Abstract
Gastro-intestinal nematodes (GINs) are still of great concern in grazing ruminants, such as camelids, ovines and caprines, affecting animal health and productivity. This is mainly due to the development of anthelmintic resistance (AR) to the compounds used long term, without much evaluation on their efficacy, including ivermectin (IVM), the most used anthelmintic drug in livestock. The aims of this study were to determine the efficacy of IVM and identify which GIN species are affecting different ruminant farms in Quebec (QC), Canada. Firstly, we collected fecal samples from six farms with different ruminant species (camelids, goats and sheep) before and after IVM treatment when applicable, analyzing them by Fecal Egg Count (FEC) and further assessments on IVM efficacy through the Fecal Egg Count Reduction Test (FECRT). In addition, molecular analyses were conducted using PCR, targeting the ITS-2 and COX-1 genes to identify GIN species. FECRT was applied only for three farms, showing that variable results with optimal efficacy (ranging from 95.5–100%) were obtained in only one farm, whereas on the other two farms, FECRT exhibited reduced efficacy, suggesting the development of IVM resistance. Among the GIN species found, Haemonchus contortus and Trichostrongylus vitrinus were identified in most of the farms, being present in sheep, goat, llama and alpaca farms, whereas Teladorsagia circumcincta was identified only in sheep and llama samples from four farms but not in alpaca samples. Trichostrongylus axei and Chabertia ovina were present in two farms (sheep and sheep and llamas). Oesophagostomum venulosum was detected in one sheep and one alpaca farm. Only one sheep farm was positive for Trichostrongylus colubriformis and Cooperia curticei. Also, Nematodirus spp. and Trichuris spp. were found in four farms, including sheep and camelids. In addition, three other species were found in camelids, including Camelostrongylus mentulatus (only in the llama samples), whereas Lamanema chavezi and Marshallagia marshalli were identified in one alpaca farm. Therefore, our work reports evidence of an uneven efficacy of IVM against GINs from ruminant farms, including the most likely emergence of IVM resistance. The diversity of GIN species found in ruminant farms in QC along with the inconsistent IVM efficacy are helpful information for veterinarians and animal producers in setting an optimal parasite management programs, including the proper use of IVM and alternative anthelmintic drugs to control these pathogens in grazing livestock.
1. Introduction
Veterinary nematodes affect grazing ruminants all around the world [1,2]. The effect of their parasitism has significantly impacted the small ruminants’ industry in several countries [3,4,5]. In North America, epidemiological studies have identified the presence of multiple species of parasitic nematodes, including the strongylid suborder in grazing livestock and ruminant wildlife [6,7,8,9]. Conventionally, the control of parasitic nematodes in agricultural systems has relied on the use of anthelmintic formulations, a practice that has contributed to the emergence of anthelmintic resistance (AR) in gastro-intestinal nematodes (GINs) [10]. In Canada, the main classes of anthelmintic drugs used to control GINs in livestock include macrocyclic lactones (MLs) and benzimidazoles (BZs) [11]. MLs are broad-spectrum anthelmintics that act by binding irreversibly to nematode Glutamate-gated chloride channels (GluCls), leading to a flaccid paralysis and further death [10], whereas BZs bind to the nematode’s β-tubulin, inducing its depolymerization and cytoskeleton disruption [10].
The diagnosis of GINs as well the assessment of drug efficacy and AR in farmed ruminants are typically performed through the fecal egg count (FEC) technique [12]. The development of new technologies has enhanced the identification of GIN species from using microscopic observation of eggs and larval morphology [13] to molecular techniques, such as PCR and deep-amplicon sequencing (DNA bar cording) [14,15]. However, besides the fecal egg count reduction test (FECRT) and assays for the SNP associated with BZ resistance, there are no standardized alternative methods or reliable markers to measure AR in GINs for other anthelmintic classes [16].
Haemonchus contortus is among the GIN species that parasitize many grazing ruminants [17]. This harmful blood-feeding helminth is the most successful species of parasitic nematode in developing AR to both old and new anthelmintic formulations [18,19,20]. H. contortus has been previously described in some parts of North America, where it affects mainly small ruminants and New World camelids, such as alpacas and llamas, and occasionally beef cattle [21,22,23]. In some provinces of Canada, research reports, mainly from sheep and goat farms, indicate the prevalence of GIN species, including H. contortus isolates, with evidence of AR to BZs [24,25]. Resistance to MLs, most commonly, resistance to ivermectin (IVM), has been identified in GINs from beef cattle in Western Canada [26].
Based on the widespread use of IVM to control parasitic nematodes in livestock raised on pastures, in the present study, we aimed to identify the GIN species that affect different grazing ruminants in Québec (Eastern Canada) and to assess the efficacy of IVM as the most common anthelmintic drug used to control parasitic nematodes.
2. Materials and Methods
2.1. Ruminant Farms and Sample Collection for IVM Efficacy Assessment
In total, 6 grazing ruminant farms were included in our study (Table 1) as follows: farm 1 contained 11 sheep of 2 years old (Y.O.), but only 9 were treated with IVM. Farm 2 corresponds to 9 sheep that were 2 Y.O., all treated with IVM. Farm 3 included 6 goats (5 adults that were 2 Y.O. and 1 kid) in addition to 2 sheep that were 2 Y.O. No animal was treated with IVM. Farm 4 has two age groups of alpacas: one group of 8 animals aged 1 Y.O. and another group of 7 animals of 2 Y.O. Both groups were treated with a monthly dose of IVM. Farm 5 had a total of 11 alpacas, divided in 9 adults that were 2 Y.O., 1 young adult being 1 Y.O. and 1 cria (6 months old), and no animal received IVM treatment. Farm 6 included 13 animals with no IVM treatment, consisting of 12 adults that were 2 Y.O. and 1 young adult aged 1 Y.O. Farms included in our study came from 3 Québec regions (Centre-Sud, Estrie and Montéregie), running the study between April 2021 and September 2022. Individual fecal samples were collected from the animal’s rectum, whereas pooled samples were collected by veterinarians, joining individual fresh samples from several animals. All fecal samples were placed on sealed and labeled plastic bags and were sent on ice on the same day or the next one (kept refrigerated at 4 °C) to the diagnostic service at FVM. Fecal samples were stored at 4 °C for 24–48 h until coprological analysis. Animals treated in our study received an oral IVM formulation (Ivomec®, Boehringer Ingelheim, Burlington, ON, Canada), based on individual weight and ruminant species dosage. Based on initial coprological results indicating a low parasite burden, farm 3 (goats and sheep) and alpaca farms 5 and 6 were not treated with IVM, based on veterinarians’ decision. Following label instructions, sheep received 0.2 mg/kg of IVM. For camelid (alpaca or llama) farms, IVM was administrated as an extra-label oral drench formulation (Ivomec®), administering 0.4 mg/kg to domestic camelid species [27]. On small ruminant farms, fecal samples were collected before and 14 days after IVM treatment individually or in pools when applicable, whereas for camelid farms, fecal samples were collected individually from each animal treated or not with IVM. Among the camelid farms, we included alpaca farm 4 that has been using IVM as a monthly preventative treatment against the meningeal worm Parelaphostrongylus tenuis, a parasitic nematode of wild ruminants in North America that could severely affect domesticated ruminants [28].

