Coprological and Molecular Analyses of Ruminant Farms in Québec, Canada, Show a Variable Efficacy of Ivermectin Against Gastro-Intestinal Nematodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Ruminant Farms and Sample Collection for IVM Efficacy Assessment
2.2. Coprological Analyses
2.3. IVM Efficacy Assessment
2.4. Molecular Identification of GIN Species
2.4.1. DNA Extraction
2.4.2. PCR Amplification of GIN Species
2.4.3. Identification of H. contortus from Recovered Nematode Eggs
3. Results
3.1. Prevalence and Identification GIN Species in Ruminant Farms
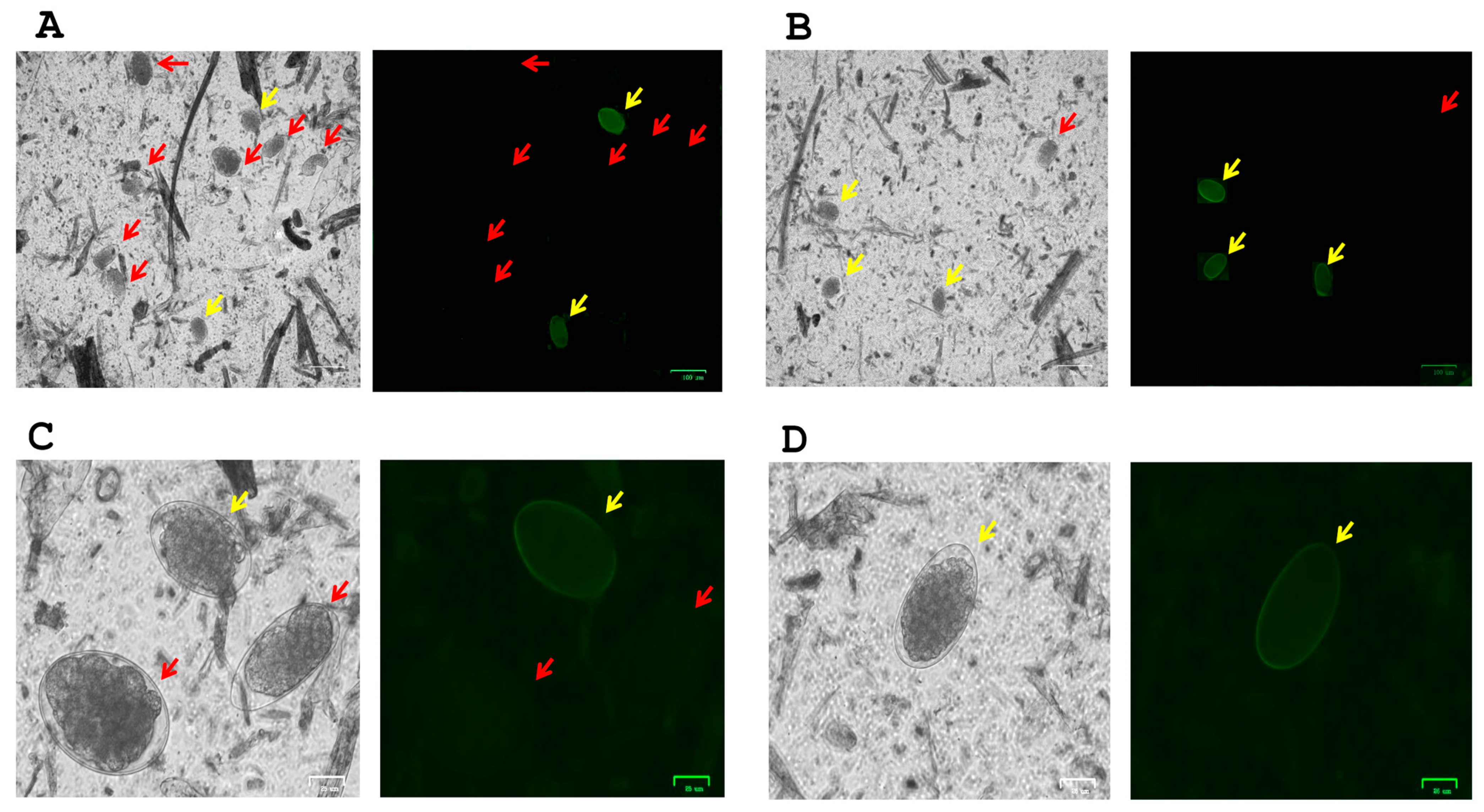
3.2. Microscopic Identification of H. contortus Eggs
3.3. FECs and IVM Efficacy on GINs from Grazing Ruminant Farms
4. Discussion
5. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Charlier, J.; van der Voort, M.; Kenyon, F.; Skuce, P.; Vercruysse, J. Chasing helminths and their economic impact on farmed ruminants. Trends Parasitol. 2014, 30, 361–367. [Google Scholar] [CrossRef]
- Playford, M.C.; Besier, R.B. Gastrointestinal nematode parasites of grazing ruminants: A comprehensive literature review of diagnostic methods for quantifying parasitism, larval differentiation and measuring anthelmintic resistance. N. Z. Vet. J. 2025, 73, 149–164. [Google Scholar] [CrossRef]
- Roeber, F.; Jex, A.R.; Gasser, R.B. Impact of gastrointestinal parasitic nematodes of sheep, and the role of advanced molecular tools for exploring epidemiology and drug resistance—An Australian perspective. Parasites Vectors 2013, 6, 153. [Google Scholar] [CrossRef]
- Charlier, J.; Rinaldi, L.; Musella, V.; Ploeger, H.W.; Chartier, C.; Vineer, H.R.; Hinney, B.; von Samson-Himmelstjerna, G.; Băcescu, B.; Mickiewicz, M.; et al. Initial assessment of the economic burden of major parasitic helminth infections to the ruminant livestock industry in Europe. Prev. Vet. Med. 2020, 182, 105103. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.; Sanyal, P.B. Epidemiological Intelligence for Grazing Management in Strategic Control of Parasitic Gastroenteritis in Small Ruminants in India—A Review. Vet. World 2011, 4, 92–96. [Google Scholar]
- Zajac, A.M.; Garza, J. Biology, Epidemiology, and Control of Gastrointestinal Nematodes of Small Ruminants. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 73–87. [Google Scholar] [CrossRef]
- Hildreth, M.B.; McKenzie, J.B. Epidemiology and Control of Gastrointestinal Nematodes of Cattle in Northern Climates. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 59–71. [Google Scholar] [CrossRef]
- Hoberg, E.P.; Abrams, A.; Pilitt, P.A.; Jenkins, E.J. Discovery and description of a new trichostrongyloid species (Nematoda: Ostertagiinae), abomasal parasites in mountain goat, Oreamnos americanus, from the Western Cordillera of North America. J. Parasitol. 2012, 98, 817–846. [Google Scholar] [CrossRef]
- Maier, G.U.; Torcal, P.; Stackhouse, J.; Davy, J.S.; Forero, L.C.; Snell, L.; Woodmansee, G. Gastrointestinal parasitic worm burdens and efficacy of deworming practices in growing beef cattle grazing California pastures. Transl. Anim. Sci. 2025, 9, txaf007. [Google Scholar] [CrossRef] [PubMed]
- Fissiha, W.; Kinde, M.Z. Anthelmintic Resistance and Its Mechanism: A Review. Infect. Drug Resist. 2021, 14, 5403–5410. [Google Scholar] [CrossRef]
- Menzies, P. Handbook for the Control of Internal Parasites of Sheep and Goats; University of Guelph: Guelph, ON, Canada, 2019. [Google Scholar]
- Paras, K.L.; George, M.M.; Vidyashankar, A.N.; Kaplan, R.M. Comparison of fecal egg counting methods in four livestock species. Vet. Parasitol. 2018, 257, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Verocai, G.G.; Chaudhry, U.N.; Lejeune, M. Diagnostic Methods for Detecting Internal Parasites of Livestock. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 125–143. [Google Scholar] [CrossRef] [PubMed]
