Evaluation of Suitable Reference Gene During the Development of Paired or Unpaired Female Schistosoma japonicum on the 18th and the 23rd Days Post Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Unisexual and Paired Infections
2.3. RNA Extraction, Amplification, and Solexa Sequencing Analysis
2.4. Gene Expression Validation via qPCR
2.5. Reaction Configuration
2.6. PCR Cycling Conditions
2.7. Data Processing
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTB | Beta-actin |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| NDUFV2 | NADH dehydrogenase (ubiquinone) flavoprotein 2 |
| PSMD4 | 26S proteasome non-ATPase regulatory subunit 4 |
| TPC2L | Trafficking protein particle complex subunit 2-like protein |
| TUBA | Tubulin alpha |
| SOD1 | Superoxide dismutase 1 |
| 18SSI | 18-day-old female worms from single-sex infections |
| 18DSI | 18-day-old female worms from double-sex infections |
| 23SSI | 23-day-old female worms from single-sex infections |
| 23DSI | 23-day-old female worms from double-sex infections |
| qPCR | Quantitative polymerase chain reaction |
References
- Amoah, I.D.; Reddy, P.; Seidu, R.; Stenström, T.A. Removal of helminth eggs by centralized and decentralized wastewater treatment plants in South Africa and Lesotho: Health implications for direct and indirect exposure to the effluents. Environ. Sci. Pollut. Res. 2018, 25, 12883–12895. [Google Scholar] [CrossRef]
- Piao, X.; Cai, P.; Liu, S.; Hou, N.; Hao, L.; Yang, F.; Wang, H.; Wang, J.; Jin, Q.; Chen, Q. Global expression analysis revealed novel gender-specific gene expression features in the blood fluke parasite Schistosoma japonicum. PLoS ONE 2011, 6, e18267. [Google Scholar] [CrossRef]
- Kuo, Y.-J.; Paras, G.; Tagami, T.; Yi, C.; Aquino, L.J.C.; Oh, H.; Rychtář, J.; Taylor, D. A compartmental model for Schistosoma japonicum transmission dynamics in the Philippines. Acta Trop. 2024, 249, 107084. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, Y.; Wang, C.; Guo, G.; Yu, X.; Dai, Y.; Liu, Y.; Wei, G.; He, X.; Jin, G.; et al. Chromosome-level genome assembly defines female-biased genes associated with sex determination and differentiation in the human blood fluke Schistosoma japonicum. Mol. Ecol. Resour. 2023, 23, 205–221. [Google Scholar] [CrossRef]
- Dinguirard, N.; Heinemann, C.; Yoshino, T.P. Mass Isolation and In Vitro Cultivation of Intramolluscan Stages of the Human Blood Fluke Schistosoma Mansoni. J. Vis. Exp. 2018, 131, e56345. [Google Scholar] [CrossRef]
- Kincaid-Smith, J.; Boissier, J.; Allienne, J.-F.; Oleaga, A.; Djuikwo-Teukeng, F.; Toulza, E. A Genome Wide Comparison to Identify Markers to Differentiate the Sex of Larval Stages of Schistosoma haematobium, Schistosoma bovis and their Respective Hybrids. PLoS Neglected Trop. Dis. 2016, 10, e0005138. [Google Scholar] [CrossRef] [PubMed]
