Epidemiological Characteristics of HSV-1 and HSV-2 in 177,599 Patients Based on PCR Testing in South Korea (2018–2022)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Source
2.2. Inclusion and Exclusion Criteria
2.3. Ethical Considerations
2.4. Nucleic Acid Extraction and PCR Testing
2.5. Statistical Analysis
3. Results
3.1. Age-Specific Distribution
3.2. Sex-Related Differences in Positivity Rates
3.3. Temporal Trends from 2018 to 2022
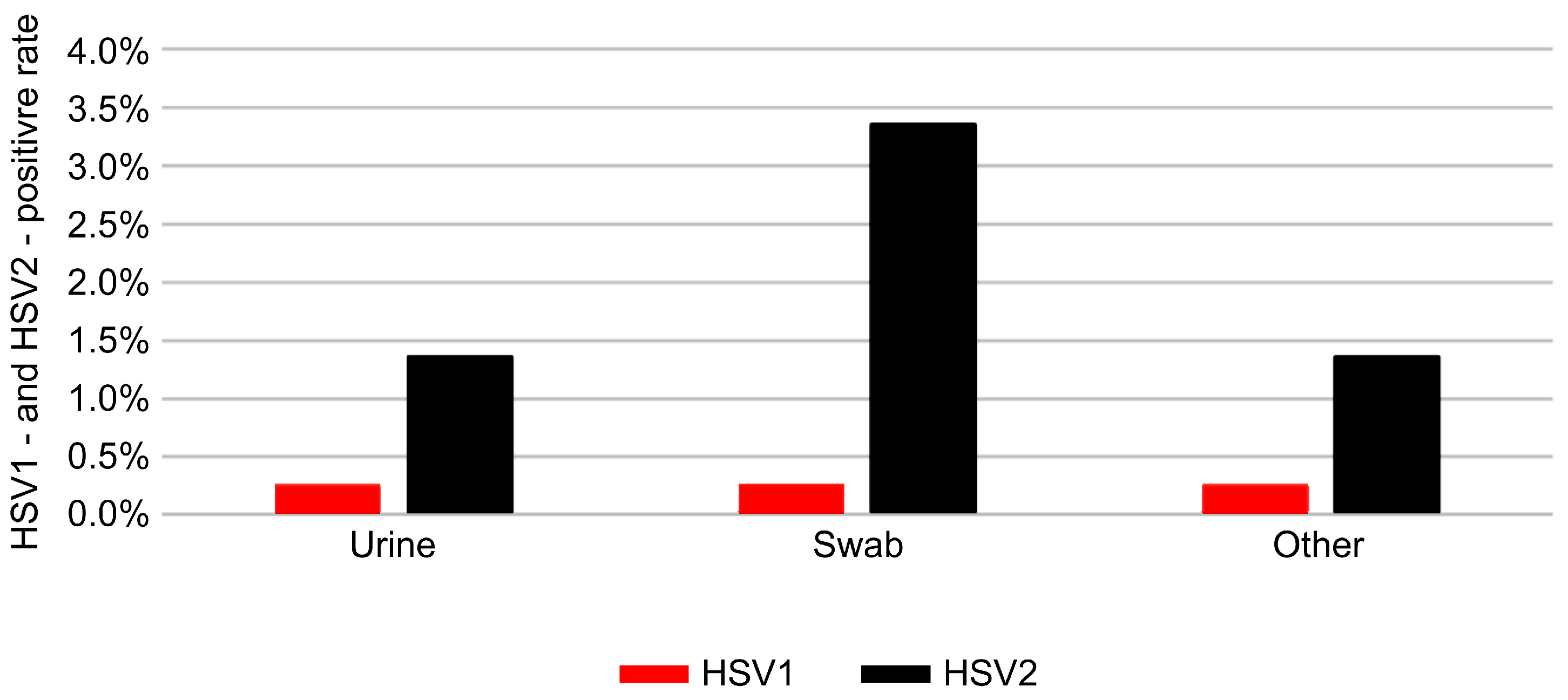
3.4. Distribution by Specimen Type
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | Confidence Interval |
| HSV | Herpes Simplex Virus |
| HSV-1 | Herpes Simplex Virus 1 |
| HSV-2 | Herpes Simplex Virus 2 |
| OR | Odds Ratio |
| Ct | Threshold Cycle |
| WHO | World Health Organization |
References
- Smith, J.S.; Robinson, N.J. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: A global review. J. Infect. Dis. 2002, 186 (Suppl. 1), S3–S28. [Google Scholar] [CrossRef] [PubMed]
- Nath, P.; Kabir, M.A.; Doust, S.K.; Ray, A. Diagnosis of herpes simplex virus: Laboratory and point-of-care techniques. Infect. Dis. Rep. 2021, 13, 518–539. [Google Scholar] [CrossRef]
- Muralidhar, S. Molecular methods in the laboratory diagnosis of sexually transmitted infections. Indian J. Sex. Transm. Dis. AIDS 2015, 36, 9–17. [Google Scholar] [CrossRef] [PubMed]
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes simplex virus: Global infection prevalence and incidence estimates, 2016. Bull. World Health Organ. 2020, 98, 315–329. [Google Scholar] [CrossRef]
