Food Safety Concerns: Anisakis spp. in Ready-to-Eat Fish from the Greek Market
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Artificial Digestion and Macroscopic Inspection
2.3. Morphological Identification
2.4. Molecular Examination
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | artificial digestion |
| RTE | ready-to-eat |
| FBOs | food business operators |
| HACCP | Hazard Analysis and Critical Control Points |
References
- Ángeles-Hernández, J.C.; Gómez-de Anda, F.R.; Reyes-Rodríguez, N.E.; Vega-Sánchez, V.; García-Reyna, P.B.; Campos-Montiel, R.G.; Calderón-Apodaca, N.L.; Salgado-Miranda, C.; Zepeda-Velázquez, A.P. Genera and species of the Anisakidae family and their geographical distribution. Animals 2020, 10, 2374. [Google Scholar] [CrossRef] [PubMed]
- Adroher-Auroux, F.J.; Benítez-Rodríguez, R. Anisakiasis and Anisakis: An underdiagnosed emerging disease and its main etiological agents. Res. Vet. Sci. 2020, 132, 535–545. [Google Scholar] [CrossRef]
- Mattiucci, S.; Palomba, M.; Cavallero, S.; D’Amelio, S. Anisakiasis. In Helminth Infections and Their Impact on Global Public Health; Springer International Publishing: Cham, Switzerland, 2022; pp. 451–495. [Google Scholar] [CrossRef]
- Martin-Carrillo, N.; García-Livia, K.; Baz-González, E.; Abreu-Acosta, N.; Dorta-Guerra, R.; Valladares, B.; Foronda, P. Morphological and molecular identification of Anisakis spp.(Nematoda: Anisakidae) in commercial fish from the Canary Islands coast (Spain): Epidemiological data. Animals 2022, 12, 2634. [Google Scholar] [CrossRef]
- Cipriani, P.; Acerra, V.; Bellisario, B.; Sbaraglia, G.L.; Cheleschi, R.; Nascetti, G.; Mattiucci, S. Larval migration of the zoonotic parasite Anisakis pegreffii (Nematoda: Anisakidae) in European anchovy, Engraulis encrasicolus: Implications to seafood safety. Food Control. 2016, 59, 148–157. [Google Scholar] [CrossRef]
- Kumas, K.; Al-Jubury, A.; Kania, P.W.; Abusharkh, T.; Buchmann, K. Location and elimination of Anisakis simplex third stage larvae in Atlantic herring Clupea harengus L. Int. J. Parasitol. Parasites Wildl. 2024, 24, 100937. [Google Scholar] [CrossRef]
- Chaligiannis, I.; Lalle, M.; Pozio, E.; Sotiraki, S. Anisakidae infection in fish of the Aegean Sea. Vet. Parasitol. 2012, 184, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Tantanasi, J.; Diakou, A.; Tamvakis, A.; Batjakas, I.E. Anisakis spp. burden in Trachurus trachurus. Helminthologia 2012, 49, 16–20. [Google Scholar] [CrossRef]
- Shamsi, S.; Barton, D.P. A critical review of anisakidosis cases occurring globally. Parasitol. Res. 2023, 122, 1733–1745. [Google Scholar] [CrossRef]
- Hochberg, N.S.; Hamer, D.H. Anisakidosis: Perils of the deep. Clin. Infect. Dis. 2010, 51, 806–812. [Google Scholar] [CrossRef]
- Nieuwenhuizen, N.E. Anisakis–immunology of a foodborne parasitosis. Parasite Immunol. 2016, 38, 548–557. [Google Scholar] [CrossRef]