Table 1.
Detailed description of grazing ruminant farms included in the study.
2.2. Coprological Analyses
Fecal samples from sheep (farms 1 and 2) were processed following the Wisconsin method, whereas the fecal samples from alpacas were analyzed through the Wisconsin (farms 4, 5 and 6) and Mini-FLOTAC method (only for farms 3 and 4 when the Mini-FLOTAC kit was available), determining FEC and further IVM efficacy [12]. Briefly, from each sample collected from small ruminant farms, three replicates of 3 g of feces were weighed and homogenized in 20 mL of water in a small plastic beaker. Then, each sample replicate was stirred in a 50 mL Falcon tube with the addition of water up to 45 mL. The prepared mix was centrifuged at 850× g for 5 min, and the supernatant was discarded. The remaining pellet was resuspended in 5 mL of a saturated sugar solution (specific gravity: 1.30) and transferred to a 15 mL Falcon tube, followed by centrifugation at 350× g for 2 min. Finally, a saturated sugar solution was added to the tubes until the formation of a positive meniscus at the top of the tube, and a coverslip was fixed on the tubes [29]. After one h of incubation at RT, the coverslip was picked up and placed on the glass slide for FEC under a compound microscope at 10× magnification. The total number of counted eggs in each slide was divided into three (the weight of feces) to achieve each replicate’s egg per gram (EPG). The mean EPG of three replicates represented the EPG for each sample pre- or post-IVM treatment when applicable.
Fecal samples from sheep and goats from farm 3 and alpacas from farm 4 were subjected to the Mini-FLOTAC method for FEC [12]. A total of 2.5 g of feces from each sample were weighed and placed in the conical collector of the fill-FLOTAC. Then, 47.5 mL of a saturated salt solution (specific gravity: 1.2) was added to the fill-FLOTAC container, and the container was screwed closed [29]. The sample was homogenized by gently pumping and circulating the homogenizer pole of the fill-FLOTAC. After, the homogenized sample was transferred to the two chambers of the Mini-FLOTAC disk, avoiding bubbles. Subsequently, the sample was incubated for 10 min, followed by a gentle turn clockwise (90°) of the Mini-FLOTAC disk and further examined under the microscope to count the GIN eggs. The total FEC in two chambers was multiplied by 10 (multiplication factor) to calculate the mean EPG of three replicates per sample.
Hinging on microscopical analyses, GIN eggs were classified into a strongylid-like form and distinct nematode eggs from the genera Nematodirus and Trichuris [30]. Only FEC from strongylid-like eggs were used to evaluate IVM efficacy [31].
2.3. IVM Efficacy Assessment
Based on the initial FEC results from the pre-treatment coprological analyses, only 2 sheep farms (farm 1 and farm 2) that were treated with IVM and only one alpaca farm (farm 4, monthly IVM treatment) were included in the efficacy analysis through FECRT. Given the variation in animal numbers among the ruminant farms subjected to IVM treatment and the paired samples (individual pre- and post-treatment for sheep farm 1 (N = 9) and alpaca farm 4 with 2 subgroups (N = 8 and N = 7)) or the pooled fecal samples at pre-and post-IVM treatment from sheep farm 2 (N = 9), we applied the “clinical protocol” criteria to analyze and interpret the FECRT results on IVM efficacy [31]. As such, we calculated EPG means with a 90% coefficient interval (CI) to validate the test, defining a target efficacy for IVM as 99% against GINs in small ruminants extended to camelids [31,32]. Furthermore, EPG mean values were manually entered in the online software tool FECRT (https://www.fecrt.com/, version 1.1.0, accessed on 23 July 2025), and we calculated the anthelmintic efficacy for each ruminant farm that received IVM with appropriate EPG values to run FECRT [31,32]. Optimal IVM efficacy was defined as 99% FECRT, whereas a “grey zone” between 98 and 90% of FECRT was considered suboptimal efficacy but not marked resistance. FECRT values ≤ 89% were considered as indicative of resistance to IVM [31].
2.4. Molecular Identification of GIN Species
2.4.1. DNA Extraction
Nematode eggs from fecal samples subjected to coprological analyses were recovered for DNA extraction. A modified version of the “Nemabiome” method [33] for nucleic acid extraction from nematode eggs was employed (https://www.nemabiome.ca/parasite, accessed on 23 July 2025). In short, approximately 200–1000 GIN eggs (with morula or larva inside) per sample were transferred to a 1.5 mL tube spin down, and 1.3 mL of a lysis buffer was added, mixed and incubated at room temperature for 5 min. After, the samples were centrifuged at 13,000× g for 4 min, discarding the supernatant and leaving the pellet, resuspension in a lysis buffer and pellet recovery for 3 times were repeated. Further, 50 μL of a lysis buffer was added for pellet resuspension. Later, the tube was incubated at 95 °C for 15 min, with vortexing every 3 min. Afterwards, the pellet was kept at −80 °C for 2 h and then thawed on ice. Next, 6 μL of proteinase K (20 mg/mL) was added to the tube and incubated at 55 °C for 2 h with regular vortexing every 1 min. Finally, the sample was incubated at 95 °C for 20 min to denature the proteinase K. The final DNA lysate was diluted at a ratio of 1:10 to use as a template for further experiments [33].
