An Update of Epidemiological Trends in Enzootic Bovine Leukosis in Italy and an Analysis of Risk Factors Associated with Infection Persistence
Abstract
1. Introduction
2. Materials and Methods
2.1. Guidelines on Surveillance Measures in the National Territory of Italy
- 1.
- Implementation of risk-based surveillance activities across the national territory to maintain a disease-free status at community level. This status can be maintained if, in the national territories, at least 99.8% of cattle farms are disease-free, and all cattle aged >24 months are tested for surveillance activities in slaughterhouses. Moreover, all cattle aged >24 months on farms identified as at risk are tested using serological analysis.
- 2.
- Implementation of adequate management of BLV infection clusters for disease eradication purposes through epidemiological surveys and a specific 3-year regional plan.An infection cluster of EBL is defined as a geographically well-delimited area within the regional territory, in which one or more of the following conditions are present: (i) the prevalence of BLV outbreak is above the threshold of 0.2% (of controllable herds), according to EU regulatory criteria; (ii) epidemiological investigations conducted in BLV outbreak demonstrate the persistence of infection for at least five years; (iii) epidemiological correlations exist among different farms, highlighting the presence of factors that may favor the persistence and circulation of the virus.The main regional plan measures for inclusion are: (i) 100% implementation of controls on the affected population, (ii) increasing the frequency of serological tests and lowering the age of the controllable population, (iii) separating infected animals, and (iv) controlling within-cluster movements and stamping out seropositive animals in the last 5 years.
- 3.
- Execution of appropriate diagnostic tests for implementing surveillance programs and eradication plans for infection clusters. The official diagnostic tests, listed in Annex III, Section 3, of Delegated Regulation (EU) 2020/689 [14], are serological tests. The ELISA (serum or milk) test is the primary tool for BLV screening programs and follow-up studies, as it offers a high degree of sensitivity and reliability, besides allowing the use of serum pools, while the AGID (serum samples) test guarantees a high degree of specificity, in accordance with the World Organization for Animal Health [15]. In particular, in three Italian