- Silva, S.; Ayoub, H.H.; Johnston, C.; Atun, R.; Abu-Raddad, L.J. Estimated economic burden of genital herpes and HIV attributable to herpes simplex virus type 2 infections in 90 low- and middle-income countries: A modeling study. PLoS Med. 2022, 19, e1003938. [Google Scholar] [CrossRef] [PubMed]
- Balaeva, T.; Grjibovski, A.M.; Sidorenkov, O.; Samodova, O.; Firsova, N.; Sannikov, A.; Klouman, E. Seroprevalence and correlates of herpes simplex virus type 2 infection among young adults in Arkhangelsk, Northwest Russia: A population-based cross-sectional study. BMC Infect. Dis. 2016, 16, 616. [Google Scholar] [CrossRef]
- Looker, K.J.; Magaret, A.S.; Turner, K.M.; Vickerman, P.; Gottlieb, S.L.; Newman, L.M. Correction: Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS ONE 2015, 10, e0128615. [Google Scholar] [CrossRef]
- Quispe Calla, N.E.; Vicetti Miguel, R.D.; Boyaka, P.N.; Hall-Stoodley, L.; Kaur, B.; Trout, W.; Pavelko, S.D.; Cherpes, T.L. Medroxyprogesterone acetate and levonorgestrel increase genital mucosal permeability and enhance susceptibility to genital herpes simplex virus type 2 infection. Mucosal Immunol. 2016, 9, 1571–1583. [Google Scholar] [CrossRef]
- Bakir, A.; Alacam, S.; Guney, M.; Yavuz, M.T. Investigation of herpes simplex virus type 2 seroprevalence in all age groups. Ann. Med. Res. 2022, 29, 346–350. [Google Scholar] [CrossRef]
- Binnicker, M.J.; Espy, M.J.; Duresko, B.; Irish, C.; Mandrekar, J. Automated processing, extraction and detection of herpes simplex virus types 1 and 2: A comparative evaluation of three commercial platforms using clinical specimens. J. Clin. Virol. 2017, 89, 30–33. [Google Scholar] [CrossRef]