2.4.2. PCR Amplification of GIN Species
Overlapping regions of the internal-transcribed-spacer genes 1 and 2 (ITS1 and ITS2, GenBank accession number: KY930444.1) were used as generic references for the identification of GIN species from the strongyloidea superfamily (https://lifemap.cnrs.fr/, accessed on 23 July 2025). Following the approach described by Bisset et al. 2014 [14], we carried out a nested PCR strategy, amplifying a first-round genetic amplicon of 370–398 bp that was used as a template with specific oligos to amplify target regions of the ITS-2 gene corresponding to the most common GIN species in grazing ruminants, such as H. contortus, Teladorsagia circumcincta, Trichostrongylus axei, Trichostrongylus colubriformis, Trichostrongylus vitrinus, Chabertia ovina, Oesophagostomum venulosum, Cooperia curticei and Camelostrongylus mentulatus [34,35]. The generic GIN amplicon was also used as a template to amplify specific regions of the ITS2 gene for Marshallagia marshalli (GenBank accession number: MT110920.1) [36]. To identify GIN species infecting domesticated camelids, we used the mitochondrial cytochrome c oxidase subunit 1 (COX-1) gene to find Lamanema chavezi (GenBank accession number: MG598421.1) in camelid farms [36]. PCR reagents (all from ThermoFischer Scientific, Burlington, ON, Canada) included MilliQ water, a 5× buffer PCR mix, 10 mM of dNTPs, 10 μM of Primers FW and RW, a DNA template comprising 1:10 dilutions and polymerase at 0.5 U/μL. Thermocycler (Bio-Rad T100TM, Saint-Laurent, QC, Canada) conditions were 95 °C for 3 min, followed by 40 cycles of the second denaturation at 98 °C for 30 s, annealing for 20 s and extension at 72 °C for 1 min, including a final extension of 10 min at 72 °C. PCR amplicons were analyzed on agarose gels and verified for their expected sizes. All primers and DNA fragment sizes for the amplification of generic or specific GIN sequences are listed in Table 2.

Table 2.
List of primers used for PCR amplification of genes from GIN species in ruminant farms.
2.4.3. Identification of H. contortus from Recovered Nematode Eggs
GIN eggs recovered from the fecal samples were subjected to both molecular identification of H. contortus (HcITS2 amplification as described before) and fluorescent staining with lectin conjugated to peanut FITC (PN-FITC) [37]. Stained eggs were then observed by fluorescent microscopy at 470/480 excitation and 527/530 emission, confirming the presence of H. contortus by both methods. Fluorescence and phase-contrast images were analyzed with the Image J software version 1.54g: https://imagej.net/, accessed on 23 July 2025.
3. Results
3.1. Prevalence and Identification GIN Species in Ruminant Farms
Through microscopical and molecular analyses, we identified multiple GIN species in most of the grazing ruminant farms included in the study. Table 3 summarizes the subset of GIN species found on each farm through molecular analyses, while Table 4 presents the nematode eggs identified from the genera Nematodirus and Trichuris spp. The most prevalent GINs found across grazing ruminant farms were H. contortus and T. vitrinus, present in 5 out of 6 farms (83.3% prevalence). Next, T. circumcincta, Nematodirus spp. and Trichuris spp. (Table 4) were present in 4 out of 6 farms (66.6%), and C. ovina and T. axei were identified in 3 out of the 6 farms (50%). Lower frequencies were found for O. venulosum and C. mentulatus (2 out of 6 farms for 33.3%), while T. colubriformis, C. curticei, L. chavezi and M. marshalli were all detected in just one farm out of 6 (16.6%).

Table 3.
GIN species identified by molecular analyses and their prevalences found in grazing ruminant farms.

Table 4.
GIN genera Nematodirus spp. and Trichuris spp., identified by microscopic analysis in grazing ruminant farms.
3.2. Microscopic Identification of H. contortus Eggs
Using peanut agglutinin (PNA) conjugated to FITC for the staining of GIN eggs, we were able to detect the presence of H. contortus in 4 out 6 farms. Complementing our molecular identification of GIN species, we confirmed the presence of H. contortus among strongylid-like eggs in sheep farms (Figure 1A,C). Most of the samples showed a greater positive staining for the presence of H. contortus in farms with one or more ruminant species, including sheep and llamas from farm 1 (Figure 1B) and alpacas from farm 4 (Figure 1D).
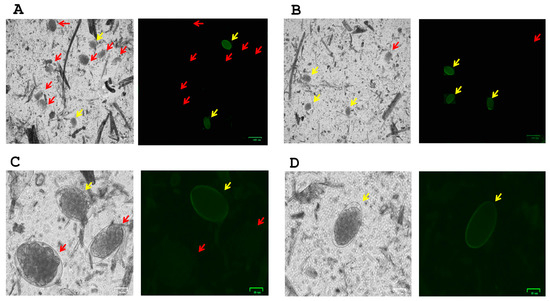
Figure 1.
Fluorescence staining of H. contortus egg (yellow arrows) screening from other GIN spp. eggs (red arrows). Peanut agglutinin (PNA) fluorescent signal on H. contortus eggs was found in most farm samples, including sheep (A,C), sheep and llamas (B) and alpacas (D). Images (A,B) in bright field and FITC signal were captured at 10× magnification (scale bar at 100 μm), and images (C,D) in bright field and FITC signal were captured at 40× magnification (scale bar at 25 μm).
3.3. FECs and IVM Efficacy on GINs from Grazing Ruminant Farms
The mean EPG with 90% CI was calculated for each sample collected before and after IVM treatment, as well as for untreated fecal samples. Strongylid-like eggs are described as “Total Strongylids”, while genera Nematodirus spp. and Trichuis spp. are reported separately. Table 5 presents the FEC values from sheep farm 1. In this farm, 11 animals older than 2 years old were included, and only 9 animals received IVM treatment, allowing for paired FECs at pre- and post-treatment comparisons. FEC values for GIN eggs showed a wide range of variation, fluctuating between 30 to more than 1000 EPG per animal at FEC only and pre-treatment samples. Egg counts for Nematodirus and Trichuris spp. maintained a low EGP, indicating a higher GIN burden of strongylid-like eggs, particularly from the Trichostrongylidae family.

Table 5.
Individual FECs from sheep farm 1. Animals were 2 years and older.
