Staphylococcus aureus Mastitis: A Time-Course Transcriptome of Immune Activation in Small-Tailed Han Sheep
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Growth Conditions
2.2. Establishment of a Model of Mastitis Induced by S. aureus in STHS
2.3. Clinical Symptom Monitoring and Sample Collection
2.4. Histopathological Examination
2.5. RNA Extraction and RNA Sequencing
2.6. Summary of the RNA-Seq Data and Bioinformatics Analysis
2.7. Differential Expression Analysis
2.8. GO and KEGG Enrichment Analysis
2.9. PPI Analysis of DEGs and Clustering Analysis of DEGs
2.10. RT-qPCR
2.11. Statistical Analysis
2.12. Ethics Statement
3. Results
3.1. Clinical Signs and Symptoms
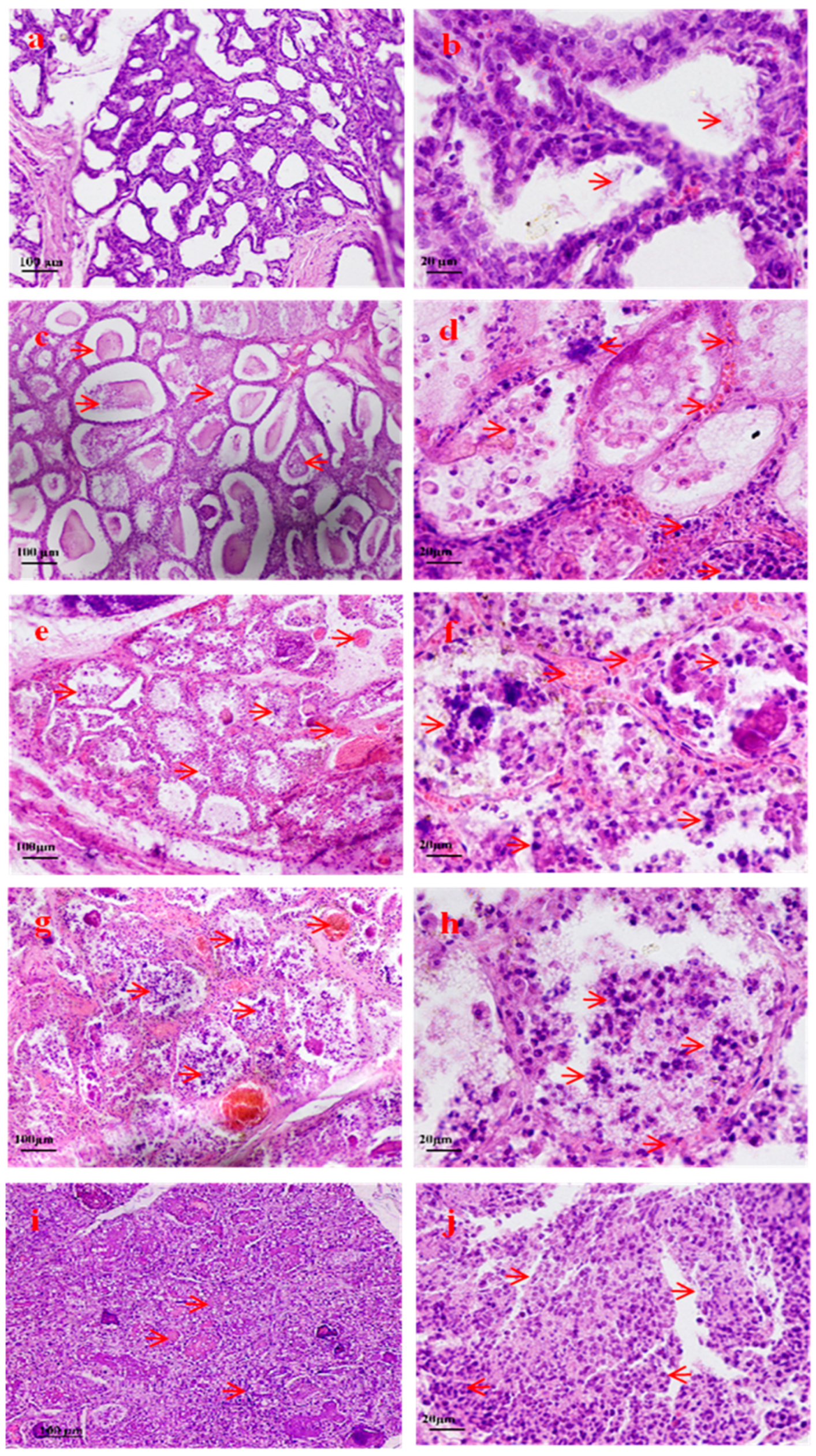
3.2. Morphologic Changes in Breast Tissue After S. aureus Infection
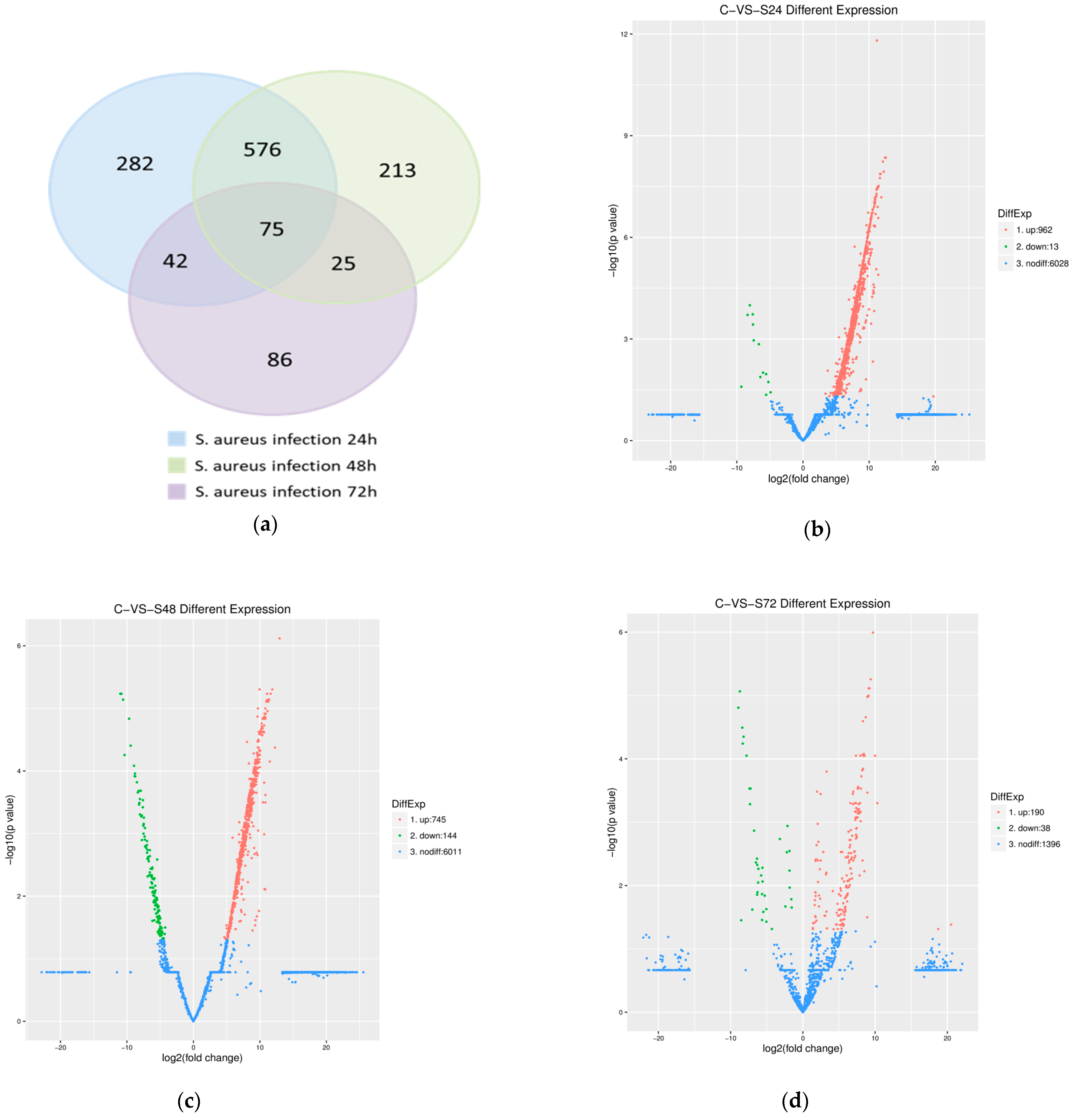
3.3. Identification of DEGs
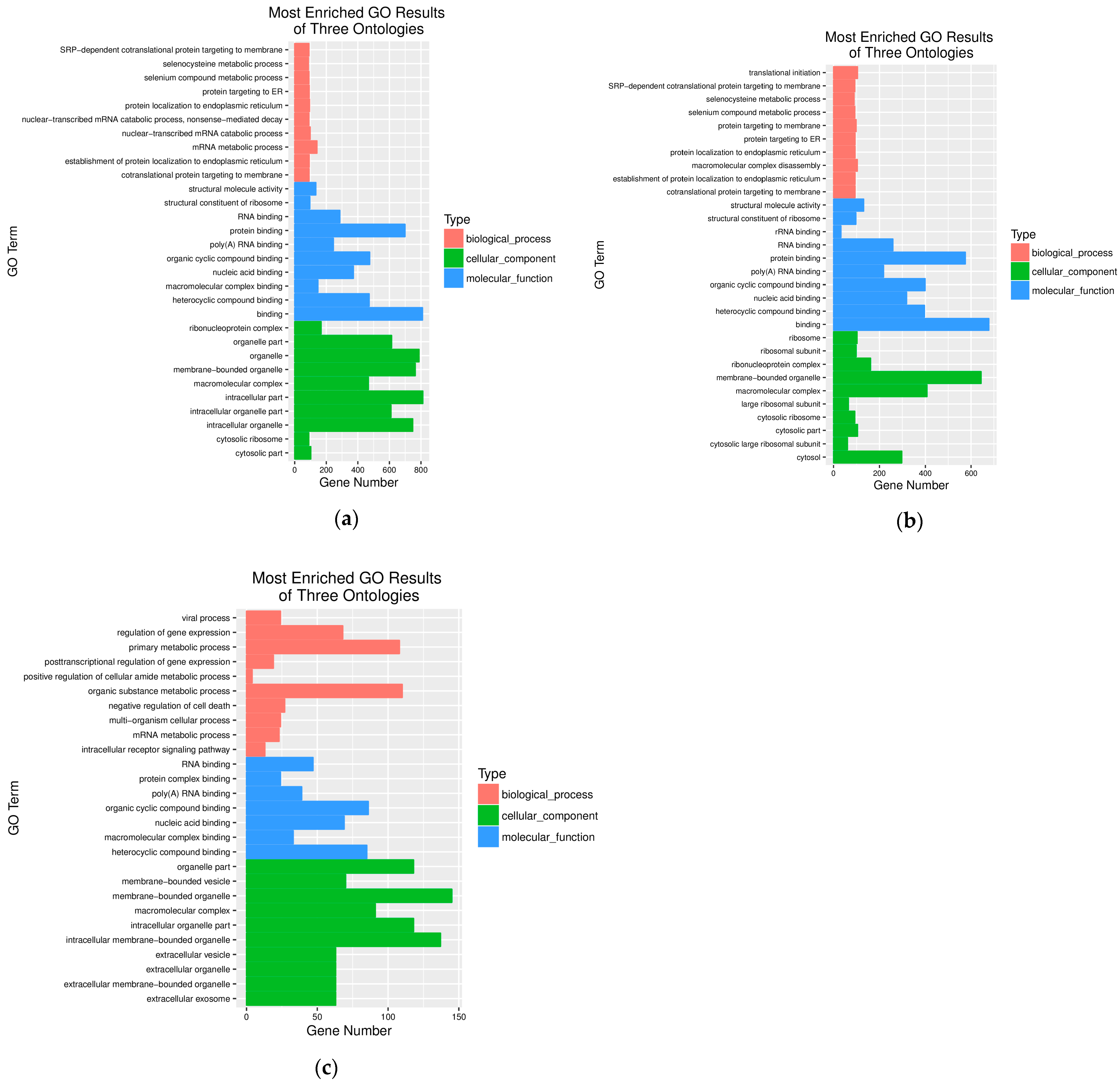
3.4. Gene Ontology (GO) Enrichment Analysis of the DEGs
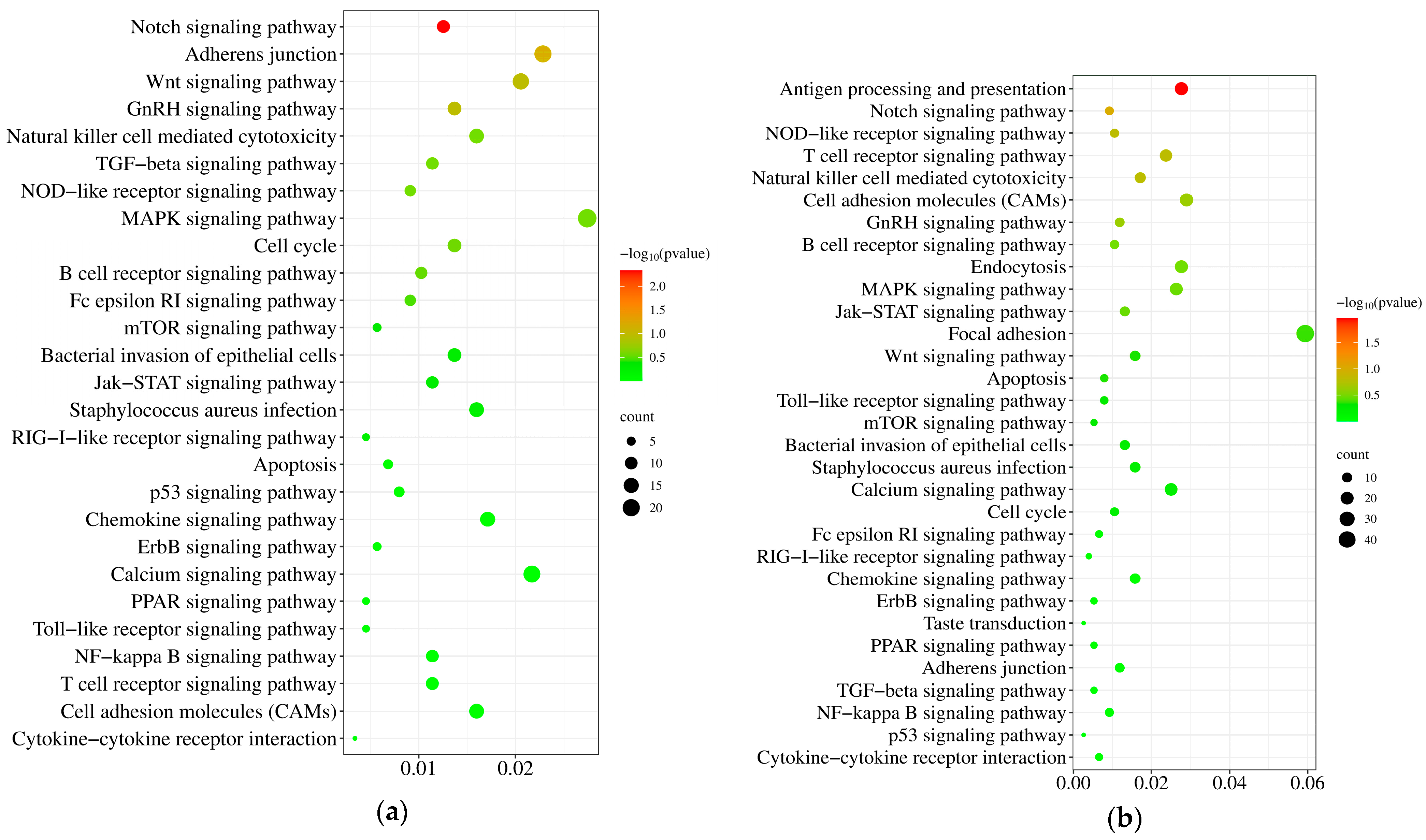
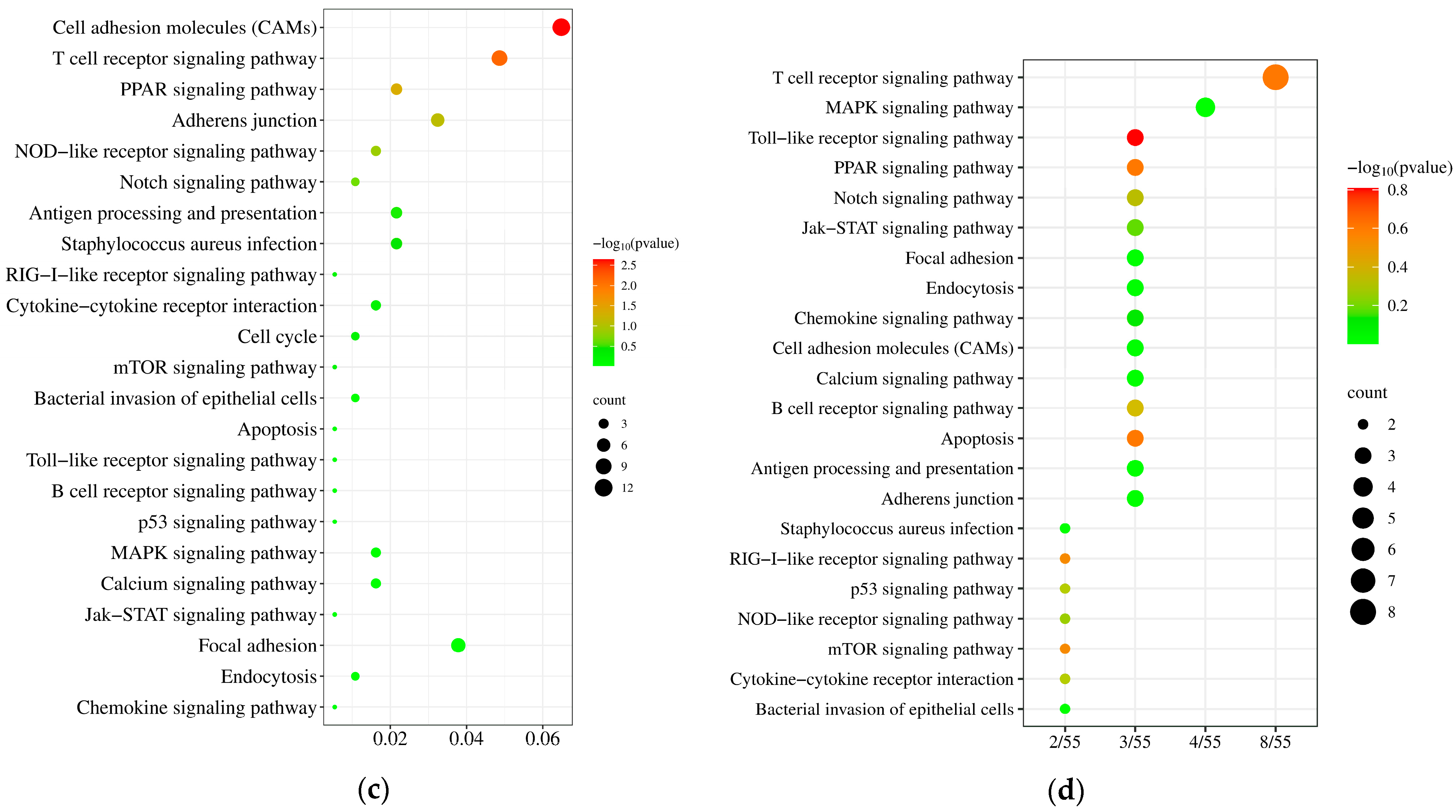
3.5. KEGG Pathway Analysis of the DEGs
3.6. Validation of RNA-Seq Results Using an RT-qPCR Approach
3.7. Detection of Expression of Inflammatory Factors at Different Stages of Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gelasakis, A.I.; Mavrogianni, V.S.; Petridis, I.G.; Vasileiou, N.G.; Fthenakis, G.C. Mastitis in sheep—The last 10 years and the future of research. Vet. Microbiol. 2015, 181, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Mørk, T.; Waage, S.; Tollersrud, T.; Kvitle, B.; Sviland, S. Clinical Mastitis in Ewes; Bacteriology, Epidemiology and Clinical Features. Acta Vet. Scand. 2007, 49, 23. [Google Scholar] [CrossRef]
- Tadee, P.; Pattanawong, W.; Manwicha, A.; Khaodang, P.; Amornlerdpison, D.; Chansakaow, S.; Tipduangta, P.; Chukiatsiri, K.; Tadee, P. Bacterial Pathogens of Bovine Mastitis: Prevalence, Antimicrobial Susceptibility, and Sensitivity to Caesalpinia sappan Both In Vitro and In Vivo Studies. Biology 2025, 14, 350. [Google Scholar] [CrossRef] [PubMed]