- Audicana, M.T.; Del Pozo, M.D.; Iglesias, R.; Ubeira, F.M. Anisakis simplex and Pseudoterranova decipiens. In International Handbook of Foodborne Pathogens; Miliotis, M.D., Bier, J.W., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 633–656. [Google Scholar] [CrossRef]
- Céspedes, M.; Saez, A.; Rodríguez, I.; Pinto, J.M.; Rodríguez, R. Chronic anisakiasis presenting as a mesenteric mass. Abdom. Imaging 2000, 25, 548–550. [Google Scholar] [CrossRef]
- Audicana, M.T.; Ansotegui, I.J.; de Corres, L.F.; Kennedy, M.W. Anisakis simplex: Dangerous—Dead and alive? Trends Parasitol. 2002, 18, 20–25. [Google Scholar] [CrossRef]
- Audicana, M.T.; Kennedy, M.W. Anisakis simplex: From obscure infectious worm to inducer of immune hypersensitivity. Clin. Microbiol. Rev. 2008, 21, 360–379. [Google Scholar] [CrossRef]
- Rahmati, A.R.; Kiani, B.; Afshari, A.; Moghaddas, E.; Williams, M.; Shamsi, S. World-wide prevalence of Anisakis larvae in fish and its relationship to human allergic anisakiasis: A systematic review. Parasitol. Res. 2020, 119, 3585–3594. [Google Scholar] [CrossRef]
- Purello-D’Ambrosio, F.; Pastorello, E.; Gangemi, S.; Lombardo, G.; Ricciardi, L.; Fogliani, O.; Merendino, R.A. Incidence of sensitivity to Anisakis simplex in a risk population of fishermen/fishmongers. Ann. Allergy Asthma Immunol. 2000, 84, 439–444. [Google Scholar] [CrossRef]
- Armentia, A.; Martin-Gil, F.J.; Pascual, C.; Martín-Esteban, M.; Callejo, A.; Martínez, C. Anisakis simplex allergy after eating chicken meat. J. Investig. Allergol. Clin. Immunol. 2006, 16, 258. [Google Scholar] [PubMed]
- Bao, M.; Pierce, G.J.; Pascual, S.; González-Muñoz, M.; Mattiucci, S.; Mladineo, I.; Cipriani, P.; Bušelić, I.; Strachan, N.J. Assessing the risk of an emerging zoonosis of worldwide concern: Anisakiasis. Sci. Rep. 2017, 7, 43699. [Google Scholar] [CrossRef] [PubMed]
- Pigłowski, M. Notifications on Anisakis spp. in the Rapid Alert System for Food and Feed (RASFF) Reported in 2001–2023. Sustainability 2024, 16, 5453. [Google Scholar] [CrossRef]
- Dinas, S.; Diakou, A.; Vasiliadis, K.; Chaintoutis, S.C.; Massa, E.; Konstantinou, G.N.; Totsi, A.; Xakis, A.; Papavasiliou, C. First case of human anisakiosis in Greece: Acute invasive infection mimicking peritoneal malignancy. Pathogens 2024, 13, 149. [Google Scholar] [CrossRef]
- European Parliament and Council of the European Union. Regulation (EC) No 853/2004 of 29 April 2004 laying down specific hygiene rules for food of animal origin. Off. J. Eur. Union 2004, L139, 55–205. [Google Scholar]
- European Commission. Commission Regulation (EC) No 2074/2005 of 5 December 2005 laying down implementing measures for certain products under Regulation (EC) No 853/2004 of the European Parliament and of the Council. Off. J. Eur. Union 2005, L338, 27–59. [Google Scholar]
- European Commission. Commission Regulation (EU) No 1276/2011 of 8 December 2011 amending Regulation (EC) No 1234/2007 as regards marketing standards for poultry meat. Off. J. Eur. Union 2011, L327, 39–40. [Google Scholar]