- Bisset, S.A.; Knight, J.S.; Bouchet, C.L. A multiplex PCR-based method to identify strongylid parasite larvae recovered from ovine faecal cultures and/or pasture samples. Vet. Parasitol. 2014, 200, 117–127. [Google Scholar] [CrossRef]
- Queiroz, C.; Levy, M.; Avramenko, R.; Redman, E.; Kearns, K.; Swain, L.; Silas, H.; Uehlinger, F.; Gilleard, J.S. The use of ITS-2 rDNA nemabiome metabarcoding to enhance anthelmintic resistance diagnosis and surveillance of ovine gastrointestinal nematodes. Int. J. Parasitol. Drugs Drug Resist. 2020, 14, 105–117. [Google Scholar] [CrossRef]
- Kotze, A.C.; Gilleard, J.S.; Doyle, S.R.; Prichard, R.K. Challenges and opportunities for the adoption of molecular diagnostics for anthelmintic resistance. Int. J. Parasitol. Drugs Drug Resist. 2020, 14, 264–273. [Google Scholar] [CrossRef]
- Emery, D.L.; Hunt, P.W.; Le Jambre, L.F. Haemonchus contortus: The then and now, and where to from here? Int. J. Parasitol. 2016, 46, 755–769. [Google Scholar] [CrossRef]
- Baltrušis, P.; Doyle, S.R.; Halvarsson, P.; Höglund, J. Genome-wide analysis of the response to ivermectin treatment by a Swedish field population of Haemonchus contortus. Int. J. Parasitol. Drugs Drug Resist. 2022, 18, 12–19. [Google Scholar] [CrossRef]
- Williamson, S.M.; Storey, B.; Howell, S.; Harper, K.M.; Kaplan, R.M.; Wolstenholme, A.J. Candidate anthelmintic resistance-associated gene expression and sequence polymorphisms in a triple-resistant field isolate of Haemonchus contortus. Mol. Biochem. Parasitol. 2011, 180, 99–105. [Google Scholar] [CrossRef]
- Raza, A.; Lamb, J.; Chambers, M.; Hunt, P.W.; Kotze, A.C. Larval development assays reveal the presence of sub-populations showing high- and low-level resistance in a monepantel (Zolvix®)-resistant isolate of Haemonchus contortus. Vet. Parasitol. 2016, 220, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Howell, S.B.; Burke, J.M.; Miller, J.E.; Terrill, T.H.; Valencia, E.; Williams, M.J.; Williamson, L.H.; Zajac, A.M.; Kaplan, R.M. Prevalence of anthelmintic resistance on sheep and goat farms in the southeastern United States. J. Am. Vet. Med. Assoc. 2008, 233, 1913–1919. [Google Scholar] [CrossRef]
- Gasbarre, L.C.; Smith, L.L.; Hoberg, E.; Pilitt, P.A. Further characterization of a cattle nematode population with demonstrated resistance to current anthelmintics. Vet. Parasitol. 2009, 166, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Edwards, E.E.; Garner, B.C.; Williamson, L.H.; Storey, B.E.; Sakamoto, K. Pathology of Haemonchus contortus in New World camelids in the southeastern United States: A retrospective review. J. Vet. Diagn. Investig. 2016, 28, 105–109. [Google Scholar] [CrossRef]
- Falzon, L.C.; Menzies, P.I.; Shakya, K.P.; Jones-Bitton, A.; Vanleeuwen, J.; Avula, J.; Stewart, H.; Jansen, J.T.; Taylor, M.A.; Learmount, J.; et al. Anthelmintic resistance in sheep flocks in Ontario, Canada. Vet. Parasitol. 2013, 193, 150–162. [Google Scholar] [CrossRef]
- Barrère, V.; Keller, K.; von Samson-Himmelstjerna, G.; Prichard, R.K. Efficiency of a genetic test to detect benzimidazole resistant Haemonchus contortus nematodes in sheep farms in Quebec, Canada. Parasitol. Int. 2013, 62, 464–470. [Google Scholar] [CrossRef]
- De Seram, E.L.; Uehlinger, F.D.; de Queiroz, C.; Redman, E.M.; Campbell, J.R.; Nooyen, D.; Morisetti, A.; Pollock, C.M.; Ekanayake, S.; Penner, G.B.; et al. Integration of ITS-2 rDNA nemabiome metabarcoding with Fecal Egg Count Reduction Testing (FECRT) reveals ivermectin resistance in multiple gastrointestinal nematode species, including hypobiotic Ostertagia ostertagi, in western Canadian beef cattle. Int. J. Parasitol. Drugs Drug Resist. 2023, 22, 27–35. [Google Scholar] [CrossRef]
- American Consortium for Small Ruminant Parasite Control. Deworming Charts for Sheep, Goats and Camelids. 2024. Available online: https://www.wormx.info/deworming (accessed on 22 March 2025).
