Mechanisms of Interleukin-10-Mediated Immunosuppression in Viral Infections
Abstract
1. Introduction
2. Methods
3. IL-10 Production During Viral Infections
3.1. The IL-10 Family of Cytokines
3.2. The Role of IL-10 During Viral Infections
| Virus | The Main Role in the Immune Response | States | Cellular Origin | Induce Viral Protein | Reference |
|---|---|---|---|---|---|
| SARS-CoV-2 | (1). Inhibition of T cell expansion (2). Enhancement of the differentiation of lung effector T cells into CD69+CD103+ tissue resident memory cells (3). Anti-viral activity and anti-inflammatory effects (4). Enhancement of ACE2 receptor mRNA expression in lung-derived and endothelial cells (5). Impairment of the MAIT cell response (6). Disease severity | Acute infection | Unknown | Unknown | [3,32,33,34,35] |
| H1N1 | (1). Anti-inflammatory (2). Prediction of progression to a fatal outcome in H1N1 | Acute infection | Unknown | Unknown | [36] |
| RSV | (1). Disease severity (2). Decrease in IFN-I secretion by alveolar macrophages (3). Inhibition of disease and inflammation (4). Dampening of effector T cell responses (5). Inhibition of pro-inflammatory cytokines and chemokines | Acute infection | CD4+ T cells, CD8+ T cells, nBreg cells | Unknown | [5,32,37,38] |
| HIV | (1). Increased of CD16high monocytes, sCD163 and sCD14 (2). Inhibition of NK cell functions (3). The development of comorbidities in patients with HIV (4). Inhibition of adaptive immune responses and inflammation | Chronic infection | Bregs, NK cells, CD8+ T cells, monocyte | Tat protein, gp41 envelope protein | [39,40,41,42,43] |
| SIV | (1). Establishment of the reservoir and persistence (2). Inhibition of the inflammation response (3). Loss of CD4+ T cells (4). Increase in virus replication | Chronic infection | B cells (B10 cells) | Unknown | [7,44] |
| HAV | Impact on the immune response and liver damage | Acute infection | Unknown | Unknown | [45] |
| HBV | (1). Increased severity of chronic HBV infection (2). Prediction of the prognosis of patients with (3). Inhibition of effector T-cells (4). Enhancement of regulatory T-cells (5). Inhibition of cytotoxic CD4+ T cell activity | Chronic infection, acute-on-chronic liver failure | Bregs, B Cells | Unknown | [46,47,48] |
| HCV | (1). Increase in susceptibility to chronic HCV (2). Predictive marker of recovery from an active HCV infection (3). Inhibition of CD4+ and CD8+ T cells | Chronic infection | B cells, CD4+ primary T cells | HCV-core protein | [49,50]. |
| HEV | Unknown | Acute infection | γδ cells | Unknown | [51] |
| LCMV | (1). Functional exhaustion of antiviral CD8+ and CD4+ T cells (2). Decrease in cytokine production (3). Inhibition of the proliferative potential of NK cells (4). Enhancement of virus replication | Chronic infection | Dendritic cells, Macrophage, T cell, NK cells | Unknown | [52,53] |
| CSFV | Unknown | Unknown | PK-15 cells, monocyte-derived dendritic cells | Erns, E1, and E2 | [54,55] |
| FMDV | (1). Lymphopenia is involved in downregulating apoptosis, trafficking, and the coinhibitory expression of lymphocytes (2). Inhibition of T cell proliferation | Acute infection | Macrophage | Unknown | [9,31,56] |
| ASFV | Interfering with immune responses by controlling antiviral IFN levels and a cell-mediated immune response | Unknown | Unknown | Unknown | [57] |
| PRRSV | (1). Immunosuppression (2). Enhancement of virus replication via enhancing CD169 expression | Unknown | MoDC, porcine alveolar macrophages | N protein, nsp2, and nsp5 | [12,17,28,58,59,60] |
| PCV2 | (1). Promotion of PCV2 persistent infection by aggravating the tissue lesions through suppression of T cell infiltration (2). The thymic depletion of pigs | Unknown | Macrophages | Rep, Cap | [29,61,62,63,64] |
| TMEV | Decrease in the kinetics of virus clearance at early times after infection and ameliorating disease at later times | Unknown | T cells | Unknown | [65] |
| HPV | (1). Immunosuppression (2). Enhancement of virus persistence | Cervical cancer cells | HPV E2, E6 and E7 proteins | [66,67] |
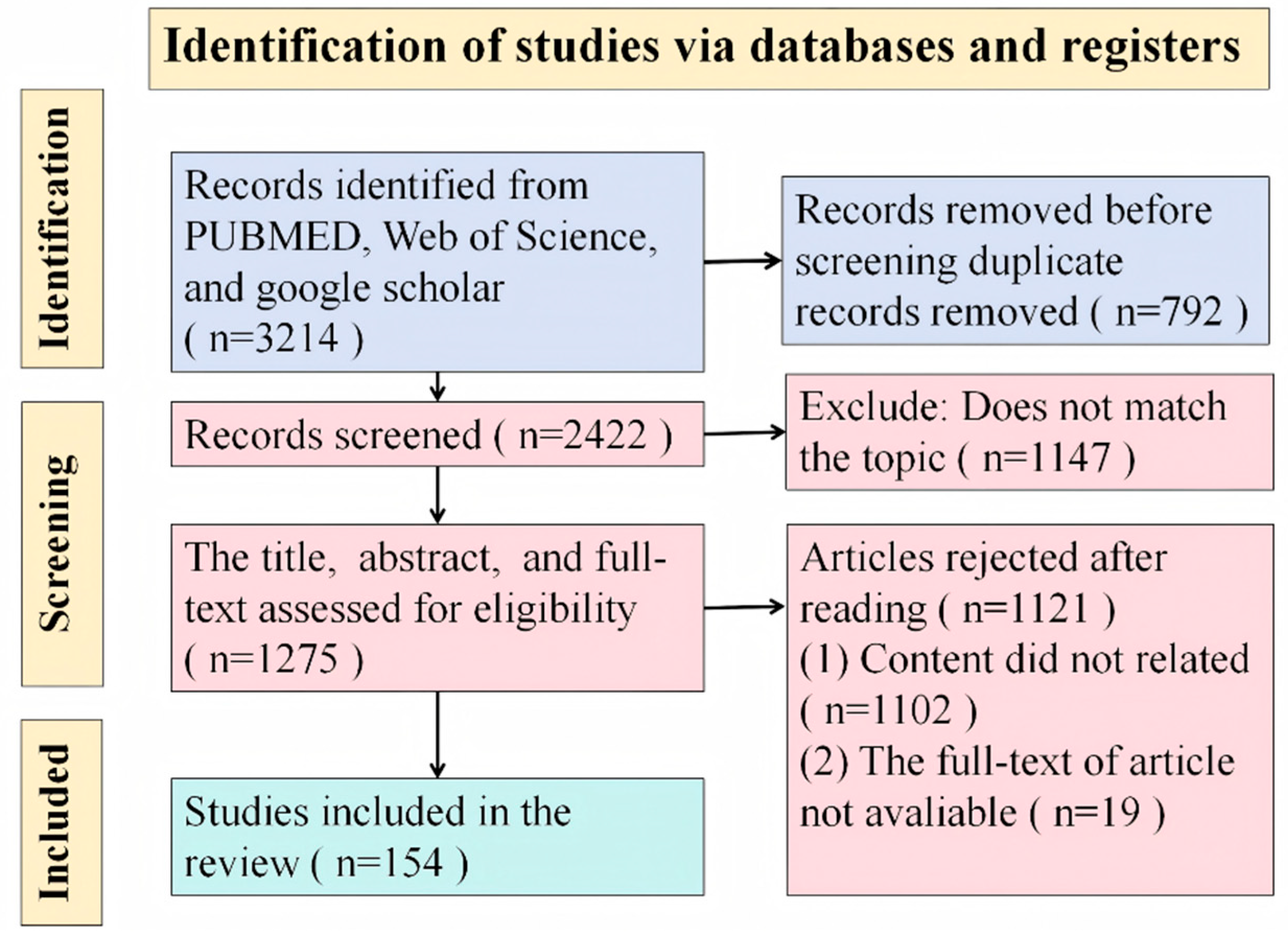
3.3. Cellular Sources and the Regulation of IL-10 Production
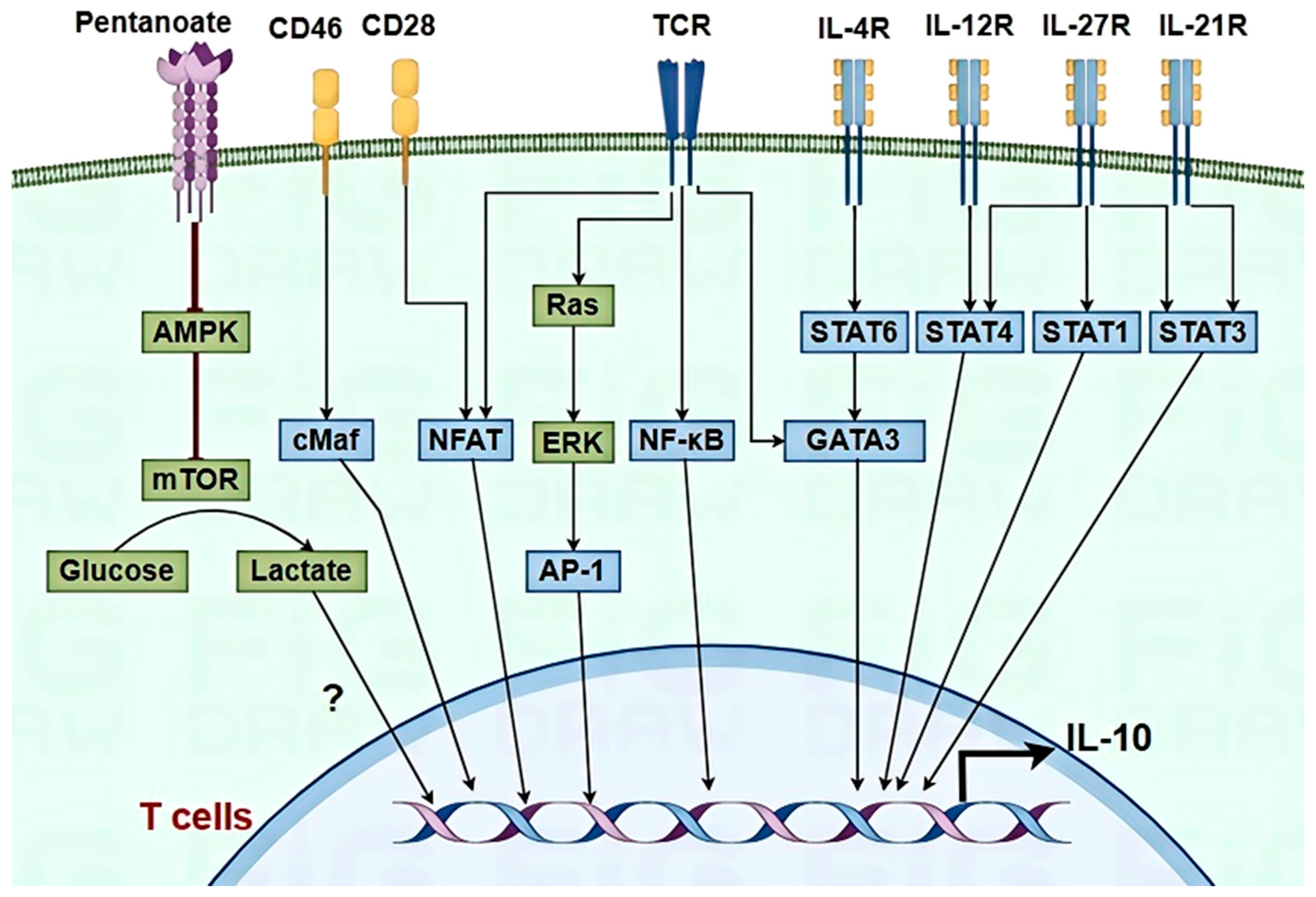
3.3.1. T Cells
3.3.2. Macrophages and DCs
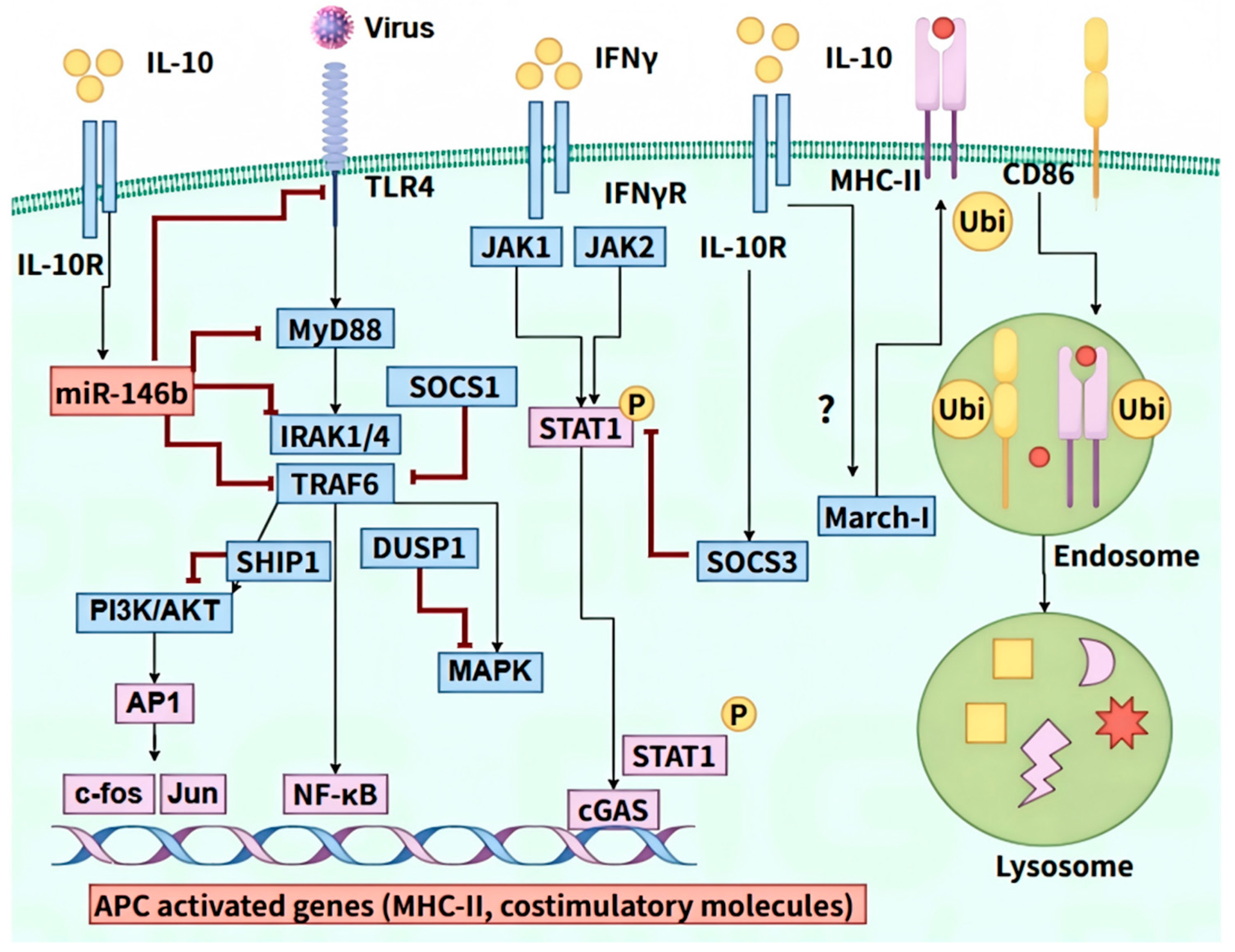
4. Mechanisms of IL-10-Mediated Immunosuppression
4.1. Reduction in Recruitment and Activation of Immune Cells
4.2. Inhibition of Antigen Presentation
4.3. Inhibition of T Cells Activation and Expansion
4.4. Modulation of Immune Cell Differentiation
4.5. Induction of Immune Cell Apoptosis
4.6. Co-Inhibitory Molecular Expression of T Cells Exhaustion
5. Therapeutic Implications and Future Directions
5.1. Utilizing IL-10 Modulation in Antiviral Therapies
5.2. Future Research Challenges and Potential Developments in IL-10 Targeting Strategies
6. Conclusion and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saraiva, M.; Vieira, P.; O’garra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 2019, 217, e20190418. [Google Scholar] [CrossRef]
- Rojas, J.M.; Avia, M.; Martín, V.; Sevilla, N. IL-10: A Multifunctional Cytokine in Viral Infections. J. Immunol. Res. 2017, 2017, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.E.; Foreman, T.W.; Fukutani, E.R.; Kauffman, K.D.; Sakai, S.; Fleegle, J.D.; Gomez, F.; Program, N.T.I.; Gould, S.T.; Le Nouën, C.; et al. IL-10 suppresses T cell expansion while promoting tissue-resident memory cell formation during SARS-CoV-2 infection in rhesus macaques. PLoS Pathog. 2024, 20, e1012339. [Google Scholar] [CrossRef]