- Ohnishi, T.; Banzai, A.; Hara-Kudo, Y.; Sugiyama, H. Prevalence and abundance of Anisakis larvae in ready-to-eat mackerel products in Japan. Int. J. Food Microbiol. 2023, 395, 110181. [Google Scholar] [CrossRef]
- Mladineo, I.; Šimat, V.; Miletić, J.; Beck, R.; Poljak, V. Molecular identification and population dynamic of Anisakis pegreffii (Nematoda: Anisakidae Dujardin, 1845) isolated from the European anchovy (Engraulis encrasicolus L.) in the Adriatic Sea. Int. J. Food Microbiol. 2012, 157, 224–229. [Google Scholar] [CrossRef]
- Guardone, L.; Nucera, D.; Rosellini, N.; Tinacci, L.; Acutis, P.L.; Guidi, A.; Armani, A. Occurrence, distribution and viability of Anisakis spp. larvae in various kind of marketed herring products in Italy. Food Control 2019, 101, 126–133. [Google Scholar] [CrossRef]
- Karl, H.; Roepstorff, A.; Huss, H.H.; Bloemsma, B. Survival of Anisakis larvae in marinated herring fillets. Int. J. Food Sci. Technol. 1995, 29, 661–670. [Google Scholar] [CrossRef]
- Sánchez-Monsalvez, I.; de Armas-Serra, C.; Martínez, J.; Dorado, M.; Sánchez, A.; Rodríguez-Caabeiro, F. A new procedure for marinating fresh anchovies and ensuring the rapid destruction of Anisakis larvae. J. Food Prot. 2005, 68, 1066–1072. [Google Scholar] [CrossRef]
- Smaldone, G.; Marrone, R.; Palma, G.; Sarnelli, P.; Anastasio, A. Preliminary study on the inactivation of anisakid larvae in baccalà prepared according to traditional methods. Ital. J. Food Saf. 2017, 6, 6964. [Google Scholar] [CrossRef]
- Šimat, V.; Trumbić, Ž. Viability of Anisakis spp. Larvae After Direct Exposure to Different Processing Media and Non-Thermal Processing in Anchovy Fillets. Fishes 2019, 4, 19. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific opinion on risk assessment of parasites in fishery products. EFSA J. 2010, 8, 1543. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bover-Cid, S.; Chemaly, M.; Bolton, D. Re-evaluation of certain aspects of the EFSA Scientific Opinion of April 2010 on risk assessment of parasites in fishery products, based on new scientific data. Part 1: ToRs1-3. EFSA J. Eur. Food Saf. 2024, 22, e8719. [Google Scholar] [CrossRef]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- European Union Reference Laboratory for Parasites Department of Infectious Diseases Unit of Foodborne and Neglected Parasitic Diseases, Istituto Superiore di Sanità. Detection of Parasites in Fish Fillet by Artificial Digestion, Standard Operating Procedure (SOP). Available online: https://www.iss.it/documents/20126/0/POP-04+(rev+3)+Artificial+digestion+of+fish+fillet.pdf/cfa9a06a-dae9-e7b8-252f-434cc1c45d32?t=1712663409737 (accessed on 1 September 2024).
- ISO 23036-2:2021; Microbiology of the Food Chain—Methods for the Detection of Anisakidae L3 Larvae in Fish and Fishery Products Part 2: Artificial Digestion Method. ISO: Geneva, Switzerland, 2021. Available online: https://www.iso.org/standard/74373.html (accessed on 1 September 2024).