- Arshad, Z.; Alturkistani, A.; Brindley, D.; Lam, C.; Foley, K.; Meinert, E. Tools for the diagnosis of herpes simplex virus 1/2: Systematic review of studies published between 2012 and 2018. JMIR Public Health Surveill. 2019, 5, e14216. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R.; Najeem, B.; Sobhanakumary, K.; Sunny, B.; Pinheiro, C.; Anukumar, B. Herpes simplex virus 1 and 2 in herpes genitalis: A polymerase chain reaction-based study from Kerala. Indian J. Dermatol. 2018, 63, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Schremser, V.; Antoniewicz, L.; Tschachler, E.; Geusau, A. Polymerase chain reaction for the diagnosis of herpesvirus infections in dermatology: Analysis of clinical data. Wien. Klin. Wochenschr. 2020, 132, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Samie, A.; Mnisi, H.P.; Ramantswana, M.N. Prevalence of HSV1 and HSV2 among HIV patients in northern South Africa as determined by real time PCR in urine samples. Am. J. Infect. Dis. 2017, 13, 3–12. [Google Scholar] [CrossRef]
- McQuillan, G.; Kruszon-Moran, D.; Flagg, E.W.; Paulose-Ram, R. Prevalence of herpes simplex virus type 1 and type 2 in persons aged 14–49: United States, 2015–2016. NCHS Data Brief 2018, 1–8. [Google Scholar]
- Chemaitelly, H.; Nagelkerke, N.; Omori, R.; Abu-Raddad, L.J. Characterizing herpes simplex virus type 1 and type 2 seroprevalence declines and epidemiological association in the United States. PLoS ONE 2019, 14, e0214151. [Google Scholar] [CrossRef]
