Surveillance of Acute Flaccid Paralysis (AFP) in Greece: 2008–2024
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimens
2.2. Treatment of Clinical Samples
2.3. Molecular Detection of Enterovirus
2.4. Cell Cultures, Viral Isolation, and Serotyping
2.5. Enterovirus Genotyping
3. Results
3.1. Patients, Clinical History and Vaccination Coverage
3.2. AFP Surveillance Indicators
3.3. EV Isolation and Typing
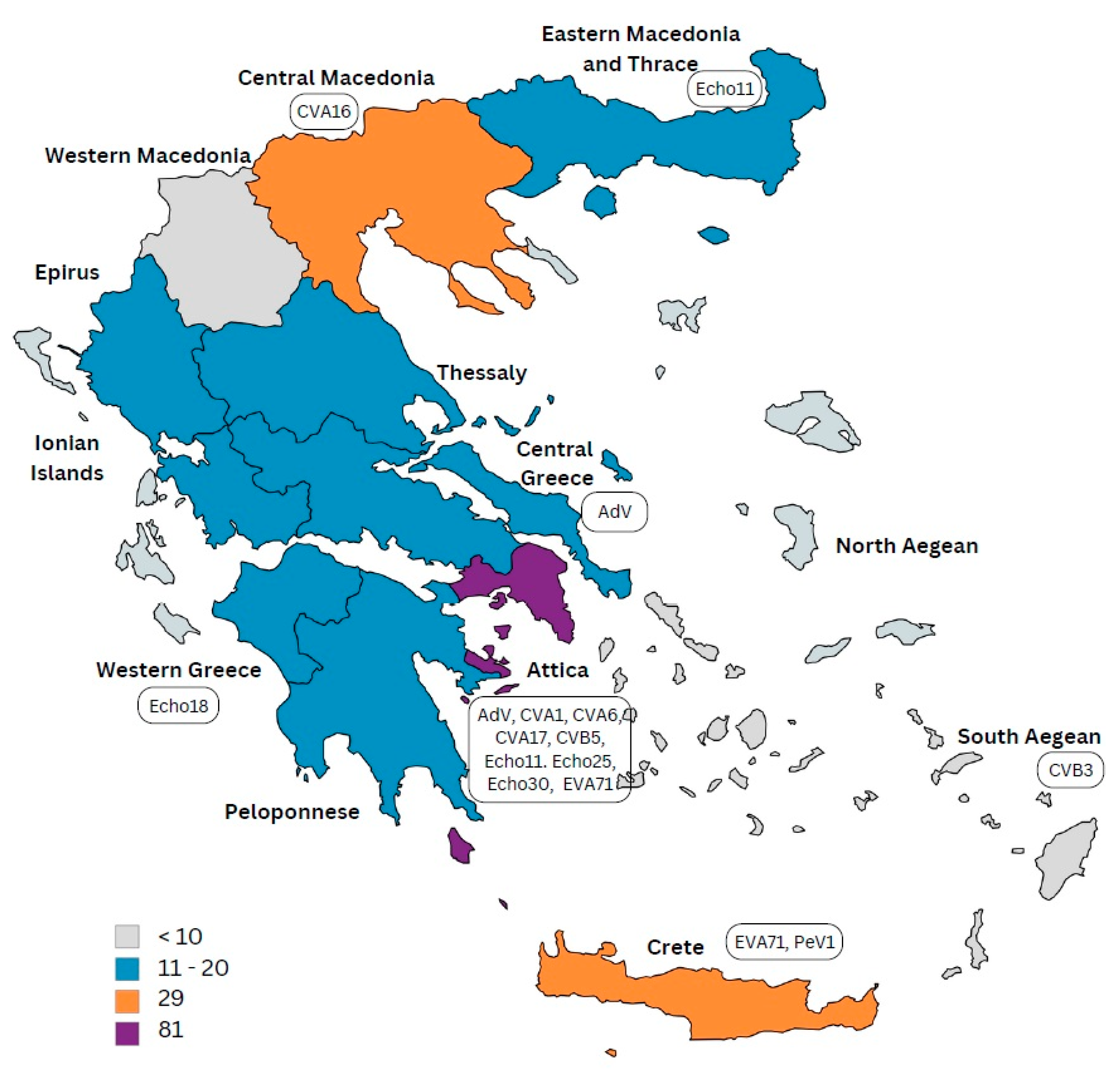
3.4. AFP Distribution
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Assembly. Global Eradication of Poliomyelitis by the Year 2000; World Health Organization: Geneva, Switzerland, 1988. Available online: https://iris.who.int/handle/10665/164531 (accessed on 20 July 2025).
