Development of Immunodetection Systems Using a Specific Antibody Against the Recombinant Coat Protein for Detecting Sugarcane Streak Mosaic Virus
Abstract
1. Introduction
2. Materials and Methods
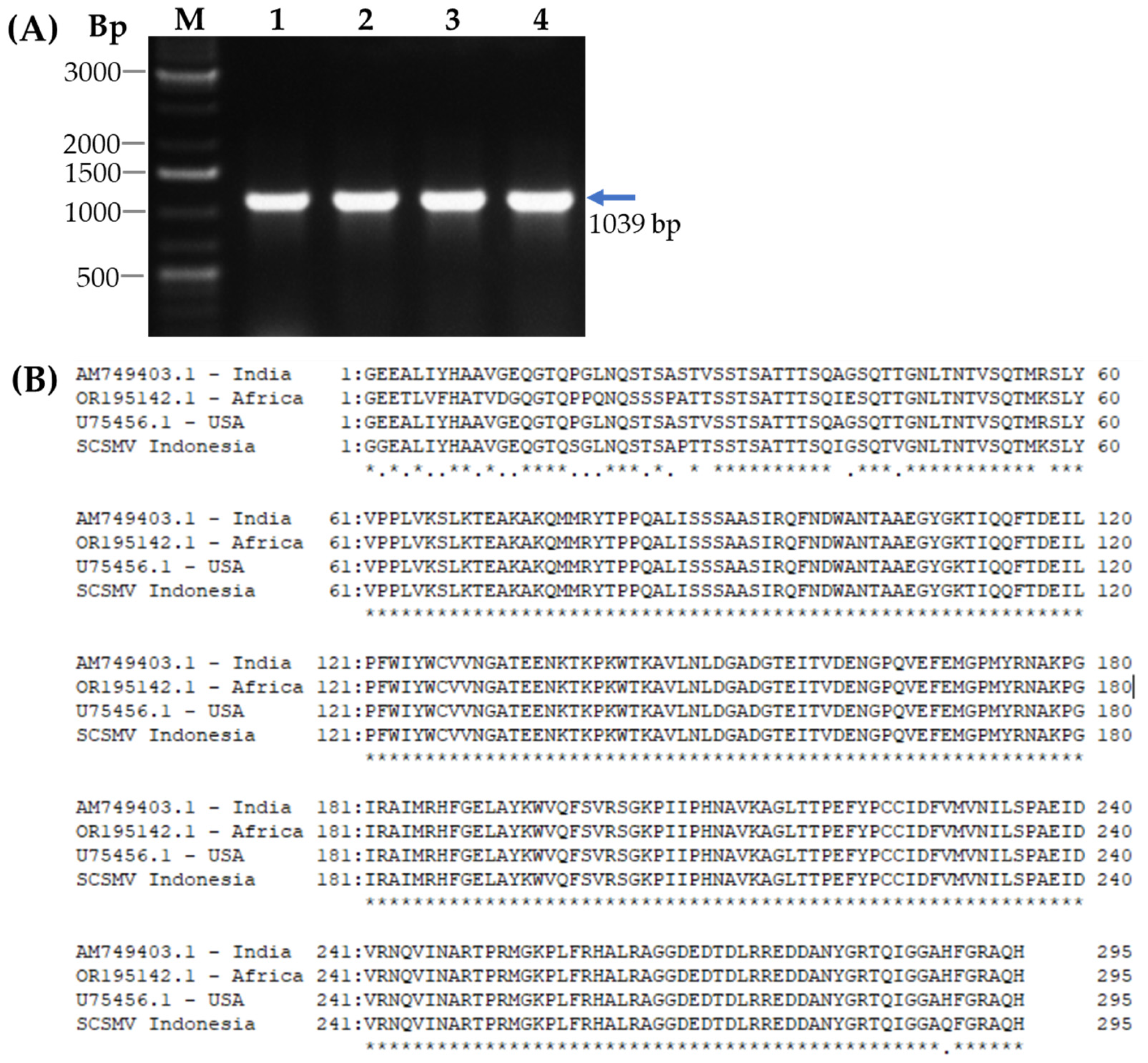
2.1. Cloning and Plasmid Construction for the Recombinant Coat Protein of SCSMV
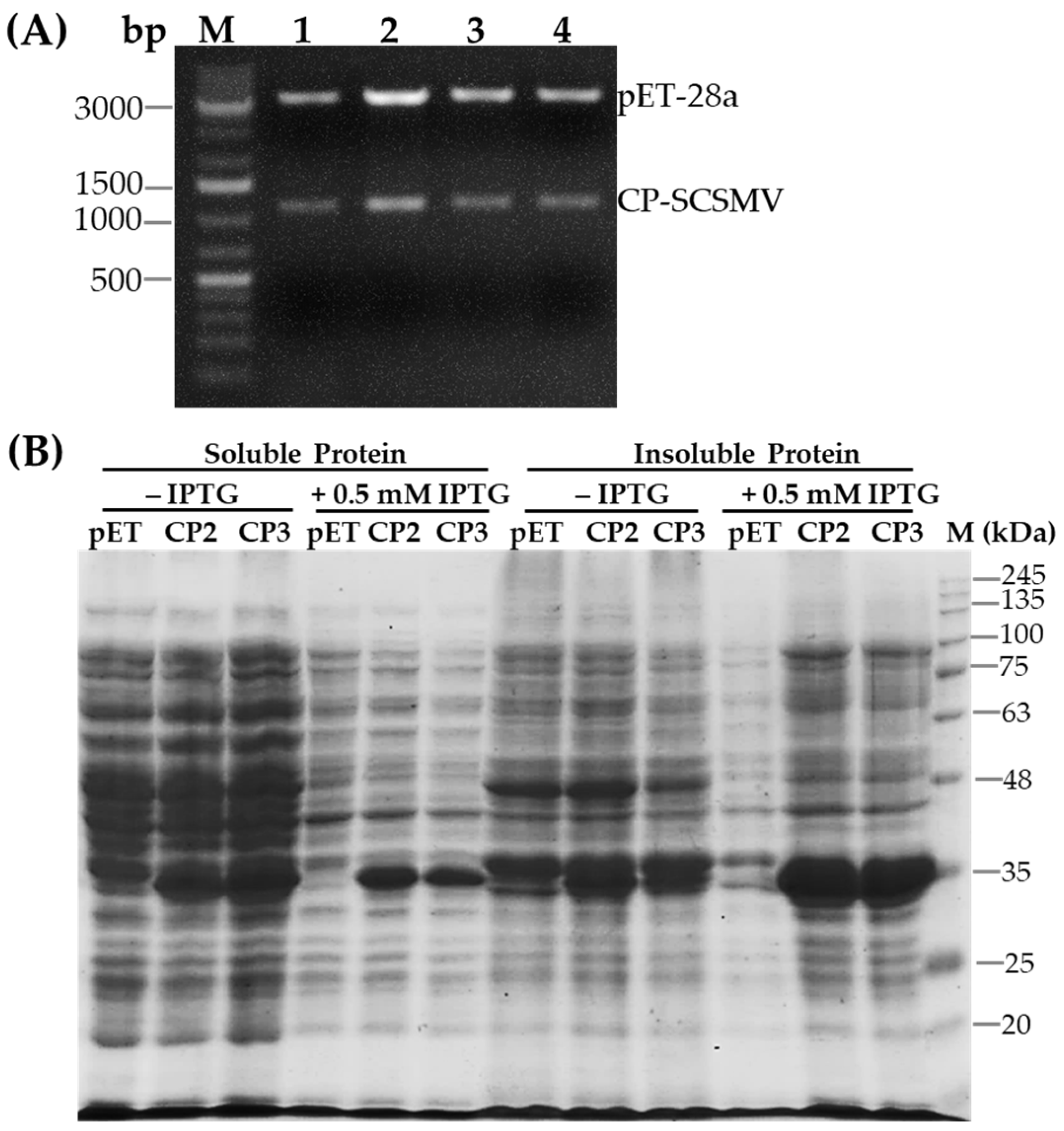
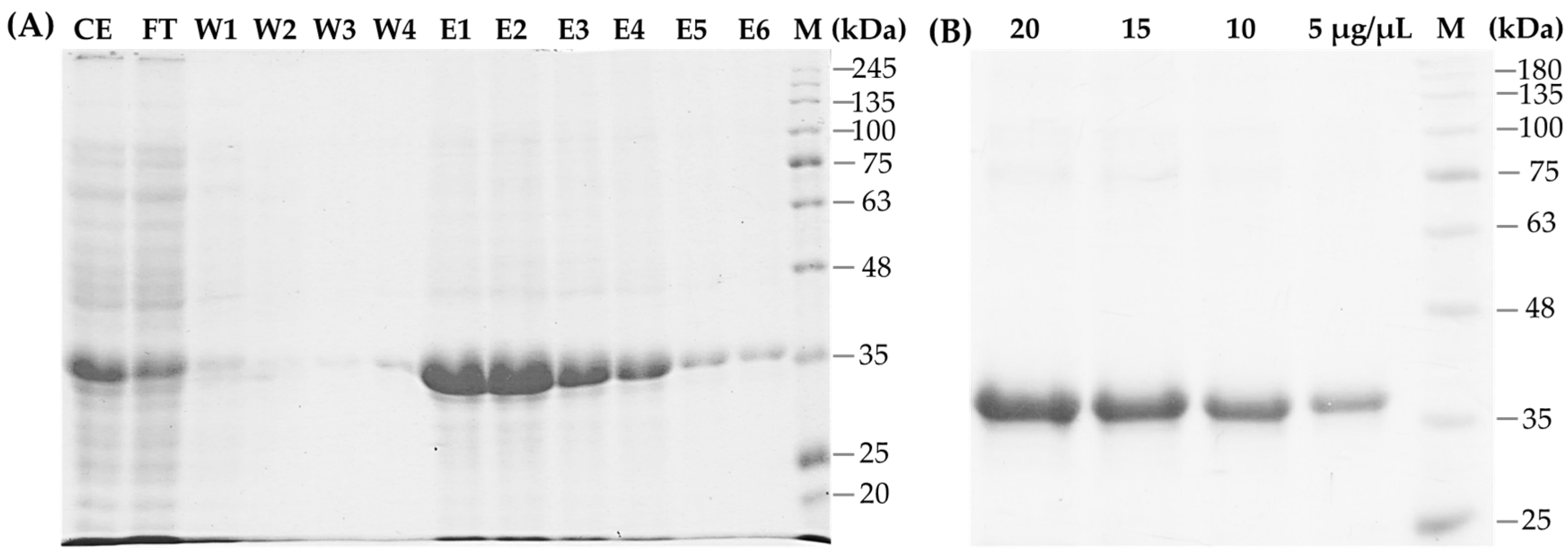
2.2. Production and Purification of the Recombinant CP-SCSMV
2.3. Development of Polyclonal Antibody
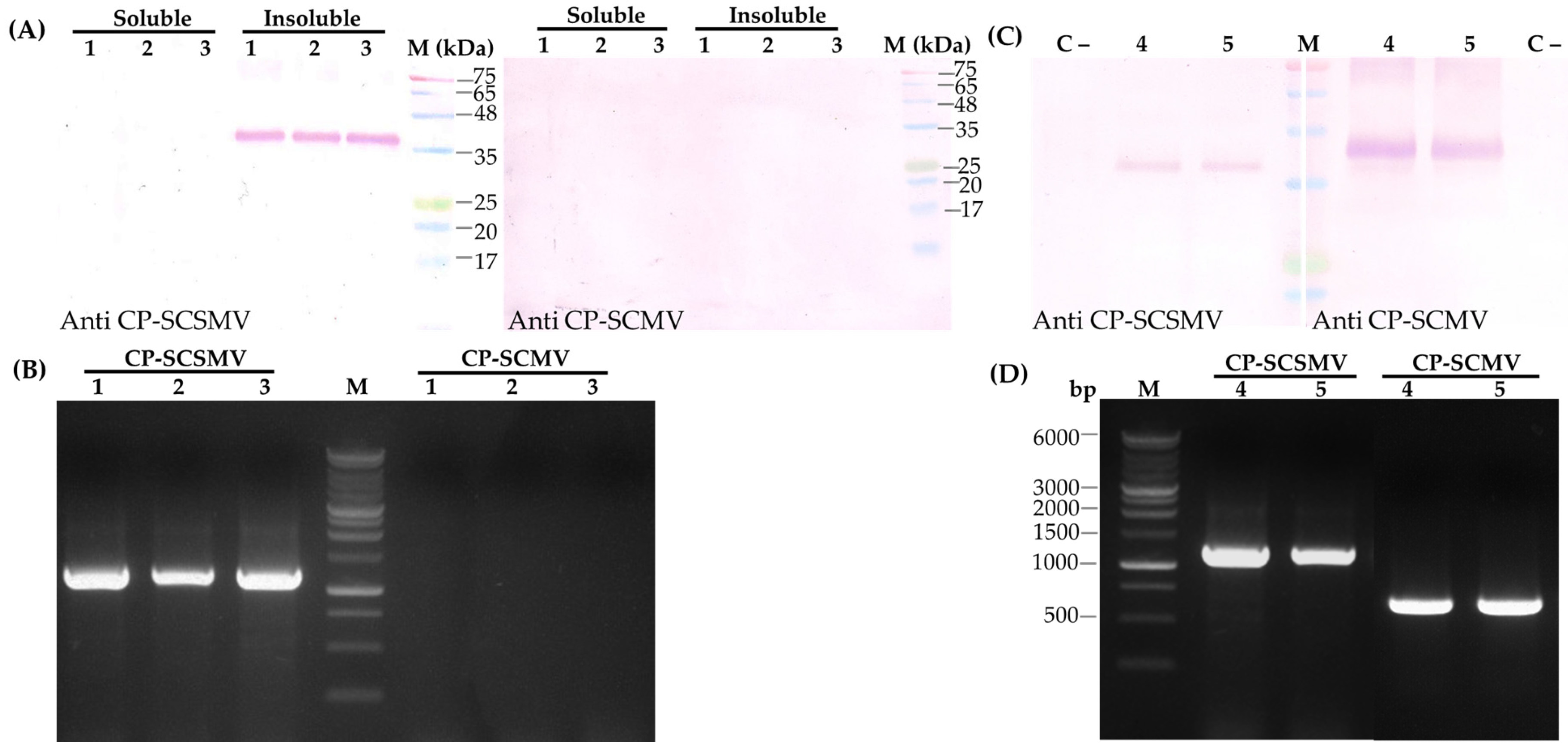
2.4. Immunoblot Analysis
2.5. Indirect ELISA (Enzyme-Linked Immunosorbent Assay) Analysis
2.6. Reverse Transcriptase-PCR (RT-PCR) Analysis
2.7. Immunocapture Reverse Transcriptase PCR (IC-RT-PCR) Analysis
3. Results
3.1. Cloning of SCSMV-CP cDNA
3.2. Expression and Purification of Recombinant SCSMV-CP
3.3. Development of the Polyclonal Antibody Against SCSMV-CP
3.4. Detection of SCSMV Infection in Sugarcane Leaves
3.4.1. Immunoblotting Analysis
3.4.2. Indirect ELISA Analysis
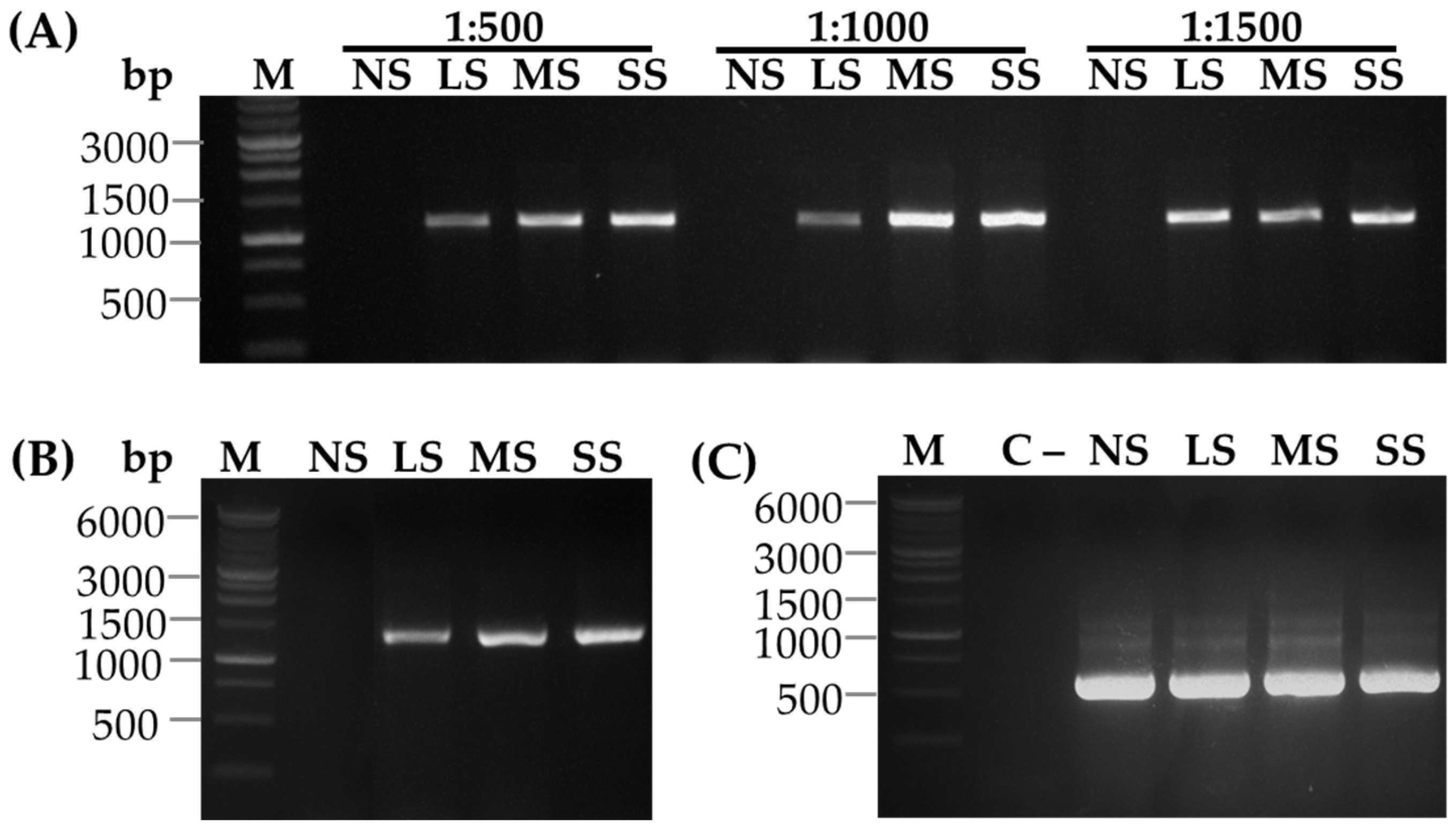
3.4.3. Immunocapture Reverse Transcription PCR Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Healey, A.L.; Garsmeur, O.; Lovell, J.T.; Shengquiang, S.; Sreedasyam, A.; Jenkins, J.; Plott, C.B.; Piperidis, N.; Pompidor, N.; Llaca, V.; et al. The Complex Polyploid