- Sun, J.; Madan, R.; Karp, C.L.; Braciale, T.J. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat. Med. 2009, 15, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Loebbermann, J.; Schnoeller, C.; Thornton, H.; Durant, L.; Sweeney, N.P.; Schuijs, M.; O’GArra, A.; Johansson, C.; Openshaw, P.J.; Chu, H.W. IL-10 Regulates Viral Lung Immunopathology during Acute Respiratory Syncytial Virus Infection in Mice. PLoS ONE 2012, 7, e32371. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, S.D.; Wherry, E.J. IL-10, T cell exhaustion and viral persistence. Trends Microbiol. 2007, 15, 143–146. [Google Scholar] [CrossRef]
- Harper, J.; Ribeiro, S.P.; Chan, C.N.; Aid, M.; Deleage, C.; Micci, L.; Pino, M.; Cervasi, B.; Raghunathan, G.; Rimmer, E.; et al. Interleukin-10 contributes to reservoir establishment and persistence in SIV-infected macaques treated with antiretroviral therapy. J. Clin. Investig. 2022, 132, e155251. [Google Scholar] [CrossRef]
- Malavige, G.N.; Jeewandara, C.; Alles, K.M.L.; Salimi, M.; Gomes, L.; Kamaladasa, A.; Jayaratne, S.D.; Ogg, G.S.; Halstead, S.B. Suppression of Virus Specific Immune Responses by IL-10 in Acute Dengue Infection. PLoS Neglected Trop. Dis. 2013, 7, e2409. [Google Scholar] [CrossRef]
- Guo, Z.; Zhao, Y.; Zhang, Z.; Li, Y. Interleukin-10-Mediated Lymphopenia Caused by Acute Infection with Foot-and-Mouth Disease Virus in Mice. Viruses 2021, 13, 2358. [Google Scholar] [CrossRef]
- De Bruyn, A.; Verellen, S.; Bruckers, L.; Geebelen, L.; Callebaut, I.; De Pauw, I.; Stessel, B.; Dubois, J. Secondary infection in COVID-19 critically ill patients: A retrospective single-center evaluation. BMC Infect. Dis. 2022, 22, 1–7. [Google Scholar] [CrossRef]
- Kekarainen, T.; Segalés, J. Porcine circovirus 2 immunology and viral evolution. Porc. Heal. Manag. 2015, 1, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mu, G.; Dang, R.; Yang, Z. Up-regulation of IL-10 upon PRRSV vaccination impacts on the immune response against CSFV. Veter- Microbiol. 2016, 197, 68–71. [Google Scholar] [CrossRef]
- Kimball, J.; Zhu, Y.; Wyatt, D.; Trabue, C.H.; Talbot, H.K. Influenza Vaccine Failure Associated with Age and Immunosuppression. J. Infect. Dis. 2020, 224, 288–293. [Google Scholar] [CrossRef]
- Mittal, S.K.; Roche, P.A. Suppression of antigen presentation by IL-10. Curr. Opin. Immunol. 2015, 34, 22–27. [Google Scholar] [CrossRef]
- Martin, N.M.; Griffin, D.E. Effect of IL-10 Deficiency on TGFβ Expression during Fatal Alphavirus Encephalomyelitis in C57Bl/6 Mice. Viruses 2022, 14, 1791. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Bi, J.; Wang, D.; Fang, L.; Zhang, L.; Li, F.; Chen, H.; Xiao, S. Porcine reproductive and respiratory syndrome virus infection activates IL-10 production through NF-κB and p38 MAPK pathways in porcine alveolar macrophages. Dev. Comp. Immunol. 2013, 39, 265–272. [Google Scholar] [CrossRef]
- Poole, E.; Neves, T.C.; Oliveira, M.T.; Sinclair, J.; da Silva, M.C.C. Human Cytomegalovirus Interleukin 10 Homologs: Facing the Immune System. Front. Cell. Infect. Microbiol. 2020, 10, 245. [Google Scholar] [CrossRef]
- Koelink, P.J.; Bloemendaal, F.M.; Li, B.; Westera, L.; Vogels, E.W.M.; van Roest, M.; Gloudemans, A.K.; Wout, A.B.v.; Korf, H.; Vermeire, S.; et al. Anti-TNF therapy in IBD exerts its therapeutic effect through macrophage IL-10 signalling. Gut 2019, 69, 1053–1063. [Google Scholar] [CrossRef]
- Walter, M.R. Structure of interleukin-10/interleukin-10R1 complex: A paradigm for class 2 cytokine activation. Immunol. Res. 2002, 26, 303–308. [Google Scholar] [CrossRef]
- Minshawi, F.; Lanvermann, S.; McKenzie, E.; Jeffery, R.; Couper, K.; Papoutsopoulou, S.; Roers, A.; Muller, W. The Generation of an Engineered Interleukin-10 Protein with Improved Stability and Biological Function. Front. Immunol. 2020, 11, 1794. [Google Scholar] [CrossRef] [PubMed]
- Josephson, K.; Logsdon, N.J.; Walter, M.R. Crystal Structure of the IL-10/IL-10R1 Complex Reveals a Shared Receptor Binding Site. Immunity 2001, 15, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Tsutsumi, N.; Su, L.L.; Abhiraman, G.C.; Mohan, K.; Henneberg, L.T.; Aduri, N.G.; Gati, C.; Garcia, K.C. Structure-based decoupling of the pro- and anti-inflammatory functions of interleukin-10. Science 2021, 371, eabc8433. [Google Scholar] [CrossRef]
- Abosalif, K.O.A.; Abdalla, A.E.; Junaid, K.; Eltayeb, L.B.; Ejaz, H. The interleukin-10 family: Major regulators of the immune response against Plasmodium falciparum infections. Saudi J. Biol. Sci. 2023, 30, 103805. [Google Scholar] [CrossRef]
- Chan, I.H.; Wu, V.; Bilardello, M.; Mar, E.; Oft, M.; Van Vlasselaer, P.; Mumm, J.B. The Potentiation of IFN-γ and Induction of Cytotoxic Proteins by Pegylated IL-10 in Human CD8 T Cells. J. Interf. Cytokine Res. 2015, 35, 948–955. [Google Scholar] [CrossRef]
- Han, H.; Ma, Q.; Li, C.; Liu, R.; Zhao, L.; Wang, W.; Zhang, P.; Liu, X.; Gao, G.; Liu, F.; et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microbes Infect. 2020, 9, 1123–1130. [Google Scholar] [CrossRef]
- McKelvey, M.; Uddin, B.; Palani, S.; Shao, S.; Sun, K. IL-10 Counteracts IFN-γ to Alleviate Acute Lung Injury in a Viral-Bacterial Superinfection Model. Am. J. Respir. Cell Mol. Biol. 2024, 71, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.P.; Strongin, Z.; Soudeyns, H.; Ten-Caten, F.; Ghneim, K.; Sanchez, G.P.; de Medeiros, G.X.; Estrada, P.M.D.R.; Pelletier, A.-N.; Hoang, T.; et al. Dual blockade of IL-10 and PD-1 leads to control of SIV viral rebound following analytical treatment interruption. Nat. Immunol. 2024, 25, 1900–1912. [Google Scholar] [CrossRef] [PubMed]
