New Light on an Old Story: Lymphocystis Disease in Copperband Butterflyfish (Chelmon rostratus) and Orbicular Batfish (Platax orbicularis)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Histology
2.2. PCR Assay
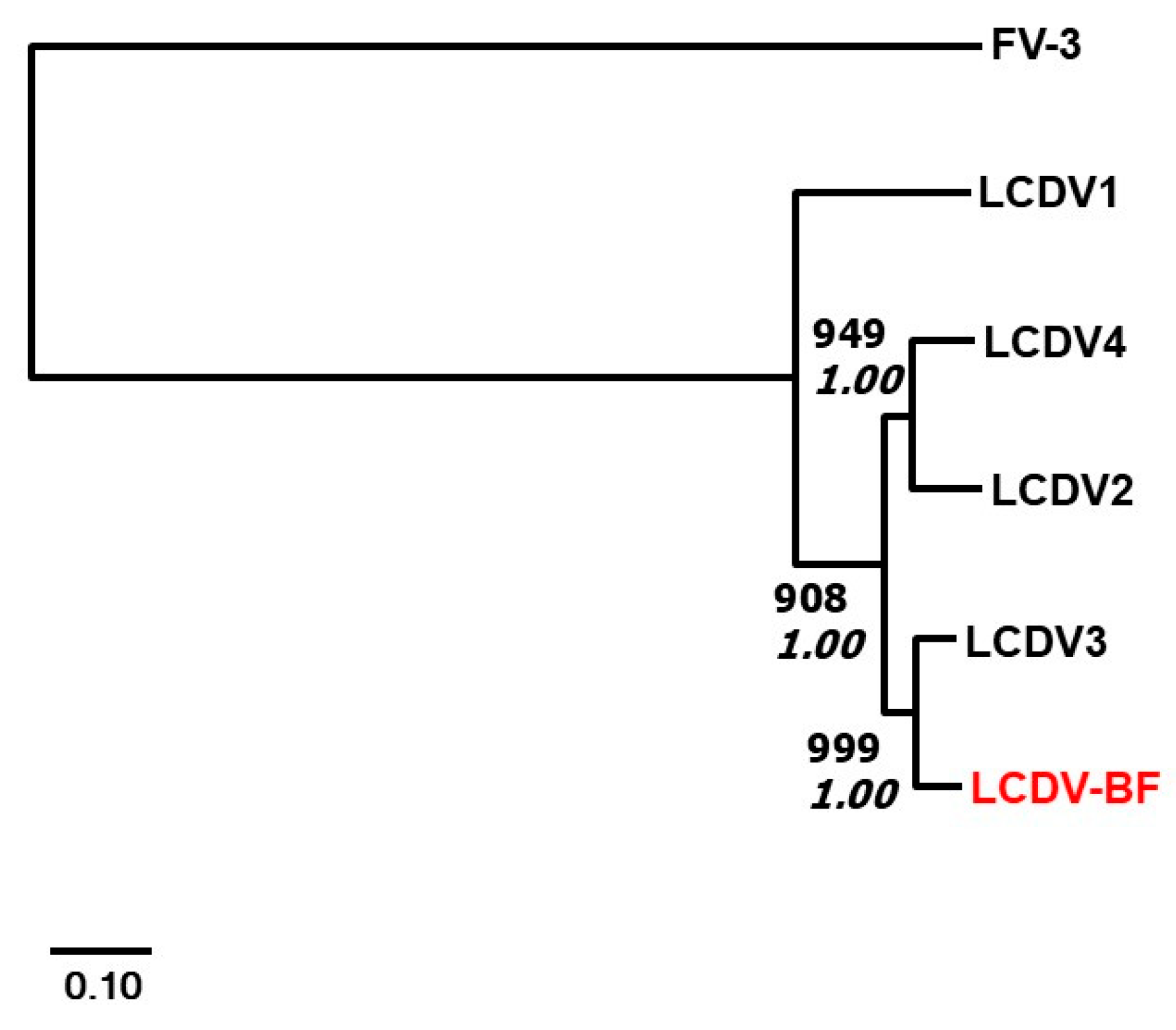
2.3. Phylogenetic Analysis
2.4. Transmission Electron Microscopy
3. Results
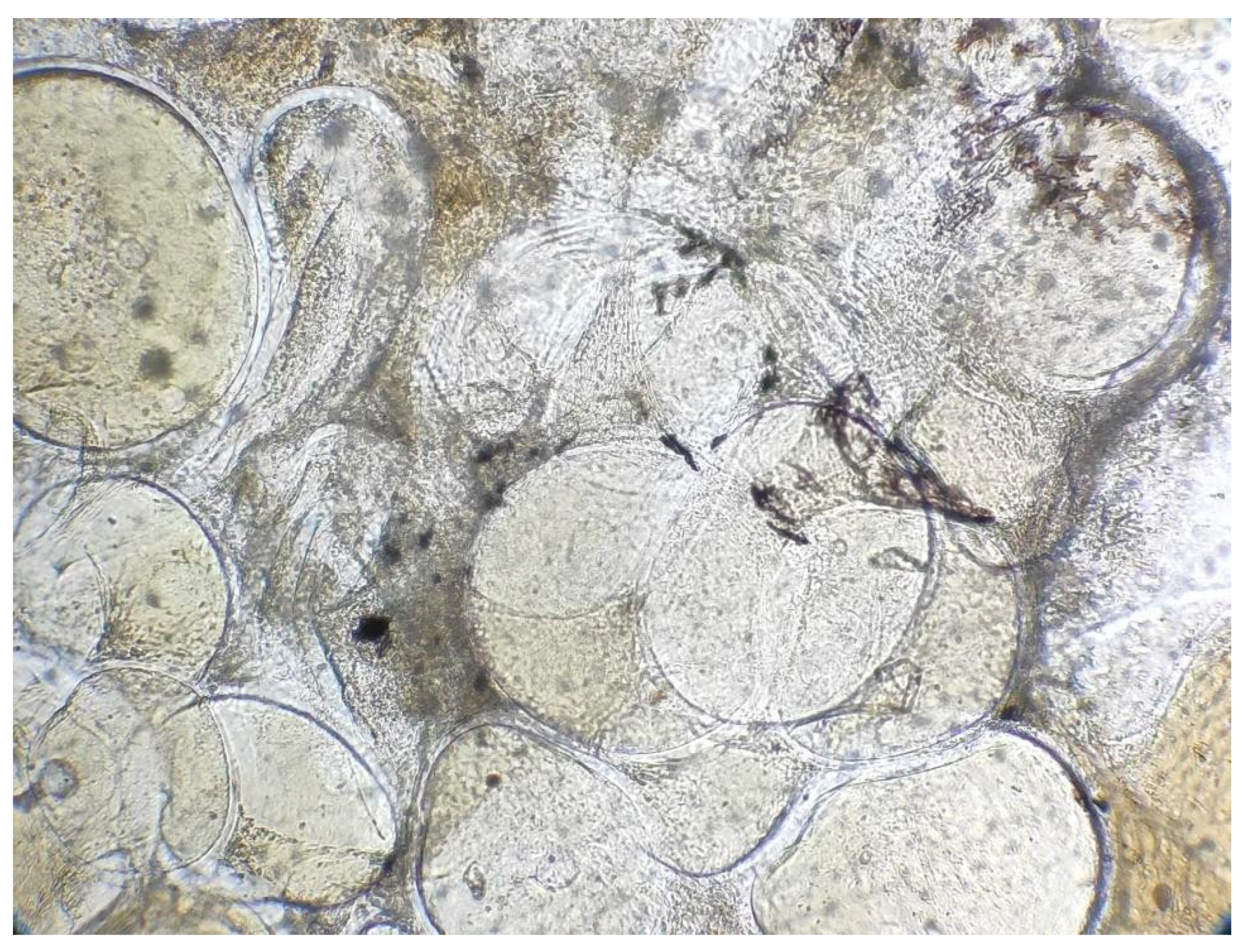
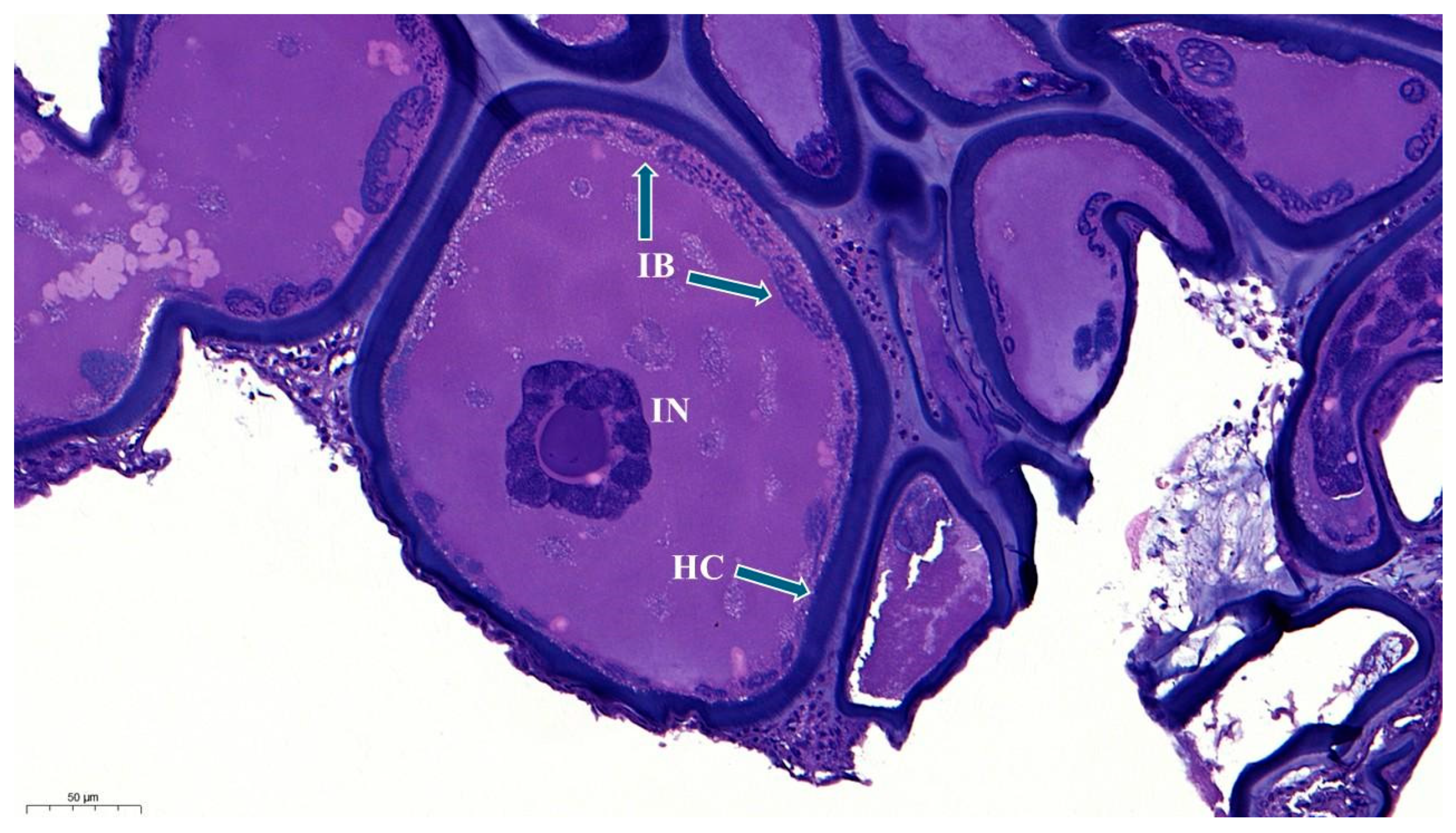
3.1. Histopathology
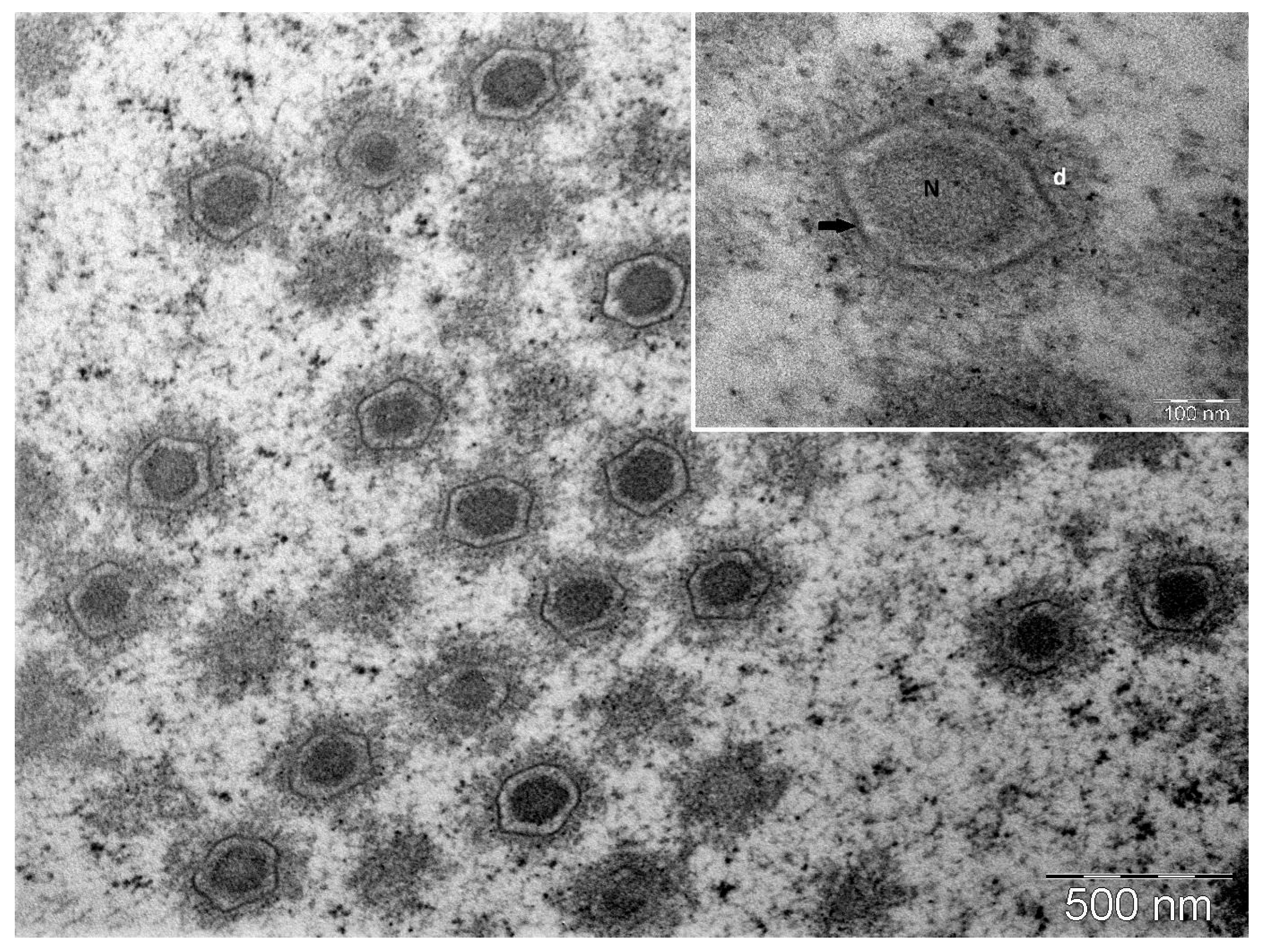
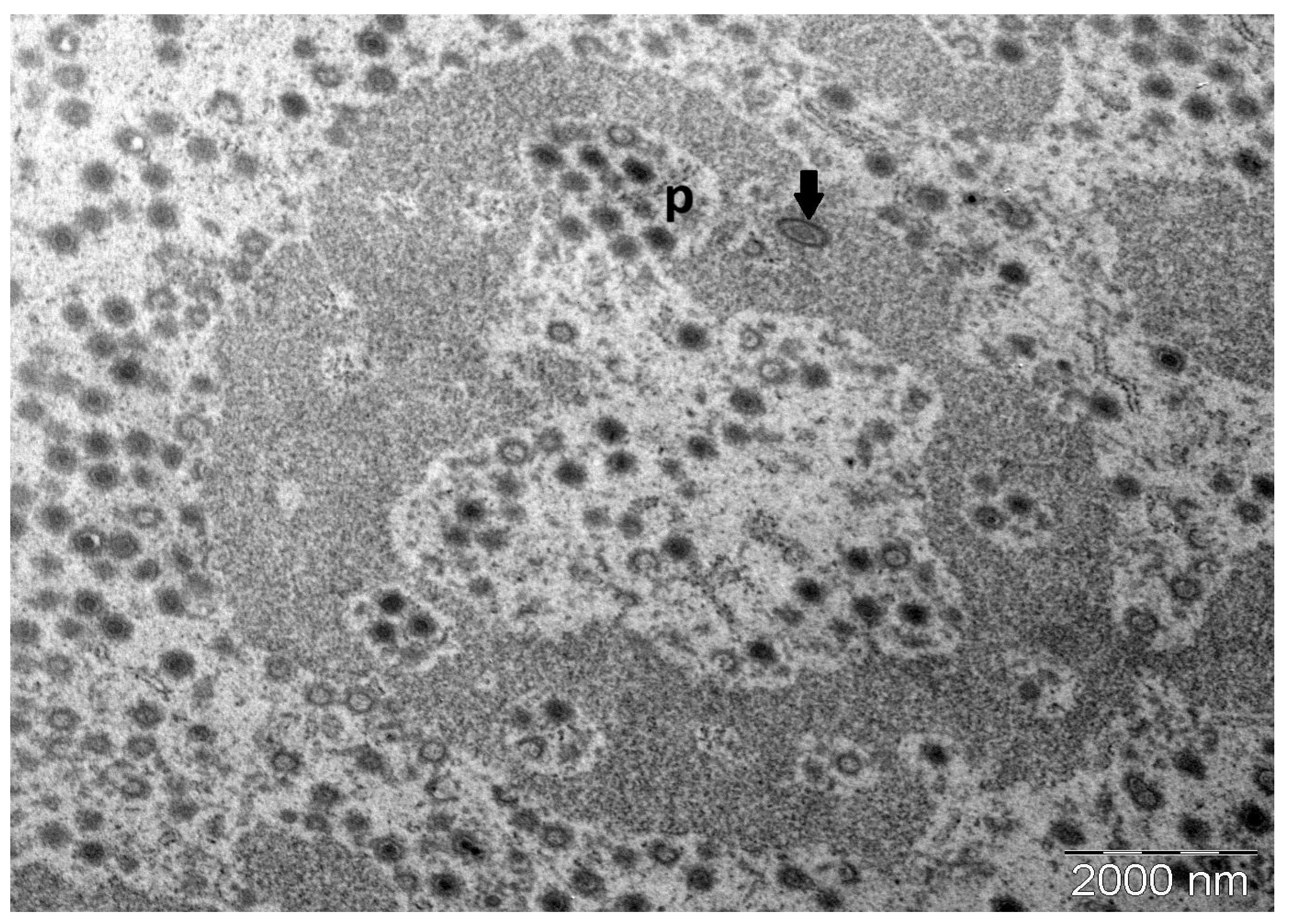
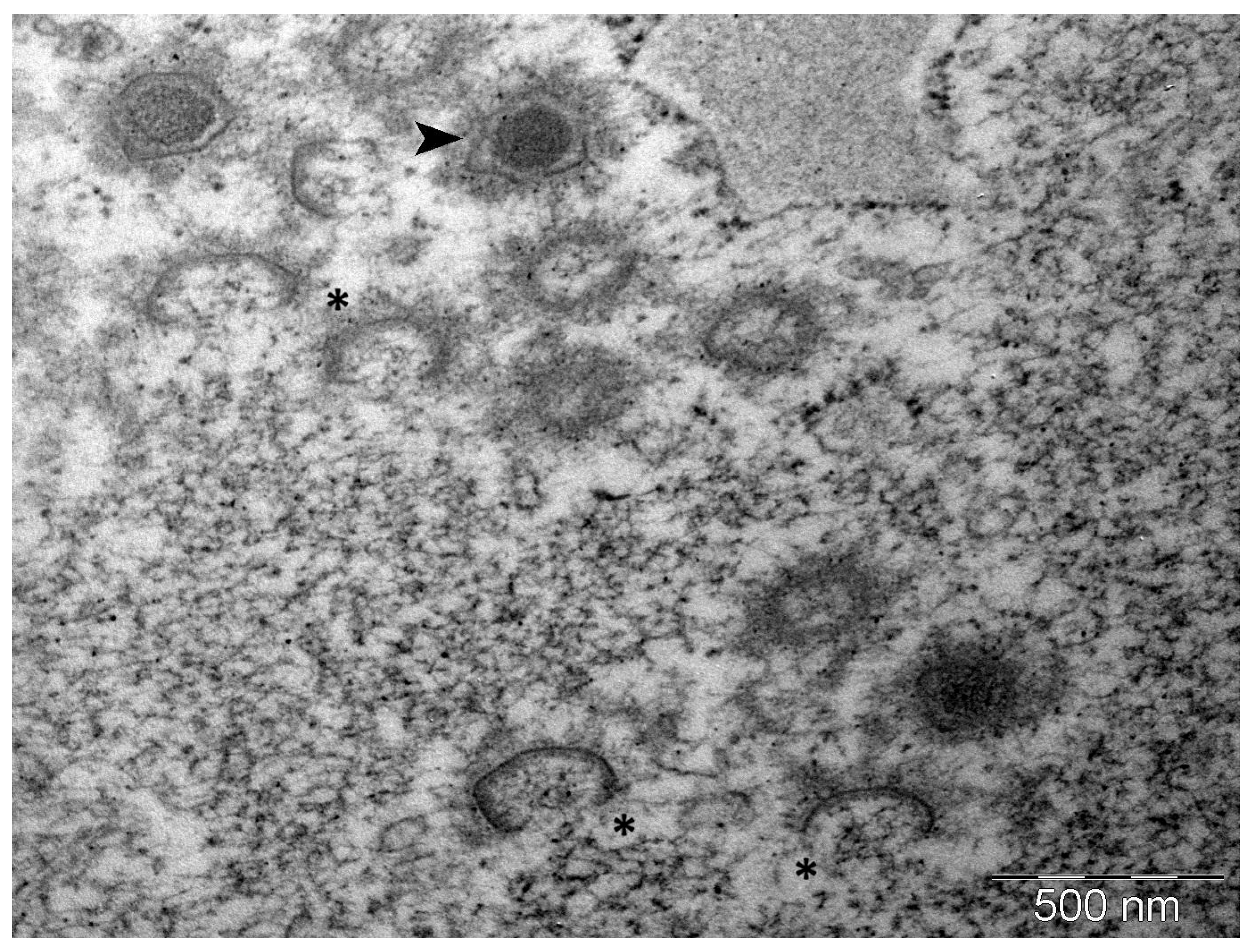
3.2. Transmission Electron Microscopy of Copperband Butterflyfish
3.3. Results from PCR Assay and Phylogenetic Analysis of Orbicular Batfish
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LCD | lymphocystis disease |
| LCDV | lymphocystis disease virus |
| LCDV-BF | batfish lymphocystis disease virus |
| TEM | transmission electron microscopy |
| ICIB | intracytoplasmic inclusion body |
| H&E | haematoxylin–eosin |
| KH | carbonite hardness |
| NO3− | nitrate ion |
| Ca | calcium |
| Mg | magnesium |
References
- Ploeg, A.; Fossaa, S.A.; Bassleer, G.; Willis, S.; Chuan, L.L. International Transport of Live Fish in the Ornamental Aquatic Industry; Ornamental Fish International: Maarssen, The Netherlands, 2012; ISBN 978-94-91354-05-2. [Google Scholar]
- Ploeg, A.; Tomey, W.; Hensen, R.; Mous, E.; Riechelman, F.; Willis, S.; Sekharan, N.M.; McLane, B. International Transport of Aquatic Plants in the Ornamental Aquatic Industry; Hollywood Import & Export, Inc.: Gainesville, FL, USA, 2014. [Google Scholar]