- Keane, C.; Marchetto, K.M.; Oliveira-Santos, L.G.R.; Wünschmann, A.; Wolf, T.M. Epidemiological Investigation of Meningeal Worm-Induced Mortalities in Small Ruminants and Camelids Over a 19 Year Period. Front. Vet. Sci. 2022, 9, 859028. [Google Scholar] [CrossRef]
- Cringoli, G.; Maurelli, M.P.; Levecke, B.; Bosco, A.; Vercruysse, J.; Utzinger, J.; Rinaldi, L. The Mini-FLOTAC technique for the diagnosis of helminth and protozoan infections in humans and animals. Nat. Protoc. 2017, 12, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Zajac, A.M. Gastrointestinal nematodes of small ruminants: Life cycle, anthelmintics, and diagnosis. Vet. Clin. N. Am. Food Anim. Pract. 2006, 22, 529–541. [Google Scholar] [CrossRef]
- Kaplan, R.M.; Denwood, M.J.; Nielsen, M.K.; Thamsborg, S.M.; Torgerson, P.R.; Gilleard, J.S.; Dobson, R.J.; Vercruysse, J.; Levecke, B. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) guideline for diagnosing anthelmintic resistance using the faecal egg count reduction test in ruminants, horses and swine. Vet. Parasitol. 2023, 318, 109936. [Google Scholar] [CrossRef] [PubMed]
- Denwood, M.J.; Kaplan, R.M.; McKendrick, I.J.; Thamsborg, S.M.; Nielsen, M.K.; Levecke, B. A statistical framework for calculating prospective sample sizes and classifying efficacy results for faecal egg count reduction tests in ruminants, horses and swine. Vet. Parasitol. 2023, 314, 109867. [Google Scholar] [CrossRef]
- Avramenko, R.W.; Redman, E.M.; Lewis, R.; Yazwinski, T.A.; Wasmuth, J.D.; Gilleard, J.S. Exploring the Gastrointestinal “Nemabiome”: Deep Amplicon Sequencing to Quantify the Species Composition of Parasitic Nematode Communities. PLoS ONE. 2015, 10, e0143559. [Google Scholar] [CrossRef]
- Redman, E.; Queiroz, C.; Bartley, D.J.; Levy, M.; Avramenko, R.W.; Gilleard, J.S. Validation of ITS-2 rDNA nemabiome sequencing for ovine gastrointestinal nematodes and its application to a large scale survey of UK sheep farms. Vet. Parasitol. 2019, 275, 108933. [Google Scholar] [CrossRef]
- Dallas, J.F.; Irvine, R.J.; Halvorsen, O.; Albon, S.D. Identification by polymerase chain reaction (PCR) of Marshallagia marshalli and Ostertagia gruehneri from Svalbard reindeer. Int. J. Parasitol. 2000, 30, 863–866. [Google Scholar] [CrossRef]
- Gliga, D.S.; Kramer, A.; Moré, G.; Frey, C.F.; Basso, W. Early Detection and Management of Lamanema chavezi infection in a llama (Lama glama) in Switzerland. Vet. Res. Commun. 2024, 48, 3365–3369. [Google Scholar] [CrossRef]
- Jurasek, M.E.; Bishop-Stewart, J.K.; Storey, B.E.; Kaplan, R.M.; Kent, M.L. Modification and further evaluation of a fluorescein-labeled peanut agglutinin test for identification of Haemonchus contortus eggs. Vet. Parasitol. 2010, 169, 209–213. [Google Scholar] [CrossRef]
- Levecke, B.; Kaplan, R.M.; Thamsborg, S.M.; Torgerson, P.R.; Vercruysse, J.; Dobson, R.J. How to improve the standardization and the diagnostic performance of the fecal egg count reduction test? Vet. Parasitol. 2018, 253, 71–78. [Google Scholar] [CrossRef]
- Coles, G.C.; Jackson, F.; Pomroy, W.E.; Prichard, R.K.; von Samson-Himmelstjerna, G.; Silvestre, A.; Taylor, M.A.; Vercruysse, J. The detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 2006, 136, 167–185. [Google Scholar] [CrossRef]
- Fowler, M.E. Camelids Are Not Ruminants. In Zoo and Wild Animal Medicine; Saunders: Stanton, KS, USA, 2008; pp. 375–385. [Google Scholar] [CrossRef]
- USDA, APHIS, Veterinary Services Strategy & Policy Protocol for the Importation of Farmed Camelids from Australia. USDA Guidelines 2006, Updated in 2020. Available online: https://www.aphis.usda.gov/sites/default/files/aus-camelid.pdf (accessed on 12 July 2025).