- Singleton, H.; Graham, S.P.; Frossard, J.-P.; Bodman-Smith, K.B.; Steinbach, F. Infection of monocytes with European porcine reproductive and respiratory syndrome virus (PRRSV-1) strain Lena is significantly enhanced by dexamethasone and IL-10. Virology 2018, 517, 199–207. [Google Scholar] [CrossRef]
- Wu, X.; Wang, X.; Shi, T.; Luo, L.; Qiao, D.; Wang, Z.; Han, C.; Du, Q.; Tong, D.; Huang, Y. Porcine Circovirus Type 2 Rep Enhances IL-10 Production in Macrophages via Activation of p38-MAPK Pathway. Viruses 2019, 11, 1141. [Google Scholar] [CrossRef]
- Han, J.; Zhang, S.; Zhang, Y.; Chen, M.; Lv, Y. Porcine circovirus type 2 increases interleukin-1beta and interleukin-10 production via the MyD88–NF-kappa B signaling pathway in porcine alveolar macrophages in vitro. J. Veter-Sci. 2017, 18, 183–191. [Google Scholar] [CrossRef]
- Segundo, F.D.-S.; Rodríguez-Calvo, T.; de Avila, A.; Sevilla, N.; Ostrowski, M.A. Immunosuppression during Acute Infection with Foot-and-Mouth Disease Virus in Swine Is Mediated by IL-10. PLoS ONE 2009, 4, e5659. [Google Scholar] [CrossRef]
- Shih, L.-J.; Yang, C.-C.; Liao, M.-T.; Lu, K.-C.; Hu, W.-C.; Lin, C.-P. An important call: Suggestion of using IL-10 as therapeutic agent for COVID-19 with ARDS and other complications. Virulence 2023, 14, 2190650. [Google Scholar] [CrossRef]
- Albini, A.; Calabrone, L.; Carlini, V.; Benedetto, N.; Lombardo, M.; Bruno, A.; Noonan, D.M. Preliminary Evidence for IL-10-Induced ACE2 mRNA Expression in Lung-Derived and Endothelial Cells: Implications for SARS-CoV-2 ARDS Pathogenesis. Front. Immunol. 2021, 12, 718136. [Google Scholar] [CrossRef] [PubMed]
- Luporini, R.L.; Rodolpho, J.M.d.A.; Kubota, L.T.; Martin, A.C.B.M.; Cominetti, M.R.; Anibal, F.d.F.; Pott-Junior, H. IL-6 and IL-10 are associated with disease severity and higher comorbidity in adults with COVID-19. Cytokine 2021, 143, 155507. [Google Scholar] [CrossRef]
- Yang, Q.; Wen, Y.; Qi, F.; Gao, X.; Chen, W.; Xu, G.; Wei, C.; Wang, H.; Tang, X.; Lin, J.; et al. Suppressive Monocytes Impair MAIT Cells Response via IL-10 in Patients with Severe COVID-19. J. Immunol. 2021, 207, 1848–1856. [Google Scholar] [CrossRef]
- Alagarasu, K.; Kaushal, H.; Shinde, P.; Kakade, M.; Chaudhary, U.; Padbidri, V.; Sangle, S.A.; Salvi, S.; Bavdekar, A.R.; D’costa, P.; et al. TNFA and IL10 Polymorphisms and IL-6 and IL-10 Levels Influence Disease Severity in Influenza A(H1N1)pdm09 Virus Infected Patients. Genes 2021, 12, 1914. [Google Scholar] [CrossRef]
- Kostadinova, E.; Angelova, S.; Tsonkova-Popova, T.; Zlateva, D.; Yordanova, R.; Stanilova, S. Systemic IL-10 and IFN-γ Levels in Respiratory Syncytial Virus- and Rhinovirus-Infected Bulgarian Children with Acute Bronchiolitis and Their Impact on Clinical Manifestation. Pathogens 2025, 14, 426. [Google Scholar] [CrossRef]
- Laubreton, D.; Drajac, C.; Eléouët, J.-F.; Rameix-Welti, M.-A.; Lo-Man, R.; Riffault, S.; Descamps, D. Regulatory B Lymphocytes Colonize the Respiratory Tract of Neonatal Mice and Modulate Immune Responses of Alveolar Macrophages to RSV Infection in IL-10-Dependant Manner. Viruses 2020, 12, 822. [Google Scholar] [CrossRef]
- Takahashi, N.; Eltalkhawy, Y.M.; Nasu, K.; Abdelnaser, R.A.; Monde, K.; Habash, S.A.; Nasser, H.; Hiyoshi, M.; Ishimoto, T.; Suzu, S. IL-10 induces activated phenotypes of monocytes observed in virally-suppressed HIV-1-infected individuals. Biochem. Biophys. Res. Commun. 2024, 729, 150342. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yang, M.; Sun, X.; Chen, X.; Ma, M.; Yin, X.; Qian, S.; Zhang, Z.; Fu, Y.; Liu, J.; et al. IL-10+ NK and TGF-β+ NK cells play negative regulatory roles in HIV infection. BMC Infect. Dis. 2018, 18, 1–10. [Google Scholar] [CrossRef]
- Cortelette, N.A.; Souza, N.D.O.; Cataldi-Rodrigues, L.; Arthur, C.; Stowell, S.R.; Dias-Baruffi, M.; Guimarães, D.A.M.; Ayres, L.R.; Pancoto, J.A.T.; Saranathan, M. Functional evaluation of immunoregulatory molecules HLA-G, galectin-1, and IL-10 in people living with HIV. Medicine 2022, 101, e28489. [Google Scholar] [CrossRef]
- Liu, J.; Zhan, W.; Kim, C.J.; Clayton, K.; Zhao, H.; Lee, E.; Cao, J.C.; Ziegler, B.; Gregor, A.; Yue, F.Y.; et al. IL-10-Producing B Cells Are Induced Early in HIV-1 Infection and Suppress HIV-1-Specific T Cell Responses. PLoS ONE 2014, 9, e89236. [Google Scholar] [CrossRef]
- Barcova, M.; Kacani, L.; Speth, C.; Dierich, M.P. gp41 Envelope Protein of Human Immunodeficiency Virus Induces Interleukin (IL)-10 in Monocytes, but Not in B, T, or NK Cells, Leading to Reduced IL-2 and Interferon- Production. J. Infect. Dis. 1998, 177, 905–913. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, M.; Zhang, M.; Xiao, Y.; Zheng, Y.; Tian, R. Dynamic changes and effects of IL-10 secretion B cells during the progression of HIV-1/SIV disease. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi = Chin. J. Cell. Mol. Immunol. 2022, 38, 391–399. [Google Scholar]
- Hussein, Z.A.; Al-Ahmar, S.D. IL-10 and IL-18: Key players in liver damage associated with hepatitis A virus infection. Hum. Antibodies 2024, 32, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, L.-S.; Wu, S.-D.; Wang, S.-Q.; Li, L.; She, W.-M.; Li, J.; Wang, J.-Y.; Jiang, W. IL-10-producing regulatory B-cells suppressed effector T-cells but enhanced regulatory T-cells in chronic HBV infection. Clin. Sci. 2016, 130, 907–919. [Google Scholar] [CrossRef]
