The Differential Early Responses of Human Leukocytes to Influenza Virus and Respiratory Syncytial Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses
2.2. Collection of PBMC and Purified Monocytes/Macrophages and Exposure of Cells to Virus
2.3. Flow Cytometry Analyses of Cell Phenotype and Activation Markers
2.4. Analysis of Calcium Mobilization by Monocytes/Macrophages
2.5. Northern Blot and Giant 2-D Gel Analyses for Cox-2 mRNA and Protein
3. Results
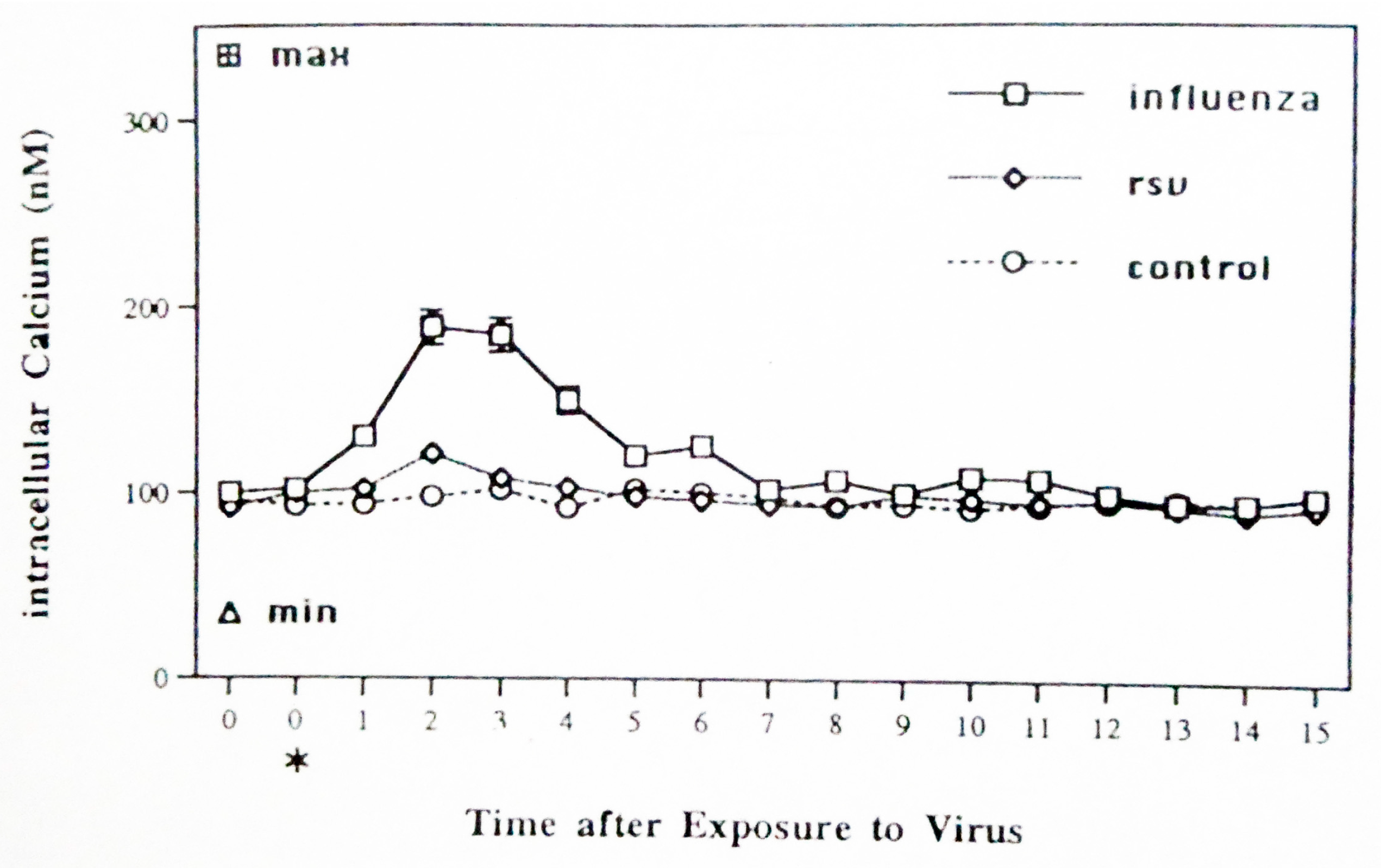
3.1. Calcium Mobilization by Sham-, Influenza-, and RSV-Exposed Monocytes/Macrophages
3.2. Cox-2 mRNA and Protein Expression by Virus-Exposed Monocytes/Macrophages
3.3. Sequential Exposure of PBMC to RSV and IAV and Analyses of Lymphocyte Activation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fonseca, W.; Lukacs, N.W.; Ptaschinski, C. Factors Affecting the Immunity to Respiratory Syncytial Virus: From Epigenetics to Microbiome. Front. Immunol. 2018, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef]
- Falsey, A.R. Respiratory syncytial virus infection in elderly and high-risk adults. Exp. Lung Res. 2005, 31 (Suppl. 1), 77. [Google Scholar] [CrossRef]
- Chatterjee, A.; Mavunda, K.; Krilov, L.R. Current State of Respiratory Syncytial Virus Disease and Management. Infect. Dis. Ther. 2021, 10, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.B.; Walsh, E.E.; Long, C.E.; Schnabel, K.C. Immunity to and frequency of reinfection with respiratory syncytial virus. J. Infect. Dis. 1991, 163, 693–698. [Google Scholar] [CrossRef]
- Domurat, F.; Roberts, N.J., Jr.; Walsh, E.E.; Dagan, R. Respiratory syncytial virus infection of human mononuclear leukocytes in vitro and in vivo. J. Infect. Dis. 1985, 152, 895–902. [Google Scholar] [CrossRef]
- Mock, D.J.; Domurat, F.; Roberts, N.J., Jr.; Walsh, E.E.; Licht, M.R.; Keng, P. Macrophages are required for influenza virus infection of human lymphocytes. J. Clin. Investig. 1987, 79, 620–624. [Google Scholar] [CrossRef]
- Mock, D.J.; Frampton, M.W.; Nichols, J.E.; Domurat, F.M.; Signs, D.J.; Roberts, N.J., Jr. Influenza Virus Infection of Human Lymphocytes Occurs in the Immune Cell Cluster of the Developing Antiviral Response. Viruses 2018, 10, 420. [Google Scholar] [CrossRef]
- Salkind, A.R.; Nichols, J.E.; Roberts, N.J., Jr. Suppressed expression of ICAM-1 and LFA-1 and abrogation of leukocyte collaboration after exposure of human mononuclear leukocytes to respiratory syncytial virus in vitro: Comparison with exposure to influenza virus. J. Clin. Investig. 1991, 88, 505–511. [Google Scholar] [CrossRef]
- Roberts, N.J., Jr. Respiratory syncytial virus suppression of the antiviral immune response: Implications for evaluation of candidate vaccines. Vaccine 2019, 37, 7451–7454. [Google Scholar] [CrossRef] [PubMed]