- Dominguez, S.R.; Pretty, K.; Hengartner, R.; Robinson, C.C. Comparison of herpes simplex virus PCR with culture for virus detection in multisource surface swab specimens from neonates. J. Clin. Microbiol. 2018, 56, e00632-18. [Google Scholar] [CrossRef]
- Talaat, A.; El-Hussein, A.R.; Babiker, A.; Elkhidir, I.M.; Enan, K.A. Molecular detection of human herpes virus types 1 and 2 among pregnant women in Khartoum State, Sudan. Int. J. Sci. Res. Sci. Eng. Technol. 2019, 4, 324–328. [Google Scholar]
- Jang, J.; Park, S.; Chun, B.C. How place shapes genital herpes simplex distribution in South Korea: A Bayesian spatial analysis using National Health Insurance Service data. BMC Public Health 2025, 25, 3023. [Google Scholar] [CrossRef]
- Oh, E.J.; Yuk, Y.S.; Kim, J.K. Laboratory investigations of herpes simplex virus-1 and −2 clinical samples in Korea. Osong Public Health Res. Perspect. 2021, 12, 385–389. [Google Scholar] [CrossRef]
- Ayoub, H.H.; Amara, I.; Awad, S.F.; Omori, R.; Chemaitelly, H.; Abu-Raddad, L.J. Analytic characterization of the herpes simplex virus Type 2 epidemic in the United States, 1950–2050. Open Forum Infect. Dis. 2021, 8, ofab218. [Google Scholar] [CrossRef] [PubMed]
- Alareeki, A.; Osman, A.M.M.; Khandakji, M.N.; Looker, K.J.; Harfouche, M.; Abu-Raddad, L.J. Epidemiology of herpes simplex virus type 2 in Europe: Systematic review, meta-analyses, and meta-regressions. Lancet Reg. Health Eur. 2023, 25, 100558. [Google Scholar] [CrossRef]