infection clusters, official laboratories perform the ELISA test by serum matrix, and in the case of positive results, CEREL also carries out the AGID test. Direct diagnostic tests (polymerase chain reaction [PCR]/real-time PCR assay, and histological examination) from the CEREL are used in addition to serological tests, mainly for testing seropositive animals slaughtered following EBL confirmation.
- 4.
- Implementation of disease control measures in establishments in cases of suspected/confirmed BLV.In establishments with suspected EBL cases: (i) notify the National Veterinary Information System on Animal Diseases (SIMAN); (ii) conduct epidemiological, clinical, and laboratory surveys; (iii) identify animals with suspected EBL and register them in the National Database (BDN); (iii) apply movement blocks; and (iv) separate animals with suspected EBL.In establishments with cases of confirmed BLV: (i) withdrawal of the disease-free status, with overall assessment derived from epidemiological and laboratory survey findings and identification of the animals not registered within 2 days of confirmation; (ii) separation and stamping out of infected animals within 15 days of confirmation; (iii) undertaken periodic checks every 60 days on cattle aged >12 months until at least two consecutive negative tests are recorded, the first after the first 4 months following the stamping out of infected animals and the second 4 months after the first test; (iv) apply movement blocks, except for immediate slaughter; (v) clean and disinfect the infected farms; and (vi) perform official diagnostic tests in farms that have had direct contact with infected animals
- 5.
- Compliance with the required information flows regarding the programming and outcomes of the VETINFO platform of the National Platform of Veterinary Information systems [1].
2.2. Italian Epidemiological Data Source
2.3. Epidemiological Indicators Considered for the Analysis
- -
- Number of EBL outbreaks reported in Italy;
- -
- Number of active EBL outbreaks (infection is still present within the affected farm) or eradicated EBL outbreaks (absence of new seropositive animals) in the Italian clusters;
- -
- Prevalence (%) of notified EBL outbreaks on controlled farms (monitored Italian farm);
- -
- Prevalence (%) of seropositive animals on infected farms;
- -
- Annual trends of outbreak prevalence (%) and incidence (%) in the Italian clusters
- -
- Comparison of controlled and controllable farms (planned farms) in affected regions;