- World Health Organization. Polio: Vaccine Preventable Diseases Surveillance Standards. 2018. Available online: https://www.who.int/publications/m/item/vaccine-preventable-diseases-surveillance-standards-polio (accessed on 20 July 2025).
- World Health Organization. Certification of Poliomyelitis Eradication: Fifteenth Meeting of the European Regional Certification Commission, Copenhagen, 19–21 June 2002; World Health Organization: Copenhagen, Denmark, 2005. Available online: https://iris.who.int/handle/10665/347456 (accessed on 20 July 2025).
- Fontana, S.; Buttinelli, G.; Fiore, S.; Amato, C.; Pataracchia, M.; Kota, M.; Aćimović, J.; Blažević, M.; Mulaomerović, M.; Nikolaeva-Glomb, L.; et al. Retrospective Analysis of Six Years of Acute Flaccid Paralysis Surveillance and Polio Vaccine Coverage Reported by Italy, Serbia, Bosnia and Herzegovina, Montenegro, Bulgaria, Kosovo, Albania, North Macedonia, Malta, and Greece. Vaccines 2021, 10, 44. [Google Scholar] [CrossRef]
- Bitnun, A.; Yeh, E.A. Acute Flaccid Paralysis and Enteroviral Infections. Curr. Infect. Dis. Rep. 2018, 20, 34. [Google Scholar] [CrossRef]
- Wieczorek, M.; Krzysztoszek, A. Molecular Characterization of Enteroviruses Isolated from Acute Flaccid Paralysis Cases in Poland, 1999–2014. Pol. J. Microbiol. 2017, 65, 443–450. [Google Scholar] [CrossRef]
- Tangermann, R.H.; Lamoureux, C.; Tallis, G.; Goel, A. The critical role of acute flaccid paralysis surveillance in the Global Polio Eradication Initiative. Int. Health 2017, 9, 156–163. [Google Scholar] [CrossRef]
- Ioulia, K.; Vasiliki, P.; Stavroula, L.; Emmanouil, A.; Andreas, M. A 5-year study of human parechoviruses in children living in bad sanitation conditions and non-polio acute flaccid paralysis children from Greece. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1907–1913. [Google Scholar] [CrossRef]
- Pogka, V.; Labropoulou, S.; Emmanouil, M.; Voulgari-Kokota, A.; Vernardaki, A.; Georgakopoulou, T.; Mentis, A.F. Laboratory Surveillance of Polio and Other Enteroviruses in High-Risk Populations and Environmental Samples. Appl. Environ. Microbiol. 2017, 83, e02872-16. [Google Scholar] [CrossRef]
- National Public Health Organization of Greece. Acute Flaccid Paralysis (AFP). Available online: https://eody.gov.gr/disease/oxeia-chalari-paralysiochp/ (accessed on 20 July 2025).
- Osterback, R.; Tevaluoto, T.; Ylinen, T.; Peltola, V.; Susi, P.; Hyypiä, T.; Waris, M. Simultaneous detection and differentiation of human rhino- and enteroviruses in clinical specimens by real-time PCR with locked nucleic Acid probes. J. Clin. Microbiol. 2013, 51, 3960–3967. [Google Scholar] [CrossRef]
- Nolan, T.; Hands, R.E.; Ogunkolade, W.; Bustin, S.A. SPUD: A quantitative PCR assay for the detection of inhibitors in nucleic acid preparations. Anal. Biochem. 2006, 351, 308–310. [Google Scholar] [CrossRef]
- World Health Organization. Polio Laboratory Manual, 4th ed.; World Health Organization: Geneva, Switzerland, 2004. Available online: http://apps.who.int/iris/bitstream/10665/68762/1/WHO_IVB_04.10.pdf (accessed on 20 July 2025).