- Staff, T.P.P. Correction: MicroRNAs Are Involved in the Regulation of Ovary Development in the Pathogenic Blood Fluke Schistosoma japonicum. PLOS Pathog. 2016, 12, e1005582. [Google Scholar] [CrossRef]
- Lawlor, H.; Meunier, A.; McDermott, N.; Lynch, T.H.; Marignol, L. Identification of suitable endogenous controls for gene and miRNA expression studies in irradiated prostate cancer cells. Tumor Biol. 2015, 36, 6019–6028. [Google Scholar] [CrossRef]
- Miao, H.; Ye, Z.; Hu, G.; Qin, Y. Comparative transcript profiling of gene expression between self-incompatible and self-compatible mandarins by suppression subtractive hybridization and cDNA microarray. Mol. Breed. 2015, 35, 47. [Google Scholar] [CrossRef]
- Rozenberg, A.; Parida, M.; Leese, F.; Weiss, L.C.; Tollrian, R.; Manak, J.R. Transcriptional profiling of predator-induced phenotypic plasticity in Daphnia pulex. Front. Zoöl. 2015, 12, 18. [Google Scholar] [CrossRef]
- Grimes, J.E.; Croll, D.; E Harrison, W.; Utzinger, J.; Freeman, M.C.; Templeton, M.R. The roles of water, sanitation and hygiene in reducing schistosomiasis: A review. Parasites Vectors 2015, 8, 156. [Google Scholar] [CrossRef]
- Li, X.; Cheng, J.; Zhang, J.; da Silva, J.A.T.; Wang, C.; Sun, H. Validation of Reference Genes for Accurate Normalization of Gene Expression in Lilium davidii var. unicolor for Real Time Quantitative PCR. PLoS ONE 2015, 10, e0141323. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, Q.-P.; Ye, Q.; Xiong, T.; Tang, C.-L.; Dong, H.-F.; Jiang, M.-S. Cloning and characterization of a bone morphogenetic protein homologue of Schistosoma japonicum. Exp. Parasitol. 2013, 135, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Nazari, F.; Parham, A.; Maleki, A.F. GAPDH, β-actin and β2-microglobulin, as three common reference genes, are not reliable for gene expression studies in equine adipose- and marrow-derived mesenchymal stem cells. J. Anim. Sci. Technol. 2015, 57, 18. [Google Scholar] [CrossRef] [PubMed]
- Katarzyńska-Banasik, D.; Grzesiak, M.; Sechman, A. Selection of reference genes for quantitative real-time PCR analysis in chicken ovary following silver nanoparticle treatment. Environ. Toxicol. Pharmacol. 2017, 56, 186–190. [Google Scholar] [CrossRef]
- Zhang, P.; Feng, G.; Zhang, W.-N.; Zhang, Y.-Y.; Liu, C.-S. Effect of RNA interference on WD101 gene of Schistosoma japonicum. Asian Pac. J. Trop. Med. 2018, 11, 136–140. [Google Scholar] [CrossRef]
- Sun, J.; Li, C.; Wang, S. Organism-like formation of Schistosoma hemozoin and its function suggest a mechanism for anti-malarial action of artemisinin. Sci. Rep. 2016, 6, 34463. [Google Scholar] [CrossRef]
- Taft, A.S.; Norante, F.A.; Yoshino, T.P. The identification of inhibitors of Schistosoma mansoni miracidial transformation by incorporating a medium-throughput small-molecule screen. Exp. Parasitol. 2010, 125, 84–94. [Google Scholar] [CrossRef]