- -
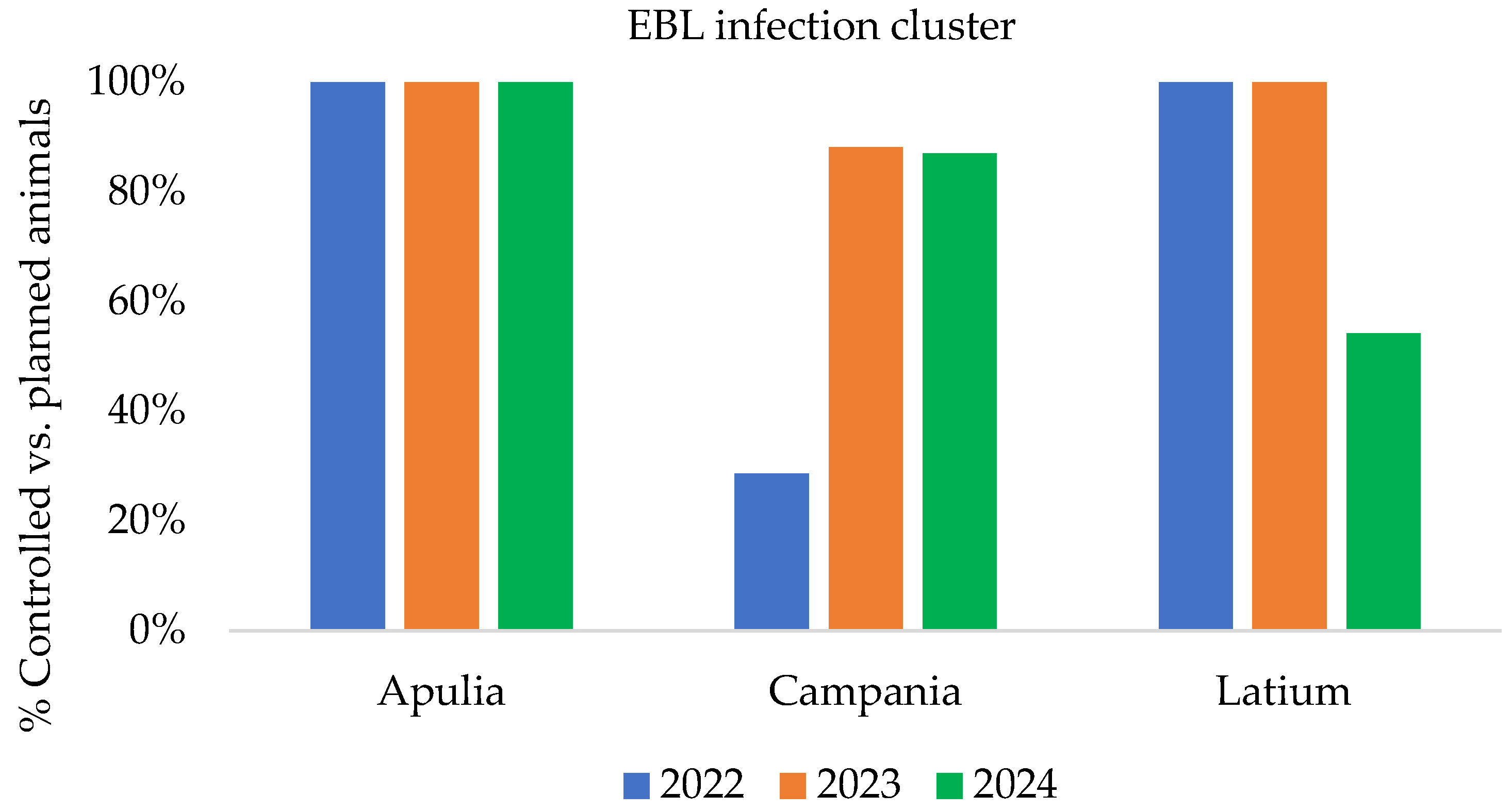
- Comparison of controlled and controllable animals (planned animals) in the affected regions and EBL infection clusters;
- -
- Surveillance activities in the establishment of assembly operations (EAO) located in Italian clusters.
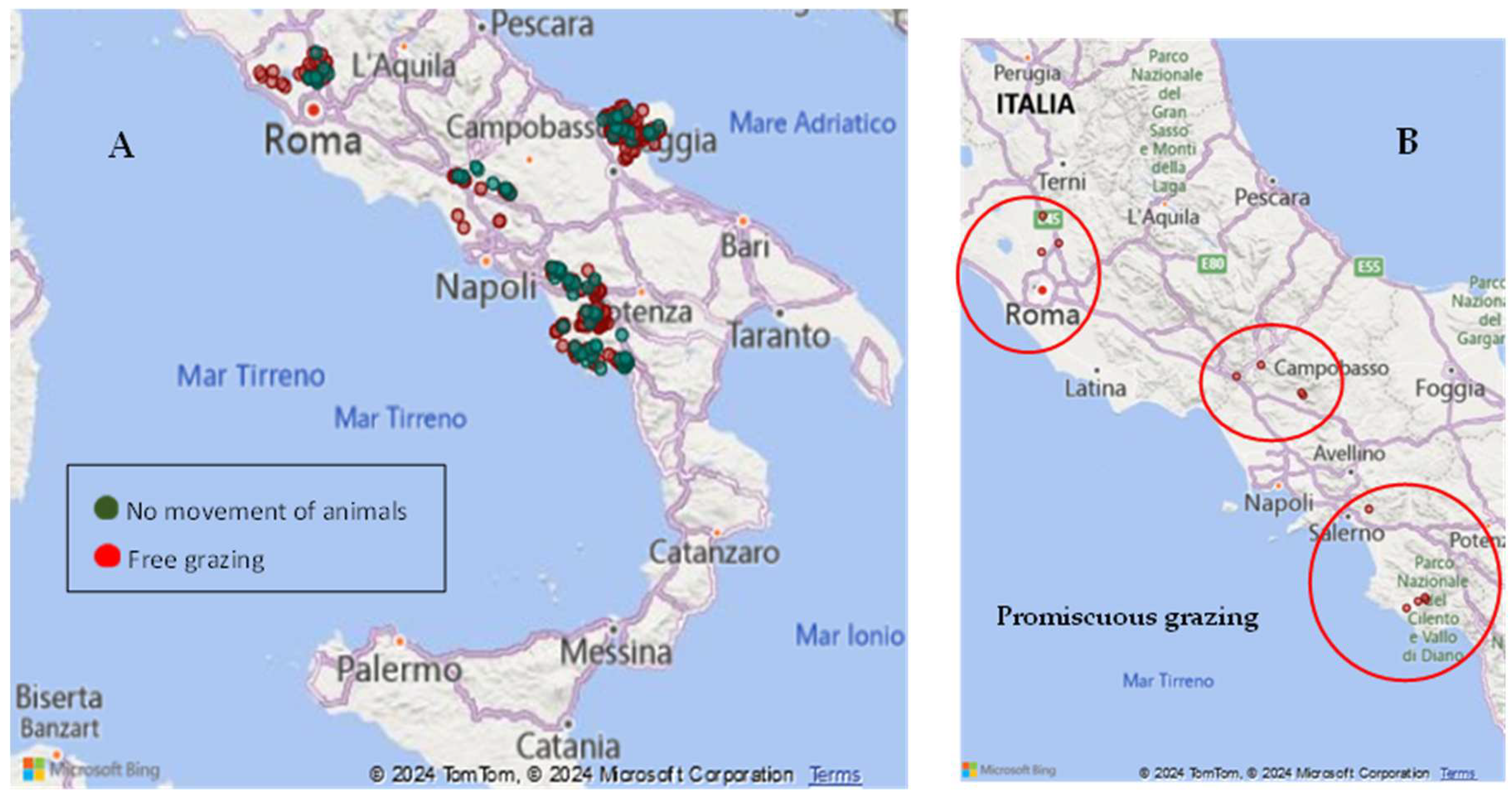
2.4. Risk Factors Analysis in the National Surveillance System
- -
- Free-or semi-free-range establishments and/or those practicing common grazing;
- -
- Movement between pastures within each infection cluster region;
- -
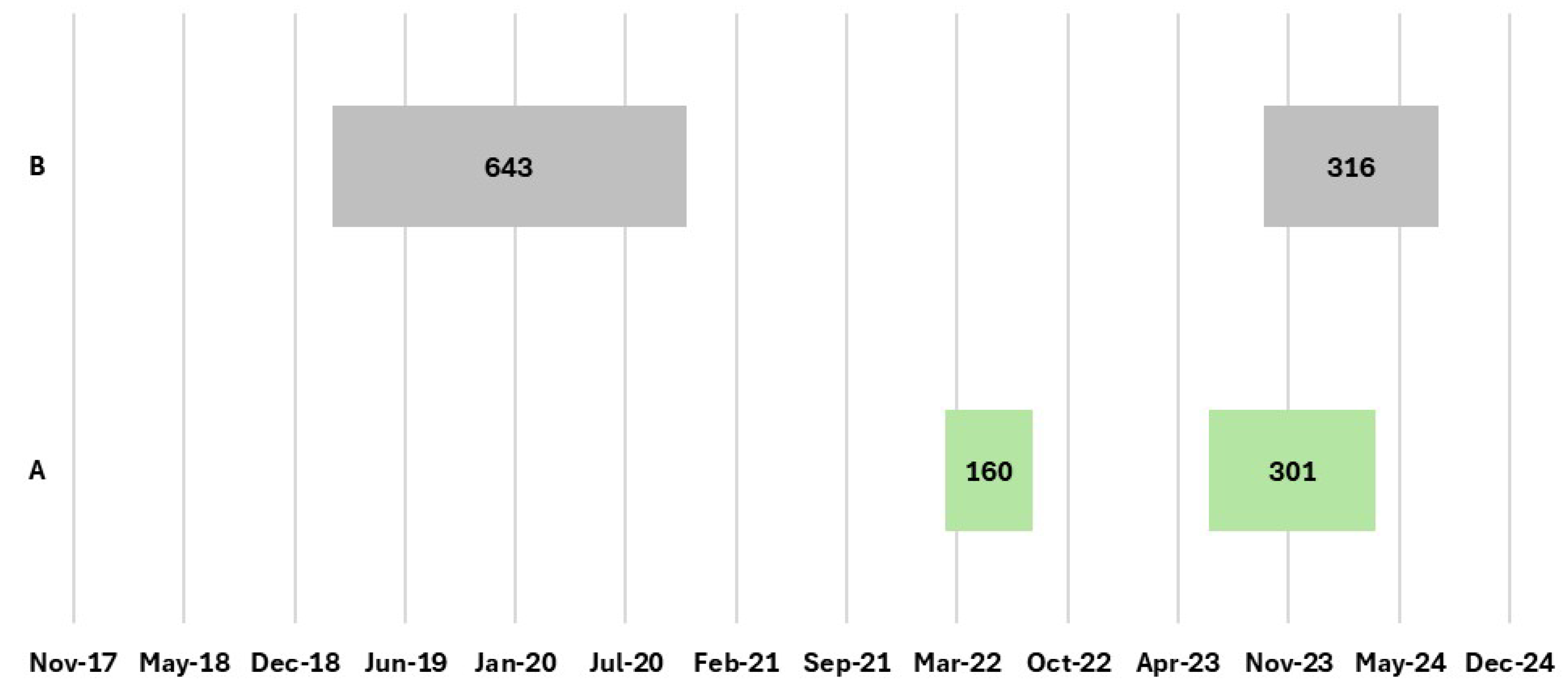
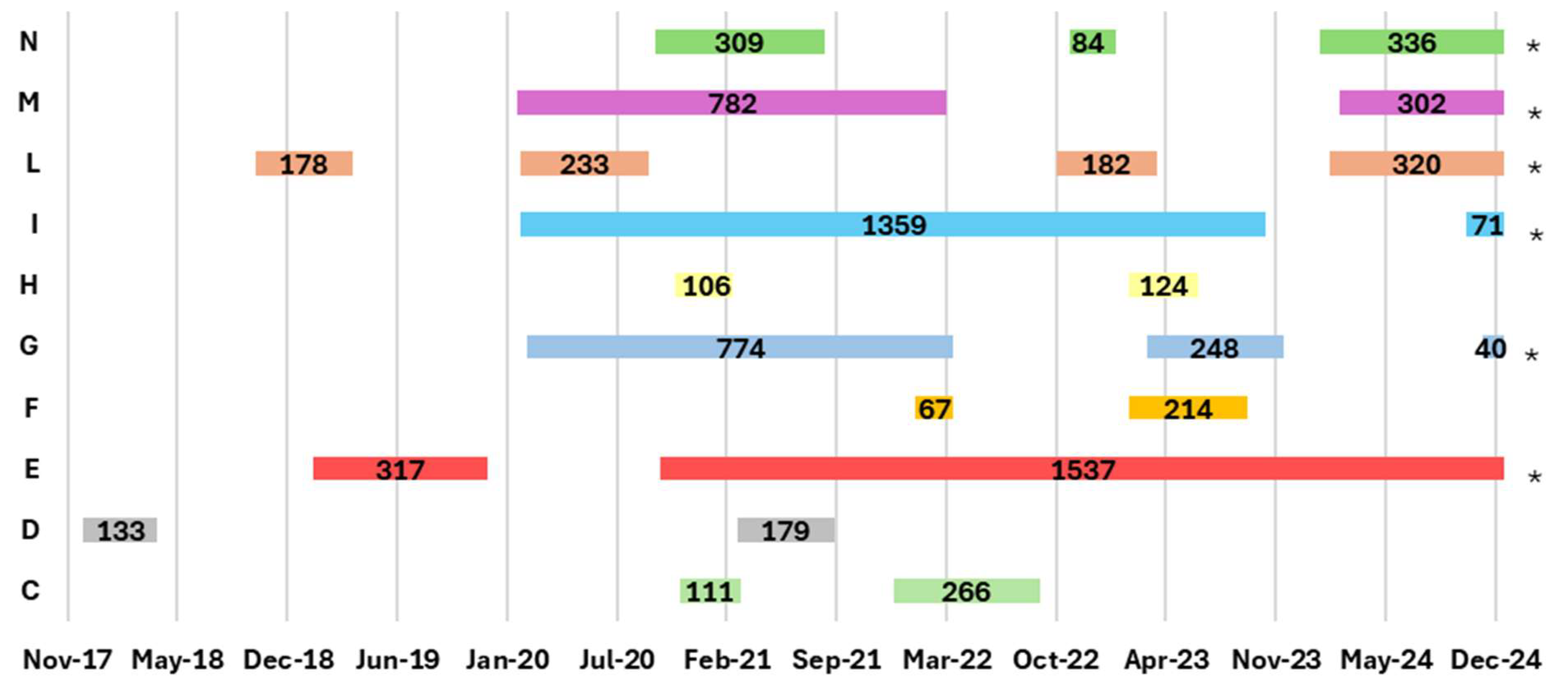
- Establishments that registered outbreaks in the last 5 years;
- -
- The length of time registered from confirmation to eradication of cluster outbreaks;
- -
- The length of time from eradication to re-emergence of infection on the same farm;
- -
- Establishments with a suspended EBL-free status in the last 12 months;
- -
- Epidemiological correlations with a confirmed disease case in the last 2 years.
3. Results
3.1. Italian Epidemiological Data Associated with Assessment Risk Indicators
3.2. Italian Epidemiological Data Associated with Assessment Risk Factors Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Righi, C.; Iscaro, C.; Petrini, S.; Lomolino, R.; Feliziani, F. Enzootic bovine leukosis in Italy: Epidemiological issues after free status recognition and measures applied to tackle the last persistent clusters. Pathogens 2021, 10, 1475. [Google Scholar] [CrossRef] [PubMed]
- Benitez, O.J.; Roberts, J.N.; Norby, B.; Bartlett, P.C.; Takeshima, S.N.; Watanuki, S.; Aida, Y.; Grooms, D.L. Breeding bulls as a potential source of bovine leukemia virus transmission in beef herds. JAVMA J. Am. Vet. Med. Assoc. 2019, 254, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Ott, S.L.; Johnson, R.; Wells, S.J. Association between bovine-leukosis virus seroprevalence and herd-level productivity on US dairy farms. Prev. Vet. Med. 2003, 61, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Nekouei, O.; Vanleeuwen, J.; Sanchez, J.; Kelton, D.; Tiwari, A.; Keefe, G. Herd-level risk factors for infection with bovineleukemia virus in Canadian dairy herds. Prev. Vet. Med. 2015, 119, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Kobayashi, S.; Konishi, M.; Kameyama, K.-I.; Tsutsui, T. Nationwide survey of bovine leukemia virus infection among dairy and beef breeding cattle in Japan from 2009–2011. J. Veter. Med. Sci. 2013, 75, 1123–1126. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Gong, Q.; Sheng, C.; Liu, Y.; Ge, G.; Li, D.; Diao, N.; Shi, K.; Li, J.; Sun, Z.; et al. Prevalence of bovine leukemia in 1983–2019 in China: A systematic review and meta-analysis. Microb. Pathog. 2021, 150, 104681. [Google Scholar] [CrossRef] [PubMed]