- Kerro Dego, O.; van Dijk, J.E.; Nederbragt, H. Factors involved in the early pathogenesis of bovine Staphylococcus aureus mastitis with emphasis on bacterial adhesion and invasion. A review. Vet. Q. 2002, 24, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Hermann, C.; Spreitzer, I.; Schröder, N.W.; Morath, S.; Lehner, M.D.; Fischer, W.; Schütt, C.; Schumann, R.R.; Hartung, T. Cytokine induction by purified lipoteichoic acids from various bacterial species—Role of LBP, sCD14, CD14 and failure to induce IL-12 and subsequent IFN-gamma release. Eur. J. Immunol. 2002, 32, 541–551. [Google Scholar] [CrossRef]
- Bannerman, D.D.; Paape, M.J.; Lee, J.W.; Zhao, X.; Hope, J.C.; Rainard, P. Escherichia coli and Staphylococcus aureus elicit differential innate immune responses following intramammary infection. Clin. Diagn. Lab. Immunol. 2004, 11, 463–472. [Google Scholar] [CrossRef]
- Martins, K.B.; Faccioli-Martins, P.Y.; Riboli, D.F.; Pereira, V.C.; Fernandes, S.; Oliveira, A.A.; Dantas, A.; Zafalon, L.F.; da Cunha, M.d.L. Clonal profile, virulence and resistance of Staphylococcus aureus isolated from sheep milk. Braz. J. Microbiol. 2015, 46, 535–543. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, H.; Hickford, J.G.H.; Hao, Z.; Shen, J.; Luo, Y.; Hu, J.; Liu, X.; Li, S. Comparison of the Transcriptome of the Ovine Mammary Gland in Lactating and Non-lactating Small-Tailed Han Sheep. Front. Genet. 2020, 11, 472. [Google Scholar] [CrossRef]
- Omoshaba, E.O.; Ojo, O.E.; Oyekunle, M.A.; Sonibare, A.O.; Adebayo, A.O. Methicillin-resistant Staphylococcus aureus (MRSA) isolated from raw milk and nasal swabs of small ruminants in Abeokuta, Nigeria. Trop. Anim. Health Prod. 2020, 52, 2599–2608. [Google Scholar] [CrossRef]