- Stevens, C.H.; Croft, D.P.; Paull, G.C.; Tyler, C.R. Stress and Welfare in Ornamental Fishes: What Can Be Learned from Aquaculture? J. Fish Biol. 2017, 91, 409–428. [Google Scholar] [CrossRef]
- Hargreaves, V. The Complete Book of the Marine Aquarium, 1st ed.; Thunder Bay Press: San Diego, CA, USA, 2002; ISBN 978-1-57145-762-2. [Google Scholar]
- Noga, E.J. Problems 77 Through 88. In Fish Disease; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; pp. 269–303. ISBN 978-1-118-78675-8. [Google Scholar]
- Lieske, E.; Myers, R. Coral Reef Fishes: Caribbean, Indian Ocean and Pacific Ocean Including the Red Sea; HarperCollins: Broadway, NY, USA, 1994; ISBN 978-0-00-219974-2. [Google Scholar]
- Randall, J.E.; Allen, G.R.; Steene, R.C. Fishes of the Great Barrier Reef and Coral Sea, Revised and Expanded Edition; University of Hawaii Press: Honolulu, HI, USA, 1998; ISBN 978-0-8248-1895-1. [Google Scholar]
- Kuiter, R.H.; Rudie, H.; Debelius, H. Surgeonfishes, Rabbitfishes and Their Relatives: A Comprehensive Guide to Acanthuroidei; The marine fish families series; TMC Publishing: Hertfordshire, UK, 2001. [Google Scholar]
- Leis, J.; Carson-Ewart, B. The Larvae of Indo-Pacific Coastal Fishes: An Identification Guide to Marine Fish Larvae; BRILL: Leiden, The Netherlands, 2021; ISBN 978-90-04-47485-7. [Google Scholar]
- Myers, R.F. Micronesian Reef Fishes: A Practical Guide to the Identification of the Coral Reef Fishes of the Tropical Central and Western Pacific, 2nd ed.; Coral Graphics: Hicksville, NY, USA, 1991; ISBN 978-0-9621564-2-7. [Google Scholar]