- Ladronka, R.M.; Ainsworth, S.; Wilkins, M.J.; Norby, B.; Byrem, T.M.; Bartlett, P.C. Prevalence of bovine leukemia virus antibodies in US dairy cattle. Vet. Med. Int. 2018, 2018, 5831278. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.; Wang, J.; Lian, S.; Wang, H.; Wu, R. The global epidemiology of bovine leukemia virus: Current trends and future implications. Animals 2024, 14, 297. [Google Scholar] [CrossRef] [PubMed]
- Commission Implementing Regulation (EU) 2021/620 of 15 April 2021 Laying Down Rules for the Application of Regulation (EU) 2016/429 of the European Parliament and of the Council as Regards the Approval of the Disease-Free and Non-Vaccination Status of Certain Member States or Zones or Compartments thereof as Regards Certain Listed Diseases and the Approval of Eradication Programmes for Those Listed Diseases. Available online: http://data.europa.eu/eli/reg_impl/2021/620/oj (accessed on 26 August 2024).
- OIE Platform WAHIS. Available online: https://wahis.oie.int/#/dashboards/country-or-disease-dashboard (accessed on 31 December 2024).
- EFSA AHAW Panel (European Food Safety Authority Panel on Animal Health and Welfare). Enzootic bovine leukosis. EFSA J. 2015, 13, 4188. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2016/429 of the European Parliament and of the Council of 9 March 2016 on Transmissible Animal Diseases and Amending and Repealing Certain Acts in the Area of Animal Health (‘Animal Health Law’). Available online: http://data.europa.eu/eli/reg/2016/429/oj (accessed on 26 August 2024).
- Commission Implementing Decision (EU) 2017/1910 of 17 October 2017 Amending Decision 93/52/EEC as Regards the Brucellosis (B. melitensis)-Free Status of Certain Regions of Spain, Decision 2003/467/EC as Regards the Official Bovine Brucellosis-Free Status of Cyprus and of Certain Regions of Spain, and as Regards the Official Enzootic-Bovine-Leucosis-Free Status of Italy, and Decision 2005/779/EC as Regards the Swine Vesicular Disease-Free Status of the Region of Campania of Italy. Available online: http://data.europa.eu/eli/dec_impl/2017/1910/oj (accessed on 26 August 2024).