- Ntuli, V.; Sibanda, T.; Elegbeleye, A.; Mugadza, T.; Seifu, E.; Buys, M. Dairy production: Microbial safety of raw milk and processed milk products. In Present Knowledge in Food Safety; Academic Press: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Godden, S.M.; Royster, E.; Timmerman, J.; Rapnicki, P.; Green, H. Evaluation of an automated milk leukocyte differential test and the California Mastitis Test for detecting intramammary infection in early- and late-lactation quarters and cows. J. Dairy Sci. 2017, 100, 6527–6544. [Google Scholar] [CrossRef]
- Buckley, M.P.; Hayman, K.P. Mastitis Therapy in Small Ruminants. Vet. Clin. N. Am. Food Anim. Pract. 2025, 1, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Sezzi, E.; Fanelli, R.; Gobbi, D.; Scandurra, P.; Mannucci, V.; Usai, I.; Ragionieri, G.; Mezher, Z.; Fichi, G.l. An Investigation of Virulence Genes of Staphylococcus aureus in Autologous Vaccines Against Sheep Mastitis. Animals 2024, 14, 3172. [Google Scholar] [CrossRef] [PubMed]
- Alalaf, A.S.; Taha, A.H.; Sheet, O.H. Genotypic characteristics and phylogenetic tree analysis of Staphylococcus aureus isolates from sheep subclinical mastitis milk. Open Vet. J. 2025, 15, 289–299. [Google Scholar] [CrossRef]
- Wang, M.; Bissonnette, N.; Laterrière, M.; Gagné, D.; Dudemaine, P.L.; Roy, J.P.; Sirard, M.A.; Ibeagha-Awemu, E.M. Genome-Wide DNA Methylation and Transcriptome Integration Associates DNA Methylation Changes with Bovine Subclinical Mastitis Caused by Staphylococcus chromogenes. Int. J. Mol. Sci. 2023, 24, 10369. [Google Scholar] [CrossRef]
- Chen, Q.; Mi, S.; Xing, Y.; An, S.; Chen, S.; Tang, Y.; Wang, Y.; Yu, Y. Transcriptome analysis identifies the NR4A subfamily involved in the alleviating effect of folic acid on mastitis induced by high concentration of Staphylococcus aureus lipoteichoic acid. BMC Genom. 2024, 25, 1051. [Google Scholar] [CrossRef]