- Cain, J.L.; Gianechini, L.S.; Vetter, A.L.; Davis, S.M.; Britton, L.N.; Myka, J.L.; Slusarewicz, P. Rapid, automated quantification of Haemonchus contortus ova in sheep faecal samples. Int. J. Parasitol. 2024, 54, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Flay, K.J.; Hill, F.I.; Muguiro, D.H. A Review: Haemonchus contortus Infection in Pasture-Based Sheep Production Systems, with a Focus on the Pathogenesis of Anaemia and Changes in Haematological Parameters. Animals 2022, 12, 1238. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.F.V.; Monteiro, J.P.; Almeida, T.M.; Molento, M.B. A systematic review of the molecular mechanisms related to anthelmintic resistance in Haemonchus contortus: A contemporary narrative. Vet. Parasitol. 2025, 334, 110394. [Google Scholar] [CrossRef] [PubMed]
- Ghatee, M.A.; Malek Hosseini, S.A.A.; Marashifard, M.; Karamian, M.; Taylor, W.R.; Jamshidi, A.; Mobedi, I.; Azarmehr, H. Phylogenetic analysis of Trichostrongylus vitrinus isolates from southwest Iran. Parasites Vectors 2020, 13, 553. [Google Scholar] [CrossRef]
- Bailey, J.N.; Kahn, L.P.; Walkden-Brown, S.W. The relative contributions of T. colubriformis, T. vitrinus, T. axei and T. rugatus to sheep infected with Trichostrongylus spp. on the northern tablelands of New South Wales. Vet. Parasitol. 2009, 165, 88–95. [Google Scholar] [CrossRef]
- Eysker, M. Regulation of Trichostrongylus vitrinus and T colubriformis populations in naturally infected sheep in the Netherlands. Res. Vet. Sci. 1987, 42, 267–271. [Google Scholar] [CrossRef]
- Mizani, A.; Gill, P.; Daryani, A.; Sarvi, S.; Amouei, A.; Katrimi, A.B.; Soleymani, E.; Mirshafiee, S.; Gholami, S.; Hosseini, S.A.; et al. A multiplex restriction enzyme-PCR for unequivocal identification and differentiation of Trichostrongylus species in human samples. Acta Trop. 2017, 173, 180–184. [Google Scholar] [CrossRef]
- Mederos, A.; Fernández, S.; VanLeeuwen, J.; Peregrine, A.S.; Kelton, D.; Menzies, P.; LeBoeuf, A.; Martin, R. Prevalence and distribution of gastrointestinal nematodes on 32 organic and conventional commercial sheep farms in Ontario and Quebec, Canada (2006–2008). Vet. Parasitol. 2010, 170, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Torres-Acosta, J.F.; Mendoza-de-Gives, P.; Aguilar-Caballero, A.J.; Cuéllar-Ordaz, J.A. Anthelmintic resistance in sheep farms: Update of the situation in the American continent. Vet. Parasitol. 2012, 189, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Beleckė, A.; Kupčinskas, T.; Stadalienė, I.; Höglund, J.; Thamsborg, S.M.; Stuen, S.; Petkevičius, S. Anthelmintic resistance in small ruminants in the Nordic-Baltic region. Acta Vet. Scand. 2021, 63, 18. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, R.A.; Williamson, L.H.; Terrill, T.H.; Kaplan, R.M. Efficacy of anthelmintics on South American camelid (llama and alpaca) farms in Georgia. Vet. Parasitol. 2010, 172, 168–171. [Google Scholar] [CrossRef]
- Jabbar, A.; Campbell, A.J.D.; Charles, J.A.; Gasser, R.B. First report of anthelmintic resistance in Haemonchus contortus in alpacas in Australia. Parasites Vectors 2013, 6, 243. [Google Scholar] [CrossRef]
- Rashid, M.H.; Vaughan, J.L.; Stevenson, M.A.; Campbell, A.J.D.; Beveridge, I.; Jabbar, A. Anthelmintic resistance in gastrointestinal nematodes of alpacas (Vicugna pacos) in Australia. Parasites Vectors 2018, 11, 388. [Google Scholar] [CrossRef]