- Commission Delegated Regulation (EU) 2020/689 of 17 December 2019 Supplementing Regulation (EU) 2016/429 of the European Parliament and of the Council as Regards Rules for Surveillance, Eradication Programmes, and Disease-Free Status for Certain Listed and Emerging Diseases. Available online: http://data.europa.eu/eli/reg_del/2020/689/oj (accessed on 26 August 2024).
- World Organization for Animal Health. Chapter 3.4.9 Enzootic bovine leukosis. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; World Organization for Animal Health: Paris, France, 2018; ISBN 978-92-95108-18-9. [Google Scholar]
- VETINFO. Available online: https://www.vetinfo.it/ (accessed on 31 December 2024).
- Miquel, S.; Porta and International Epidemiological Association (Eds.) A Dictionary of Epidemiology, 5th ed; Oxford University Press: Oxford, UK; New York, NY, USA, 2008. [Google Scholar]
- Lancheros-Buitrago, D.J.; Bulla-Castañeda, D.M.; Giraldo-Forero, J.C.; Pulido-Medellin, M.O. Risk factors associated with enzootic bovine leukosis in Boyacá and Cundinamarca municipalities, Colombia. Open Vet. J. 2023, 13, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Panei, C.J.; Pérez Aguirreburualde, M.S.; Echeverría, M.G.; Galosi, C.M.; Torres, A.; Silva, H.J.E. Seroprevalencia de infección por el virus de leucosis bovina durante 2015 en rodeos de críade la Zona Deprimida del Río Salado, provincia de Buenos Aires, Argentina. Analecta Vet. 2017, 37, 65–68. [Google Scholar] [CrossRef][Green Version]