- Pelayo, R.; Gutiérrez-Gil, B.; Marina, H.; Fonseca, P.A.S.; Alonso-García, M.; Arranz, J.J.; Suárez-Vega, A. Unraveling dynamic transcriptomic changes in sheep’s lactating mammary gland following Esch. erichia coli lipopolysaccharide exposure. J. Dairy Sci. 2024, 107, 11269–11282. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Wang, M.L.; Cai, M.; Nan, X.M.; Zhao, Y.G.; Xiong, B.H.; Yang, L. Protein Expression Profiles in Exosomes of Bovine Mammary Epithelial Cell Line MAC-T Infected with Staphylococcus aureus. Appl. Environ. Microbiol. 2023, 89, e0174322. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Yang, Y.; Ma, X.; Wei, L.; Wan, X.; Zhang, Z.; Ding, J.; Peng, J.; Liu, G.; Gou, H.; et al. Effect of Two Different Drug-Resistant Staphylococcus aureus Strains on the Physiological Proper ties of MAC-T Cells and Their Transcriptome Analysis. Front. Vet. Sci. 2022, 9, 818928. [Google Scholar] [CrossRef]
- Pisoni, G.; Moroni, P.; Genini, S.; Stella, A.; Boettcher, P.J.; Cremonesi, P.; Scaccabarozzi, L.; Giuffra, E.; Castiglioni, B. Differentially expressed genes associated with Staphylococcus aureus mastitis in dairy goats. Vet. Immunol. Immunopathol. 2010, 135, 208–217. [Google Scholar] [CrossRef]
- Goldstein, L.D.; Cao, Y.; Pau, G.; Lawrence, M.; Wu, T.D.; Seshagiri, S.; Gentleman, R. Prediction and Quantification of Splice Events from RNA-Seq Data. PLoS ONE 2016, 11, e0156132. [Google Scholar] [CrossRef]
- Parkhomchuk, D.; Borodina, T.; Amstislavskiy, V.; Banaru, M.; Hallen, L.; Krobitsch, S.; Lehrach, H.; Soldatov, A. Transcriptome analysis by strand-specific sequencing of complementary DNA. Nucleic Acids Res. 2009, 37, e123. [Google Scholar] [CrossRef] [PubMed]
- Kotzamanidis, C.; Vafeas, G.; Giantzi, V.; Anastasiadou, S.; Mygdalias, S.; Malousi, A.; Loukia, E.; Daniel, S.; Zdragas, A. Staphylococcus aureus Isolated from Ruminants with Mastitis in Northern Greece Dairy Herds: Genetic Relatedness and Phenotypic and Genotypic Characterization. Toxins 2021, 13, 176. [Google Scholar] [CrossRef] [PubMed]