- Taft, A.S.; Vermeire, J.J.; Bernier, J.; Birkeland, S.R.; Cipriano, M.J.; Papa, A.R.; McARTHUR, A.G.; Yoshino, T.P. Transcriptome analysis of Schistosoma mansoni larval development using serial analysis of gene expression (SAGE). Parasitology 2009, 136, 469–485. [Google Scholar] [CrossRef]
- Ranasinghe, S.L.; Fischer, K.; Gobert, G.N.; McManus, D.P. Functional expression of a novel Kunitz type protease inhibitor from the human blood fluke Schistosoma mansoni. Parasites Vectors 2015, 8, 408. [Google Scholar] [CrossRef] [PubMed]
- Long, T.; Neitz, R.J.; Beasley, R.; Kalyanaraman, C.; Suzuki, B.M.; Jacobson, M.P.; Dissous, C.; McKerrow, J.H.; Drewry, D.H.; Zuercher, W.J.; et al. Structure-Bioactivity Relationship for Benzimidazole Thiophene Inhibitors of Polo-Like Kinase 1 (PLK1), a Potential Drug Target in Schistosoma mansoni. PLoS Neglected Trop. Dis. 2016, 10, e0004356. [Google Scholar] [CrossRef] [PubMed]
- Urbatzka, R.; Galante-Oliveira, S.; Rocha, E.; Castro, L.; Cunha, I. Normalization strategies for gene expression studies by real-time PCR in a marine fish species, Scophthalmus maximus. Mar. Genom. 2013, 10, 17–25. [Google Scholar] [CrossRef]
- Hu, S.; Yang, L.; Wu, Z.; Wong, C.S.; Fung, M.C. Suppression of adaptive immunity to heterologous antigens by SJ16 of Schistosoma japonicum. J. Parasitol. 2012, 98, 274–283. [Google Scholar] [CrossRef]
- Ma, Y.-J.; Sun, X.-H.; Xu, X.-Y.; Zhao, Y.; Pan, Y.-J.; Hwang, C.-A.; Wu, V.C.H. Investigation of Reference Genes in Vibrio parahaemolyticus for Gene Expression Analysis Using Quantitative RT-PCR. PLoS ONE 2015, 10, e0144362. [Google Scholar] [CrossRef]
- Nolan, T.; Hands, R.E.; Bustin, S.A. Quantification of mRNA using real-time RT-PCR. Nat. Protoc. 2006, 1, 1559–1582. [Google Scholar] [CrossRef]
- Xu, Y.; Li, H.; Li, X.; Lin, J.; Wang, Z.; Yang, Q.; Chang, Y. Systematic selection and validation of appropriate reference genes for gene expression studies by quantitative real-time PCR in pear. Acta Physiol. Plant. 2015, 37, 40. [Google Scholar] [CrossRef]
- Yang, J.; Lu, Y.B.; Chi, M.H.; Wang, Q.M.; Deng, S.N. Analysis on stability of reference genes in different developmental stages of seeds from Carthamus tinctorius. Chin. Tradit. Herbal. Drugs 2017, 48, 1845–1850. [Google Scholar]
- Ren, J.; Zhang, N.; Li, X.; Sun, X.; Song, J. Identification of reference genes for gene expression studies among different developmental stages of murine hearts. BMC Dev. Biol. 2021, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; Li, J.; Liu, H.; Ernst, C.W.; Liu, X.; Liu, G.; Xi, Y.; Lei, M. Evaluation of housekeeping genes for normalizing real-time quantitative PCR assays in pig skeletal muscle at multiple developmental stages. Gene 2015, 565, 235–241. [Google Scholar] [CrossRef]