- Petter, A.J.; Maillard, C. Larves d’Ascarides parasites de Poissons en Méditerranée occidentale. Bull. Muséum Natl. Hist. Nat. 1988, 10, 347–369. [Google Scholar] [CrossRef]
- Smith, J.W. Anisakis simplex (Nematoda: Ascaridae): Morphology and morphometry of larvae from euphasiids and fish and a review of the life history and ecology. J. Helminthol. 1983, 57, 205–224. [Google Scholar] [CrossRef]
- Hartwich, G. Keys to genera of the Ascaridoidea. In CIH Keys to the Nematode Parasites of Vertebrates; Anderson, R.C., Chabaud, A.G., Wilmott, S., Eds.; Commonwealth Agricultural Bureaux, Farnham Royal: Slough, UK, 1974; pp. 1–15. [Google Scholar] [CrossRef]
- Chassalevris, T.; Chaintoutis, S.C.; Apostolidi, E.D.; Giadinis, N.D.; Vlemmas, I.; Brellou, G.D.; Dovas, C.I. A highly sensitive semi-nested real-time PCR utilizing oligospermine-conjugated degenerate primers for the detection of diverse strains of small ruminant lentiviruses. Mol. Cell. Probes 2020, 51, 101528. [Google Scholar] [CrossRef]
- Dimzas, D.; Rubiola, S.; Pacifico, L.; Veneziano, V.; Chiesa, F.; Chassalevris, T.; Diakou, A. Microscopic detection and molecular characterization of Sarcocystis miescheriana in wild boars (Sus scrofa): First report from Greece. Parasitol. Res. 2024, 123, 234. [Google Scholar] [CrossRef]
- Mattiucci, S.; Paoletti, M.; Borrini, F.; Palumbo, M.; Palmieri, R.M.; Gomes, V.; Casati, A.; Nascetti, G. First molecular identification of the zoonotic parasite Anisakis pegreffii (Nematoda: Anisakidae) in a paraffin-embedded granuloma taken from a case of human intestinal anisakiasis in Italy. BMC Infect. Dis. 2011, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evoluationary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Myers, B.J. Research then and now on the Anisakidae nematodes. Trans. Am. Microsc. Soc. 1976, 95, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, H.; Shiroyama, M.; Yamamoto, I.; Ishikawa, T.; Morishima, Y. Anisakiasis annual incidence and causative species, Japan, 2018–2019. Emerg. Infect. Dis. 2022, 28, 2105. [Google Scholar] [CrossRef]