- Abril, A.G.; Villa, T.; Barros-Velázquez, J.; Cañas, B.; Sánchez-Pérez, A.; Calo-Mata, P.; Carrera, M. Staphylococcus aureus Exotoxins and Their Detection in the Dairy Industry and Mastitis. Toxins 2020, 12, 537. [Google Scholar] [CrossRef] [PubMed]
- Rahmatalla, S.A.; Arends, D.; Said Ahmed, A.; Hassan, L.M.A.; Krebs, S.; Reissmann, M.; Brockmann, G.A. Capture Sequencing to Explore and Map Rare Casein Variants in Goats. Front Genet. 2021, 12, 620253. [Google Scholar] [CrossRef]
- Crisà, A.; Ferrè, F.; Chillemi, G.; Moioli, B. RNA-Sequencing for profiling goat milk transcriptome in colostrum and mature milk. BMC Vet. Res. 2016, 12, 264. [Google Scholar] [CrossRef][Green Version]
- Jatav, P.; Sodhi, M.; Sharma, A.; Mann, S.; Kishore, A.; Shandilya, U.K.; Mohanty, A.K.; Kataria, R.S.; Yadav, P.; Verma, P.; et al. Identification of internal control genes in milk-derived mammary epithelial cells during lactation cycle of Indian zebu cow. Anim. Sci. J. 2016, 87, 344–353. [Google Scholar] [CrossRef]
- Chang, D.W.; Xing, Z.; Capacio, V.L.; Peter, M.E.; Yang, X. Interdimer processing mechanism of procaspase-8 activation. EMBO J. 2003, 22, 4132–4142. [Google Scholar] [CrossRef]
- Pang, J.; Vince, J.E. The role of caspase-8 in inflammatory signalling and pyroptotic cell death. Semin. Immunol. 2023, 70, 101832. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, C.; Liao, Y.; Zhou, M.; Xu, W.; Zou, Z. Caspase-8 in inflammatory diseases: A potential therapeutic target. Cell Mol. Biol. Lett. 2024, 29, 130. [Google Scholar] [CrossRef]
- Vince, J.E.; De Nardo, D.; Gao, W.; Vince, A.J.; Hall, C.; McArthur, K.; Simpson, D.; Vijayaraj, S.; Lindqvist, L.M.; Bouillet, P.; et al. The Mitochondrial Apoptotic Effectors BAX/BAK Activate Caspase-3 and -7 to Trigger NLRP3 Inflammasome and Caspase-8 Driven IL-1β Activation. Cell Rep. 2018, 25, 2339–2353. [Google Scholar] [CrossRef]
- Faulconnier, Y.; Boby, C.; Coulpier, F.; Lemoine, S.; Martin, P.; Leroux, C. Transcriptome analysis of goat (Capra hircus) adipose tissue reveals physiological regulation of body reserve recovery after the peak of lactation. Comp. Biochem. Physiol. Part. D Genom. Proteom. 2022, 41, 100956. [Google Scholar] [CrossRef] [PubMed]