- Ageeb, R.A.; Harfouche, M.; Chemaitelly, H.; Abu-Raddad, L.J. Epidemiology of herpes simplex virus type 1 in the United States: Systematic review, meta-analyses, and meta-regressions. iScience 2024, 27, 110652. [Google Scholar] [CrossRef]
- Harfouche, M.; AlMukdad, S.; Alareeki, A.; Osman, A.M.M.; Gottlieb, S.; Rowley, J.; Abu-Raddad, L.J.; Looker, K.J. Estimated global and regional incidence and prevalence of herpes simplex virus infections and genital ulcer disease in 2020: Mathematical modelling analyses. Sex. Transm. Infect. 2025, 101, 214–223. [Google Scholar] [CrossRef]
- Johnston, C.; Magaret, A.; Son, H.; Stern, M.; Rathbun, M.; Renner, D.; Szpara, M.; Gunby, S.; Ott, M.; Jing, L.; et al. Viral shedding 1 year following first-episode genital HSV-1 infection. JAMA 2022, 328, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Kim, K.S.; Han, C.H.; Bae, S. Recent changes in sexually transmitted infection in Korea: A population-based analysis. J. Clin. Med. 2025, 14, 5145. [Google Scholar] [CrossRef] [PubMed]
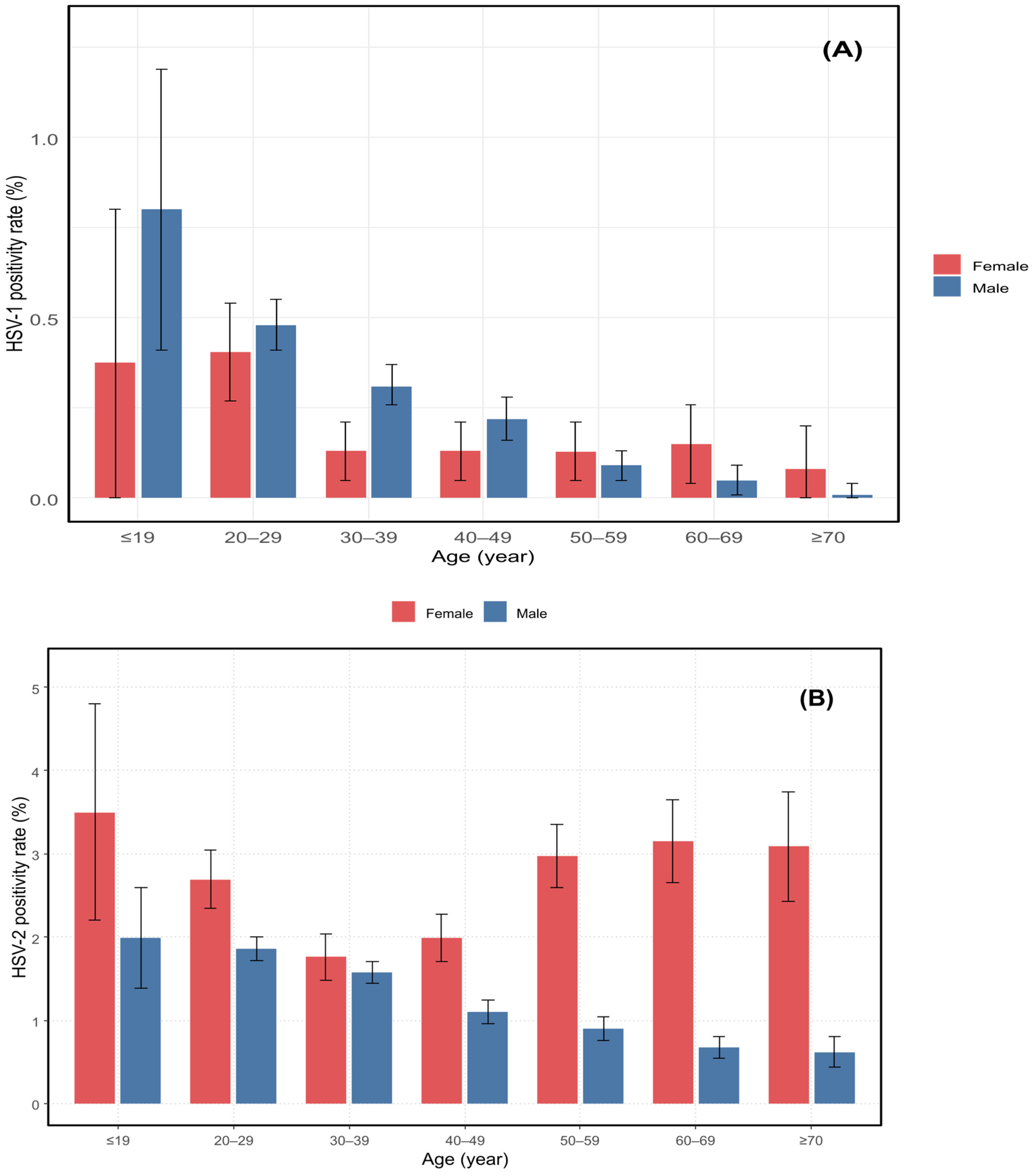
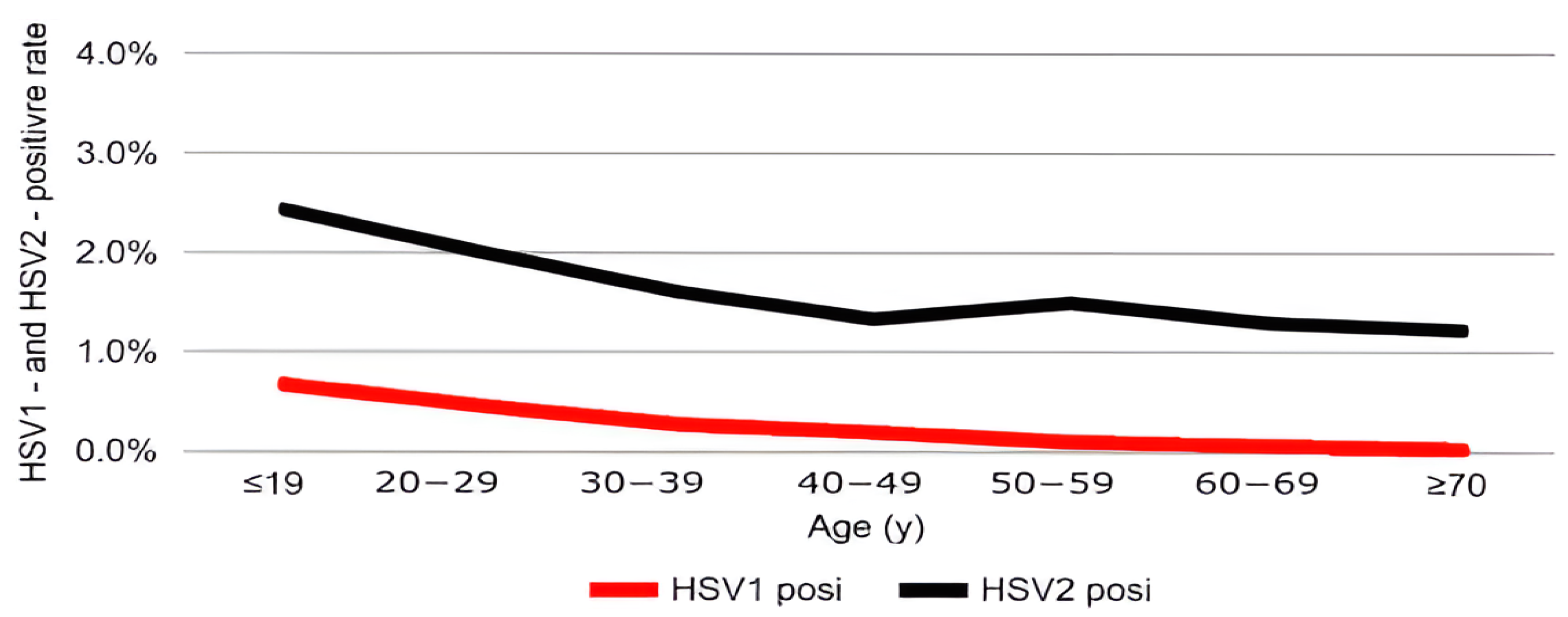
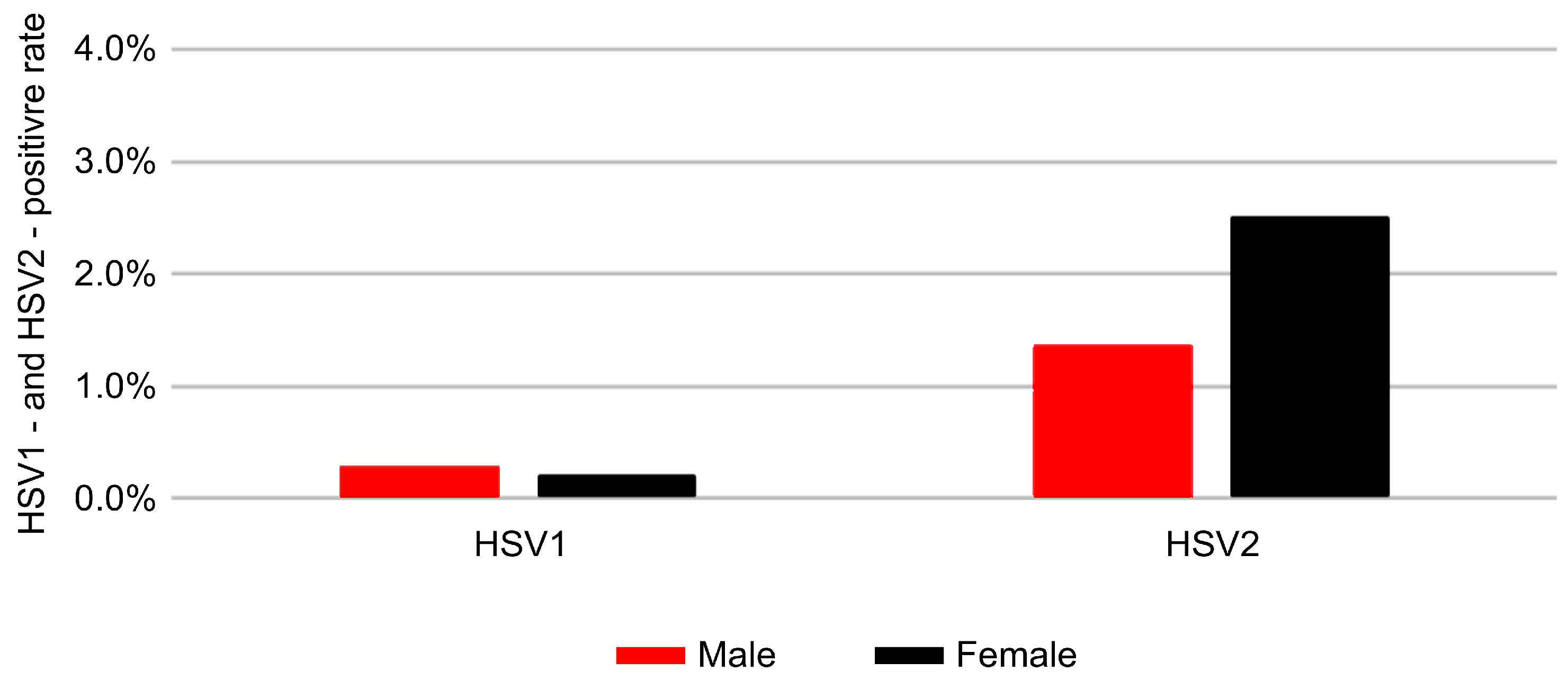
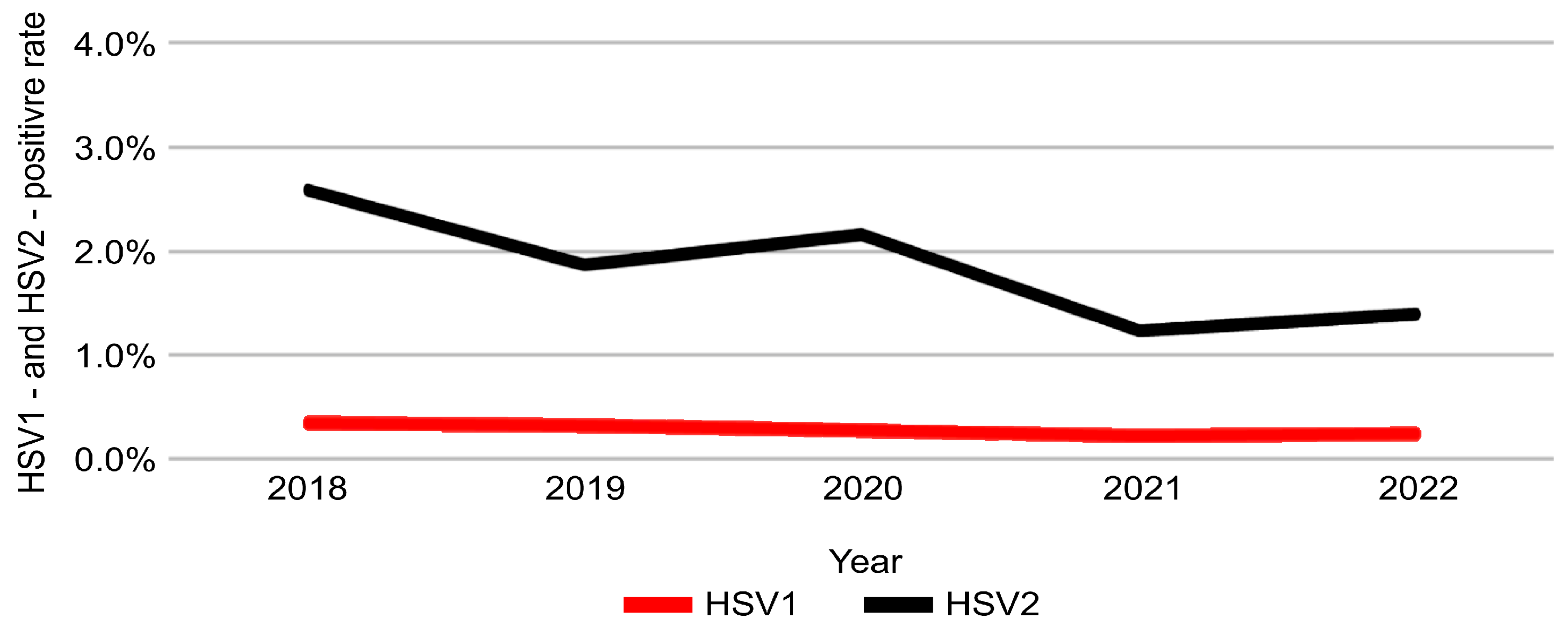
| Age (Years) | ≤19 (n = 2801) | 20–29 (n = 43,877) | 30–39 (n = 43,793) | 40–49 (n = 32,072) | 50–59 (n = 26,705) | 60–69 (n = 18,674) | ≥70 (n = 9677) | χ2 | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| HSV-1, n (%) (95% CI) | 19 (0.68) (0.37–0.98) | 205 (0.47) (0.40–0.53) | 122 (0.28) (0.23–0.33) | 63 (0.20) (0.15–0.24) | 27 (0.10) (0.06–0.14) | 14 (0.07) (0.04–0.11) | 3 (0.03) (0.00–0.07) | 170.41 | <0.001 *** |