- Gao, L.; Chen, X.; Zhang, L.; Wu, D.; Zhao, H.; Niu, J. Association of IL-10 polymorphisms with hepatitis B virus infection and outcome in Han population. Eur. J. Med Res. 2016, 21, 1–6. [Google Scholar] [CrossRef]
- Xue, H.; Lin, F.; Tan, H.; Zhu, Z.-Q.; Zhang, Z.-Y.; Zhao, L.; Gray, C.M. Overrepresentation of IL-10-Expressing B Cells Suppresses Cytotoxic CD4+ T Cell Activity in HBV-Induced Hepatocellular Carcinoma. PLoS ONE 2016, 11, e0154815. [Google Scholar] [CrossRef] [PubMed]
- Owusu, D.O.; Phillips, R.; Owusu, M.; Sarfo, F.S.; Frempong, M. Increased levels of circulating IL-10 in persons recovered from hepatitis C virus (HCV) infection compared with persons with active HCV infection. BMC Res. Notes 2020, 13, 1–7. [Google Scholar] [CrossRef]
- Fernandez-Ponce, C.; Dominguez-Villar, M.; Aguado, E.; Garcia-Cozar, F.; Unutmaz, D. CD4+ Primary T Cells Expressing HCV-Core Protein Upregulate Foxp3 and IL-10, Suppressing CD4 and CD8 T Cells. PLoS ONE 2014, 9, e85191. [Google Scholar] [CrossRef]
- Barragué, H.; Fontaine, J.; Abravanel, F.; Mauré, E.; Péron, J.-M.; Alric, L.; Dubois, M.; Izopet, J.; Champagne, E. Mobilization of γδ T Cells and IL-10 Production at the Acute Phase of Hepatitis E Virus Infection in Cytomegalovirus Carriers. J. Immunol. 2021, 206, 1027–1038. [Google Scholar] [CrossRef]
- Richter, K.; Perriard, G.; Behrendt, R.; Schwendener, R.A.; Sexl, V.; Dunn, R.; Kamanaka, M.; Flavell, R.A.; Roers, A.; Oxenius, A.; et al. Macrophage and T Cell Produced IL-10 Promotes Viral Chronicity. PLoS Pathog. 2013, 9, e1003735. [Google Scholar] [CrossRef]
- Hackstein, C.-P.; Spitzer, J.; Symeonidis, K.; Horvatic, H.; Bedke, T.; Steglich, B.; Klein, S.; Assmus, L.M.; Odainic, A.; Szlapa, J.; et al. Interferon-induced IL-10 drives systemic T-cell dysfunction during chronic liver injury. J. Hepatol. 2023, 79, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liang, D.; Tian, Y.; Liang, J.; Li, X.; Liu, C.; Liang, J.; Luo, T.R.; Li, X. Classical Swine Fever Virus Envelope Glycoproteins Erns, E1, and E2 Activate IL-10-STAT1-MX1/OAS1 Antiviral Pathway via Replacing Classical IFNα/β. Biomolecules 2025, 15, 200. [Google Scholar] [CrossRef]
- Chen, L.-J.; Dong, X.-Y.; Shen, H.-Y.; Zhao, M.-Q.; Ju, C.-M.; Yi, L.; Zhang, X.-T.; Kang, Y.-M.; Chen, J.-D. Classical swine fever virus suppresses maturation and modulates functions of monocyte-derived dendritic cells without activating nuclear factor kappa B. Res. Veter-Sci. 2011, 93, 529–537. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, F.; Zhao, S.; Zhang, Z.; Zhang, H.; Bai, L.; Zhang, Z.; Li, Y. IL-10 Promotes CXCL13 Expression in Macrophages Following Foot-and-Mouth Disease Virus Infection. Int. J. Mol. Sci. 2023, 24, 6322. [Google Scholar] [CrossRef]
- Barroso-Arévalo, S.; Barasona, J.A.; Cadenas-Fernández, E.; Sánchez-Vizcaíno, J.M. The Role of Interleukine-10 and Interferon-γ as Potential Markers of the Evolution of African Swine Fever Virus Infection in Wild Boar. Pathogens 2021, 10, 757. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Zeng, K.; Lei, Y.-F.; Chen, X.-H.; Ying, S.-C.; Lv, X.-B.; Wang, Z.; Gao, R. Knockdown expression of IL-10Rα gene inhibits PRRSV replication and elevates immune responses in PBMCs of Tibetan pig in vitro. Veter- Res. Commun. 2017, 42, 11–18. [Google Scholar] [CrossRef]
- Yu, J.; Liu, Y.; Zhang, Y.; Zhu, X.; Ren, S.; Guo, L.; Liu, X.; Sun, W.; Chen, Z.; Cong, X.; et al. The integrity of PRRSV nucleocapsid protein is necessary for up-regulation of optimal interleukin-10 through NF-κB and p38 MAPK pathways in porcine alveolar macrophages. Microb. Pathog. 2017, 109, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Burgara-Estrella, A.; Díaz, I.; Rodríguez-Gómez, I.M.; Essler, S.E.; Hernández, J.; Mateu, E. Predicted Peptides from Non-Structural Proteins of Porcine Reproductive and Respiratory Syndrome Virus Are Able to Induce IFN-γ and IL-10. Viruses 2013, 5, 663–677. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Zhang, H.; He, M.; Zhao, X.; He, J.; Cui, B.; Yang, X.; Tong, D.; Huang, Y. Interleukin-10 Promotes Porcine Circovirus Type 2 Persistent Infection in Mice and Aggravates the Tissue Lesions by Suppression of T Cell Infiltration. Front. Microbiol. 2019, 10, 2050. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Huang, Y.; Wang, T.; Zhang, X.; Chen, Y.; Cui, B.; Li, D.; Zhao, X.; Zhang, W.; Chang, L.; et al. Porcine circovirus type 2 activates PI3K/Akt and p38 MAPK pathways to promote interleukin-10 production in macrophages via Cap interaction of gC1qR. Oncotarget 2016, 7, 17492–17507. [Google Scholar] [CrossRef]
- Doster, A.R.; Subramaniam, S.; Yhee, J.-Y.; Kwon, B.-J.; Yu, C.-H.; Kwon, S.-Y.; Osorio, F.A.; Sur, J.-H. Distribution and characterization of IL-10-secreting cells in lymphoid tissues of PCV2-infected pigs. J. Veter-Sci. 2010, 11, 177–183. [Google Scholar] [CrossRef]
- Kekarainen, T.; Montoya, M.; Mateu, E.; Segalés, J. Porcine circovirus type 2-induced interleukin-10 modulates recall antigen responses. J. Gen. Virol. 2008, 89, 760–765. [Google Scholar] [CrossRef]
- Perlman, S.; Zhao, J. Roles of regulatory T cells and IL-10 in virus-induced demyelination. J. Neuroimmunol. 2017, 308, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Berti, F.C.B.; Pereira, A.P.L.; Cebinelli, G.C.M.; Trugilo, K.P.; de Oliveira, K.B. The role of interleukin 10 in human papilloma virus infection and progression to cervical carcinoma. Cytokine Growth Factor Rev. 2017, 34, 1–13. [Google Scholar] [CrossRef]