- Bernoth, E.-M.; Crane, M. Viral Diseases of Aquarium Fish. Semin. Avian Exot. Pet Med. 1995, 4, 103–110. [Google Scholar] [CrossRef]
- Borzák, R.; Sellyei, B.; Baska, F.; Székely, C.; Doszpoly, A. Detection of Cyprinid Herpesvirus 1 (CyHV-1) in Barbel (Barbus Barbus): First Molecular Evidence for the Presence of CyHV-1 in Fish Other than Carp (Cyprinus Carpio). Acta Vet. Hung. 2020, 68, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Pirarat, N.; Pratakpiriya, W.; Jongnimitpaiboon, K.; Sajjawiriyakul, K.; Rodkhum, C.; Chansue, N. Lymphocystis Disease in Cultured False Clown Anemonefish (Amphiprion Ocellaris). Aquaculture 2011, 315, 414–416. [Google Scholar] [CrossRef]
- Sriwanayos, P.; Francis-Floyd, R.; Stidworthy, M.F.; Petty, B.D.; Kelley, K.; Waltzek, T.B. Megalocytivirus Infection in Orbiculate Batfish Platax Orbicularis. Dis. Aquat. Org. 2013, 105, 1–8. [Google Scholar] [CrossRef]
- Tarján, Z.L.; Doszpoly, A.; Eszterbauer, E.; Benkő, M. Partial Genetic Characterisation of a Novel Alloherpesvirus Detected by PCR in a Farmed Wels Catfish (Silurus Glanis). Acta Vet. Hung. 2022, 70, 321–327. [Google Scholar] [CrossRef]
- Yu, X.-D.; Ke, F.; Zhang, Q.-Y.; Gui, J.-F. Genome Characteristics of Two Ranavirus Isolates from Mandarin Fish and Largemouth Bass. Pathogens 2023, 12, 730. [Google Scholar] [CrossRef] [PubMed]