- Nix, W.A.; Oberste, M.S.; Pallansch, M.A. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J. Clin. Microbiol. 2006, 44, 2698–2704. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Kroneman, A.; Vennema, H.; Deforche, K.V.D.; Avoort, H.V.D.; Peñaranda, S.; Oberste, M.S.; Vinjé, J.; Koopmans, M. An automated genotyping tool for enteroviruses and noroviruses. J. Clin. Virol. 2011, 51, 121–125. [Google Scholar] [CrossRef]
- Anwar, H.B.; Mazumder, Y.; Nujhat, S.; Islam, B.Z.; Kalbarczyk, A.; Alonge, O.; Sarker, M. The evolution, facilitators, barriers, and additional activities of acute flaccid paralysis surveillance platform in polio eradication programme Bangladesh: A mixed-method study. Glob. Health Action 2024, 17, 2370096. [Google Scholar] [CrossRef]
- Bessing, B.; Dagoe, E.A.; Tembo, D.; Mwangombe, A.; Kanyanga, M.K.; Manneh, F.; Matapo, B.B.; Bobo, P.M.; Chipoya, M.; Eboh, V.A.; et al. Evaluation of the Acute flaccid paralysis surveillance indicators in Zambia from 2015–2021: A retrospective analysis. BMC Public Health 2023, 23, 2227. [Google Scholar] [CrossRef]
- D’Errico, M.M.; Barbadoro, P.; Bacelli, S.; Esposto, E.; Moroni, V.; Scaccia, F.; Tantucci, L.; Prospero, E.; AFP Study Group. Surveillance of acute flaccid paralysis in the Marches region (Italy): 1997–2007. BMC Infect. Dis. 2008, 8, 135. [Google Scholar] [CrossRef]
- Fontana, S.; Buttinelli, G.; Fiore, S.; Mulaomerovic, M.; Aćimović, J.; Amato, C.; Delogu, R.; Rezza, G.; Stefanelli, P. Acute flaccid paralysis surveillance in bosnia and herzegovina: Recent isolation of two sabin like type 2 poliovirus. J. Med. Virol. 2017, 89, 1678–1681. [Google Scholar] [CrossRef]
- Momen, A.A.; Shakurnia, A.; Momen, M. Eleven-year surveillance of acute flaccid paralysis in southwestern Iran. Turk. J. Pediatr. 2019, 61, 544–551. [Google Scholar] [CrossRef]
- Marx, A.; Glass, J.D.; Sutter, R.W. Surveillance of acute flaccid paralysis in The Netherlands, 1992-94. Bull. World Health Organ. 1998, 76, 55–62. [Google Scholar]
- Pellegrinelli, L.; Galli, C.; Primache, V.; Bubba, L.; Buttinelli, G.; Stefanelli, P.; Pariani, E.; Binda, S. Emerging Non-Polio Enteroviruses recognized in the framework of the Acute Flaccid Paralyses (AFP) surveillance system in Northern Italy, 2016–2018. Int. J. Infect. Dis. 2021, 106, 36–40. [Google Scholar] [CrossRef]