| HSV-2, n (%) (95% CI) | 68 (2.42) (1.86–3.00) | 884 (2.01) (1.88–2.15) | 705 (1.60) (1.49–1.73) | 430 (1.34) (1.21–1.47) | 401 (1.50) (1.36–1.65) | 242 (1.29) (1.13–1.46) | 120 (1.24) (1.02–1.46) | 94.11 | <0.001 *** |
| Sex | Male, n (%) (n = 137,027) | Female, n (%) (n = 40,572) | χ2 | p-Value |
|---|---|---|---|---|
| HSV-1 | 376 (0.3) | 77 (0.2) | 8.809 | 0.003 * |
| HSV-2 | 1837 (1.37) | 1015 (2.50) | 267.950 | <0.001 *** |
| Variable | Category | Reference | aOR | 95% CI | p-Value |
|---|---|---|---|---|---|
| HSV-2 | |||||
| Sex | Female | Male | 1000 | 0.88–1.13 | 0.969 |
| Specimen type | Swab | Urine | 2.30 | 2.01–2.62 | <0.001 |
| Other | Urine | 0.88 | 0.75–1.03 | 0.109 | |
| Age group | 30–39 | ≤19 | 0.69 | 0.53–0.88 | 0.003 |
| 40–49 | ≤19 | 0.55 | 0.43–0.72 | <0.001 | |
| 50–59 | ≤19 | 0.64 | 0.50–0.84 | <0.001 | |
| 60–69 | ≤19 | 1.3 | 0.99–1.71 | 0.062 | |
| ≥70 | ≤19 | 0.55 | 0.41–0.75 | <0.001 | |
| HSV-1 | |||||
| Sex | Female | Male | 0.14 | 0.10–0.19 | <0.001 |
| Specimen type | Swab | Urine | 0.88 | 0.61–1.26 | 0.478 |
| Other | Urine | 0.19 | 0.13–0.28 | <0.001 | |
| Age group | 20–29 | ≤19 | 5.81 | 3.59–9.39 | <0.001 |
| 30–39 | ≤19 | 0.36 | 0.22–0.59 | <0.001 | |
| 40–49 | ≤19 | 0.27 | 0.16–0.45 | <0.001 | |
| 50–59 | ≤19 | 0.8 | 0.44–1.47 | 0.476 | |
| 60–69 | ≤19 | 0.51 | 0.24–1.10 | 0.087 | |
| ≥70 | ≤19 | 0.05 | 0.01–0.16 | <0.001 |