- Sun, J.; Wang, S.-W.; Li, C.; Hu, W.; Ren, Y.-J.; Wang, J.-Q. Transcriptome profilings of female Schistosoma japonicum reveal significant differential expression of genes after pairing. Parasitol. Res. 2013, 113, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Blomquist, D.; Fernández, J.R.; Miranda, J.; Bello, C.; Silva, J.A.; Estrada, R.C.; Novoa, L.I.; Palenzuela, D.; Bello, I. Selection of reference genes for use in quantitative reverse transcription PCR assays when using interferons in U87MG. Mol. Biol. Rep. 2012, 39, 11167–11175. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, J.; Wang, Y.; Bao, Z.; Zhao, H. Identification of Suitable Reference Genes for Gene Expression Normalization in the Quantitative Real-Time PCR Analysis of Sweet Osmanthus (Osmanthus fragrans Lour.). PLoS ONE 2015, 10, e0136355. [Google Scholar] [CrossRef] [PubMed]
- Kanakachari, M.; Solanke, A.U.; Prabhakaran, N.; Ahmad, I.; Dhandapani, G.; Jayabalan, N.; Kumar, P.A. Evaluation of Suitable Reference Genes for Normalization of qPCR Gene Expression Studies in Brinjal (Solanum melongena L.) During Fruit Developmental Stages. Appl. Biochem. Biotechnol. 2015, 178, 433–450. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Li, T.; Huang, B.; Cheng, H.; Ding, H.; Dong, W.; Xiao, M.; Liu, L.; Wang, Z. The use of laser microdissection in the identification of suitable reference genes for normalization of quantitative real-time PCR in human FFPE epithelial ovarian tissue samples. PLoS ONE 2014, 9, e95974. [Google Scholar] [CrossRef]
- Johnson, G.; Nour, A.A.; Nolan, T.; Huggett, J.; Bustin, S. Minimum information necessary for quantitative real-time PCR experiments. Methods Mol. Biol. 2014, 1160, 5–17. [Google Scholar] [CrossRef]
- Fenwick, A. Schistosomiasis research and control since the retirement of Sir Patrick Manson in 1914. Trans. R. Soc. Trop. Med. Hyg. 2017, 111, 191–198. [Google Scholar] [CrossRef]
- Kong, F.; Cao, M.; Sun, P.; Liu, W.; Mao, Y. Selection of reference genes for gene expression normalization in Pyropia yezoensis using quantitative real-time PCR. J. Appl. Phycol. 2015, 27, 1003–1010. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, X.; Chen, S.; Zheng, L.; He, X.; Liu, M.; Qiao, G.; Wang, Y.; Zhuo, R. Selection of suitable reference genes for quantitative real-time PCR gene expression analysis in Salix matsudana under different abiotic stresses. Sci. Rep. 2017, 7, 40290. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, R34. [Google Scholar] [CrossRef]
- Martínez-Escauriaza, R.; Lozano, V.; Pérez-Parallé, M.L.; Pazos, A.J.; Sánchez, J.L. Validation of Reference Genes in Mussel Mytilus galloprovincialis Tissues under the Presence of Okadaic Acid. J. Shellfish. Res. 2018, 37, 93–101. [Google Scholar] [CrossRef]