- Hamer, K.; Bartley, D.; Jennings, A.; Morrison, A.; Sargison, N. Lack of efficacy of monepantel against trichostrongyle nematodes in a UK sheep flock. Vet. Parasitol. 2018, 257, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Prichard, R.; Ménez, C.; Lespine, A. Moxidectin and the avermectins: Consanguinity but not identity. Int. J. Parasitol. Drugs Drug Resist. 2012, 2, 134–153. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.M.F.; Ghazy, A.A. Advances in diagnosis and control of anthelmintic resistant gastrointestinal helminths infecting ruminants. J. Parasit. Dis. 2022, 46, 901–915. [Google Scholar] [CrossRef] [PubMed]
| Farm ID | Ruminant Species | Number of Animals and Age/Sex | Type of Fecal Sample Collected and Sampling Intervals | Number of Animals That Received IVM Treatment |
|---|---|---|---|---|
| 1 | Sheep and llamas (raised together) | 11 sheep, 2 Y.O. (7 females and 4 males) 2 llamas, 2 Y.O. (2 males) N total: 11 | Individual samples from 11 sheep (10 g each), pre- and 14 days post-IVM treatment. Individual samples from 4 llamas (20 g each), including 2 samples from 1 llama at pre- and post-IVM treatment. | 9 sheep and 1 llama |
| 2 | Sheep | 9 sheep, 2 Y.O. (9 females) N total: 9 | Two pooled samples containing 10 g of feces from each animal. Each pooled sample corresponded to pre-and post-IVM treatment. | 9 sheep |
| 3 | Goats and sheep (raised together) | 5 goats, 2 Y.O. (5 females) 1 kid, 6 months old 2 sheep, 2 Y.O. (2 females) N total: 8 | One pooled sample from goats, with 10 g from each animal. A total of 2–10 g of individual samples from each sheep. | No animal received treatment |
| 4 | Alpacas | 2 groups: Group 1: 8 youngs, 1 Y.O. (4 females and 4 males) Group 2: 7 adults 2 Y.O. (6 females and 1 male) N total: 15 | Individual samples (20 g) from each animal at pre- and 14 days post-IVM treatment. Pre-treatment samples were collected once at 30–31 days between IVM treatments. | 15 alpacas |
| 5 | Alpacas | 3 groups: Group 1: 6 female adults, 2 Y.O. Group 2: 3 male adults, 2 Y.O. Group 3: 1 young, 1 Y.O. Group 4: 1 cria, 6 months old N total: 11 | Pooled samples from age groups 1 and 2, containing at least 5 g from each animal. Age groups 3 and 4 were collected individual samples (5 g). | No animal received treatment |
| 6 | Alpacas | 3 groups: Group 1: 5 male adults, 2 Y.O. Group 2: 3 male adults, 2 Y.O. Group 3: 4 female adults, 2 Y.O. Group 4; 1 young, 1 Y.O. N total: 13 | Pooled samples from each group, containing at least 5 g from each animal. | No animal received treatment |
| GIN Species, Gene and Reference | Sequence (5′ → 3′) | PCR Product Size (bp) | Annealing Temp | |
|---|---|---|---|---|
| GIN generic ITS2 [35] | FW | CACGAATTGCAGACGCTTAG | 370–398 | 53 °C |
| RV | GCTAAATGATATGCTTAAGTTCAGC | |||
| H. contortus (1) ITS2 [35] | FW | CACGAATTGCAGACGCTTAG | 170 | 53 °C |
| RV | CTTGAACTGAAATGGGAATTGTCT | |||
| H. contortus ITS2 (2) [36] | FW | GTTACAATTTCATAACATCACGT | 321 | 55 °C |
| RV | TTTACAGTTTGCAGAACTTA | |||
| T. circumcincta ITS2 [36] | FW | ATACCGCATGGTGTGTACGG | 421 | 58 °C |
| RV | CAGGAACGTTACGACGGTAAT | |||
| T. axei ITS2 [36] | FW | AGGGATATTAATGTCGTTCA | 67 | 56 °C |
| RV | TGATAATTCCCATTTTAGTTT | |||
| T. colubriformis ITS2 [36] | FW | CCCGTTAGAGCTCTGTATA | 165 | 59 °C |
| RV | TGCGTACTCAACCACCACTAT | |||
| T. vitrinus ITS2 [36] | FW | AGGAACATTAATGTCGTTACA | 100 | 54 °C |
| RV | CTGTTTGTCGAATGGTTATTA | |||
| Ch. ovina ITS2 [36] | FW | CATGTGTGATCCTCGTACTAGATAAGA | 158 | 54 °C |
| RV | ATGAACCGTACACCGTTGTCA | |||
| O. venulosum ITS2 [36] | FW | TGTTTACTACAGTGTGGCTTG | 280 | 54 °C |
| RV | CGGTTGTCTCATTTCACAGGC | |||
| C. curticei ITS2 [36] | FW | TATACTACAGTGTGGCTAGCG | 143 | 54 °C |
| RV | TCATACCATTCAGAAATGTTC | |||
| C. mentulatus ITS2 (this study) | FW | CTTCGGCACGTCTGGTTCAG | 278 | 55 °C |
| RV | TGAGCTCAGGTTGCAATACAAA | |||