- Pellegrinelli, L.; Primache, V.; Fiore, L.; Amato, C.; Fiore, S.; Bubba, L.; Pariani, E.; Amendola, A.; Barbi, M.; Binda, S. Surveillance of acute flaccid paralysis (AFP) in Lombardy, Northern Italy, from 1997 to 2011 in the context of the national AFP surveillance system. Hum. Vaccin. Immunother. 2015, 11, 277–281. [Google Scholar] [CrossRef][Green Version]
- Yoon, Y.; Lee, Y.P.; Lee, D.Y.; Kim, H.J.; Lee, J.W.; Lee, S.; Kang, C.; Choi, W.; Bin, J.H.; Kim, Y.H.; et al. Non-Polio Enteroviruses from Acute Flaccid Paralysis Surveillance in Korea, 2012–2019. Viruses 2021, 13, 411. [Google Scholar] [CrossRef]
- Cavounidis, J. The migration experience of Greece and the impact of the economic crisis on its migrant and native populations. Eur. J. Public Health 2018, 28 (Suppl. S5), 20–23. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.; Forgie, S.; Robinson, J. Non-polio Enterovirus detection with acute flaccid paralysis: A systematic review. J. Med. Virol. 2018, 90, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Pogka, V.; Emmanouil, M.; Labropoulou, S.; Voulgari-Kokota, A.; Angelakis, E.; Mentis, A.F. Molecular characterization of enteroviruses among hospitalized patients in Greece, 2013–2015. J. Clin. Virol. 2020, 127, 104349. [Google Scholar] [CrossRef]
- Soldatou, A.; Vartzelis, G.; Vorre, S.; Papa, A.; Voudris, K.; Garoufi, A. A toddler with acute flaccid paralysis due to West Nile virus infection. Pediatr. Infect. Dis. J. 2013, 32, 1023. [Google Scholar] [CrossRef] [PubMed]
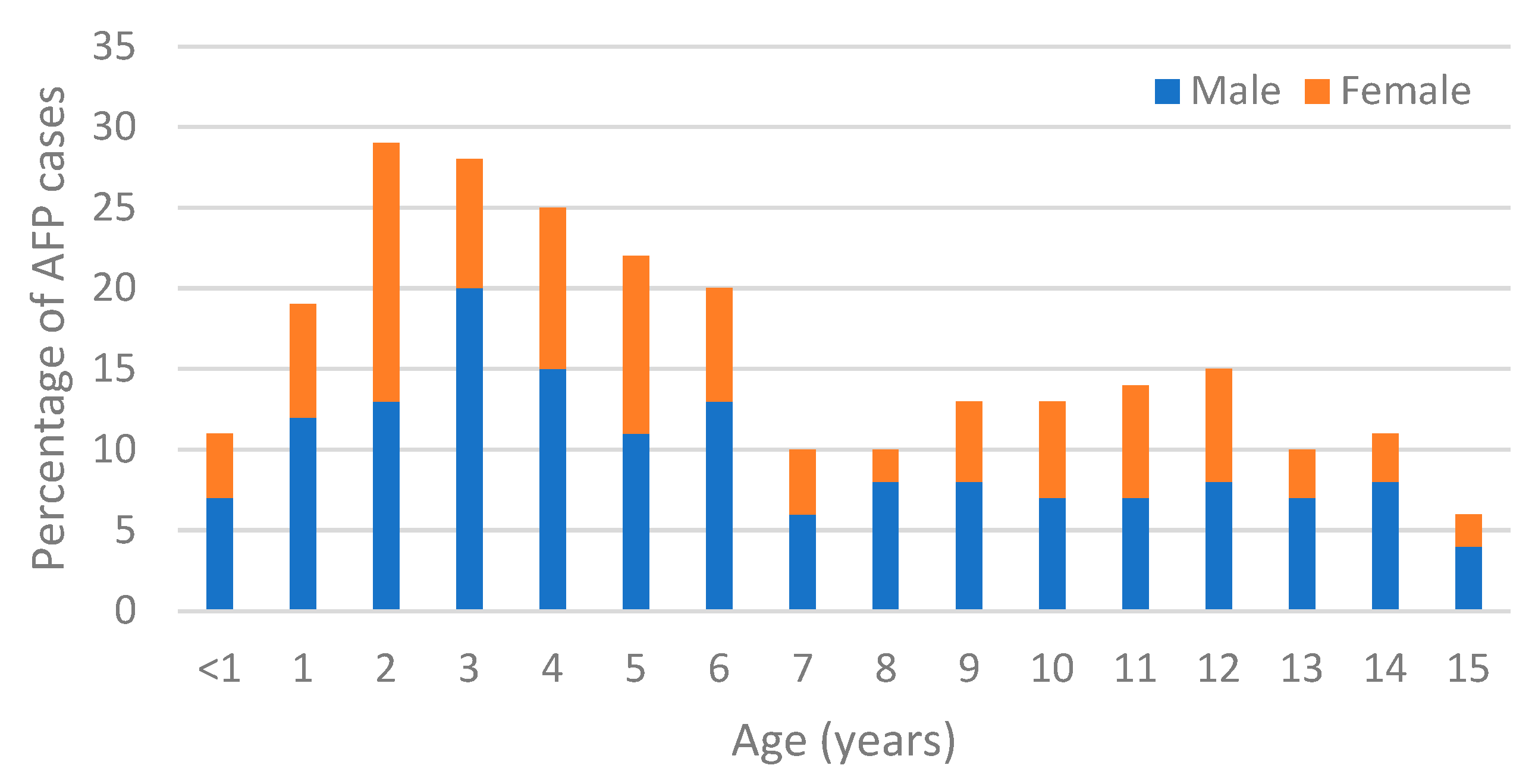
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of AFP cases | 18 | 16 | 20 | 24 | 16 | 23 | 15 | 18 | 18 | 10 | 17 | 11 | 7 | 4 | 18 | 9 | 12 | 256 |
| Gender | ||||||||||||||||||
| Male | 10 (56%) | 14 (88%) | 13 (65%) | 17 (71%) | 10 (63%) | 13 (57%) | 10 (67%) | 6 (33%) | 10 (56%) | 5 (50%) | 11 (65%) | 6 (55%) | 6 (85%) | 1 (25%) | 9 (50%) | 6 (67%) | 7 (60%) | 154 (60%) |
| Female | 8 (44%) | 2 (12%) | 7 (35%) | 7 (29%) | 6 (37%) | 10 (43%) | 5 (33%) | 12 (67%) | 8 (44%) | 5 (50%) | 6 (35%) | 5 (45%) | 1 (15%) | 3 (75%) | 9 (50%) | 3 (33%) | 5 (40%) | 102 (40%) |
| Age (years) | ||||||||||||||||||