Genome Architecture of Sugarcane. Nature 2024, 628, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, F.; Cao, Z.; Zhang, S.; Gan, Y.; Cai, W.; Peng, L.; Wu, Y.; Wang, W.; Yang, B. Factors Affecting the Production of Sugarcane Yield and Sucrose Accumulation: Suggested Potential Biological Solutions. Front. Plant Sci. 2024, 15, 1374228. [Google Scholar] [CrossRef] [PubMed]
- Addy, H.; Nurmalasari; Wahyudi, A.; Sholeh, A.; Anugrah, C.; Iriyanto, F.; Darmanto, W.; Sugiharto, B. Detection and Response of Sugarcane against the Infection of Sugarcane Mosaic Virus (SCMV) in Indonesia. Agronomy 2017, 7, 50. [Google Scholar] [CrossRef]
- Neliana, I.R.; Soleha, W.; Suherman; Darsono, N.; Harmoko, R.; Sawitri, W.D.; Sugiharto, B. Alteration of Photosynthetic and Antioxidant Gene Expression in Sugarcane Infected by Multiple Mosaic Viruses. Int. J. Plant Biol. 2024, 15, 757–768. [Google Scholar] [CrossRef]
- Hema, M.; Joseph, J.; Gopinath, K.; Sreenivasulu, P.; Savithri, H.S. Molecular Characterization and Interviral Relationships of a Flexuous Filamentous Virus Causing Mosaic Disease of Sugarcane (Saccharum officinarum L.) in India. Arch. Virol. 1999, 144, 479–490. [Google Scholar] [CrossRef]
- Daugrois, J.; Roumagnac, P.; Julian, C.; Filloux, D.; Putra, L.; Mollov, D.; Rott, P. Historical Review of Sugarcane Streak Mosaic Virus That Has Recently Emerged in Africa. Phytopathology 2024, 114, 668–680. [Google Scholar] [CrossRef]