- Fleming, E.H.; Ochoa, E.E.; Nichols, J.E.; O’Banion, M.K.; Salkind, A.R.; Roberts, N.J., Jr. Reduced activation and proliferation of human lymphocytes exposed to respiratory syncytial virus compared to cells exposed to influenza virus. J. Med. Virol. 2018, 90, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Chonmaitree, T.; Roberts, N.J., Jr.; Douglas, R.G., Jr.; Hall, C.B.; Simons, R.L. Interferon production by human mononuclear leukocytes: Differences between respiratory syncytial virus and influenza viruses. Infect. Immun. 1981, 32, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Keng, P.; Li, C.K.N.; Wheeler, K.T. Characterization of the separation properties of the Beckman elutriator system. Cell Biophys. 1981, 3, 41–56. [Google Scholar] [CrossRef]
- Wahl, L.M.; Katona, I.M.; Wilder, R.L.; Winter, C.C.; Haraouri, B.; Scher, I.; Wahl, S.M. Isolation of human mononuclear cell subsets by counterflow centrifugal elutriation (CCE). I. Characterization of B-lymphocyte-, T-lymphocyte-, and monocyte-enriched fractions by flow cytometry analysis. Cell. Immunol. 1984, 85, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Wahl, G.M.; Meinkoth, J.L.; Kimmel, A.R. Northern and Southern blots. In Guide to Molecular Cloning Techniques. Methods in Enzymology; Berger, S.L., Kimmel, A.R., Eds.; Academic Press, Inc.: San Diego, CA, USA, 1987; Volume 152, pp. 572–581. [Google Scholar]
- Young, D.A.; Voris, B.P.; Maytin, E.V.; Colbert, R.A. Very-high-resolution two-dimensional electrophoretic separation of proteins on giant gels. Meth. Enzymol. 1983, 91, 190–214. [Google Scholar] [CrossRef]
- O’Banion, M.K.; Winn, V.D.; Young, D.A. cDNA cloning and functional activity of a glucocorticoid-regulated inflammatory cyclooxygenase. Proc. Natl. Acad. Sci. USA 1992, 89, 4888–4892. [Google Scholar] [CrossRef]
- Han, J.W.; Sadowski, H.; Young, D.A.; Macara, I.G. Persistent induction of cyclooxygenase in p60v-src-transformed 3T3 fibroblasts. Proc. Natl. Acad. Sci. USA 1990, 87, 3373–3377. [Google Scholar] [CrossRef]
- Murakami, T.; Ockinger, J.; Yu, J.; Byles, V.; McColl, A.; Hofer, A.M.; Horng, T. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc. Natl. Acad. Sci. USA 2012, 109, 11282–11287. [Google Scholar] [CrossRef]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef]
- O’Banion, M.K.; Sadowski, H.B.; Winn, V.; Young, D.A. A serum- and glucocorticoid-regulated 4 kilobase mRNA encodes a cyclooxygenase-related protein. J. Biol. Chem. 1991, 266, 23261–23267. [Google Scholar] [CrossRef]
- Mertin, J.; Stackpoole, A. Anti-PGE antibodies inhibit in vivo development of cell-mediated immunity. Nature 1981, 294, 456–458. [Google Scholar] [CrossRef]