| <1 | 2 (11%) | 2 (12%) | 0 (0%) | 2 (8%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (11%) | 0 (0%) | 0 (0%) | 1 (9%) | 0 (0%) | 0 (0%) | 1 (6%) | 0 (0%) | 1 (8%) | 11 (4%) |
| 1–5 | 12 (66%) | 4 (25%) | 11 (55%) | 15 (63%) | 7 (44%) | 13 (57%) | 8 (53%) | 9 (50%) | 7 (39%) | 4 (40%) | 7 (42%) | 5 (46%) | 3 (42%) | 2 (50%) | 6 (33%) | 4 (44%) | 6 (50%) | 123 (48%) |
| 6–10 | 3 (17%) | 7 (44%) | 3 (15%) | 4 (17%) | 4 (25%) | 7 (30%) | 4 (27%) | 5 (28%) | 7 (39%) | 4 (40%) | 5 (29%) | 4 (36%) | 2 (29%) | 1 (25%) | 2 (11%) | 1 (12%) | 3 (25%) | 66 (26%) |
| 11–15 | 1 (6%) | 3 (19%) | 6 (30%) | 3 (12%) | 5 (31%) | 3 (13%) | 3 (20%) | 4 (22%) | 2 (11%) | 2 (20%) | 5 (29%) | 1 (9%) | 2 (29%) | 1 (25%) | 9 (50%) | 4 (44%) | 2 (17%) | 56 (22%) |
| Vaccination history | ||||||||||||||||||
| 0 or unknown dose | 2 (11%) | 7 (44%) | 3 (15%) | 2 (8%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (6%) | 0 (0%) | 0 (0%) | 0 (0%) | 6 (33%) | 1 (11%) | 1 (8%) | 23 (9%) |
| 1–2 doses | 1 (6%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (9%) | 1 (7%) | 0 (0%) | 1 (6%) | 1 (10%) | 0 (%) | 1 (9%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (11%) | 1 (8%) | 9 (3%) |
| 3 or more doses | 15 (83%) | 9 (56%) | 17 (85%) | 22 (92%) | 16 (100%) | 21 (91%) | 14 (93%) | 18 (100%) | 17 (94%) | 9 (90%) | 16 (94%) | 10 (91%) | 7 (100%) | 4 (100%) | 12 (67%) | 7 (78%) | 10 (84%) | 224 (88%) |
| Clinical history | ||||||||||||||||||
| Fever at onset | 4 (22%) | 3 (19%) | 3 (15%) | 3 (13%) | 0 (0%) | 2 (9%) | 3 (20%) | 2 (11%) | 7 (39%) | 0 (0%) | 4 (24%) | 2 (18%) | 0 (0%) | 0 (0%) | 6 (33%) | 2 (22%) | 0 (0%) | 41 (16%) |
| Paralysis progression | 17 (94%) | 12 (75%) | 13 (65%) | 21 (88%) | 14 (88%) | 18 (78%) | 11 (73%) | 13 (72%) | 9 (50%) | 6 (60%) | 13 (76%) | 6 (55%) | 6 (86%) | 1 (25%) | 11 (61%) | 5 (55%) | 7 (58%) | 183 (71%) |
| Asymmetric paralysis | 3 (17%) | 2 (12%) | 6 (30%) | 4 (17%) | 3 (19%) | 4 (17%) | 1 (7%) | 0 (0%) | 3 (17%) | 0 (0%) | 3 (18%) | 0 (0%) | 1 (14%) | 2 (50%) | 9 (50%) | 3 (33%) | 1 (8%) | 45 (18%) |
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of expected cases in <15 children | 17 | 17 | 17 | 17 | 17 | 17 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 15 | 15 | 14 | 14 |
| Number of AFP cases | 18 | 16 | 20 | 24 | 16 | 23 | 15 | 18 | 18 | 10 | 17 | 11 | 7 | 4 | 18 | 9 | 12 |