- Hourani, H.; Abou-Jawdah, Y. Immunodiagnosis of Cucurbit Yellow Stunting Disorder Virus Using Polyclonal Antibodies Developed against Recombinant Coat Protein. J. Plant Pathol. 2003, 85, 197–204. [Google Scholar]
- Gulati-Sakhuja, A.; Sears, J.L.; Nuñez, A.; Liu, H.-Y. Production of Polyclonal Antibodies against Pelargonium Zonate Spot Virus Coat Protein Expressed in Escherichia Coli and Application for Immunodiagnosis. J. Virol. Methods 2009, 160, 29–37. [Google Scholar] [CrossRef]
- Khan, S.; Jan, A.T.; Aquil, B.; Haq, Q. Coat Protein Gene Characterization of Cucumber Mosaic Virus Isolates Infecting Banana in India. J. Phytol. 2011, 3, 94–101. [Google Scholar]
- Khatabi, B.; He, B.; Hajimorad, M.R. Diagnostic Potential of Polyclonal Antibodies Against Bacterially Expressed Recombinant Coat Protein of Alfalfa Mosaic Virus. Plant Dis. 2012, 96, 1352–1357. [Google Scholar] [CrossRef]
- Darsono, N.; Azizah, N.; Putranty, K.; Astuti, N.; Addy, H.; Darmanto, W.; Sugiharto, B. Production of a Polyclonal Antibody against the Recombinant Coat Protein of the Sugarcane Mosaic Virus and Its Application in the Immunodiagnostic of Sugarcane. Agronomy 2018, 8, 93. [Google Scholar] [CrossRef]