- Ramalho, G.C.; Silva, M.L.C.R.; Falcão, B.M.R.; Limeira, C.H.; Nogueira, D.B.; Dos Santos, A.M.; Martins, C.M.; Alves, C.J.; Clementino, I.J.; Santos, C.D.S.A. High herd-level seroprevalence and associated factors for bovine leukemia virus in the semi-arid Paraíba state, Northeast Region of Brazil. Prev. Vet. Med. 2021, 190, 105324. [Google Scholar] [CrossRef] [PubMed]
- Ordinanza Ministeriale 28 Maggio 2015, Misure Straordinarie di Polizia Veterinaria in Materia di Tubercolosi, Brucellosi Bovina e Bufalina, Brucellosi Ovi-Caprina, Leucosi Bovina Enzootica. Gazzetta Ufficiale Serie Generale n.144, 24 June 2015. Available online: https://www.gazzettaufficiale.it/eli/id/2015/06/24/15A04879/sg (accessed on 31 December 2024).
- Selim, A.; Manaa, E.A.; Alanazi, A.D.; Alyousif, M.S. Seroprevalence, risk factors and molecular identification of bovine leukemia virus in Egyptian cattle. Animals 2021, 11, 319. [Google Scholar] [CrossRef] [PubMed]
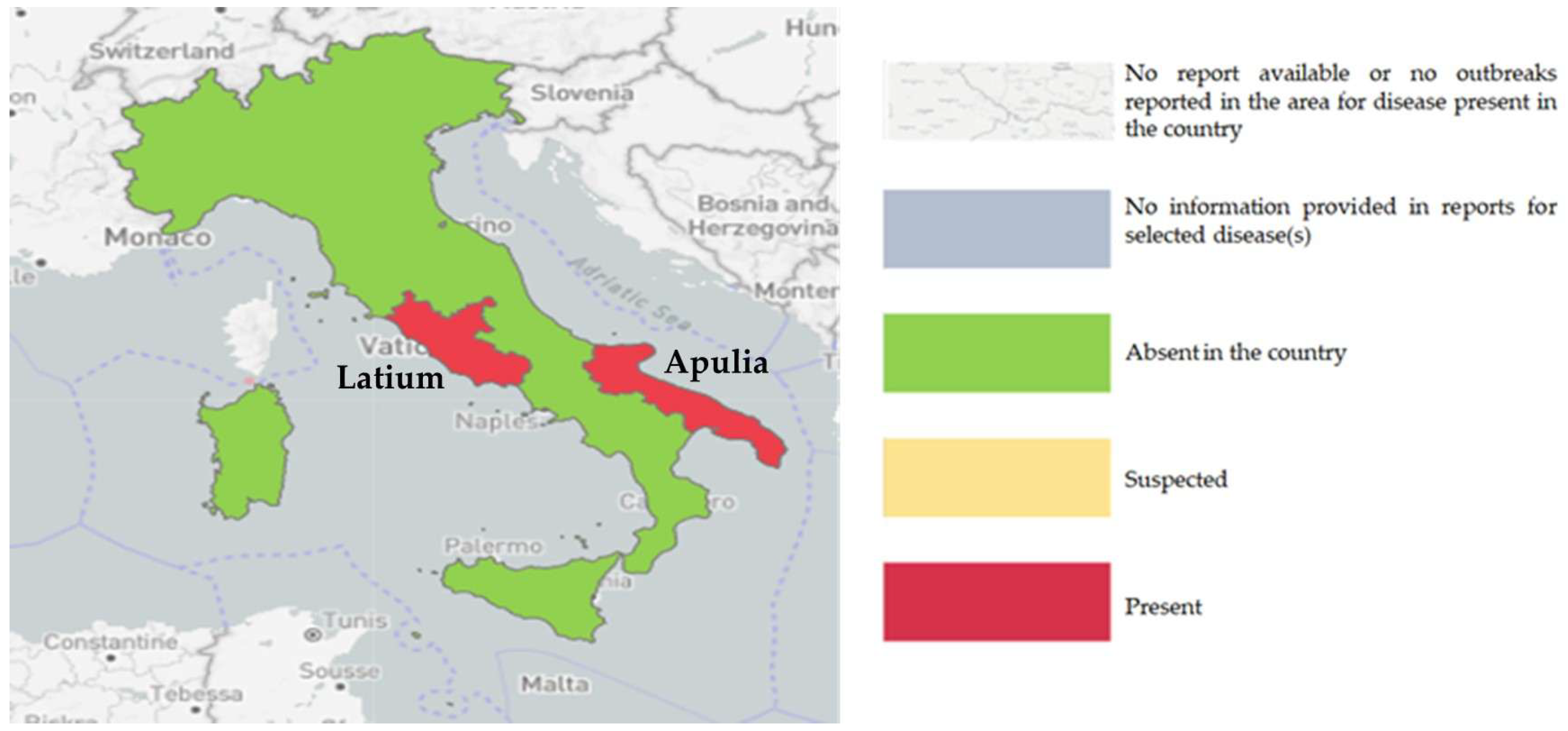
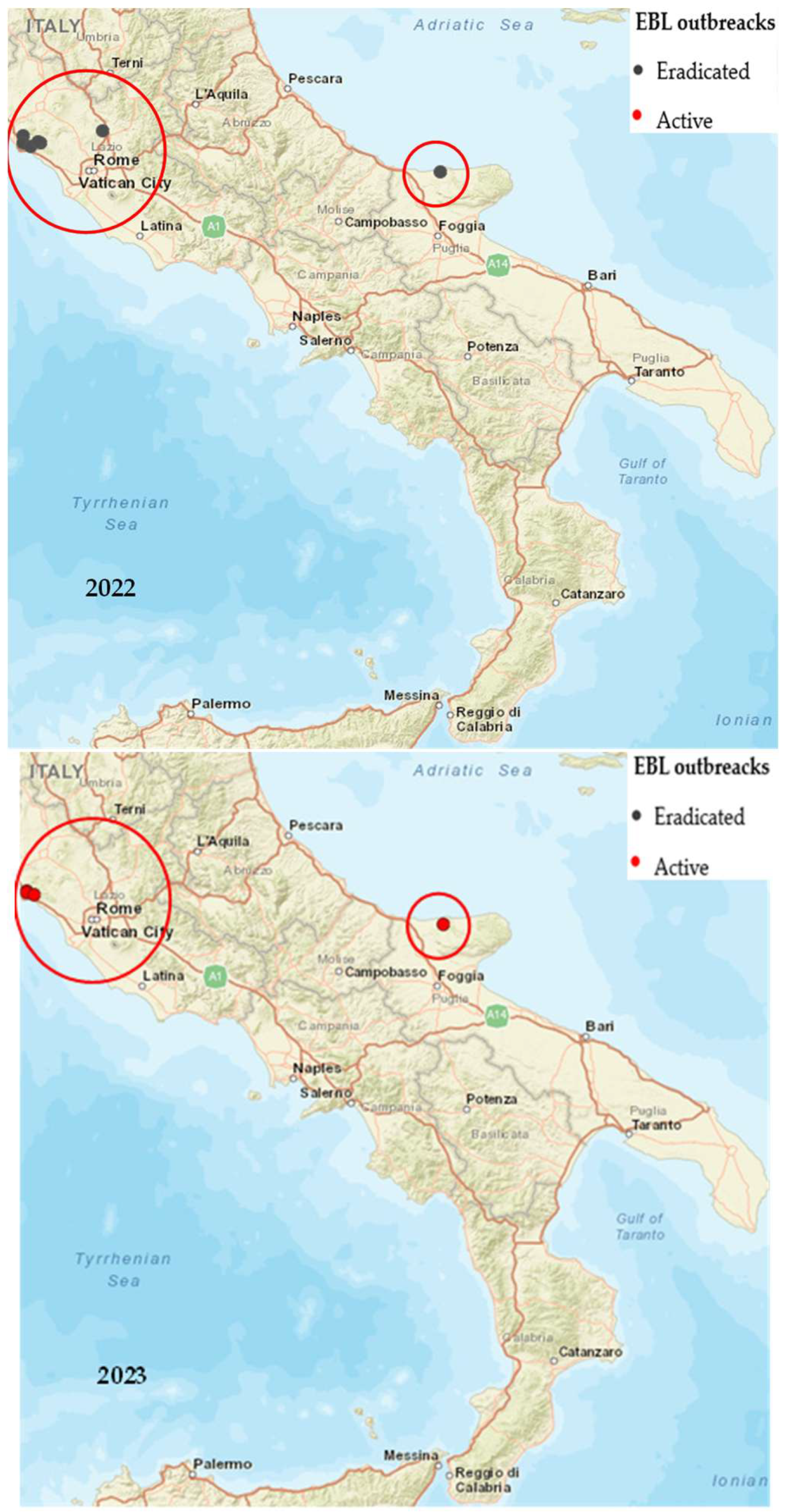


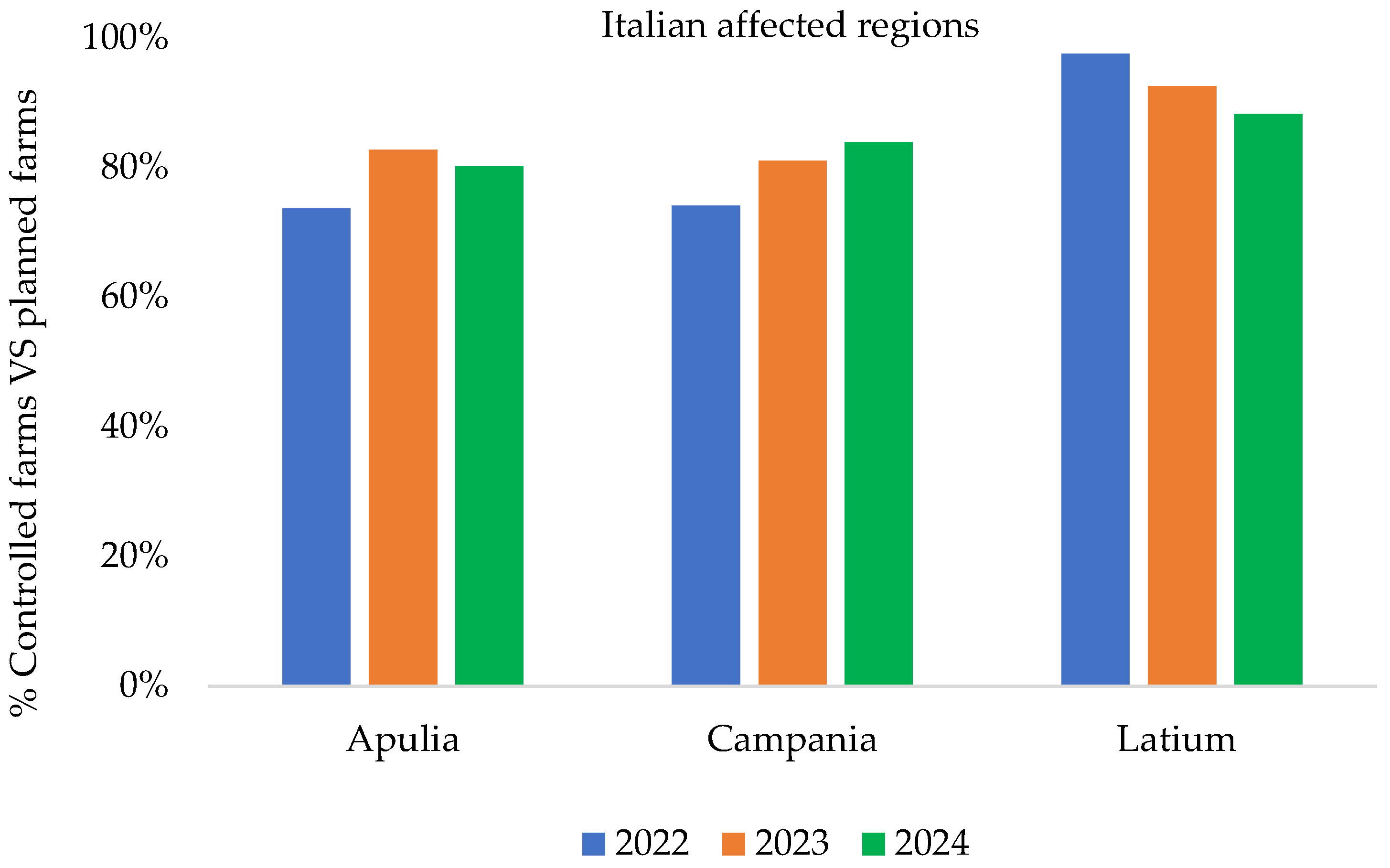
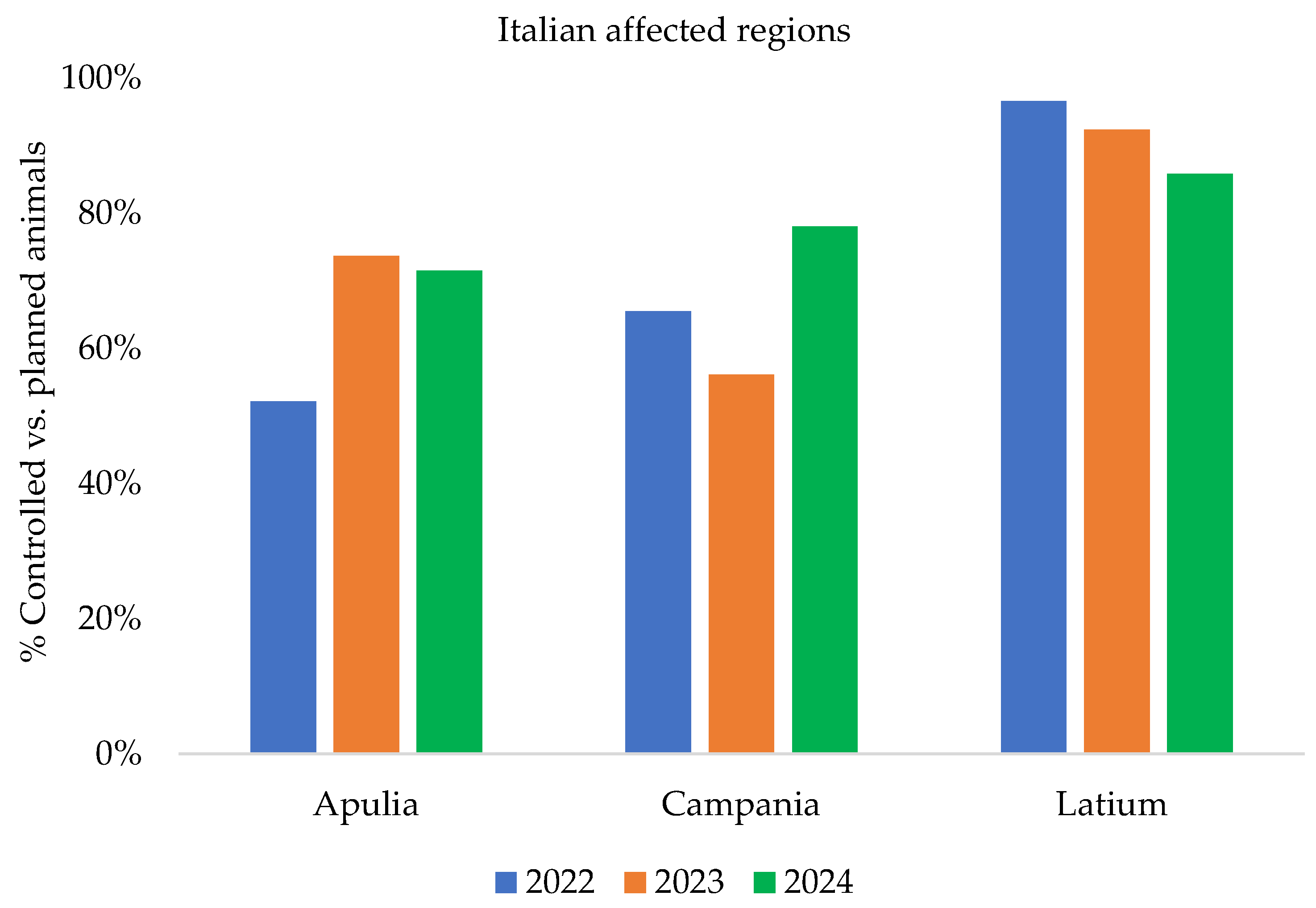
| Years | Number of EBL Outbreaks | |||
|---|---|---|---|---|
| North Italy | Central Italy | South Italy | Total | |
| 2022 | 0 | 6 | 1 | 7 |
| 2023 | 0 | 6 | 3 | 9 |
| 2024 | 0 | 6 | 1 | 7 |
| Total | 0 | 18 | 5 | 23 |
| Years | No. of Controlled Farms | Total No. of EBL Outbreaks | Prevalence of EBL Outbreaks | Total Prevalence of EBL Outbreaks | ||
|---|---|---|---|---|---|---|
| Apulia | Latium | Apulia | Latium | |||
| 2022 | 21.095 | 1 | 6 | 0.004% | 0.02% | 0.03% |
| 2023 | 19.770 | 3 | 6 | 0.01% | 0.03% | 0.04% |
| 2024 | 18.277 | 1 | 6 | 0.005% | 0.03% | 0.03% |