- Marina, M.; Bermúdez-Morales, V.H.; Peralta-Zaragoza, O.; Alcocer-González, J.M.; Moreno, J.; Madrid-Marina, V. IL-10 expression is regulated by HPV E2 protein in cervical cancer cells. Mol. Med. Rep. 2011, 4, 369–375. [Google Scholar] [CrossRef][Green Version]
- Martinez-Espinosa, I.; Serrato, J.A.; Ortiz-Quintero, B. Role of IL-10-Producing Natural Killer Cells in the Regulatory Mechanisms of Inflammation during Systemic Infection. Biomolecules 2021, 12, 4. [Google Scholar] [CrossRef]
- Golebski, K.; Layhadi, J.A.; Sahiner, U.; Steveling-Klein, E.H.; Lenormand, M.M.; Li, R.C.Y.; Bal, S.M.; Heesters, B.A.; Vilà-Nadal, G.; Hunewald, O.; et al. Induction of IL-10-producing type 2 innate lymphoid cells by allergen immunotherapy is associated with clinical response. Immunity 2021, 54, 291–307.e7. [Google Scholar] [CrossRef]
- Park, M.-J.; Lee, S.-H.; Kim, E.-K.; Lee, E.-J.; Baek, J.-A.; Park, S.-H.; Kwok, S.-K.; Cho, M.-L. Interleukin-10 produced by myeloid-derived suppressor cells is critical for the induction of Tregs and attenuation of rheumatoid inflammation in mice. Sci. Rep. 2018, 8, 3753. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.; O’GArra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef]
- Ouyang, W.; O’gArra, A. IL-10 Family Cytokines IL-10 and IL-22: From Basic Science to Clinical Translation. Immunity 2019, 50, 871–891. [Google Scholar] [CrossRef]
- Saraiva, M.; Christensen, J.R.; Veldhoen, M.; Murphy, T.L.; Murphy, K.M.; O’GArra, A. Interleukin-10 Production by Th1 Cells Requires Interleukin-12-Induced STAT4 Transcription Factor and ERK MAP Kinase Activation by High Antigen Dose. Immunity 2009, 31, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Helbig, C.; Tykocinski, L.; Kreher, S.; Koeck, J.; Niesner, U.; Radbruch, A. Expression of IL-10 in Th memory lymphocytes is conditional on IL-12 or IL-4, unless the IL-10 gene is imprinted by GATA-3. Eur. J. Immunol. 2007, 37, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Neumann, C.; Heinrich, F.; Neumann, K.; Junghans, V.; Mashreghi, M.-F.; Ahlers, J.; Janke, M.; Rudolph, C.; Mockel-Tenbrinck, N.; Kühl, A.A.; et al. Role of Blimp-1 in programing Th effector cells into IL-10 producers. J. Exp. Med. 2014, 211, 1807–1819. [Google Scholar] [CrossRef] [PubMed]
- Perucha, E.; Melchiotti, R.; Bibby, J.A.; Wu, W.; Frederiksen, K.S.; Roberts, C.A.; Hall, Z.; LeFriec, G.; Robertson, K.A.; Lavender, P.; et al. The cholesterol biosynthesis pathway regulates IL-10 expression in human Th1 cells. Nat. Commun. 2019, 10, 498. [Google Scholar] [CrossRef]
- Luu, M.; Pautz, S.; Kohl, V.; Singh, R.; Romero, R.; Lucas, S.; Hofmann, J.; Raifer, H.; Vachharajani, N.; Carrascosa, L.C.; et al. The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Zhu, A.; Chen, Z.; Yan, Q.; Jiang, M.; Liu, X.; Li, Z.; Li, N.; Tang, C.; Jian, W.; He, J.; et al. Robust mucosal SARS-CoV-2-specific T cells effectively combat COVID-19 and establish polyfunctional resident memory in patient lungs. Nat. Immunol. 2025, 26, 459–472. [Google Scholar] [CrossRef]
- Carlini, V.; Noonan, D.M.; Abdalalem, E.; Goletti, D.; Sansone, C.; Calabrone, L.; Albini, A. The multifaceted nature of IL-10: Regulation, role in immunological homeostasis and its relevance to cancer, COVID-19 and post-COVID conditions. Front. Immunol. 2023, 14, 1161067. [Google Scholar] [CrossRef]
- Nandan, D.; de Oliveira, C.C.; Moeenrezakhanlou, A.; Lopez, M.; Silverman, J.M.; Subek, J.; E Reiner, N. Myeloid Cell IL-10 Production in Response to Leishmania Involves Inactivation of Glycogen Synthase Kinase-3β Downstream of Phosphatidylinositol-3 Kinase. J. Immunol. 2012, 188, 367–378. [Google Scholar] [CrossRef]
- Gabryšová, L.; Howes, A.; Saraiva, M.; O’Garra, A. The regulation of IL-10 expression. Curr Top Microbiol Immunol. 2014, 380, 157–190. [Google Scholar] [CrossRef]
- Mishra, M.; Yadav, M.; Kumar, S.; Kumar, R.; Sen, P. TIM-3 increases the abundance of type-2 dendritic cells during Leishmania donovani infection by enhancing IL-10 production via STAT3. Cell Death Dis. 2023, 14, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Toller-Kawahisa, J.E.; Viacava, P.R.; Palsson-McDermott, E.M.; Nascimento, D.C.; Cervantes-Silva, M.P.; O’CArroll, S.M.; Zotta, A.; Damasceno, L.E.A.; Públio, G.A.; Forti, P.; et al. Metabolic reprogramming of macrophages by PKM2 promotes IL-10 production via adenosine. Cell Rep. 2025, 44, 115172. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, X.; Li, X.; Wang, W.; Huang, Y.; Qin, Y.; Liu, P.; Wu, K.; Li, B.; He, Y.; et al. Foot-and-mouth disease virus activates glycolysis and hijacks HK2 to inhibit innate immunity and promote viral replication. Veter-Res. 2025, 56, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Zhou, M.; Li, Q.; Liu, X.-Z. Chlamydia trachomatis inhibits the production of pro-inflammatory cytokines in human PBMCs through induction of IL-10. J. Med Microbiol. 2018, 67, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Sokol, C.L.; Luster, A.D. The Chemokine System in Innate Immunity. Cold Spring Harb. Perspect. Biol. 2015, 7, a016303. [Google Scholar] [CrossRef]
- Bennett, J.R.; Lateef, Z.; Fleming, S.B.; Mercer, A.A.; Wise, L.M. Orf virus IL-10 reduces monocyte, dendritic cell and mast cell recruitment to inflamed skin. Virus Res. 2016, 213, 230–237. [Google Scholar] [CrossRef]