| M. marshalli COX1 (this study) | FW | TCATGAATGACACATGCAACA | 188 | 53 °C |
| RV | TAAGTTCAGCGGGTAATCACG | |||
| L. chavezi COX-1 (this study) | FW | TTTGGGCATCCTGAGGTTTA | 157 | 53 °C |
| RV | GAGCTCAAACCACACAACCA | |||
| GIN Species | Farm 1 | Farm 2 | Farm 3 | Farm 4 | Farm 5 | Farm 6 | Prevalence (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Sheep N = 11 | Llama N = 2 | Sheep N = 9 | Sheep N = 2 | Goat N = 6 | Alpaca N = 15 | Alpaca N = 11 | Alpaca N = 13 | ||
| H. contortus | pos | pos | pos | pos | pos | pos | pos | N/A | 83.3% |
| T. circumcincta | pos | pos | pos | pos | neg | neg | neg | N/A | 66.6% |
| T. axei | pos | pos | neg | pos | neg | neg | neg | N/A | 50% |
| T. colubriformis | neg | neg | pos | neg | neg | neg | neg | N/A | 16.6% |
| T. vitrinus | pos | pos | pos | pos | pos | pos | pos | N/A | 83.3% |
| O. venulosum | pos | neg | neg | neg | neg | neg | pos | N/A | 33.3% |
| C. curticei | pos | neg | neg | neg | neg | neg | neg | N/A | 16.6% |
| C. ovina | pos | pos | neg | pos | neg | neg | neg | N/A | 50% |
| C. mentulatus | N/A | neg | N/A | N/A | N/A | pos | pos | N/A | 33.3% |
| L. chavezi | N/A | neg | N/A | N/A | N/A | pos | neg | N/A | 16.6% |
| M. marshalli | N/A | neg | N/A | N/A | N/A | pos | neg | N/A | 16.6% |
| Genera | Farm 1 | Farm 2 | Farm 3 | Farm 4 | Farm 5 | Farm 6 | Prevalence (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Sheep N = 11 | Llama N = 2 | Sheep N = 9 | Sheep N = 2 | Goat N = 6 | Alpaca N = 15 | Alpaca N = 11 | Alpaca N = 13 | ||
| Nematodirus spp. | pos | pos | neg | neg | neg | pos | pos | pos | 66.6% |
| Trichuris spp. | pos | pos | neg | pos | neg | pos | pos | neg | 66.6% |
| Farm | Sample ID | Type | Total Strongylids | Nematodirus spp. | Trichuris spp. | |||
|---|---|---|---|---|---|---|---|---|
| EPG Mean | SD (90% CI) | EPG Mean | SD (90% CI) | EPG Mean | SD (90% CI) | |||
| 1 | SHP 11 | FEC | 138.6 | ±22.2 (102.0–175.1) | 3.7 | ±2.1 | 0 | N/A |
| 1 | SHP 12 | FEC | 22.3 | ±4.4 (15.0–29.5) | 0.7 | ±1.2 | 0 | N/A |
| 1 | SHP 13 | pre-treatment | 61.7 | ±34.5 (4.9–118.4) | 0.3 | ±0.3 (−0.1–0.7) | 0.1 | ±0.2 |
| post-treatment | 12.2 | ±3.6 (6.1–18.2) | 0 | N/A | 0 | N/A | ||
| 1 | SHP 14 | pre-treatment | 120.4 | ±28.2 (74.0–166.7) | 2.3 | ±0.3 (1.8–2.7) | 1.6 | ±0.7 (0.4–2.7) |
| post-treatment | 5.4 | ±1.4 (3.0–7.7) | 0 | N/A | 0 | N/A | ||
| 1 | SHP 15 | pre-treatment | 31.9 | ±14.8 (7.5–56.2) | 0 | N/A | 0 | N/A |
| post-treatment | 0.6 | ±0.2 (0.2–0.9) | 0 | N/A | 0 | N/A | ||
| 1 | SHP 16 | pre-treatment | 35.2 | ±22 (−0.9–71.3) | 0 | N/A | 0 | N/A |
| post-treatment | 16.3 | ±5.6 (7.0–25.5) | 0.3 | ±0.34 (−0.2–1.8) | 0 | NA | ||
| 1 | SHP 17 | pre-treatment | 1013.2 | ±139.7 (783.4–1242.9) | 0 | NA | 0 | N/A |
| post-treatment | 31.9 | ±10 (14.3–49.5) | 0 | N/A | 0 | N/A | ||
| 1 | SHP 18 | pre-treatment | 248 | ±33.4 (193.0–302.9) | 0.3 | ±0.34 (−0.2–1.8) | 0.4 | ±0.57 (−0.5–1.3) |
| post-treatment | 1.8 | ±1.1 (−0.0–3.6) | 0 | N/A | 0 | N/A | ||
| 1 | SHP 19 | pre-treatment | 239.4 | ±239 (−153.7–632.5) | 0 | N/A | 0 | N/A |
| post-treatment | 0.1 | ±0.2 (−0.2–0.4) | 0 | N/A | 0 | N/A | ||
| 1 | SHP 20 | pre-treatment | 594.1 | ±175.8 (304.9–883.2) | 3.1 | ±0.4 (2.4–3.7) | 0.6 | ±0.2 (0.2–0.9) |
| post-treatment | 337 | ±110.9 (171.0–502.9) | 7.1 | ±2.5 (2.9–11.2) | 0.3 | ±0.3 (−0.1–0.7) | ||
| 1 | SHP 21 | pre-treatment | 1019.1 | ±268.3 (577.7–1460.4) | 61.1 | ±13.6 (38.7–83.4) | 1.8 | ±0.4 (1.1–2.4) |
| post-treatment | 335.8 | ±355.1 (−248.2–919.8) | 0.8 | ±1.1 (−1.0–2.6) | 0 | N/A | ||
| Farm | Sample ID | Type | Total Strongylids | Nematodirus spp. | Trichuris spp. | |||
|---|---|---|---|---|---|---|---|---|
