Trop. Paediatr. 2012, 32, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Bur, R.; Suwarto, S.; Pohan, H.T.; Prihartono, J.; Harahap, A.R.; Dewi, B.E.; Sadikin, M.; Rachman, A.; Yusuf, H. Early intervention of 5% albumin shown superior control of vascular integrity and function compared to ringer’s lactatein hospitalized adult with grade I & II Dengue hemorrhagic fever: A multicenter randomized controlled trial in Indonesia. Trop. Dis. Travel Med. Vaccines 2024, 10, 20. [Google Scholar] [CrossRef]
- Hanley, J.P.; Tu, H.A.; Dragon, J.A.; Dickson, D.M.; del Rio-Guerra, R.; Tighe, S.W.; Eckstrom, K.M.; Selig, N.; Scarpino, S.V.; Whitehead, S.S.; et al. Immunotranscriptomic profiling the acute and clearance phases of a human challenge dengue virus serotype 2 infection model. Nat. Commun. 2021, 12, 3054. [Google Scholar] [CrossRef]
- Nhi, D.M.; Huy, N.T.; Ohyama, K.; Kimura, D.; Lan, N.T.P.; Uchida, L.; Van Thuong, N.; Nhon, C.T.M.; Phuc, L.H.; Mai, N.T.; et al. A Proteomic Approach Identifies Candidate Early Biomarkers to Predict Severe Dengue in children. PLoS Neglected Trop. Dis. 2016, 10, e0004435. [Google Scholar] [CrossRef]
- Han, L.; Ao, X.; Lin, S.; Guan, S.; Zheng, L.; Han, X.; Ye, H. Quantitative Comparative Proteomics Reveal Biomarkers for Dengue Disease Severity. Front. Microbiol. 2019, 10, 2836. [Google Scholar] [CrossRef]
- Yadav, A.; Shamim, U.; Ravi, V.; Devi, P.; Kumari, P.; Maurya, R.; Das, P.; Somani, M.; Budhiraja, S.; Tarai, B.; et al. Early transcriptomic host response signatures in the serum of dengue patients provides insights into clinical pathogenesis and disease severity. Sci. Rep. 2023, 13, 14170. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Yan, Q.; Li, Z.; Wang, X.; Wu, P.; Liao, F.; Lao, Z.; Jiang, Y.; Liu, X.; Zhan, S.; et al. Liver transcriptomics reveals features of the host response in a mouse model of dengue virus infection. Front. Immunol. 2022, 13, 892469. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, A.; Mondal, S.; Sadhukhan, S.; Sadhukhan, P.C. Dengue Virus and the Host Immune System: A Battle of Immune Modulation, Response and Evasion. Pathogens 2025, 14, 1132. https://doi.org/10.3390/pathogens14111132
Ghosh A, Mondal S, Sadhukhan S, Sadhukhan PC. Dengue Virus and the Host Immune System: A Battle of Immune Modulation, Response and Evasion. Pathogens. 2025; 14(11):1132. https://doi.org/10.3390/pathogens14111132
Chicago/Turabian StyleGhosh, Anwesha, Sudipta Mondal, Soumyodip Sadhukhan, and Provash Chandra Sadhukhan. 2025. "Dengue Virus and the Host Immune System: A Battle of Immune Modulation, Response and Evasion" Pathogens 14, no. 11: 1132. https://doi.org/10.3390/pathogens14111132
APA StyleGhosh, A., Mondal, S., Sadhukhan, S., & Sadhukhan, P. C. (2025). Dengue Virus and the Host Immune System: A Battle of Immune Modulation, Response and Evasion. Pathogens, 14(11), 1132. https://doi.org/10.3390/pathogens14111132