- Aydin, C.; Pekmezci, G.Z. Molecular identification and infection levels of Anisakis species (Nematoda: Anisakidae) in the red scorpionfish Scorpaena scrofa (Scorpaenidae) from the Aegean Sea. Parasitol. Int. 2023, 92, 102691. [Google Scholar] [CrossRef]
- Roca-Geronès, X.; Segovia, M.; Godínez-González, C.; Fisa, R.; Montoliu, I. Anisakis and Hysterothylacium species in Mediterranean and North-East Atlantic fishes commonly consumed in Spain: Epidemiological, molecular and morphometric discriminant analysis. Int. J. Food Microbiol. 2020, 325, 108642. [Google Scholar] [CrossRef]
- Roca-Geronès, X.; Alcover, M.M.; Godínez-González, C.; Montoliu, I.; Fisa, R. Hybrid genotype of Anisakis simplex (ss) and A. pegreffii identified in third-and fourth-stage larvae from sympatric and allopatric Spanish marine waters. Animals 2021, 11, 2458. [Google Scholar] [CrossRef] [PubMed]
- Mattiucci, S.; Cipriani, P.; Levsen, A.; Paoletti, M.; Nascetti, G. Molecular epidemiology of Anisakis and anisakiasis: An ecological and evolutionary road map. Adv. Parasitol. 2018, 99, 93–263. [Google Scholar] [CrossRef]
- Gómez-Mateos, M.; Merino-Espinosa, G.; Corpas-López, V.; Valero-López, A.; Martín-Sánchez, J. A multi-restriction fragment length polymorphism genotyping approach including the beta-tubulin gene as a new differential nuclear marker for the recognition of the cryptic species Anisakis simplex ss and Anisakis pegreffii and their hybridization events. Vet. Parasitol. 2020, 283, 109162. [Google Scholar] [CrossRef] [PubMed]
- Šimat, V.; Miletić, J.; Bogdanović, T.; Poljak, V.; Mladineo, I. Role of biogenic amines in the post-mortem migration of Anisakis pegreffii (Nematoda: Anisakidae Dujardin, 1845) larvae into fish fillets. Int. J. Food Microbiol. 2015, 214, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, P.; Giulietti, L.; Bao, M.; Palomba, M.; Mattiucci, S.; Levsen, A. Post-mortem tissue migration of Anisakis simplex (ss) larvae (Nematoda: Anisakidae) in three commercially harvested fish species from the Northeast Atlantic: The role of storage time and temperature. Food Control 2024, 157, 110162. [Google Scholar] [CrossRef]