| Years | Animals Controlled | Seropositive Animals | Prevalence of EBL-Seropositive Animals | Total Prevalence of EBL- Seropositive Animals | ||
|---|---|---|---|---|---|---|
| Apulia | Latium | Apulia | Latium | |||
| 2022 | 509.400 | 2 | 8 | 0.0003% | 0.001% | 0.001% |
| 2023 | 569.300 | 6 | 7 | 0.001% | 0.001% | 0.002% |
| 2024 | 550.753 | 4 | 8 | 0.0007% | 0.001% | 0.002% |
| Years | Region | No. of EAOs Under the Eradication Plan | No. of Checked EAOs | % EAO Coverage |
|---|---|---|---|---|
| 2022 | Apulia | 0 | 0 | 0% |
| Campania | 10 | 9 | 90% | |
| Latium | 1 | 0 | 0% | |
| 2023 | Apulia | 0 | 0 | 0% |
| Campania | 9 | 5 | 55.5% | |
| Latium | 1 | 0 | 0% | |
| 2024 | Apulia | 0 | 0 | 0% |
| Campania | 6 | 6 | 100% | |
| Latium | 0 | 0 | 0% |
| Years | Clusters Regions | Percentage of Pastures with Herds’ Movements Within the Own EBL Cluster Region |
|---|---|---|
| 2022 | Apulia | 17.6% |
| Campania | 23.2% | |
| Latium | 15.7% | |
| 2023 | Apulia | 13.6% |
| Campania | 21.5% | |
| Latium | 16.9% | |
| 2024 | Apulia | 12.6% |
| Campania | 22% | |
| Latium | 18.3% |
| Years | EBL Outbreaks Farms in the EBL Cluster Regions with Recurrence in the Previous 5 Years | ||
|---|---|---|---|
| Apulia * | Campania | Latium ** | |
| 2017 | - | - | D |
| 2018 | - | - | D, L |
| 2019 | B | - | E, L |
| 2020 | B | - | C, E, G, H, I, L, M, N |
| 2021 | - | - | C, D, G, H, I, M, N |
| 2022 | A | - | C, F, G, H, I, L, M, N |
| 2023 | A, B | - | F, G, H, I, L, N |
| 2024 | A, B | - | G, I, L, M, N |