| EPG Mean | SD (90% CI) | EPG Mean | SD (90% CI) | EPG Mean | SD (90% CI) | |||
| 2 | SHP 30a Adults N = 9 | pre-treatment | 714.2 | ±142.4 (479.9–948.4) | 0 | N/A | 0 | N/A |
| post-treatment | 0 | NA | 0 | N/A | 0 | N/A | ||
| Farm | Sample ID | Type | Total Strongylids | Nematodirus spp. | Trichuris spp. | |||
|---|---|---|---|---|---|---|---|---|
| EPG Mean | SD (90% CI) | EPG Mean | SD (90% CI) | EPG Mean | SD (90% CI) | |||
| 3 | GOT 11 (5 females and 1 kid) N = 6 | FEC | 416 | ±35.1 (393.1–440.2) | 0.0 | N/A | 0.0 | N/A |
| 3 | Sheep 11 (1 adult, 2-years old) | FEC | 113.3 | ±55.1 (22.6–203.9) | 0 | N/A | 0 | N/A |
| 3 | Sheep 12 (1 adult, 2-years old) | FEC | 196.7 | ±47.3 (118.8–274.5) | 0.8 | ±1.24 (−1.2–2.8) | 0 | N/A |
| Farm | Sample ID | Type | Total Strongylids | Nematodirus spp. | Trichuris spp. | |||
|---|---|---|---|---|---|---|---|---|
| EPG Mean | SD (90% CI) | EPG Mean | SD (90% CI) | EPG Mean | SD (90% CI) | |||
| 1 | LAM 11 | FEC | 76.4 | ±27.0 (31.8–120.8) | 2.0 | ±1.0 (0.3–3.6) | 0.0 | N/A |
| FEC | 21.3 | ±11.9 (1.7–40.8) | 1.8 | ±1.2 (−0.1–3.7) | 0.1 | ±0.2 (−0.2–0.4) | ||
| 1 | LAM 12 | pre-treatment | 526.6 | ±347.4 (−44.8–1098.0) | 1.0 | ±1.2 (−0.9–2.9) | 0.1 | ±0.2 (−0.2–0.4) |
| post-treatment | 185.2 | ±109.8 (4.5–365.8) | 0.0 | N/A | 0.0 | N/A | ||
| Farm | Sample ID | Type | Total Strongylids | Nematodirus spp. | Trichuris spp. | |||
|---|---|---|---|---|---|---|---|---|
| EPG Mean | SD (90% CI) | EPG Mean | SD (90% CI) | EPG Mean | SD (90% CI) | |||
| 4 | ALP 11 | pre-treatment | 96.7 | ±67 (−13.2–207.2) | 6.7 | ±5.7 (−2.6–16.0) | 7 | ±5.9 (−2.7–16.7) |
| post-treatment | 153.3 | ±50 (71.0–235.5) | 20 | ±10 (3.5–36.4) | 10 | ±10 (−6.4–26.4) | ||
| 4 | ALP 12 | pre-treatment | 30 | ±17 (2.0–57.9) | 0 | N/A | 20 | ±17.3 (−15.5–50.1) |
| post-treatment | 23.3 | ±12.1 (3.3–43.2) | 0 | N/A | 10 | ±10 (−6.4–26.4) | ||
| 4 | ALP 13 | pre-treatment | 3.3 | ±6.2 (−7.1–13.1) | 0 | N/A | 0 | N/A |
| post-treatment | 0 | N/A | 0 | N/A | 0 | N/A | ||
| 4 | ALP 14 | pre-treatment | 146.7 | ±49.4 (65.4–227.9) | 3.3 | ±5.7 (−6.0–12.6) | 23.3 | ±10 (−6.4–26.4) |
| post-treatment | 6.7 | ±5.7 (−2.6–16.0) | 0 | N/A | 3.3 | ±5.7 (−6.0–12.6) | ||
| 4 | ALP 15 | pre-treatment | 16.7 | ±5.7 (7.3–26.0) | 0 | N/A | 0 | N/A |
| post-treatment | 16.7 | ±11.5 (−2.2–35.6) | 0 | N/A | 3.3 | ±5.7 (−6.0–12.6) | ||
| 4 | ALP 16 | pre-treatment | 23.3 | ±32 (−29.3–75.9) | 20 | ±34.6 (−36.9–76.9) | 0 | NA |
| post-treatment | 13.3 | ±5.7 (3.9–22.6) | 10 | N/A | 3.3 | ±5.7 (−6.0–12.6) | ||
| 4 | ALP 17 | pre-treatment | 45 | ±35.3 (−13.0–103.0) | 10 | ±14.1 (−13.1–33.1) | 10 | NA |
| post-treatment | 53.3 | ±32.1 (0.5–106.1) | 40 | ±30.5 (−10.1–90.1) | 10 | ±10 (−6.4–26.4) | ||
| 4 | ALP 18 | pre-treatment | 6.7 | ±5.7 (−2.6–16.0) | 3.3 | ±5.7 (−6.0–12.6) | 3.3 | ±5.7 (−6.0–12.6) |
| post-treatment | 26.7 | ±15.2 (1.6–51.7) | 23.3 | ±11.5 (4.3–42.2) | 3.3 | ±5.7 (−6.0–12.6) | ||
| Farm | Sample ID | Type | Total Strongylids | Nematodirus spp. | Trichuris spp. | |||
|---|---|---|---|---|---|---|---|---|
| EPG Mean | SD (90% CI) | EPG Mean | SD (90% CI) | EPG Mean | SD (90% CI) | |||
| 4 | ALP 20 | pre-treatment | 0 | N/A | 0 | N/A | 0 | N/A |
| post-treatment | 6.7 | ±5.7 (−2.6–16.0) | 0 | N/A | 0 | N/A | ||
| 4 | ALP 21 | pre-treatment | 10.2 | NA | 0 | NA | 0 | N/A |
| post-treatment | 27.1 | ±11.5 (12.2–25.6) | 3.3 | ±5.7 (−6.0–12.6) | 0 | N/A | ||
| 4 | ALP 22 | pre-treatment | 32.8 | NA | 0 | N/A | 0 | N/A |
| post-treatment | 56.7 | NA | 0 | N/A | 0 | N/A | ||
| 4 | ALP 23 | pre-treatment | 43.3 | ±25.1 (2.0–84.5) | 0 | N/A | 0 | N/A |
| post-treatment | 23.3 | NA | 0 | N/A | 0 | N/A | ||