- Karl, H.; Baumann, F.; Ostermeyer, U.; Kuhn, T.; Klimpel, S. Anisakis simplex (ss) larvae in wild Alaska salmon: No indication of post-mortem migration from viscera into flesh. Dis. Aquat. Org. 2011, 94, 201–209. [Google Scholar] [CrossRef]
- Sciortino, C.; Giamporcaro, G.; Sgroi, F.; Costantino, S.; Giuffrida, A.; Virga, A.N.; Modica, F. Fish consumption and Anisakis Risk: An exploratory study of Sicilian consumer awareness. Food Humanit. 2025, 5, 100668. [Google Scholar] [CrossRef]
- Simsek, E.; Pekmezci, G.Z.; Yildirim, A.; Duzlu, O.; Onder, Z.; Ciloglu, A.; Sursal, N.; Yilmaz, E.; Gonulalan, Z.; Inci, A. Investigation of Anisakis larvae in different products of ready-to-eat fish meat and imported frozen fish in Turkey. Int. J. Food Microbiol. 2020, 333, 108829. [Google Scholar] [CrossRef]
- Guardone, L.; Nucera, D.; Lodola, L.B.; Tinacci, L.; Acutis, P.L.; Guidi, A.; Armani, A. Anisakis spp. larvae in different kinds of ready to eat products made of anchovies (Engraulis encrasicolus) sold in Italian supermarkets. Int. J. Food Microbiol. 2018, 268, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Smaldone, G.; Ambrosio, R.L.; Marrone, R.; Ceruso, M.; Anastasio, A. Anisakis spp. Larvae in Deboned, in-Oil Fillets Made of Anchovies (Engraulis encrasicolus) and Sardines (Sardina pilchardus) Sold in EU Retailers. Animals 2020, 10, 1807. [Google Scholar] [CrossRef]
- Sánchez-Alonso, I.; Rodríguez, S.; Tejada, M.; Navas, A.; González-Muñoz, M.; Careche, M. The artificial digestion method underestimates the viability of Anisakis simplex (sl) L3 present in processed fish products. Food Waterborne Parasitol. 2021, 23, e00121. [Google Scholar] [CrossRef]
- Anastasio, A.; Smaldone, G.; Cacace, D.; Marrone, R.; Voi, A.L.; Santoro, M.; Pozio, E. Inactivation of Anisakis pegreffii larvae in anchovies (Engraulis encrasicolus) by salting and quality assessment of finished product. Food Control 2016, 64, 115–119. [Google Scholar] [CrossRef]