- Lawler, A.R.; Ogle, J.T.; Donnes, C. Dascyllus spp.: New Hosts for Lymphocystis, and a List Of Recent Hosts. J. Fish Dis. 1977, 13, 307–312. [Google Scholar] [CrossRef]
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kannan, S.; Khovanskaya, R.; Leipe, D.; Mcveigh, R.; O’Neill, K.; Robbertse, B.; et al. NCBI Taxonomy: A Comprehensive Update on Curation, Resources and Tools. Database 2020, 2020, baaa062. [Google Scholar] [CrossRef]
- Cheng, M.-C.; See, M.S.; Wang, P.-C.; Kuo, Y.-T.; Ho, Y.-S.; Chen, S.-C.; Tsai, M.-A. Lymphocystis Disease Virus Infection in Clownfish Amphiprion Ocellaris and Amphiprion Clarkii in Taiwan. Animals 2023, 13, 153. [Google Scholar] [CrossRef]
- Ferguson, H.W. Skin. In Systemic Pathology of Fish: A Text and Atlas of Normal Tissues in Teleosts and Their Responses in Disease; Scotian Press: London, UK, 2006; pp. 65–89. ISBN 978-0-9553037-0-8. [Google Scholar]
- Russell, P.H. Lymphocystis in Wild Plaice Pleuronectes platessa (L.), and Flounder, Platichthys flesus (L.), in British Coastal Waters: A Histopathological and Serological Study. J. Fish Biol. 1974, 6, 771–778. [Google Scholar] [CrossRef]
- Cano, I.; Ferro, P.; Alonso, M.C.; Bergmann, S.M.; Römer-Oberdörfer, A.; Garcia-Rosado, E.; Castro, D.; Borrego, J.J. Development of Molecular Techniques for Detection of Lymphocystis Disease Virus in Different Marine Fish Species. J. Appl. Microbiol. 2007, 102, 32–40. [Google Scholar] [CrossRef]