Table 6 presents the FEC data from sheep farm 2. These values correspond to a pooled sample of 9 animals, aged 2 years and older, all of which were treated with IVM. Notably, the FECs revealed a high EPG mean at pre-treatment FEC for strongylid-like eggs, which was totally reduced (0 EPG) at post-treatment FEC. No eggs of Nematodirus or Trichuris spp. were detected.

Table 6.
Pooled FECs from sheep farm 2. Animals were 2 years and older.
Table 7 corresponds to the FECs from farm 3, including sheep and goats. Animals were not treated with IVM. Due to the small group sizes for both sheep and goats, we applied the Mini-FLOTAC method, which has a higher sensitivity to detect GIN infection in ruminants, compared with the FEC results from the Wisconsin method (see Supplementary Data S1). The FEC values indicate some moderate GIN burden, with each small ruminant group comprising more than 100 EGP means of strongylid-like eggs. Other nematode genera were scarcely detected by the Mini-FLOTAC method.

Table 7.
Pooled FECs from goat and individual FECs from sheep farm 3.
Table 8 summarizes the FECs from 2 llamas that were present in farm 1 that were also grazing with sheep. One llama (ID LAM 11) presented a moderate EPG for strongylid-like eggs at two FECs. The other llama (ID LAM12) showed an initial high EPG (pre-treatment) that was subjected to IVM treatment. However, comparing pre- and post-treatment samples for strongylid-like eggs, the difference in EPG means is reduced but not significant. For the other nematode genera as well, both animals show very low FECs.

Table 8.
Individual FECs from llamas from farm 1. Animals were 2 years old.
FEC results from alpaca farm 4 that received a monthly treatment of IVM were divided by age. In Table 9, there are the individual FECs from the 1-year-old alpacas. EPG values fluctuated between pre- and post-treatment samples, independent of the method used (Mini-FLOTAC or Wisconsin; see Supplementary Data S2); however, due to the higher sensitivity to detecting Nematodirus and Trichuris eggs, we present the FECs from the Mini-FLOTAC method. Most animals exhibited low EPG values at post-treatment, which are recorded in the column labeled as “Total Strongylids”. Table 10 presents the FEC data for 2-year-old alpacas, which show non-significant EPG values overall, with only a few animals excreting more than 40 EPG during the pre-treatment assessment. Interestingly, in both age groups of alpacas, eggs of Nematodirus and Trichuris spp. were detected in 13 animals, with modest FECs (generally below 40 EPG). However, this represents a higher prevalence of these two nematode genera compared to the other farms included in our study.

Table 9.
Individual FECs from farm 4 corresponding to 1-year-old alpacas that received a monthly treatment with IVM.

Table 10.
Individual FECs from farm 4 corresponding to 2-year-old alpacas that received a monthly treatment with IVM.
We also included the FECs from pooled samples corresponding to two alpaca farms (farms 5 and 6, on Table 11) with different age subgroups. These two alpaca farms had no documented history of parasitism by GINs. After performing the coprology examinations, we found not significant FEC values for strongylid-like eggs nor for Nematodirus spp. and Trichuris spp. eggs. Based on this evidence, veterinarians and producers decided not to carry out an IVM treatment, opting instead to monitor using coprology analyses later in the season, beyond the timeframe of our study.

Table 11.
Pooled FECs from alpaca farms 5 and 6.
IVM efficacies were considered for only 3 out the 6 farms that received an IVM treatment and where the FEC values were suitable to calculate FECRT. Table 12 shows the efficacy results and classification from the FECRT analysis using the online software tool http://www.fecrt.com/, accessed on 23 July 2025. The results indicate that in farm 1 (sheep), there was a resistant status from GINs, as IVM treatment failed to significantly reduce the expected number of strongylid-like eggs. Both Delta and the classic WAAVP methods identified resistance through different statistical analyses. Nonetheless, the CI 90% upper limits of the tests are over 90%, placing the results in the “grey zone” or suboptimal efficacy, rather than clear resistance. Farm 2 (sheep) showed optimal IVM efficacy as the FECRT by the Delta and the BNB methods gave a clearly susceptible outcome for the GIN population subjected to IVM treatment. Although farm 2 corresponds to a pooled sample of sheep (N = 9, Table 6), the drastic reduction in EPG for the post-treatment sample was significant and validated by both Delta and BNB methods (Table 12).

Table 12.
Efficacy of IVM against GINs (strongylid-like eggs only) based on FECRT *.
In the case of both age groups from alpaca farm 4 (receiving monthly IVM treatment), notwithstanding the presence of an overall small EPG mean per animal, the FECRT analysis showed resistance for this farm, validated by the Delta and WAAVP methods, which showed that both CI 90% upper limits were below the threshold for the optimal efficacy window. We have included the full reports from the FECRT analyses run on the http://www.fecrt.com/, accessed on 23 July 2025. website as Supplementary Data (Data S3 for farm 1 (sheep); Data S4 are from farm 2 (sheep) and from alpaca farm 4 (monthly IVM treatment) for age groups of 1 year old (Data S5) and 2 years old (Data S6).
4. Discussion
Parasitic nematodes continue to hamper animal health in farm animals, such as grazing ruminants. Taxonomically and anatomically, ruminants such as cattle, sheep and goats are in a different Artiodactyla suborder from south American camelids (SACs, e.g., alpacas) based on the number of stomachs [40]. Nonetheless, from veterinary care and regulatory perspectives, SACs are included in the “ruminant” group, as they are raised in pastures for livestock production and share common infectious diseases, as illustrated by their susceptibility to endo-parasitoses for gastro-intestinal nematodes (GINs) [41]. Our study has examined the efficacy of ivermectin (IVM), the most common and available antiparasitic drug against GINs in livestock worldwide. In the ruminant farms included in our study, we identified several species of GINs affecting the animals and a varied efficacy of IVM to control these pathogens.
Our epidemiological data across most of the ruminant farms that included sheep, goats, llamas and alpacas reveal the presence of H. contortus and T. vitrinus as the most prominent GIN species infecting these ruminants. This is in line with the description of H. contortus as one of the most widespread endoparasites in ruminant livestock worldwide [42,43] including North America [23] Moreover, H. contortus has been extensively documented as one of the GINs that has developed anthelmintic resistance (AR) to all anthelmintic drug families, including the macrocyclic lactones (MLs) [44]. T. vitrinus has been described in small ruminants at different latitudes, such as Europe, the Middle East and Oceania [45,46,47], but there is no documented research on SACs such as that reported in our study. T. vitrinus has been described as a zoonotic helminth transmitted from ruminants to humans [48].