- Apriasti, R.; Widyaningrum, S.; Hidayati, W.N.; Sawitri, W.D.; Darsono, N.; Hase, T.; Sugiharto, B. Full Sequence of the Coat Protein Gene Is Required for the Induction of Pathogen-Derived Resistance against Sugarcane Mosaic Virus in Transgenic Sugarcane. Mol. Biol. Rep. 2018, 45, 2749–2758. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Hu, P.; Wang, W.; Lan, Y.; Du, L.; Zhou, Y.; Zhou, T. Rice Stripe Virus Coat Protein-Mediated Virus Resistance Is Associated With RNA Silencing in Arabidopsis. Front. Microbiol. 2020, 11, 591619. [Google Scholar] [CrossRef] [PubMed]
- Ntui, V.O.; Kynet, K.; Khan, R.S.; Ohara, M.; Goto, Y.; Watanabe, M.; Fukami, M.; Nakamura, I.; Mii, M. Transgenic Tobacco Lines Expressing Defective CMV Replicase-Derived dsRNA Are Resistant to CMV-O and CMV-Y. Mol. Biotechnol. 2014, 56, 50–63. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, M.-J.; Pak, J.H.; Im, H.H.; Lee, D.H.; Kim, K.-H.; Lee, J.-H.; Kim, D.-H.; Choi, H.K.; Jung, H.W.; et al. RNAi-Mediated Soybean Mosaic Virus (SMV) Resistance of a Korean Soybean Cultivar. Plant Biotechnol. Rep. 2016, 10, 257–267. [Google Scholar] [CrossRef]
- Widyaningrum, S.; Pujiasih, D.R.; Sholeha, W.; Harmoko, R.; Sugiharto, B. Induction of Resistance to Sugarcane Mosaic Virus by RNA Interference Targeting Coat Protein Gene Silencing in Transgenic Sugarcane. Mol. Biol. Rep. 2021, 48, 3047–3054. [Google Scholar] [CrossRef]