- Porichis, F.; Hart, M.G.; Zupkosky, J.; Barblu, L.; Kwon, D.S.; McMullen, A.; Brennan, T.; Ahmed, R.; Freeman, G.J.; Kavanagh, D.G.; et al. Differential Impact of PD-1 and/or Interleukin-10 Blockade on HIV-1-Specific CD4 T Cell and Antigen-Presenting Cell Functions. J. Virol. 2014, 88, 2508–2518. [Google Scholar] [CrossRef]
- Mishra, B.; Bachu, M.; Yuan, R.; Wingert, C.; Chaudhary, V.; Brauner, C.; Bell, R.; Ivashkiv, L.B. IL-10 targets IRF transcription factors to suppress IFN and inflammatory response genes by epigenetic mechanisms. Nat. Immunol. 2025, 26, 748–759. [Google Scholar] [CrossRef]
- Murray, P. Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr. Opin. Pharmacol. 2006, 6, 379–386. [Google Scholar] [CrossRef]
- El Kasmi, K.C.; Smith, A.M.; Williams, L.; Neale, G.; Panopoulos, A.D.; Watowich, S.S.; Häcker, H.; Foxwell, B.M.J.; Murray, P.J. Cutting Edge: A Transcriptional Repressor and Corepressor Induced by the STAT3-Regulated Anti-Inflammatory Signaling Pathway. J. Immunol. 2007, 179, 7215–7219. [Google Scholar] [CrossRef]
- Smith, A.M.; Qualls, J.E.; O’BRien, K.; Balouzian, L.; Johnson, P.F.; Schultz-Cherry, S.; Smale, S.T.; Murray, P.J. A Distal Enhancer in Il12b Is the Target of Transcriptional Repression by the STAT3 Pathway and Requires the Basic Leucine Zipper (B-ZIP) Protein NFIL3. J. Biol. Chem. 2011, 286, 23582–23590. [Google Scholar] [CrossRef]
- Hammer, M.; Mages, J.; Dietrich, H.; Schmitz, F.; Striebel, F.; Murray, P.J.; Wagner, H.; Lang, R. Control of dual-specificity phosphatase-1 expression in activated macrophages by IL-10. Eur. J. Immunol. 2005, 35, 2991–3001. [Google Scholar] [CrossRef]
- Schaljo, B.; Kratochvill, F.; Gratz, N.; Sadzak, I.; Sauer, I.; Hammer, M.; Vogl, C.; Strobl, B.; Müller, M.; Blackshear, P.J.; et al. Tristetraprolin Is Required for Full Anti-Inflammatory Response of Murine Macrophages to IL-10. J. Immunol. 2009, 183, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- McCoy, C.E.; Sheedy, F.J.; Qualls, J.E.; Doyle, S.L.; Quinn, S.R.; Murray, P.J.; O’NEill, L.A. IL-10 Inhibits miR-155 Induction by Toll-like Receptors. J. Biol. Chem. 2010, 285, 20492–20498. [Google Scholar] [CrossRef] [PubMed]
- Rajbhandari, P.; Thomas, B.J.; Feng, A.-C.; Hong, C.; Wang, J.; Vergnes, L.; Sallam, T.; Wang, B.; Sandhu, J.; Seldin, M.M.; et al. IL-10 Signaling Remodels Adipose Chromatin Architecture to Limit Thermogenesis and Energy Expenditure. Cell 2018, 172, 218–233.e17. [Google Scholar] [CrossRef] [PubMed]
- Antoniv, T.T.; Ivashkiv, L.B. Interleukin-10-induced gene expression and suppressive function are selectively modulated by the PI3K-Akt-GSK3 pathway. Immunology 2011, 132, 567–577. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Brown, J.R.; Sag, D.; Zhang, L.; Suttles, J. Adenosine 5′-Monophosphate–Activated Protein Kinase Regulates IL-10–Mediated Anti-Inflammatory Signaling Pathways in Macrophages. J. Immunol. 2015, 194, 584–594. [Google Scholar] [CrossRef]
- Emmerich, J.; Mumm, J.B.; Chan, I.H.; LaFace, D.; Truong, H.; McClanahan, T.; Gorman, D.M.; Oft, M. IL-10 Directly Activates and Expands Tumor-Resident CD8+ T Cells without De Novo Infiltration from Secondary Lymphoid Organs. Cancer Res. 2012, 72, 3570–3581. [Google Scholar] [CrossRef]
- York, A.G.; Skadow, M.H.; Oh, J.; Qu, R.; Zhou, Q.D.; Hsieh, W.-Y.; Mowel, W.K.; Brewer, J.R.; Kaffe, E.; Williams, K.J.; et al. IL-10 constrains sphingolipid metabolism to limit inflammation. Nature 2024, 627, 628–635. [Google Scholar] [CrossRef]
- Sundstrom, J.B.; Ansari, A.A. Comparative study of the role of professional versus semiprofessional or nonprofessional antigen presenting cells in the rejection of vascularized organ allografts. Transpl. Immunol. 1995, 3, 273–289. [Google Scholar] [CrossRef]
- Linde, M.H.; Fan, A.C.; Köhnke, T.; Trotman-Grant, A.C.; Gurev, S.F.; Phan, P.; Zhao, F.; Haddock, N.L.; Nuno, K.A.; Gars, E.J.; et al. Reprogramming Cancer into Antigen-Presenting Cells as a Novel Immunotherapy. Cancer Discov. 2023, 13, 1164–1185. [Google Scholar] [CrossRef]
- Roche, P.A.; Furuta, K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat. Rev. Immunol. 2015, 15, 203–216. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Q.; Han, S.; Song, G.; Wang, B.; Wang, Y.; Cai, C. Human cytomegalovirus-IE2 suppresses antigen presentation of macrophage through the IL10/STAT3 signalling pathway in transgenic mouse. PLoS ONE 2025, 20, e0322334. [Google Scholar] [CrossRef]
- Curtale, G.; Mirolo, M.; Renzi, T.A.; Rossato, M.; Bazzoni, F.; Locati, M. Negative regulation of Toll-like receptor 4 signaling by IL-10–dependent microRNA-146b. Proc. Natl. Acad. Sci. USA 2013, 110, 11499–11504. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Kunkel, S.L.; Chang, C.-H. Negative regulation of MyD88-dependent signaling by IL-10 in dendritic cells. Proc. Natl. Acad. Sci. USA 2009, 106, 18327–18332. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, Q.; Wu, W.; Huang, X.; Broering, R.; Werner, M.; Roggendorf, M.; Yang, D.; Lu, M. TLR2 Stimulation Strengthens Intrahepatic Myeloid-Derived Cell-Mediated T Cell Tolerance through Inducing Kupffer Cell Expansion and IL-10 Production. J. Immunol. 2018, 200, 2341–2351. [Google Scholar] [CrossRef]
- Ito, S.; Ansari, P.; Sakatsume, M.; Dickensheets, H.; Vazquez, N.; Donnelly, R.P.; Larner, A.C.; Finbloom, D.S. Interleukin-10 inhibits expression of both interferon alpha- and interferon gamma- induced genes by suppressing tyrosine phosphorylation of STAT1. Blood J. Am. Soc. Hematol. 1999, 93, 1456–1463. [Google Scholar] [CrossRef]