- Saleem, A.; Mumtaz, P.T.; Saleem, S.; Manzoor, T.; Taban, Q.; Dar, M.A.; Bhat, B.; Ahmad, S.M. Comparative transcriptome analysis of E. coli & Staphylococcus aureus infected goat mammary epithelial cells reveals genes associated with infection. Int. Immunopharmacol. 2024, 126, 111213. [Google Scholar] [CrossRef] [PubMed]
- Darang, E.; Pezeshkian, Z.; Mirhoseini, S.Z.; Ghovvati, S. Identification of Key Genes and Potential Pathways Associated with Mastitis Induced by E. coli. Biochem. Genet. 2023, 61, 202–220. [Google Scholar] [CrossRef]
- Suárez-Vega, A.; Gutiérrez-Gil, B.; Arranz, J.J. Transcriptome expression analysis of candidate milk genes affecting cheese-related traits in 2 sheep breeds. J. Dairy Sci. 2016, 99, 6381–6390. [Google Scholar] [CrossRef]
- Dai, W.T.; Zou, Y.X.; White, R.R.; Liu, J.X.; Liu, H.Y. Transcriptomic profiles of the bovine mammary gland during lactation and the dry period. Funct. Integr. Genom. 2018, 18, 125–140. [Google Scholar] [CrossRef]
- Jiang, K.F.; Zhao, G.; Deng, G.Z.; Wu, H.C.; Yin, N.N.; Chen, X.Y.; Qiu, C.W.; Peng, X.L. Polydatin ameliorates Staphylococcus aureus-induced mastitis in mice via inhibiting TLR2-mediated activation of the p38 MAPK/NF-κB pathway. Acta Pharmacol. Sin. 2017, 38, 211–222. [Google Scholar] [CrossRef]
- Hu, C.; Xie, J.; Fei, X.; Sun, Y.; Lei, S.; Yan, X.; Ran, C. Overexpressed RPS6KA1 and its potential diagnostic value in head and neck squamous cell carcinoma. Discov. Oncol. 2025, 16, 60. [Google Scholar] [CrossRef]
- He, S.; Wang, X.; Liu, Z.; Zhang, W.; Fang, J.; Xue, J.; Bao, H. Hydroxysafflor Yellow A Inhibits Staphylococcus aureus-Induced Mouse Endometrial Inflammation via TLR2-Mediated NF-kB and MAPK Pathway. Inflammation 2021, 44, 835–845. [Google Scholar] [CrossRef]