Besides H. contortus and T. vitrinus, our molecular analyses identified 9 other GIN species with strongylid-like eggs. In general, our results on the identification of GIN species from grazing ruminants have both similarities and contrasts with an earlier study specifically on sheep farms in Ontario and Québec [49]. Mederos et al. identified GIN species from larval cultures and morphological validation along with identification of adult worms from necropsies, whereas our study performed molecular analyses to establish GIN species in small ruminant and camelid farms. Although our study only comprised 6 ruminant farms, including 3 sheep farms with and without SACs, in both studies, H. contortus was found to be the most prevalent GIN species in sheep, whereas the other GIN species are described in the two studies at different prevalences.
Our assessment on the IVM efficacy against GINs in different ruminant farms showed a variable efficacy of this drug. The efficacy was determined through FECRT, including only suitable data from individual animals treated with IVM (Ivomec®) and counting with a minimal arithmetic mean of 40 EPG at pre-treatment FECs [31]. In the case of lower EPG means per animal, we considered a minimum of 5 animals per group, with an overall arithmetic mean of 15 EPG at pre-treatment FECs, as established by the WAAVP guidelines for anthelmintic efficacy field studies in ruminants [31]. Taking these parameters into consideration, we carried out an analysis of IVM efficacy through the FECRT online software (http://www.fecrt.com/, accessed on 23 July 2025) for only three farms treated with IVM. We found optimal IVM efficacy in only one sheep farm (farm 2 ≥ 99% FECRT, Table 12), whereas in another sheep farm (farm 1, Table 12), IVM efficacy was non-optimal and reported as IVM resistance; however, the CI 90% upper limits for two FECRT methods were between 90–99%, which is interpreted as being in the “grey zone” [31]. Results were validated through two statistical analyses (Table 12). This finding of loss of IVM efficacy in sheep farms has been well documented in North America and elsewhere [44,50,51].
Of interest, our study included an alpaca farm that administrates a monthly IVM treatment to camelids as a prophylactic against the meningeal worm P. tenuis. This practice is controversial in camelid farms in Eastern North America, including the US and Canada, as it may lead to selection of resistance of GINs to IVM and other MLs [52]. Our findings identified H. contortus and T. vitrinus, which have been under IVM selection, decreasing anthelmintic efficacy by up to a resistant status (Table 12, farm 4, both alpaca age groups). Resistance in H. contortus to MLs, including IVM, has been an emerging problem in the last 15 years in alpaca farms [53,54]. Trichostrongylus spp. resistance to IVM and to other anthelmintic classes has also been described in alpaca farms [55]. On the alpaca farm with monthly IVM treatment, we found limited IVM efficacy, indicating a selection process allowing for both H. contortus and T. vitrinus to develop an IVM-resistant phenotypes. Several studies have examined the basis of ML resistance in GINs, including H. contortus [16], but there is still no consensus; this is explained in part by the multigenic mechanism underlying AR to ML anthelmintics in veterinary nematodes [56]. The absence of molecular markers for resistance to MLs is another limitation in establishing a clear phenotype in ML-resistant parasitic helminths [16]. For these reasons, FECRT remains the most straightforward approach in field studies to assess anthelmintic efficacy against GINs in animal health, including farm ruminants raised in pastures.
5. Limitations of the Study
Although our study found the presence of IVM resistance in GINs from grazing ruminant farms in QC, Canada, using approved standardized analyses, we were careful about making assumptions about a general resistant process in the province’s livestock farms that are exposed to GINs, such H. contortus. Firstly, we only considered 6 ruminant farms, and further, only 3 have suitable data to assess IVM efficacy through FECRT. In addition, due to the overall medium–low EPG means per farm, we did not have enough parasite material to run the two coprological methods (Wisconsin and Mini-FLOTAC) and molecular analyses in all farms, such as farm 6 (alpacas), or to have parasite cultures to perform a bioassay to confirm IVM resistance in GINs through a larval developmental assay (LDA) or a larval migration assay (LMA) [57]. Another constraint of our study is that we applied the FECRT parameters from sheep to assess anthelmintic efficacy/AR in camelid farms. This is not ideal, but currently, there are no specific guidelines for studies of anthelmintic efficacy on SACs. Future studies should include a larger number of grazing ruminant farms and apply a consistent methodology (e.g., include only individual fecal samples vs pooled fecal samples) to validate anthelmintic efficacy studies by FECRT combined with pharmacological in vitro assays to clearly establish the presence of optimal drug efficacy or the presence of AR.
6. Conclusions
Our study has shown that grazing ruminants in Québec farms are exposed to several GINs, including H. contortus, a parasitic helminth species that may be becoming resistant to IVM. We found a variable efficacy of IVM against GINs, suggesting a process of parasite selection for resistance to IVM, and this phenomenon is independent of the ruminant species and age. All this information requires attention from veterinarians and livestock producers to direct applications of the best control strategies against these pathogens. Opting for the right choice of anthelmintic drug when deworming ruminants, coupled with alternative methods (refugia and pasture rotation, for instance), will help in the control of parasitic nematodes in grazing animal farms.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens14100984/s1, Data S1. FEC data from farm 3 (goats and sheep) using Wisconsin method (pdf). Data S2. FEC data from farm 4 (alpacas), both group ages (1 Y.O) and 2 Y.O, at both pre- and post-IVM treatment. (pdf). Data S3. Full FECRT report on farm 1 (sheep) from the online software http://fecrt.com/ (pdf). Data S4. Full FECRT report on farm 2 (sheep) from the online software http://fecrt.com/ (pdf). Data S5. Full FECRT report on farm 4 (alpaca, 1 Y.O. group) from the online software http://fecrt.com/ (pdf). Data S6. Full FECRT report on farm 4 (alpaca, 2 Y.O. group) from the online software http://fecrt.com/ (pdf).
Author Contributions
B.R.-D.: experimental work, methodology, data curation and analysis and manuscript writing. L.A.: project administration, manuscript writing, and data analysis and review. M.R.: sample collection and methodology. P.G.: conceptualization, project administration, resources, data analysis and manuscript writing and review. All authors have read and agreed to the published version of the manuscript.