- Rahmati-Holasoo, H.; Marandi, A.; Mousavi, H.E.; Arabkhazaeli, F.; Shokrpoor, S.; Ziafati Kafi, Z. Clinical, Histopathological and Phylogenetic Analysis of Myxobolus Lentisturalis (Myxozoa: Myxobolidae) Infecting the Musculature of Farmed Population of Goldfish (Carassius Auratus) in Iran: 2021–2022. BMC Vet. Res. 2024, 20, 361. [Google Scholar] [CrossRef]
- Roberts, R.J. The Virology of Teleosts Fish Pathology; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 1-118-22296-2. [Google Scholar]
- Barton, B.A. Stress in Fishes: A Diversity of Responses with Particular Reference to Changes in Circulating Corticosteroids1. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef]
- Masud, N.; Ellison, A.; Cable, J. A Neglected Fish Stressor: Mechanical Disturbance during Transportation Impacts Susceptibility to Disease in a Globally Important Ornamental Fish. Dis. Aquat. Org. 2019, 134, 25–32. [Google Scholar] [CrossRef]
- Sampaio, F.D.F.; Freire, C.A. An Overview of Stress Physiology of Fish Transport: Changes in Water Quality as a Function of Transport Duration. Fish Fish. 2016, 17, 1055–1072. [Google Scholar] [CrossRef]
- Kitamura, S.-I.; Jung, S.-J.; Kim, W.-S.; Nishizawa, T.; Yoshimizu, M.; Oh, M.-J. A New Genotype of Lymphocystivirus, LCDV-RF, from Lymphocystis Diseased Rockfish. Arch. Virol. 2006, 151, 607–615. [Google Scholar] [CrossRef]
- Kvitt, H.; Heinisch, G.; Diamant, A. Detection and Phylogeny of Lymphocystivirus in Sea Bream Sparus Aurata Based on the DNA Polymerase Gene and Major Capsid Protein Sequences. Aquaculture 2008, 275, 58–63. [Google Scholar] [CrossRef]