- Hull, R. Matthews’ Plant Virology; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar] [CrossRef]
- Kunert, R.; Reinhart, D. Advances in Recombinant Antibody Manufacturing. Appl. Microbiol. Biotechnol. 2016, 100, 3451–3461. [Google Scholar] [CrossRef]
- Frenzel, A.; Hust, M.; Schirrmann, T. Expression of Recombinant Antibodies. Front. Immunol. 2013, 4, 217. [Google Scholar] [CrossRef]
- Dong, N.; Wang, Z.; Sun, Q.; Chen, X.; Zhang, H.; Zheng, J.; Zhang, X.; Qiu, Y.; Li, Z.; Li, B.; et al. Establishment and Application of an Indirect ELISA for the Detection of Antibodies to Porcine Streptococcus Suis Based on a Recombinant GMD Protein. Animals 2023, 13, 719. [Google Scholar] [CrossRef]
- Nehring, M.; Pugh, S.; Dihle, T.; Gallichotte, E.; Nett, T.; Weber, E.; Mayo, C.; Lynn, L.; Ebel, G.; Fosdick, B.K.; et al. Laboratory-Based SARS-CoV-2 Receptor Binding Domain Serologic Assays Perform with Equivalent Sensitivity and Specificity to Commercial FDA-EUA Approved Tests. Viruses 2022, 15, 106. [Google Scholar] [CrossRef]
- Liu, Q.; Niu, X.; Jiang, L.; Zhang, G.; Wang, P.; Zhang, S.; Gao, W.; Guo, H.; Wang, Y.; Li, Y. Establishment of an Indirect ELISA Method for Detecting Bovine Coronavirus Antibodies Based on N Protein. Front. Vet. Sci. 2025, 12, 1530870. [Google Scholar] [CrossRef]