Funding
Dr. Godoy received a “Louis-Phillipe Phaneuf” grant (FMV # RH000748) for this study, along with start-up funding resources at FMV from U. of Montreal.
Institutional Review Board Statement
The study design and procedures for fecal sample collection from grazing animals included in this study were fully reviewed and approved by CÉUA (Comité d’éthique de l’utilisation des animaux) at FMV from U. of Montréal, protocol # 21-Rech-2159.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data included from this research is enclosed in this research article.
Acknowledgments
The authors acknowledge all animal producers from the ruminant farms involved in the study. We extend our gratitude for facilitating the fecal sample collection to Richard Bourassa and Nancy Meunier from Clinique Vétérinaire Sherbrooke, Sherbrooke, QC, Canada; Véronique Lebel from Clinique Ambulatoire Saint-Narcisse, Saint-Narcisse, QC, Canada; and Joanie Desrochers at Clinique Vétérinaire de l’Érable, Plessisville, QC, Canada. We also appreciate the help received for sample reception at FMV by the personnel at the laboratory of Parasitology from the Centre de diagnostic vétérinaire de l’Université de Montréal (CDVUM) and the technical support of Frédéric Berthiaume at FMV. Finally, we acknowledge Malcolm Whiteway at Concordia University in Montreal, QC, Canada, for language revisions and scientific discussion.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Charlier, J.; van der Voort, M.; Kenyon, F.; Skuce, P.; Vercruysse, J. Chasing helminths and their economic impact on farmed ruminants. Trends Parasitol. 2014, 30, 361–367. [Google Scholar] [CrossRef]
- Playford, M.C.; Besier, R.B. Gastrointestinal nematode parasites of grazing ruminants: A comprehensive literature review of diagnostic methods for quantifying parasitism, larval differentiation and measuring anthelmintic resistance. N. Z. Vet. J. 2025, 73, 149–164. [Google Scholar] [CrossRef]
- Roeber, F.; Jex, A.R.; Gasser, R.B. Impact of gastrointestinal parasitic nematodes of sheep, and the role of advanced molecular tools for exploring epidemiology and drug resistance—An Australian perspective. Parasites Vectors 2013, 6, 153. [Google Scholar] [CrossRef]
- Charlier, J.; Rinaldi, L.; Musella, V.; Ploeger, H.W.; Chartier, C.; Vineer, H.R.; Hinney, B.; von Samson-Himmelstjerna, G.; Băcescu, B.; Mickiewicz, M.; et al. Initial assessment of the economic burden of major parasitic helminth infections to the ruminant livestock industry in Europe. Prev. Vet. Med. 2020, 182, 105103. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.; Sanyal, P.B. Epidemiological Intelligence for Grazing Management in Strategic Control of Parasitic Gastroenteritis in Small Ruminants in India—A Review. Vet. World 2011, 4, 92–96. [Google Scholar]
- Zajac, A.M.; Garza, J. Biology, Epidemiology, and Control of Gastrointestinal Nematodes of Small Ruminants. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 73–87. [Google Scholar] [CrossRef]
- Hildreth, M.B.; McKenzie, J.B. Epidemiology and Control of Gastrointestinal Nematodes of Cattle in Northern Climates. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 59–71. [Google Scholar] [CrossRef]
- Hoberg, E.P.; Abrams, A.; Pilitt, P.A.; Jenkins, E.J. Discovery and description of a new trichostrongyloid species (Nematoda: Ostertagiinae), abomasal parasites in mountain goat, Oreamnos americanus, from the Western Cordillera of North America. J. Parasitol. 2012, 98, 817–846. [Google Scholar] [CrossRef]
- Maier, G.U.; Torcal, P.; Stackhouse, J.; Davy, J.S.; Forero, L.C.; Snell, L.; Woodmansee, G. Gastrointestinal parasitic worm burdens and efficacy of deworming practices in growing beef cattle grazing California pastures. Transl. Anim. Sci. 2025, 9, txaf007. [Google Scholar] [CrossRef] [PubMed]
- Fissiha, W.; Kinde, M.Z. Anthelmintic Resistance and Its Mechanism: A Review. Infect. Drug Resist. 2021, 14, 5403–5410. [Google Scholar] [CrossRef]
- Menzies, P. Handbook for the Control of Internal Parasites of Sheep and Goats; University of Guelph: Guelph, ON, Canada, 2019. [Google Scholar]
- Paras, K.L.; George, M.M.; Vidyashankar, A.N.; Kaplan, R.M. Comparison of fecal egg counting methods in four livestock species. Vet. Parasitol. 2018, 257, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Verocai, G.G.; Chaudhry, U.N.; Lejeune, M. Diagnostic Methods for Detecting Internal Parasites of Livestock. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 125–143. [Google Scholar] [CrossRef] [PubMed]
- Bisset, S.A.; Knight, J.S.; Bouchet, C.L. A multiplex PCR-based method to identify strongylid parasite larvae recovered from ovine faecal cultures and/or pasture samples. Vet. Parasitol. 2014, 200, 117–127. [Google Scholar] [CrossRef]
- Queiroz, C.; Levy, M.; Avramenko, R.; Redman, E.; Kearns, K.; Swain, L.; Silas, H.; Uehlinger, F.; Gilleard, J.S. The use of ITS-2 rDNA nemabiome metabarcoding to enhance anthelmintic resistance diagnosis and surveillance of ovine gastrointestinal nematodes. Int. J. Parasitol. Drugs Drug Resist. 2020, 14, 105–117. [Google Scholar] [CrossRef]
- Kotze, A.C.; Gilleard, J.S.; Doyle, S.R.; Prichard, R.K. Challenges and opportunities for the adoption of molecular diagnostics for anthelmintic resistance. Int. J. Parasitol. Drugs Drug Resist. 2020, 14, 264–273. [Google Scholar] [CrossRef]
- Emery, D.L.; Hunt, P.W.; Le Jambre, L.F. Haemonchus contortus: The then and now, and where to from here? Int. J. Parasitol. 2016, 46, 755–769. [Google Scholar] [CrossRef]
- Baltrušis, P.; Doyle, S.R.; Halvarsson, P.; Höglund, J. Genome-wide analysis of the response to ivermectin treatment by a Swedish field population of Haemonchus contortus. Int. J. Parasitol. Drugs Drug Resist. 2022, 18, 12–19. [Google Scholar] [CrossRef]
- Williamson, S.M.; Storey, B.; Howell, S.; Harper, K.M.; Kaplan, R.M.; Wolstenholme, A.J. Candidate anthelmintic resistance-associated gene expression and sequence polymorphisms in a triple-resistant field isolate of Haemonchus contortus. Mol. Biochem. Parasitol. 2011, 180, 99–105. [Google Scholar] [CrossRef]