- Jordan, P.M.; Gunther, K.; Nischang, V.; Ning, Y.; Deinhardt-Emmer, S.; Ehrhardt, C.; Werz, O. Influenza A virus selectively elevates prostaglandin E(2) formation in pro-resolving macrophages. iScience 2024, 27, 108775. [Google Scholar] [CrossRef]
- Phipps, R.P.; Stein, S.H.; Roper, R.L. A new view of prostaglandin E regulation of the immune response. Immunol. Today 1991, 12, 349–352. [Google Scholar] [CrossRef]
- Testi, R.; Phillips, J.H.; Lanier, L.L. Leu 23 induction as an early marker of functional CD3/T cell antigen receptor triggering. Requirement for receptor cross-linking, prolonged elevation of intracellular [Ca++] and stimulation of protein kinase C. J. Immunol. 1989, 142, 1854–1860. [Google Scholar] [CrossRef]
- Bezouska, K.; Nepovim, A.; Horvath, O.; Pospisil, M.; Hamann, J.; Feizi, T. CD 69 antigen of human lymphocytes is a calcium-dependent carbohydrate- binding protein. Biochem. Biophys. Res. Commun. 1995, 208, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Santis, A.G.; Lopez-Cabrera, M.; Hamann, J.; Strauss, M.; Sanchez-Madrid, F. Structure of the gene coding for the human early lymphocyte activation antigen CD69: A C-type lectin receptor evolutionarily related with the gene families of natural killer cell-specific receptors. Eur. J. Immunol. 1994, 24, 1692–1697. [Google Scholar] [CrossRef] [PubMed]
- Hamann, J.; Fiebig, H.; Strauss, M. Expression cloning of the early activation antigen CD69, a type II integral membrane protein with a C-type lectin domain. J. Immunol. 1993, 150, 4920–4927. [Google Scholar] [CrossRef] [PubMed]
- Cosulich, M.E.; Rubartelli, A.; Risso, A.; Cozzolino, F.; Bargellesi, A. Functional characterization of an antigen involved in an early step of T-cell activation. Proc. Natl. Acad. Sci. USA 1987, 84, 4205–4209. [Google Scholar] [CrossRef]
- Wyde, P.R.; Wilson, M.R.; Cate, T.R. Interferon production by leukocytes infiltrating the lungs of mice during primary influenza virus infection. Infect. Immun. 1982, 38, 1249–1255. [Google Scholar] [CrossRef]
- Wyde, P.R.; Cate, T.R. Cellular changes in lungs of mice infected with influenza virus: Characterization of the cytotoxic responses. Infect. Immun. 1978, 22, 423–429. [Google Scholar] [CrossRef]
- Wyde, P.R.; Peavy, D.L.; Cate, T.R. Morphological and cytochemical characterization of cells infiltrating mouse lungs after influenza infection. Infect. Immun. 1978, 21, 140–146. [Google Scholar] [CrossRef]
- MacKenzie, C.D.; Taylor, P.M.; Askonas, B.A. Rapid recovery of lung histology correlates with clearance of influenza virus by specific CD8+ cytotoxic T cells. Immunology 1989, 67, 375–381. [Google Scholar]