- Vasselon, T.; Hanlon, W.A.; Wright, S.D.; Detmers, P.A. Toll-like receptor 2 (TLR2) mediates activation of stress-activated MAP kinase p38. J. Leukoc. Biol. 2002, 71, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, F.; Li, L.; Yan, L.; Badri, T.; Lv, C.; Yu, D.; Zhang, M.; Jang, X.; Li, J. Three Novel Players: PTK2B, SYK, and TNFRSF21 Were Identified to Be Involved in the Regulation of Bovine Mastitis Susceptibility via GWAS and Post-transcriptional Analysis. Front. Immunol. 2019, 10, 1579. [Google Scholar] [CrossRef]
- Lu, B.L.; Williams, G.M.; Brimble, M.A. TLR2 agonists and their structure-activity relationships. Org. Biomol. Chem. 2020, 18, 5073–5094. [Google Scholar] [CrossRef]
- Fan, L.; Zhou, P.; Hong, Q.; Chen, A.X.; Liu, G.Y.; Yu, K.D.; Shao, Z.M. Toll-like receptor 3 acts as a suppressor gene in breast cancer initiation and progression: A two-stage association study and functional investigation. Oncoimmunology 2019, 8, e1593801. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, J.; Zhao, Y.; Ma, X.; Yi, H. Toll-like receptor 3 (TLR3) regulation mechanisms and roles in antiviral innate immune responses. J. Zhejiang Univ. Sci. B 2021, 22, 609–632. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Derbigny, W.A. TLR3 Deficiency Leads to a Dysregulation in the Global Gene-Expression Profile in Murine Oviduct Epithelial Cells Infected with Chlamydia muridarum. Int. J. Microbiol. Curr. Res. 2019, 1, 1–13. [Google Scholar] [CrossRef][Green Version]
- Cavassani, K.A.; Ishii, M.; Wen, H.; Schaller, M.A.; Lincoln, P.M.; Lukacs, N.W.; Hogaboam, C.M.; Kunkel, S.L. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J. Exp. Med. 2008, 205, 2609–2621. [Google Scholar] [CrossRef]
- Zablocki-Thomas, L.; Menzies, S.A.; Lehner, P.J.; Manel, N.; Benaroch, P. A genome-wide CRISPR screen identifies regulation factors of the TLR3 signalling pathway. Innate Immun. 2020, 26, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gong, P.; Zhang, X.; Wang, X.; Zhang, X.; Zhang, N.; Yu, Y.; Ma, Y.; Zhang, H.; Zhang, X. TLR3 activation by Clonorchis sinensis infection alleviates the fluke-induced liver fibrosis. PLoS Negl. Trop. Dis. 2023, 17, e0011325. [Google Scholar] [CrossRef]