- Chan, L.L.Y.; Cheung, B.K.W.; Li, J.C.B.; Lau, A.S.Y. A role for STAT3 and cathepsin S in IL-10 down-regulation of IFN-γ-induced MHC class II molecule on primary human blood macrophages. J. Leukoc. Biol. 2010, 88, 303–311. [Google Scholar] [CrossRef]
- Herrero, C.; Hu, X.; Li, W.P.; Samuels, S.; Sharif, M.N.; Kotenko, S.; Ivashkiv, L.B. Reprogramming of IL-10 Activity and Signaling by IFN-γ. J. Immunol. 2003, 171, 5034–5041. [Google Scholar] [CrossRef]
- Furuta, K.; Walseng, E.; Roche, P.A. Internalizing MHC class II–peptide complexes are ubiquitinated in early endosomes and targeted for lysosomal degradation. Proc. Natl. Acad. Sci. USA 2013, 110, 20188–20193. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.; Garcia, W.; Godwin, M.J.; Spencer, J.V.; Stern, J.L.; Abendroth, A.; Slobedman, B. Immunomodulatory Properties of a Viral Homolog of Human Interleukin-10 Expressed by Human Cytomegalovirus during the Latent Phase of Infection. J. Virol. 2008, 82, 3736–3750. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Akdis, M.; Joss, A.; Akkoç, T.; Wenig, R.; Colonna, M.; Daigle, I.; Flory, E.; Blaser, K.; Akdis, C.A. IL-10 inhibits CD28 and ICOS costimulations of T cells via src homology 2 domain—Containing protein tyrosine phosphatase 1. J. Allergy Clin. Immunol. 2007, 120, 76–83. [Google Scholar] [CrossRef]
- Taylor, A.; Verhagen, J.; Akkoç, T.; Wenig, R.; Flory, E.; Blaser, K.; Akdis, M.; Akdis, C.A. IL-10 suppresses CD2-mediated T cell activation via SHP-1. Mol. Immunol. 2009, 46, 622–629. [Google Scholar] [CrossRef]
- Niesen, E.; Schmidt, J.; Flecken, T.; Thimme, R. Suppressive Effect of Interleukin 10 on Priming of Naive Hepatitis C Virus–Specific CD8+ T Cells. J. Infect. Dis. 2014, 211, 821–826. [Google Scholar] [CrossRef][Green Version]
- Yang, Y.; Sun, Z.; Li, J.; Song, Y.; Xu, W. Neutrophil-derived IL-10 increases CVB3-induced acute pancreatitis pathology via suppressing CD8+T cell activation while increasing macrophage STAT3-IL-6 cascade. Cytokine 2024, 184, 156784. [Google Scholar] [CrossRef]
- Kong, G.; Chen, Y.; Liu, Z.; Wang, Y.; Li, H.; Guo, C. Adenovirus-IL-10 relieves chronic rejection after mouse heart transplantation by inhibiting miR-155 and activating SOCS5. Int. J. Med Sci. 2023, 20, 172–185. [Google Scholar] [CrossRef]
- Boehler, R.; Kuo, R.; Shin, S.; Goodman, A.; Pilecki, M.; Leonard, J.; Shea, L. Lentivirus delivery of IL-10 to promote and sustain macrophage polarization towards an anti-inflammatory phenotype. Biotechnol. Bioeng. 2013, 111, 1210–1221. [Google Scholar] [CrossRef]
- Deng, B.; Wehling-Henricks, M.; Villalta, S.A.; Wang, Y.; Tidball, J.G. IL-10 Triggers Changes in Macrophage Phenotype That Promote Muscle Growth and Regeneration. J. Immunol. 2012, 189, 3669–3680. [Google Scholar] [CrossRef]
- Sun, Z.; Xu, Y.; Shao, B.; Dang, P.; Hu, S.; Sun, H.; Chen, C.; Wang, C.; Liu, J.; Liu, Y.; et al. Exosomal circPOLQ promotes macrophage M2 polarization via activating IL-10/STAT3 axis in a colorectal cancer model. J. Immunother. Cancer 2024, 12, e008491. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Saeed, A.F.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in immunoregulation and therapeutics. Signal Transduct. Target. Ther. 2023, 8, 1–35. [Google Scholar] [CrossRef]
- Fernandes, T.L.; Gomoll, A.H.; Lattermann, C.; Hernandez, A.J.; Bueno, D.F.; Amano, M.T. Macrophage: A Potential Target on Cartilage Regeneration. Front. Immunol. 2020, 11, 111. [Google Scholar] [CrossRef]
- Peda, J.D.; Salah, S.M.; Wallace, D.P.; Fields, P.E.; Grantham, C.J.; Fields, T.A.; Swenson-Fields, K.I. Autocrine IL-10 activation of the STAT3 pathway is required for pathological macrophage differentiation in polycystic kidney disease. Dis. Model. Mech. 2016, 9, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Ma, T.; Zhang, Q.; Wang, Y.; Song, C.; Lai, M.; Zhang, C.; Fang, X.; Chen, X. Porcine Reproductive and Respiratory Syndrome Virus Modulates the Switch of Macrophage Polarization from M1 to M2 by Upregulating MoDC-Released sCD83. Viruses 2023, 15, 773. [Google Scholar] [CrossRef] [PubMed]
- Laidlaw, B.J.; Cui, W.; A Amezquita, R.; Gray, S.M.; Guan, T.; Lu, Y.; Kobayashi, Y.; A Flavell, R.; Kleinstein, S.H.; Craft, J.; et al. Production of IL-10 by CD4+ regulatory T cells during the resolution of infection promotes the maturation of memory CD8+ T cells. Nat. Immunol. 2015, 16, 871–879. [Google Scholar] [CrossRef]
- Brooks, D.G.; Walsh, K.B.; Elsaesser, H.; Oldstone, M.B.A. IL-10 directly suppresses CD4 but not CD8 T cell effector and memory responses following acute viral infection. Proc. Natl. Acad. Sci. USA 2010, 107, 3018–3023. [Google Scholar] [CrossRef]
- Sun, Q.; Zhao, X.; Li, R.; Liu, D.; Pan, B.; Xie, B.; Chi, X.; Cai, D.; Wei, P.; Xu, W.; et al. STAT3 regulates CD8+ T cell differentiation and functions in cancer and acute infection. J. Exp. Med. 2023, 220, e20220686. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Nishiyama, C. IL-10 in Mast Cell-Mediated Immune Responses: Anti-Inflammatory and Proinflammatory Roles. Int. J. Mol. Sci. 2021, 22, 4972. [Google Scholar] [CrossRef]
- Bailey, D.P.; Kashyap, M.; Bouton, L.A.; Murray, P.J.; Ryan, J.J. Interleukin-10 induces apoptosis in developing mast cells and macrophages. J. Leukoc. Biol. 2006, 80, 581–589. [Google Scholar] [CrossRef]