- Raza, A.; Lamb, J.; Chambers, M.; Hunt, P.W.; Kotze, A.C. Larval development assays reveal the presence of sub-populations showing high- and low-level resistance in a monepantel (Zolvix®)-resistant isolate of Haemonchus contortus. Vet. Parasitol. 2016, 220, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Howell, S.B.; Burke, J.M.; Miller, J.E.; Terrill, T.H.; Valencia, E.; Williams, M.J.; Williamson, L.H.; Zajac, A.M.; Kaplan, R.M. Prevalence of anthelmintic resistance on sheep and goat farms in the southeastern United States. J. Am. Vet. Med. Assoc. 2008, 233, 1913–1919. [Google Scholar] [CrossRef]
- Gasbarre, L.C.; Smith, L.L.; Hoberg, E.; Pilitt, P.A. Further characterization of a cattle nematode population with demonstrated resistance to current anthelmintics. Vet. Parasitol. 2009, 166, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Edwards, E.E.; Garner, B.C.; Williamson, L.H.; Storey, B.E.; Sakamoto, K. Pathology of Haemonchus contortus in New World camelids in the southeastern United States: A retrospective review. J. Vet. Diagn. Investig. 2016, 28, 105–109. [Google Scholar] [CrossRef]
- Falzon, L.C.; Menzies, P.I.; Shakya, K.P.; Jones-Bitton, A.; Vanleeuwen, J.; Avula, J.; Stewart, H.; Jansen, J.T.; Taylor, M.A.; Learmount, J.; et al. Anthelmintic resistance in sheep flocks in Ontario, Canada. Vet. Parasitol. 2013, 193, 150–162. [Google Scholar] [CrossRef]
- Barrère, V.; Keller, K.; von Samson-Himmelstjerna, G.; Prichard, R.K. Efficiency of a genetic test to detect benzimidazole resistant Haemonchus contortus nematodes in sheep farms in Quebec, Canada. Parasitol. Int. 2013, 62, 464–470. [Google Scholar] [CrossRef]
- De Seram, E.L.; Uehlinger, F.D.; de Queiroz, C.; Redman, E.M.; Campbell, J.R.; Nooyen, D.; Morisetti, A.; Pollock, C.M.; Ekanayake, S.; Penner, G.B.; et al. Integration of ITS-2 rDNA nemabiome metabarcoding with Fecal Egg Count Reduction Testing (FECRT) reveals ivermectin resistance in multiple gastrointestinal nematode species, including hypobiotic Ostertagia ostertagi, in western Canadian beef cattle. Int. J. Parasitol. Drugs Drug Resist. 2023, 22, 27–35. [Google Scholar] [CrossRef]
- American Consortium for Small Ruminant Parasite Control. Deworming Charts for Sheep, Goats and Camelids. 2024. Available online: https://www.wormx.info/deworming (accessed on 22 March 2025).
- Keane, C.; Marchetto, K.M.; Oliveira-Santos, L.G.R.; Wünschmann, A.; Wolf, T.M. Epidemiological Investigation of Meningeal Worm-Induced Mortalities in Small Ruminants and Camelids Over a 19 Year Period. Front. Vet. Sci. 2022, 9, 859028. [Google Scholar] [CrossRef]
- Cringoli, G.; Maurelli, M.P.; Levecke, B.; Bosco, A.; Vercruysse, J.; Utzinger, J.; Rinaldi, L. The Mini-FLOTAC technique for the diagnosis of helminth and protozoan infections in humans and animals. Nat. Protoc. 2017, 12, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Zajac, A.M. Gastrointestinal nematodes of small ruminants: Life cycle, anthelmintics, and diagnosis. Vet. Clin. N. Am. Food Anim. Pract. 2006, 22, 529–541. [Google Scholar] [CrossRef]
- Kaplan, R.M.; Denwood, M.J.; Nielsen, M.K.; Thamsborg, S.M.; Torgerson, P.R.; Gilleard, J.S.; Dobson, R.J.; Vercruysse, J.; Levecke, B. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) guideline for diagnosing anthelmintic resistance using the faecal egg count reduction test in ruminants, horses and swine. Vet. Parasitol. 2023, 318, 109936. [Google Scholar] [CrossRef] [PubMed]
- Denwood, M.J.; Kaplan, R.M.; McKendrick, I.J.; Thamsborg, S.M.; Nielsen, M.K.; Levecke, B. A statistical framework for calculating prospective sample sizes and classifying efficacy results for faecal egg count reduction tests in ruminants, horses and swine. Vet. Parasitol. 2023, 314, 109867. [Google Scholar] [CrossRef]
- Avramenko, R.W.; Redman, E.M.; Lewis, R.; Yazwinski, T.A.; Wasmuth, J.D.; Gilleard, J.S. Exploring the Gastrointestinal “Nemabiome”: Deep Amplicon Sequencing to Quantify the Species Composition of Parasitic Nematode Communities. PLoS ONE. 2015, 10, e0143559. [Google Scholar] [CrossRef]
- Redman, E.; Queiroz, C.; Bartley, D.J.; Levy, M.; Avramenko, R.W.; Gilleard, J.S. Validation of ITS-2 rDNA nemabiome sequencing for ovine gastrointestinal nematodes and its application to a large scale survey of UK sheep farms. Vet. Parasitol. 2019, 275, 108933. [Google Scholar] [CrossRef]
- Dallas, J.F.; Irvine, R.J.; Halvorsen, O.; Albon, S.D. Identification by polymerase chain reaction (PCR) of Marshallagia marshalli and Ostertagia gruehneri from Svalbard reindeer. Int. J. Parasitol. 2000, 30, 863–866. [Google Scholar] [CrossRef]
- Gliga, D.S.; Kramer, A.; Moré, G.; Frey, C.F.; Basso, W. Early Detection and Management of Lamanema chavezi infection in a llama (Lama glama) in Switzerland. Vet. Res. Commun. 2024, 48, 3365–3369. [Google Scholar] [CrossRef]
- Jurasek, M.E.; Bishop-Stewart, J.K.; Storey, B.E.; Kaplan, R.M.; Kent, M.L. Modification and further evaluation of a fluorescein-labeled peanut agglutinin test for identification of Haemonchus contortus eggs. Vet. Parasitol. 2010, 169, 209–213. [Google Scholar] [CrossRef]
- Levecke, B.; Kaplan, R.M.; Thamsborg, S.M.; Torgerson, P.R.; Vercruysse, J.; Dobson, R.J. How to improve the standardization and the diagnostic performance of the fecal egg count reduction test? Vet. Parasitol. 2018, 253, 71–78. [Google Scholar] [CrossRef]
- Coles, G.C.; Jackson, F.; Pomroy, W.E.; Prichard, R.K.; von Samson-Himmelstjerna, G.; Silvestre, A.; Taylor, M.A.; Vercruysse, J. The detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 2006, 136, 167–185. [Google Scholar] [CrossRef]
- Fowler, M.E. Camelids Are Not Ruminants. In Zoo and Wild Animal Medicine; Saunders: Stanton, KS, USA, 2008; pp. 375–385. [Google Scholar] [CrossRef]
- USDA, APHIS, Veterinary Services Strategy & Policy Protocol for the Importation of Farmed Camelids from Australia. USDA Guidelines 2006, Updated in 2020. Available online: https://www.aphis.usda.gov/sites/default/files/aus-camelid.pdf (accessed on 12 July 2025).