- Suresh, M.V.; Dolgachev, V.A.; Zhang, B.; Balijepalli, S.; Swamy, S.; Mooliyil, J.; Kralovich, G.; Thomas, B.; Machado-Aranda, D.; Karmakar, M.; et al. TLR3 absence confers increased survival with improved macrophage activity against pneumonia. JCI Insight 2019, 4, e131195. [Google Scholar] [CrossRef] [PubMed]



| Symptom Rate Time | Activity | Feed and Water Intake | Fever | The Degree of Swelling | The Amount of Milk | The Color of Milk | The Consistency of Milk |
|---|---|---|---|---|---|---|---|
| 24 h | +++ | +++ | ++ | + | +++ | + | + |
| 48 h | ++ | ++ | +++ | ++ | ++ | ++ | ++ |
| 72 h | ++ | ++ | +++ | +++ | + | ++ | ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wang, L.; Chen, W.; Song, X.; Wang, M.; Ma, X.; Yan, L.; Wang, C. Staphylococcus aureus Mastitis: A Time-Course Transcriptome of Immune Activation in Small-Tailed Han Sheep. Pathogens 2025, 14, 1133. https://doi.org/10.3390/pathogens14111133
Zhang X, Wang L, Chen W, Song X, Wang M, Ma X, Yan L, Wang C. Staphylococcus aureus Mastitis: A Time-Course Transcriptome of Immune Activation in Small-Tailed Han Sheep. Pathogens. 2025; 14(11):1133. https://doi.org/10.3390/pathogens14111133
Chicago/Turabian StyleZhang, Xiaoli, Li Wang, Wenzhe Chen, Xiaoyu Song, Meng Wang, Xiaojun Ma, Lijiao Yan, and Chuan Wang. 2025. "Staphylococcus aureus Mastitis: A Time-Course Transcriptome of Immune Activation in Small-Tailed Han Sheep" Pathogens 14, no. 11: 1133. https://doi.org/10.3390/pathogens14111133
APA StyleZhang, X., Wang, L., Chen, W., Song, X., Wang, M., Ma, X., Yan, L., & Wang, C. (2025). Staphylococcus aureus Mastitis: A Time-Course Transcriptome of Immune Activation in Small-Tailed Han Sheep. Pathogens, 14(11), 1133. https://doi.org/10.3390/pathogens14111133