- Oh, S.R.; Zhang, C.Y.; Kim, T.I.; Hong, S.J.; Ju, I.S.; Lee, S.H.; Ha, S.D. Inactivation of Anisakis larvae in salt-fermented squid and pollock tripe by freezing, salting, and combined treatment with chlorine and ultrasound. Food Control. 2014, 40, 46–49. [Google Scholar] [CrossRef]
- Polimeno, L.; Lisanti, M.T.; Rossini, M.; Giacovazzo, E.; Polimeno, L.; Debellis, L.; Ballini, A.; Topi, S.; Santacroce, L. Anisakis Allergy: Is Aquacultured Fish a Safe and Alternative Food to Wild-Capture Fisheries for Anisakis simplex-Sensitized Patients? Biology 2021, 10, 106. [Google Scholar] [CrossRef] [PubMed]
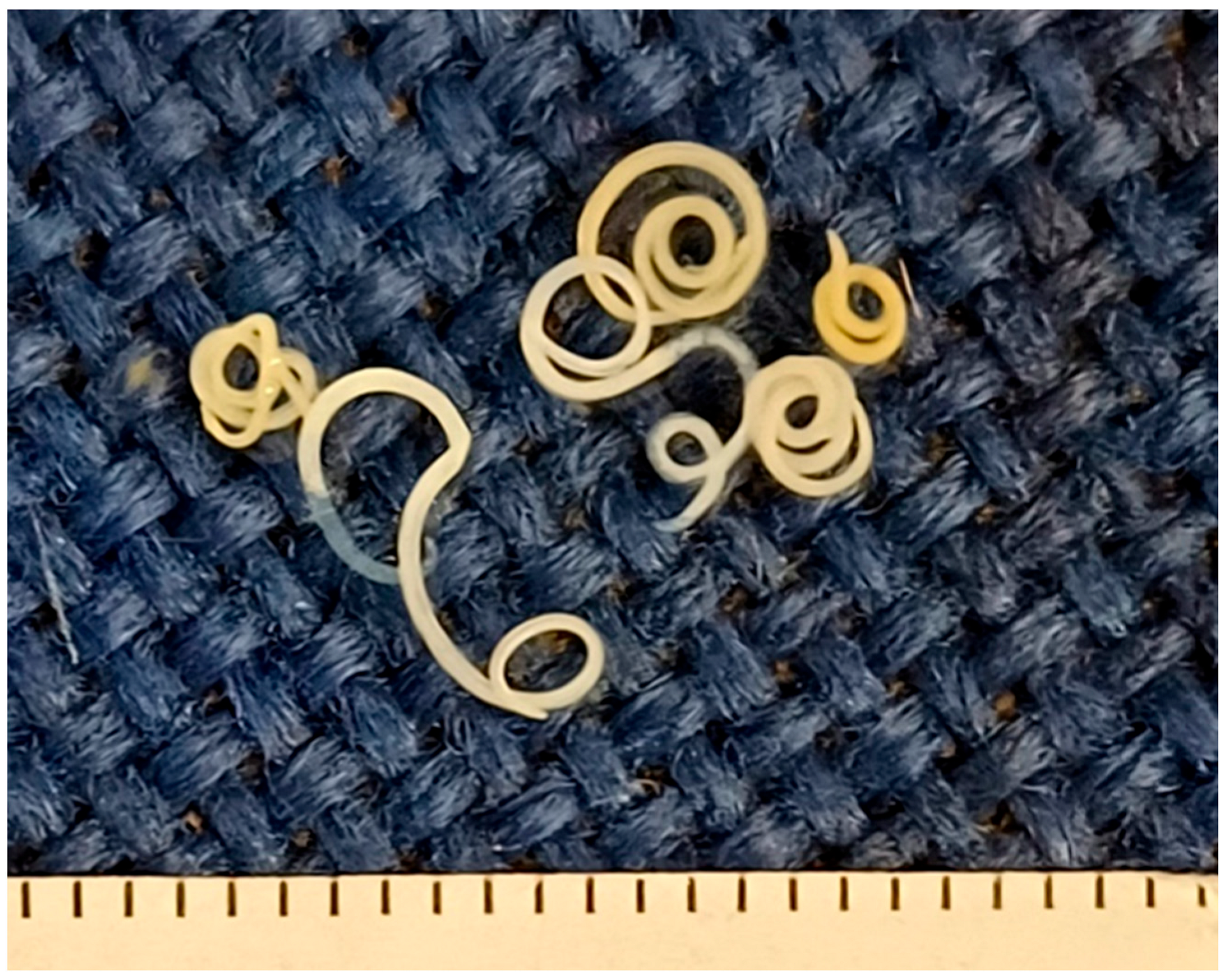
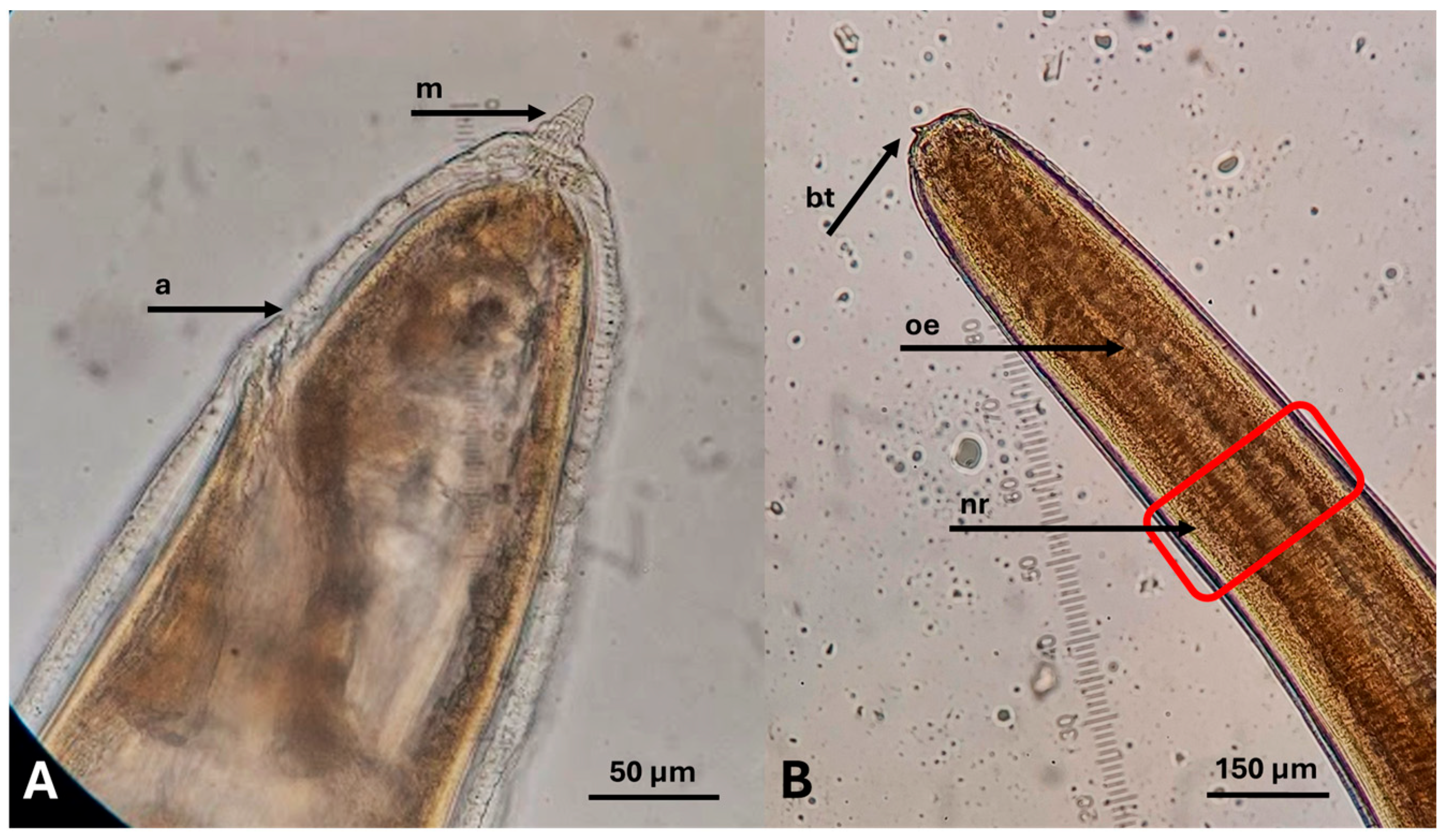
| Type of Product | No. of Products | Packaging Net Weight (on Average) | Fishing Area |
|---|---|---|---|
| Smoked whole herring | 12 | 12 × 200 g | FAO 27 |
| Smoked herring fillet in oil. | 9 | 9 × 110 g | FAO 27 |
| Smoked whole mackerel | 15 | 15 × 300 g | FAO 27 |
| Smoked mackerel fillet in oil | 11 | 11 × 100 g | FAO 27 |
| Salted whole Atlantic chub mackerel | 17 | 17 × 100 g | FAO 37 |
| Salted whole anchovies | 10 | 10 × 100 g | FAO 37 |
| Marinated anchovies fillet in oil | 16 | 16 × 50 g | FAO 37/FAO 87 |
| Salted whole sardines in oil | 5 | 5 × 70 g | FAO 37 |
| Salted pollock fillet in oil | 5 | 5 × 80 g | FAO 27 |
| Smoked albacore fillet in oil | 3 | 3 × 100 g | FAO 37/FAO 47 |
| Smoked Skipjack tuna fillet in water | 5 | 5 × 80 g | FAO 71 |
| Type of Product | No. of Samples | Prevalence (%) | No. of Retained Larvae | Mean Intensity * | No. of Larvae per Sample |
|---|---|---|---|---|---|
| Smoked whole herring | 12 | 11 (91.6) | 62 | 5.63 | 9–15 |
| Smoked herring fillet in oil | 9 | 1 (11.1) | 1 | 1 | 1 |
| Smoked whole mackerel | 15 | 7 (46.6) | 17 | 2.42 | 1–6 |
| Smoked mackerel fillet in oil | 11 | 2 (18.2) | 3 | 1.5 | 1–2 |
| Salted whole Atlantic chub mackerel | 17 | 8 (47.0) | 60 | 7.5 | 5–11 |
| Salted whole anchovies | 10 | 1 (10.0) | 1 | 1 | 1 |
| Marinated anchovies fillet in oil | 16 | 2 (12.5) | 3 | 1.5 | 1–2 |
| Salted whole sardines in oil | 5 | 0 (0) | 0 | na | na |
| Salted pollock fillet in oil | 5 | 0 (0) | 0 | na | na |
| Smoked albacore fillet in oil | 3 | 0 (0) | 0 | na | na |