| Year | 2018 (n = 5912) | 2019 (n = 25,394) | 2020 (n = 33,885) | 2021 (n = 42,764) | 2022 (n = 69,644) | χ2 | p-Value |
|---|---|---|---|---|---|---|---|
| HSV1, n (%) | 20 (0.3) | 80 (0.3) | 94 (0.3) | 94 (0.2) | 165 (0.2) | 8.855 | 0.065 |
| HSV2, n (%) | 153 (2.6) | 474 (1.9) | 731 (2.2) | 524 (1.2) | 968 (1.4) | 170.083 | <0.001 *** |
| Virus | Statistical Model | Adjusted Odds Ratio (aOR) per Year | 95% Confidence Interval | p-Value |
|---|---|---|---|---|
| HSV-2 | Logistic regression (per 1-year increase) | 0.86 | 0.83–0.88 | < 0.001 |
| HSV-1 | Logistic regression (per 1-year increase) | 0.9 | 0.84–0.98 | 0.009 |
| Sex | Age (Years) | Specimen Type | Total Tests (n) | HSV-1 Positive (n) | HSV-2 Positive (n) |
|---|---|---|---|---|---|
| Male | ≤19 | Urine | 1815 | 11 | 36 |
| Swab | 25 | 1 | 2 | ||
| Other | 161 | 4 | 2 | ||
| 20–29 | Urine | 33,334 | 153 | 582 | |
| Swab | 232 | 4 | 32 | ||
| Other | 2185 | 15 | 51 | ||
| 30–39 | Urine | 32,722 | 100 | 484 | |
| Swab | 216 | 6 | 35 | ||
| Other | 2448 | 5 | 38 | ||
| 40–49 | Urine | 21,596 | 48 | 227 | |
| Swab | 120 | 1 | 16 | ||
| Other | 1937 | 3 | 19 | ||
| 50–59 | Urine | 17,032 | 15 | 139 | |
| Swab | 88 | 0 | 15 | ||
| Other | 1856 | 2 | 19 | ||
| 60–69 | Urine | 12,505 | 7 | 78 | |
| Swab | 31 | 0 | 9 | ||
| Other | 1474 | 0 | 8 | ||
| ≥70 | Urine | 6666 | 0 | 36 | |
| Swab | 14 | 0 | 6 | ||
| Other | 570 | 1 | 3 | ||
| Female | ≤19 | Urine | 308 | 1 | 6 |
| Swab | 461 | 2 | 22 | ||
| Other | 31 | 0 | 0 | ||
| 20–29 | Urine | 3288 | 15 | 64 | |
| Swab | 4572 | 17 | 151 | ||
| Other | 266 | 3 | 4 | ||