| 4 | ALP 24 | pre-treatment | 10 | ±10 (−6.4–26.4) | 0 | N/A | 10 | ±10 (−6.4–26.4) |
| post-treatment | 3.3 | ±5.7 (−6.0–12.6) | 0 | N/A | 3.3 | ±5.7 (−6.0–12.6) | ||
| 4 | ALP 25 | pre-treatment | 43.3 | ±15.2 (18.2–68.3) | 36.7 | ±11.5 (17.7–55.6) | 0 | N/A |
| post-treatment | 20 | NA | 10 | ±10 (−6.4–26.4) | 0 | N/A | ||
| 4 | ALP 26 | pre-treatment | 23.3 | ±20.8 (−10.9–57.5) | 0 | N/A | 16.7 | ±15.2 (−8.3–41.7) |
| post-treatment | 10 | ±17.3 (−18.4–38.4) | 0 | N/A | 3.3 | ±5.7 (−6.0–12.6) | ||
| Farm | Sample ID | Type | Total Strongylids | Nematodirus spp. | Trichuris spp. | |||
|---|---|---|---|---|---|---|---|---|
| EPG Mean | SD (90% CI) | EPG Mean | SD (90% CI) | EPG Mean | SD (90% CI) | |||
| 5 | ALP (females, 2 Y.O.) N = 6 | FEC | 35.7 | ±6.3 (25.3–46.0) | 2.2 | ±1.2 (0.2–4.1) | 0.2 | ±0.1 (0.0–0.3) |
| 5 | ALP (old males, 2 Y.O.) N = 3 | FEC | 2.8 | ±1.1 (0.9–4.6) | 0.6 | ±0.6 (−0.3–1.5) | 0.1 | ±0.1 (−0.0–0.2) |
| 5 | ALP (young male, 1 Y.O.) N = 1 | FEC | 1.3 | ±0.5 (0.4–2.1) | 0.7 | ±0.4 (0.0–1.3) | 0.1 | ±0.1 (−0.0–0.2) |
| 5 | ALP (crias, <1 Y.O.) N = 1 | FEC | 1 | ±1.1 (−0.8–2.8) | 0.6 | ±0.6 (−0.3–1.5) | 0.2 | ±0.1 (−0.0–0.3) |
| 6 | ALP (young, 1 Y.O.) N = 5 | FEC | 8 | ±1.0 (6.3–9.6) | 0 | NA | 0.3 | ±0.3 (−0.1–0.7) |
| 6 | ALP males (1) (2 Y.O) N = 5 | FEC | 11.9 | ±6.8 (0.7–23.0) | 0.1 | ±0.1 (−0.0–0.2) | 0.1 | ±0.1 (−0.0–0.2) |
| 6 | ALP males (2) (2 Y.O) N = 3 | FEC | 1.6 | ±1.5 (−0.8–4.0) | 0 | NA | 1.3 | ±1.3 (−0.8–3.4) |
| 6 | ALP (females, 2 Y.O.) N = 4 | FEC | 4.1 | ±1.7 (1.3–6.8) | 0.1 | ±0.1 (−0.1–0.3) | 3 | ±1.1 (1.1–4.8) |
| Efficacy Classification | Expected Efficacy for IVM (MLs) ** | Delta Method *** (Levecke et al., 2018) [38] | WAAVP Method *** (Coles et al., 2006) [39] | BNB Method *** (Denwood et al., 2023) [31] |
|---|---|---|---|---|
| Farm 1 (adult sheep) N = 9 | 99% with lower threshold of 90% | Resistant 90% CI = 48.8–95.9 | Resistant 90% CI = 21–93.9 | Unavailable |
| Farm 2 (adult sheep) N = 9 | 99% with lower threshold of 90% | Susceptible 90% CI = NA | Unavailable | Susceptible Version B #: p < 0.001 |
| Farm 4 (alpacas, 1 Y.O.) N = 8 | 99% with lower threshold of 90% | Resistant 90% CI = −82.1–79.8 | Resistant 90% CI = −162.7–73.4 | Unavailable |
| Farm 4 (alpacas, 2 Y.O.) N = 6 | 99% with lower threshold of 90% | Resistant 90% CI = −49.7–61.4 | Resistant 90% CI = −92.9–61.3 | Unavailable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezanezhad-Dizaji, B.; Abrahamyan, L.; Rousseau, M.; Godoy, P. Coprological and Molecular Analyses of Ruminant Farms in Québec, Canada, Show a Variable Efficacy of Ivermectin Against Gastro-Intestinal Nematodes. Pathogens 2025, 14, 984. https://doi.org/10.3390/pathogens14100984
Rezanezhad-Dizaji B, Abrahamyan L, Rousseau M, Godoy P. Coprological and Molecular Analyses of Ruminant Farms in Québec, Canada, Show a Variable Efficacy of Ivermectin Against Gastro-Intestinal Nematodes. Pathogens. 2025; 14(10):984. https://doi.org/10.3390/pathogens14100984
Chicago/Turabian StyleRezanezhad-Dizaji, Behrouz, Levon Abrahamyan, Marjolaine Rousseau, and Pablo Godoy. 2025. "Coprological and Molecular Analyses of Ruminant Farms in Québec, Canada, Show a Variable Efficacy of Ivermectin Against Gastro-Intestinal Nematodes" Pathogens 14, no. 10: 984. https://doi.org/10.3390/pathogens14100984
APA StyleRezanezhad-Dizaji, B., Abrahamyan, L., Rousseau, M., & Godoy, P. (2025). Coprological and Molecular Analyses of Ruminant Farms in Québec, Canada, Show a Variable Efficacy of Ivermectin Against Gastro-Intestinal Nematodes. Pathogens, 14(10), 984. https://doi.org/10.3390/pathogens14100984