- Perretta, A.; Doszpoly, A.; Puentes, R.; Bessonart, M. Diagnosis of Lymphocystis Disease in a Novel Host, the Whitemouth Croaker Micropogonias Furnieri, Associated with a Putatively Novel Lymphocystivirus Species (LCDV-WC). Dis. Aquat. Org. 2020, 137, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Pikor, L.A.; Enfield, K.S.; Cameron, H.; Lam, W.L. DNA Extraction from Paraffin Embedded Material for Genetic and Epigenetic Analyses. J. Vis. Exp. JoVE 2011, 49, 2763. [Google Scholar]
- Kuraku, S.; Zmasek, C.M.; Nishimura, O.; Katoh, K. aLeaves Facilitates On-Demand Exploration of Metazoan Gene Family Trees on MAFFT Sequence Alignment Server with Enhanced Interactivity. Nucleic Acids Res. 2013, 41, W22–W28. [Google Scholar] [CrossRef]
- Hulsenbeck, J.P.; Ronquist, F. MrBayes: Bayesian Inference of Phylogeny. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Milne, I.; Wright, F.; Rowe, G.; Marshall, D.F.; Husmeier, D.; McGuire, G. TOPALi: Software for Automatic Identification of Recombinant Sequences within DNA Multiple Alignments. Bioinformatics 2004, 20, 1806–1807. [Google Scholar] [CrossRef]
- Marcello, G.M.; Szabó, L.E.; Sótonyi, P.; Rácz, B. Quantitative Electron Microscopic Assay Using Random Sampling from Single Sections to Test Plastic Synaptic Changes in Hippocampus. Bio-protocol 2018, 8, e2946. [Google Scholar] [CrossRef]
- Sheng, X.; Zhan, W.; Xu, S.; Cheng, S. Histopathological Observation of Lymphocystis Disease and Lymphocystis Disease Virus (LCDV) Detection in Cultured Diseased Sebastes Schlegeli. J. Ocean. Univ. China 2007, 6, 378–382. [Google Scholar] [CrossRef]