| 30–39 | Urine | 2473 | 1 | 33 | |
| Swab | 5637 | 10 | 111 | ||
| Other | 297 | 0 | 4 | ||
| 40–49 | Urine | 2920 | 5 | 48 | |
| Swab | 5039 | 6 | 112 | ||
| Other | 460 | 0 | 8 | ||
| 50–59 | Urine | 3825 | 3 | 102 | |
| Swab | 3333 | 4 | 121 | ||
| Other | 571 | 1 | 7 | ||
| 60–69 | Urine | 2820 | 4 | 66 | |
| Swab | 1520 | 3 | 75 | ||
| Other | 324 | 0 | 6 | ||
| ≥70 | Urine | 1739 | 1 | 43 | |
| Swab | 591 | 1 | 29 | ||
| Other | 97 | 0 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.H.; Jang, S.H.; Han, J.S.; Jeon, J.-S.; Kim, J.K. Epidemiological Characteristics of HSV-1 and HSV-2 in 177,599 Patients Based on PCR Testing in South Korea (2018–2022). Pathogens 2025, 14, 1107. https://doi.org/10.3390/pathogens14111107
Kim HH, Jang SH, Han JS, Jeon J-S, Kim JK. Epidemiological Characteristics of HSV-1 and HSV-2 in 177,599 Patients Based on PCR Testing in South Korea (2018–2022). Pathogens. 2025; 14(11):1107. https://doi.org/10.3390/pathogens14111107
Chicago/Turabian StyleKim, Hyeong Ho, Sung Hun Jang, Jeong Su Han, Jae-Sik Jeon, and Jae Kyung Kim. 2025. "Epidemiological Characteristics of HSV-1 and HSV-2 in 177,599 Patients Based on PCR Testing in South Korea (2018–2022)" Pathogens 14, no. 11: 1107. https://doi.org/10.3390/pathogens14111107
APA StyleKim, H. H., Jang, S. H., Han, J. S., Jeon, J.-S., & Kim, J. K. (2025). Epidemiological Characteristics of HSV-1 and HSV-2 in 177,599 Patients Based on PCR Testing in South Korea (2018–2022). Pathogens, 14(11), 1107. https://doi.org/10.3390/pathogens14111107