- Lundy, S.K.; Boros, D.L. Fas Ligand-Expressing B-1a Lymphocytes Mediate CD4+-T-Cell Apoptosis during Schistosomal Infection: Induction by Interleukin 4 (IL-4) and IL-10. Infect. Immun. 2002, 70, 812–819. [Google Scholar] [CrossRef]
- Brosseau, C.; Durand, M.; Colas, L.; Durand, E.; Foureau, A.; Cheminant, M.-A.; Bouchaud, G.; Castan, L.; Klein, M.; Magnan, A.; et al. CD9+ Regulatory B Cells Induce T Cell Apoptosis via IL-10 and Are Reduced in Severe Asthmatic Patients. Front. Immunol. 2018, 9, 3034. [Google Scholar] [CrossRef]
- Xiaofan, Y.; Bin, S.; Huijuan, W.; Cheng, Y.; Xiaole, W.; Xiaohui, J. Increased serum IL-10 in lupus patients promotes apoptosis of T cell subsets via the caspase 8 pathway initiated by Fas signaling. J. Biomed. Res. 2015, 29, 232–240. [Google Scholar] [CrossRef]
- Qiao, J.; Liu, Z.; Dong, C.; Luan, Y.; Zhang, A.; Moore, C.; Fu, K.; Peng, J.; Wang, Y.; Ren, Z.; et al. Targeting Tumors with IL-10 Prevents Dendritic Cell-Mediated CD8+ T Cell Apoptosis. Cancer Cell 2019, 35, 901–915.e4. [Google Scholar] [CrossRef]
- Gary-Gouy, H.; Harriague, J.; Bismuth, G.; Platzer, C.; Schmitt, C.; Dalloul, A.H. Human CD5 promotes B-cell survival through stimulation of autocrine IL-10 production. Blood 2002, 100, 4537–4543. [Google Scholar] [CrossRef]
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef]
- Fenwick, C.; Joo, V.; Jacquier, P.; Noto, A.; Banga, R.; Perreau, M.; Pantaleo, G. T-cell exhaustion in HIV infection. Immunol. Rev. 2019, 292, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Zheng, X.; Yang, W. The Role of Metabolic Dysfunction in T-Cell Exhaustion During Chronic Viral Infection. Front. Immunol. 2022, 13, 843242. [Google Scholar] [CrossRef]
- Im, S.J.; Ha, S.-J. Re-defining T-Cell Exhaustion: Subset, Function, and Regulation. Immune Netw. 2020, 20, e2. [Google Scholar] [CrossRef] [PubMed]
- Osuch, S.; Metzner, K.J.; Cortés, K.C. Reversal of T Cell Exhaustion in Chronic HCV Infection. Viruses 2020, 12, 799. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Suprunenko, T.; Ashhurst, T.; Marbach, F.; Raifer, H.; Wolff, S.; Strecker, T.; Viengkhou, B.; Jung, S.R.; Obermann, H.-L.; et al. IRF9 Prevents CD8+ T Cell Exhaustion in an Extrinsic Manner during Acute Lymphocytic Choriomeningitis Virus Infection. J. Virol. 2017, 91, e01219-17. [Google Scholar] [CrossRef]
- Sharov, K.S. HIV/SARS-CoV-2 co-infection: T cell profile, cytokine dynamics and role of exhausted lymphocytes. Int. J. Infect. Dis. 2021, 102, 163–169. [Google Scholar] [CrossRef]
- Richter, K.; Perriard, G.; Oxenius, A. Reversal of chronic to resolved infection by IL-10 blockade is LCMV strain dependent. Eur. J. Immunol. 2013, 43, 649–654. [Google Scholar] [CrossRef]
- Jubel, J.M.; Barbati, Z.R.; Burger, C.; Wirtz, D.C.; Schildberg, F.A. The Role of PD-1 in Acute and Chronic Infection. Front. Immunol. 2020, 11, 487. [Google Scholar] [CrossRef]
- Chen, S.; Crabill, G.A.; Pritchard, T.S.; McMiller, T.L.; Wei, P.; Pardoll, D.M.; Pan, F.; Topalian, S.L. Mechanisms regulating PD-L1 expression on tumor and immune cells. J. Immunother. Cancer 2019, 7, 305. [Google Scholar] [CrossRef]
- Schorer, M.; Rakebrandt, N.; Lambert, K.; Hunziker, A.; Pallmer, K.; Oxenius, A.; Kipar, A.; Stertz, S.; Joller, N. TIGIT limits immune pathology during viral infections. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.M.; Griffin, D.E.; Dermody, T.S. Interleukin-10 Modulation of Virus Clearance and Disease in Mice with Alphaviral Encephalomyelitis. J. Virol. 2018, 92, e01517-17. [Google Scholar] [CrossRef] [PubMed]
- Bu, T.; Li, Z.; Hou, Y.; Sun, W.; Zhang, R.; Zhao, L.; Wei, M.; Yang, G.; Yuan, L. Exosome-mediated delivery of inflammation-responsive Il-10 mRNA for controlled atherosclerosis treatment. Theranostics 2021, 11, 9988–10000. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.T.; Oldstone, M.B.A. IL-10: Achieving balance during persistent viral infection. Curr Top Microbiol Immunol. 2014, 380, 129–144. [Google Scholar] [CrossRef]
- Ni, G.; Wang, T.; Walton, S.; Zhu, B.; Chen, S.; Wu, X.; Wang, Y.; Wei, M.Q.; Liu, X. Manipulating IL-10 signalling blockade for better immunotherapy. Cell. Immunol. 2015, 293, 126–129. [Google Scholar] [CrossRef]
- Levast, B.; Li, Z.; Madrenas, J. The role of IL-10 in microbiome-associated immune modulation and disease tolerance. Cytokine 2015, 75, 291–301. [Google Scholar] [CrossRef]
- Panchal, R.G.; Mourich, D.V.; Bradfute, S.; Hauck, L.L.; Warfield, K.L.; Iversen, P.L.; Bavari, S. Induced IL-10 Splice Altering Approach to Antiviral Drug Discovery. Nucleic Acid Ther. 2014, 24, 179–185. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; He, Q.; Zhang, Y.; Li, Y.; Zhang, Z. Mechanisms of Interleukin-10-Mediated Immunosuppression in Viral Infections. Pathogens 2025, 14, 989. https://doi.org/10.3390/pathogens14100989
Guo Z, He Q, Zhang Y, Li Y, Zhang Z. Mechanisms of Interleukin-10-Mediated Immunosuppression in Viral Infections. Pathogens. 2025; 14(10):989. https://doi.org/10.3390/pathogens14100989
Chicago/Turabian StyleGuo, Zijing, Qifu He, Yan Zhang, Yuling Li, and Zhidong Zhang. 2025. "Mechanisms of Interleukin-10-Mediated Immunosuppression in Viral Infections" Pathogens 14, no. 10: 989. https://doi.org/10.3390/pathogens14100989
APA StyleGuo, Z., He, Q., Zhang, Y., Li, Y., & Zhang, Z. (2025). Mechanisms of Interleukin-10-Mediated Immunosuppression in Viral Infections. Pathogens, 14(10), 989. https://doi.org/10.3390/pathogens14100989