- Hema, M.; Kirthi, N.; Sreenivasulu, P.; Savithri, H.S. Development of Recombinant Coat Protein Antibody Based IC-RT-PCR for Detection and Discrimination of Sugarcane Streak Mosaic Virus Isolates from Southern India. Arch. Virol. 2003, 148, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Mumford, R.A.; Seal, S.E. Rapid Single-Tube Immunocapture RT-PCR for the Detection of Two Yam Potyviruses. J. Virol. Methods 1997, 69, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Putra, L.K.; Kristini, A.; Achadian, E.M.; Damayanti, T.A. Sugarcane Streak Mosaic Virus in Indonesia: Distribution, Characterisation, Yield Losses and Management Approaches. Sugar Tech 2014, 16, 392–399. [Google Scholar] [CrossRef]
- Adams, I.P.; Miano, D.W.; Kinyua, Z.M.; Wangai, A.; Kimani, E.; Phiri, N.; Reeder, R.; Harju, V.; Glover, R.; Hany, U.; et al. Use of Next-generation Sequencing for the Identification and Characterization of MAize Chlorotic Mottle Virus and SUgarcane Mosaic Virus Causing Maize Lethal Necrosis in KEnya. Plant Pathol. 2013, 62, 741–749. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Nie, K.; Zhang, C.; Zhang, Y.; Wang, J.; Niu, P.; Ma, X. VIP: An Integrated Pipeline for Metagenomics of Virus Identification and Discovery. Sci. Rep. 2016, 6, 23774. [Google Scholar] [CrossRef]