- Maclachlan, N.J.; Dubovi, E.J. Laboratory Diagnosis of Viral Infections. In Fenner’s Veterinary Virology, 5th ed.; Academic Press: Amsterdam, The Netherlands, 2016; ISBN 978-0-12-800946-8. [Google Scholar]
- Valverde, E.J.; Labella, A.M.; Borrego, J.J.; Castro, D. Artemia Spp., a Susceptible Host and Vector for Lymphocystis Disease Virus. Viruses 2019, 11, 506. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.R.; Sun, X.Q.; Xing, M.Q.; Liu, H. Immune Response of DNA Vaccine against Lymphocystis Disease Virus and Expression Analysis of Immune-Related Genes after Vaccination. Aquac. Res. 2010, 41, 1444–1451. [Google Scholar] [CrossRef]
- Eaton, H.E.; Metcalf, J.; Penny, E.; Tcherepanov, V.; Upton, C.; Brunetti, C.R. Comparative Genomic Analysis of the Family Iridoviridae: Re-Annotating and Defining the Core Set of Iridovirus Genes. Virol. J. 2007, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Doszpoly, A.; Kaján, G.L.; Puentes, R.; Perretta, A. Complete Genome Sequence and Analysis of a Novel Lymphocystivirus Detected in Whitemouth Croaker (Micropogonias Furnieri): Lymphocystis Disease Virus 4. Arch. Virol. 2020, 165, 1215–1218. [Google Scholar] [CrossRef]



| LCDV-BF | LCDV-1 | LCDV-2 | LCDV-3 | LCDV-4 |
|---|---|---|---|---|
| MCP (451 aa) | 88.22% | 95.38% | 97.69% | 93.76% |
| ATPase (244 aa) | 61.07% | 87.30% | 93.03% | 85.25% |
| DNApol (139 aa) | 52.38% | 84.68% | 88.71% | 85.48% |
| RNA pol (692 aa) | 67.58% | 81.79% | 91.91% | 83.24% |
| Core gene 20 (376 aa) | 68.09% | 88.56% | 93.09% | 90.69% |