| Smoked Skipjack tuna fillet in water | 5 | 0 (0) | 0 | na | na |
| Total | 108 | 32 (29.62) | 147 | na | na |
| Type of Product | No. of Samples | Molecular Analysis (AD Sediments) | No. of Positive Samples by AD (Presence of L3) | Molecular Analysis (Larvae) |
|---|---|---|---|---|
| Smoked whole herring | 12 | Neg. | 11 + 2 * | A. simplex s.s. |
| Smoked herring fillet in oil | 9 | Neg. | 1 | A. simplex s.s. |
| Smoked whole mackerel | 15 | Neg. | 7 | A. simplex s.s. |
| Smoked mackerel fillet in oil | 11 | Neg. | 2 | A. simplex s.s. |
| Salted whole Atlantic chub mackerel | 17 | Neg. | 8 | A. pegreffii |
| Salted whole anchovies | 10 | Neg. | 1 | A. pegreffii |
| Marinated anchovies fillet in oil | 16 | Neg. | 2 | A. pegreffii |
| Salted whole sardines in oil | 5 | Neg. | 0 | N/A ** |
| Salted pollock fillet in oil | 5 | Neg. | 0 | N/A ** |
| Smoked albacore fillet in oil | 3 | Neg. | 0 | N/A ** |
| Smoked Skipjack tuna fillet in water | 5 | Neg. | 0 | N/A ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papapostolou, E.N.; Chaintoutis, S.C.; Gousia, P.; Karpouza, A.; Kachrimanidou, M.; Diakou, A. Food Safety Concerns: Anisakis spp. in Ready-to-Eat Fish from the Greek Market. Pathogens 2025, 14, 981. https://doi.org/10.3390/pathogens14100981
Papapostolou EN, Chaintoutis SC, Gousia P, Karpouza A, Kachrimanidou M, Diakou A. Food Safety Concerns: Anisakis spp. in Ready-to-Eat Fish from the Greek Market. Pathogens. 2025; 14(10):981. https://doi.org/10.3390/pathogens14100981
Chicago/Turabian StylePapapostolou, Evangelia N., Serafeim C. Chaintoutis, Panagiota Gousia, Aggeliki Karpouza, Melania Kachrimanidou, and Anastasia Diakou. 2025. "Food Safety Concerns: Anisakis spp. in Ready-to-Eat Fish from the Greek Market" Pathogens 14, no. 10: 981. https://doi.org/10.3390/pathogens14100981
APA StylePapapostolou, E. N., Chaintoutis, S. C., Gousia, P., Karpouza, A., Kachrimanidou, M., & Diakou, A. (2025). Food Safety Concerns: Anisakis spp. in Ready-to-Eat Fish from the Greek Market. Pathogens, 14(10), 981. https://doi.org/10.3390/pathogens14100981