- Lee, S.H.; Oh, T.-K.; Oh, S.; Kim, S.; Noh, H.B.; Vinod, N.; Lee, J.Y.; Moon, E.S.; Choi, C.W. Development of a Kit for Rapid Immunochromatographic Detection of Sacbrood Virus Infecting Apis Cerana (AcSBV) Based on Polyclonal and Monoclonal Antibodies Raised against Recombinant VP1 and VP2 Expressed in Escherichia Coli. Viruses 2021, 13, 2439. [Google Scholar] [CrossRef]
- Zhao, Z.; Tian, Y.; Xu, C.; Xing, Y.; Yang, L.; Qian, G.; Hua, X.; Gong, W.; Hu, B.; Wang, L. A Monoclonal Antibody-Based Immunochromatographic Test Strip and Its Application in the Rapid Detection of Cucumber Green Mottle Mosaic Virus. Biosensors 2023, 13, 199. [Google Scholar] [CrossRef]


| Sugarcane Leaves Samples | Coating Protein Concentration μg/mL | Absorbance of Buffer Extraction Abs450 | Absorbance of Crude Extract Protein Abs450 | P/N Ratio |
|---|---|---|---|---|
| No Symptom [NS] | 2 | 0.125 | 0.218 | 1.74 |
| 1 | 0.106 | 0.177 | 1.66 | |
| 0.5 | 0.089 | 0.126 | 1.43 | |
| 0.3 | 0.072 | 0.106 | 1.46 | |
| Low Symptomatic [LS] | 2 | 0.125 | 0.663 | 5.31 |
| 1 | 0.106 | 0.447 | 4.20 | |
| 0.5 | 0.089 | 0.215 | 2.43 | |
| 0.3 | 0.072 | 0.156 | 2.16 | |
| Middle Symptomatic [MS] | 2 | 0.125 | 0.721 | 5.77 |
| 1 | 0.106 | 0.473 | 4.45 | |
| 0.5 | 0.089 | 0.253 | 2.86 | |
| 0.3 | 0.072 | 0.168 | 2.33 | |
| Severe Symptomatic [SS] | 2 | 0.125 | 0.734 | 5.87 |