| Non polio AFP rate | 1.05 | 0.94 | 1.17 | 1.41 | 0.94 | 1.35 | 0.94 | 1.12 | 1.1 | 0.63 | 1.06 | 0.69 | 0.44 | 0.27 | 1.2 | 0.64 | 0.86 |
| Adequate specimen | 94% | 88% | 65% | 88% | 100% | 91% | 100% | 94% | 100% | 100% | 94% | 100% | 85% | 100% | 72% | 56% | 75% |
| Surveillance index | 0.94 | 0.82 | 0.65 | 0.88 | 0.94 | 0.91 | 0.94 | 0.94 | 1 | 0.63 | 0.94 | 0.69 | 0.38 | 0.27 | 0.72 | 0.35 | 0.65 |
| 60-day follow-up | 89% | 100% | 100% | 96% | 100% | 96% | 87% | 100% | 100% | 100% | 100% | 100% | 85% | 25% | 100% | 78% | 58% |
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nο. of AFP cases | 18 | 16 | 20 | 24 | 16 | 23 | 15 | 18 | 18 | 10 | 17 | 11 | 7 | 4 | 18 | 9 | 12 |
| Enterovirus cause | 1 | 0 | 1 | 1 | 0 | 2 | 2 | 1 | 2 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 |
| NPEV type | Echo 30 | - | CoxB3 | Echo 11 | - | CoxA17 CoxA6 | Echo 25 CoxA1 | Echo 18 | EVA71 | - | - | CVA16 | - | - | CoxB5 | Echo 11 | - |
| Other causative | - | - | - | 3 | - | - | 1 | 2 | - | - | - | - | - | - | - | - | - |
| Type | 2 Adenoviruses, West Nile virus | Adenovirus | Adenovirus Parechovirus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labropoulou, S.; Georgakopoulou, T.; Baniasadi, V.; Mpizta, G.; Vorre, S.; Theodoridou, M.; Emmanouil, M.; Angelakis, E. Surveillance of Acute Flaccid Paralysis (AFP) in Greece: 2008–2024. Pathogens 2025, 14, 976. https://doi.org/10.3390/pathogens14100976
Labropoulou S, Georgakopoulou T, Baniasadi V, Mpizta G, Vorre S, Theodoridou M, Emmanouil M, Angelakis E. Surveillance of Acute Flaccid Paralysis (AFP) in Greece: 2008–2024. Pathogens. 2025; 14(10):976. https://doi.org/10.3390/pathogens14100976
Chicago/Turabian StyleLabropoulou, Stavroula, Theano Georgakopoulou, Vahid Baniasadi, Giota Mpizta, Stella Vorre, Maria Theodoridou, Mary Emmanouil, and Emmanouil Angelakis. 2025. "Surveillance of Acute Flaccid Paralysis (AFP) in Greece: 2008–2024" Pathogens 14, no. 10: 976. https://doi.org/10.3390/pathogens14100976
APA StyleLabropoulou, S., Georgakopoulou, T., Baniasadi, V., Mpizta, G., Vorre, S., Theodoridou, M., Emmanouil, M., & Angelakis, E. (2025). Surveillance of Acute Flaccid Paralysis (AFP) in Greece: 2008–2024. Pathogens, 14(10), 976. https://doi.org/10.3390/pathogens14100976