- Cain, J.L.; Gianechini, L.S.; Vetter, A.L.; Davis, S.M.; Britton, L.N.; Myka, J.L.; Slusarewicz, P. Rapid, automated quantification of Haemonchus contortus ova in sheep faecal samples. Int. J. Parasitol. 2024, 54, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Flay, K.J.; Hill, F.I.; Muguiro, D.H. A Review: Haemonchus contortus Infection in Pasture-Based Sheep Production Systems, with a Focus on the Pathogenesis of Anaemia and Changes in Haematological Parameters. Animals 2022, 12, 1238. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.F.V.; Monteiro, J.P.; Almeida, T.M.; Molento, M.B. A systematic review of the molecular mechanisms related to anthelmintic resistance in Haemonchus contortus: A contemporary narrative. Vet. Parasitol. 2025, 334, 110394. [Google Scholar] [CrossRef] [PubMed]
- Ghatee, M.A.; Malek Hosseini, S.A.A.; Marashifard, M.; Karamian, M.; Taylor, W.R.; Jamshidi, A.; Mobedi, I.; Azarmehr, H. Phylogenetic analysis of Trichostrongylus vitrinus isolates from southwest Iran. Parasites Vectors 2020, 13, 553. [Google Scholar] [CrossRef]
- Bailey, J.N.; Kahn, L.P.; Walkden-Brown, S.W. The relative contributions of T. colubriformis, T. vitrinus, T. axei and T. rugatus to sheep infected with Trichostrongylus spp. on the northern tablelands of New South Wales. Vet. Parasitol. 2009, 165, 88–95. [Google Scholar] [CrossRef]
- Eysker, M. Regulation of Trichostrongylus vitrinus and T colubriformis populations in naturally infected sheep in the Netherlands. Res. Vet. Sci. 1987, 42, 267–271. [Google Scholar] [CrossRef]
- Mizani, A.; Gill, P.; Daryani, A.; Sarvi, S.; Amouei, A.; Katrimi, A.B.; Soleymani, E.; Mirshafiee, S.; Gholami, S.; Hosseini, S.A.; et al. A multiplex restriction enzyme-PCR for unequivocal identification and differentiation of Trichostrongylus species in human samples. Acta Trop. 2017, 173, 180–184. [Google Scholar] [CrossRef]
- Mederos, A.; Fernández, S.; VanLeeuwen, J.; Peregrine, A.S.; Kelton, D.; Menzies, P.; LeBoeuf, A.; Martin, R. Prevalence and distribution of gastrointestinal nematodes on 32 organic and conventional commercial sheep farms in Ontario and Quebec, Canada (2006–2008). Vet. Parasitol. 2010, 170, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Torres-Acosta, J.F.; Mendoza-de-Gives, P.; Aguilar-Caballero, A.J.; Cuéllar-Ordaz, J.A. Anthelmintic resistance in sheep farms: Update of the situation in the American continent. Vet. Parasitol. 2012, 189, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Beleckė, A.; Kupčinskas, T.; Stadalienė, I.; Höglund, J.; Thamsborg, S.M.; Stuen, S.; Petkevičius, S. Anthelmintic resistance in small ruminants in the Nordic-Baltic region. Acta Vet. Scand. 2021, 63, 18. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, R.A.; Williamson, L.H.; Terrill, T.H.; Kaplan, R.M. Efficacy of anthelmintics on South American camelid (llama and alpaca) farms in Georgia. Vet. Parasitol. 2010, 172, 168–171. [Google Scholar] [CrossRef]
- Jabbar, A.; Campbell, A.J.D.; Charles, J.A.; Gasser, R.B. First report of anthelmintic resistance in Haemonchus contortus in alpacas in Australia. Parasites Vectors 2013, 6, 243. [Google Scholar] [CrossRef]
- Rashid, M.H.; Vaughan, J.L.; Stevenson, M.A.; Campbell, A.J.D.; Beveridge, I.; Jabbar, A. Anthelmintic resistance in gastrointestinal nematodes of alpacas (Vicugna pacos) in Australia. Parasites Vectors 2018, 11, 388. [Google Scholar] [CrossRef]
- Hamer, K.; Bartley, D.; Jennings, A.; Morrison, A.; Sargison, N. Lack of efficacy of monepantel against trichostrongyle nematodes in a UK sheep flock. Vet. Parasitol. 2018, 257, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Prichard, R.; Ménez, C.; Lespine, A. Moxidectin and the avermectins: Consanguinity but not identity. Int. J. Parasitol. Drugs Drug Resist. 2012, 2, 134–153. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.M.F.; Ghazy, A.A. Advances in diagnosis and control of anthelmintic resistant gastrointestinal helminths infecting ruminants. J. Parasit. Dis. 2022, 46, 901–915. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).