| Core gene 9 (154 aa) | 76.62% | 91.56% | 94.16% | 93.51% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoitsy, M.; Sós, E.; Gál, J.; Ziszisz, Á.; Baska, F.; Folkman, L.A.; Marcello, G.M.; Bali, K.; Mitró, G.; Doszpoly, A. New Light on an Old Story: Lymphocystis Disease in Copperband Butterflyfish (Chelmon rostratus) and Orbicular Batfish (Platax orbicularis). Pathogens 2025, 14, 988. https://doi.org/10.3390/pathogens14100988
Hoitsy M, Sós E, Gál J, Ziszisz Á, Baska F, Folkman LA, Marcello GM, Bali K, Mitró G, Doszpoly A. New Light on an Old Story: Lymphocystis Disease in Copperband Butterflyfish (Chelmon rostratus) and Orbicular Batfish (Platax orbicularis). Pathogens. 2025; 14(10):988. https://doi.org/10.3390/pathogens14100988
Chicago/Turabian StyleHoitsy, Márton, Endre Sós, János Gál, Árisz Ziszisz, Ferenc Baska, Lars August Folkman, Giuseppe Mark Marcello, Krisztina Bali, Gergő Mitró, and Andor Doszpoly. 2025. "New Light on an Old Story: Lymphocystis Disease in Copperband Butterflyfish (Chelmon rostratus) and Orbicular Batfish (Platax orbicularis)" Pathogens 14, no. 10: 988. https://doi.org/10.3390/pathogens14100988
APA StyleHoitsy, M., Sós, E., Gál, J., Ziszisz, Á., Baska, F., Folkman, L. A., Marcello, G. M., Bali, K., Mitró, G., & Doszpoly, A. (2025). New Light on an Old Story: Lymphocystis Disease in Copperband Butterflyfish (Chelmon rostratus) and Orbicular Batfish (Platax orbicularis). Pathogens, 14(10), 988. https://doi.org/10.3390/pathogens14100988