| Clusters Regions | No. of Farms with EBL Outbreaks in the Last 5 Years | No. of Farms With EBL-Free Status Suspended in the Last 12 Months | No. of Epidemiological Links with Confirmed EBL Case in the Last 2 Years |
|---|---|---|---|
| Apulia | 2 | 2 | 1 |
| Campania | 0 | 0 | 0 |
| Latium | 10 | 6 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Righi, C.; Iscaro, C.; Petrini, S.; Scoccia, E.; Pirani, S.; Fiorucci, A.; Lomolino, R.; Feliziani, F. An Update of Epidemiological Trends in Enzootic Bovine Leukosis in Italy and an Analysis of Risk Factors Associated with Infection Persistence. Pathogens 2025, 14, 1088. https://doi.org/10.3390/pathogens14111088
Righi C, Iscaro C, Petrini S, Scoccia E, Pirani S, Fiorucci A, Lomolino R, Feliziani F. An Update of Epidemiological Trends in Enzootic Bovine Leukosis in Italy and an Analysis of Risk Factors Associated with Infection Persistence. Pathogens. 2025; 14(11):1088. https://doi.org/10.3390/pathogens14111088
Chicago/Turabian StyleRighi, Cecilia, Carmen Iscaro, Stefano Petrini, Eleonora Scoccia, Silvia Pirani, Alessandro Fiorucci, Roberto Lomolino, and Francesco Feliziani. 2025. "An Update of Epidemiological Trends in Enzootic Bovine Leukosis in Italy and an Analysis of Risk Factors Associated with Infection Persistence" Pathogens 14, no. 11: 1088. https://doi.org/10.3390/pathogens14111088
APA StyleRighi, C., Iscaro, C., Petrini, S., Scoccia, E., Pirani, S., Fiorucci, A., Lomolino, R., & Feliziani, F. (2025). An Update of Epidemiological Trends in Enzootic Bovine Leukosis in Italy and an Analysis of Risk Factors Associated with Infection Persistence. Pathogens, 14(11), 1088. https://doi.org/10.3390/pathogens14111088