- Guan, F.; Wang, R.; Yi, Z.; Luo, P.; Liu, W.; Xie, Y.; Liu, Z.; Xia, Z.; Zhang, H.; Cheng, Q. Tissue macrophages: Origin, heterogenity, biological functions, diseases and therapeutic targets. Signal Transduct. Target. Ther. 2025, 10, 93. [Google Scholar] [CrossRef] [PubMed]
- Ettensohn, D.B.; Lalor, P.A.; Roberts, N.J., Jr. Human alveolar macrophage regulation of lymphocyte proliferation. Am. Rev. Respir. Dis. 1986, 133, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Ettensohn, D.B.; Frampton, M.W.; Nichols, J.E.; Roberts, N.J., Jr. Human Alveolar Macrophages May Not Be Susceptible to Direct Infection by a Human Influenza Virus. J. Infect. Dis. 2016, 214, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Wen, B.; Liu, W.; Zhang, J.; Liu, C.; Fan, C.; Qu, X. Altered regulatory cytokine profiles in cases of pediatric respiratory syncytial virus infection. Cytokine 2018, 103, 57–62. [Google Scholar] [CrossRef]
- Bohmwald, K.; Galvez, N.M.S.; Canedo-Marroquin, G.; Pizarro-Ortega, M.S.; Andrade-Parra, C.; Gomez-Santander, F.; Kalergis, A.M. Contribution of Cytokines to Tissue Damage During Human Respiratory Syncytial Virus Infection. Front. Immunol. 2019, 10, 452. [Google Scholar] [CrossRef]
- Roberts, N.J., Jr.; Hiscott, J.; Signs, D.J. The limited role of the human interferon system response to respiratory syncytial virus challenge: Analysis and comparison to influenza virus. Microb. Pathog. 1992, 12, 409–414. [Google Scholar] [CrossRef]
- Patel, J.A.; Nair, S.; Ochoa, E.E.; Huda, R.; Roberts, N.J.; Chonmaitree, T. Interleukin-6(-)(1)(7)(4) and tumor necrosis factor alpha(-)(3)(0)(8) polymorphisms enhance cytokine production by human macrophages exposed to respiratory viruses. J. Interferon Cytokine Res. 2010, 30, 917–921. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roberts, N.J., Jr.; O’Banion, M.K. The Differential Early Responses of Human Leukocytes to Influenza Virus and Respiratory Syncytial Virus. Pathogens 2025, 14, 974. https://doi.org/10.3390/pathogens14100974
Roberts NJ Jr., O’Banion MK. The Differential Early Responses of Human Leukocytes to Influenza Virus and Respiratory Syncytial Virus. Pathogens. 2025; 14(10):974. https://doi.org/10.3390/pathogens14100974
Chicago/Turabian StyleRoberts, Norbert J., Jr., and M. Kerry O’Banion. 2025. "The Differential Early Responses of Human Leukocytes to Influenza Virus and Respiratory Syncytial Virus" Pathogens 14, no. 10: 974. https://doi.org/10.3390/pathogens14100974
APA StyleRoberts, N. J., Jr., & O’Banion, M. K. (2025). The Differential Early Responses of Human Leukocytes to Influenza Virus and Respiratory Syncytial Virus. Pathogens, 14(10), 974. https://doi.org/10.3390/pathogens14100974