| 1 | 0.106 | 0.486 | 4.57 | |
| 0.5 | 0.089 | 0.286 | 3.23 | |
| 0.3 | 0.072 | 0.204 | 2.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neliana, I.R.; Sugiharto, B.; Harmoko, R.; Fanata, W.I.D. Development of Immunodetection Systems Using a Specific Antibody Against the Recombinant Coat Protein for Detecting Sugarcane Streak Mosaic Virus. Pathogens 2025, 14, 1106. https://doi.org/10.3390/pathogens14111106
Neliana IR, Sugiharto B, Harmoko R, Fanata WID. Development of Immunodetection Systems Using a Specific Antibody Against the Recombinant Coat Protein for Detecting Sugarcane Streak Mosaic Virus. Pathogens. 2025; 14(11):1106. https://doi.org/10.3390/pathogens14111106
Chicago/Turabian StyleNeliana, Intan Ria, Bambang Sugiharto, Rikno Harmoko, and Wahyu Indra Duwi Fanata. 2025. "Development of Immunodetection Systems Using a Specific Antibody Against the Recombinant Coat Protein for Detecting Sugarcane Streak Mosaic Virus" Pathogens 14, no. 11: 1106. https://doi.org/10.3390/pathogens14111106
APA StyleNeliana, I. R., Sugiharto, B., Harmoko, R., & Fanata, W. I. D. (2025). Development of Immunodetection Systems Using a Specific Antibody Against the Recombinant Coat Protein for Detecting Sugarcane Streak Mosaic Virus. Pathogens, 14(11), 1106. https://doi.org/10.3390/pathogens14111106