- E Hernandez-Santana, Y.; Ontoria, E.; Gonzalez-García, A.C.; Quispe-Ricalde, M.A.; Larraga, V.; Valladares, B.; Carmelo, E. The Challenge of Stability in High-Throughput Gene Expression Analysis: Comprehensive Selection and Evaluation of Reference Genes for BALB/c Mice Spleen Samples in the Leishmania infantum Infection Model. PLoS ONE 2016, 11, e0163219. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Zhang, X.; Pan, L.; Tian, X.; Dong, P. Identification of Key Pathways and Genes in Advanced Coronary Atherosclerosis Using Bioinformatics Analysis. BioMed Res. Int. 2017, 2017, 4323496. [Google Scholar] [CrossRef]
- Qureshi, R.; Sacan, A. A novel method for the normalization of microRNA RT-PCR data. BMC Med. Genom. 2013, 6 (Suppl. S1), S14. [Google Scholar] [CrossRef]
- Stoffel, K.; van Leeuwen, H.; Kozik, A.; Caldwell, D.; Ashrafi, H.; Cui, X.; Tan, X.; Hill, T.; Reyes-Chin-Wo, S.; Truco, M.-J.; et al. Development and application of a 6.5 million feature Affymetrix Genechip® for massively parallel discovery of single position polymorphisms in lettuce (Lactuca spp.). BMC Genom. 2012, 13, 185. [Google Scholar] [CrossRef]
- Radonić, A.; Thulke, S.; Mackay, I.M.; Landt, O.; Siegert, W.; Nitsche, A. Guideline to reference gene selection for quantitative real-time PCR. Biochem. Biophys. Res. Commun. 2004, 313, 856–862. [Google Scholar] [CrossRef]
- Liu, S.; Cai, P.; Hou, N.; Piao, X.; Wang, H.; Hung, T.; Chen, Q. Genome-wide identification and characterization of a panel of house-keeping genes in Schistosoma japonicum. Mol. Biochem. Parasitol. 2012, 182, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Juan, P.H.; Guang, C.X.; Hua, L.; Hong, Z.X.; Man, S.S.; Zhang, W.X. Analysis of the expression characteristics of several novel genes of Schistosoma japonicum. Chin. J. Zoonoses 2003, 19, 46–49. [Google Scholar]
- Wu, X.; Huang, A.; Xu, M.; Wang, C.; Jia, Z.; Wang, G.; Niu, J. Variation of expression levels of seven housekeeping genes at different life-history stages in Porphyra yezoensis. PLoS ONE 2013, 8, e60740. [Google Scholar] [CrossRef] [PubMed]
- Bhat, I.A.; Dubiel, M.M.; Rodriguez, E.; Jónsson, Z.O. Insights into Early Ontogenesis of Salmo salar: RNA Extraction, Housekeeping Gene Validation and Transcriptional Expression of Important Primordial Germ Cell and Sex-Determination Genes. Animals 2023, 13, 1094. [Google Scholar] [CrossRef]
- Narita, M.; Nishida, H.; Asahina, R.; Nakata, K.; Yano, H.; Ueda, T.; Inden, M.; Akiyoshi, H.; Maeda, S.; Kamishina, H. Identification of reference genes for microRNAs of extracellular vesicles isolated from plasma samples of healthy dogs by ultracentrifugation, precipitation, and membrane affinity chromatography methods. Am. J. Vet. Res. 2019, 80, 449–454. [Google Scholar] [CrossRef]
- Kloubert, V.; Rink, L. Selection of an inadequate housekeeping gene leads to misinterpretation of target gene expression in zinc deficiency and zinc supplementation models. J. Trace Elem. Med. Biol. 2019, 56, 192–197. [Google Scholar] [CrossRef] [PubMed]
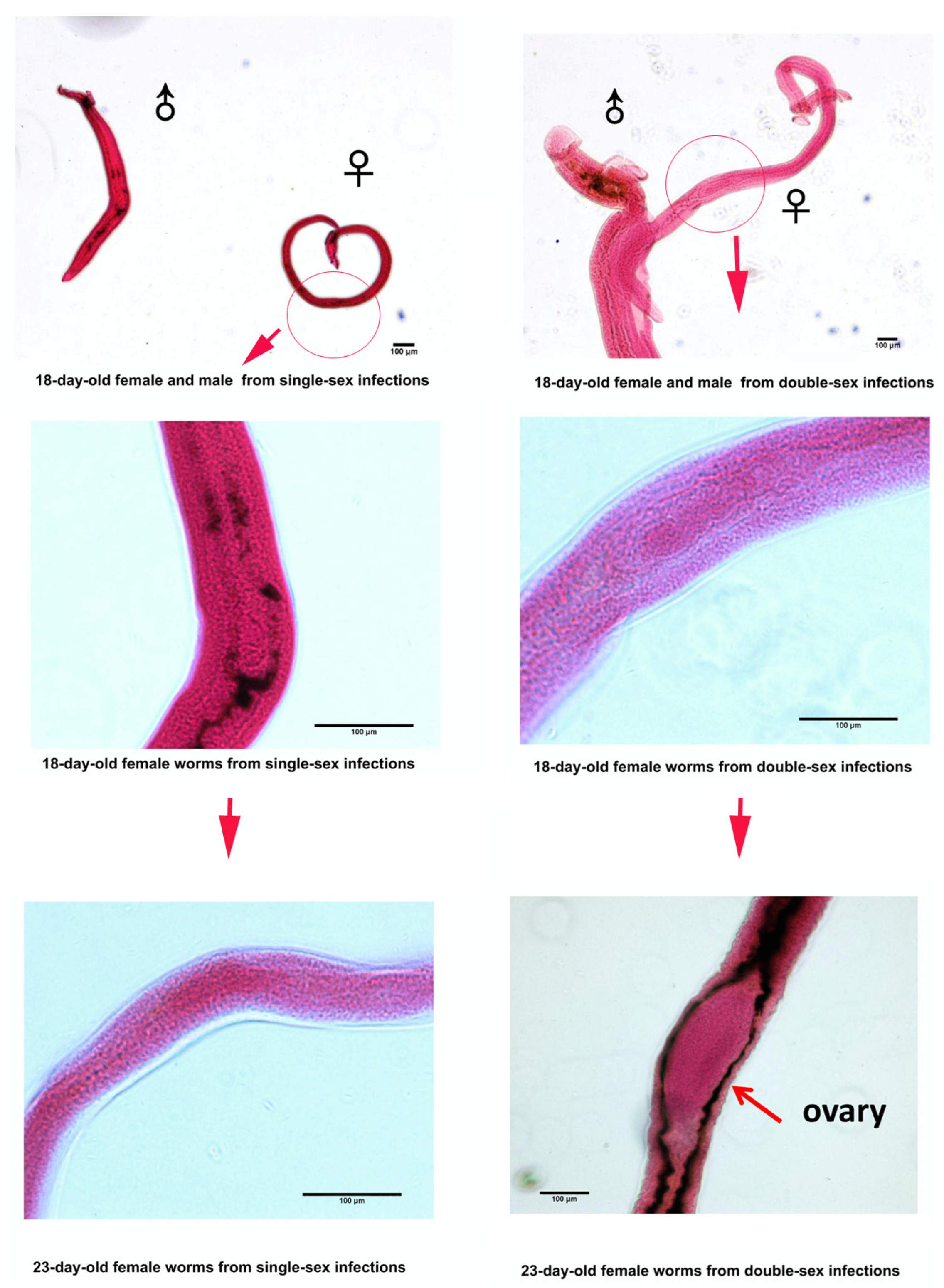

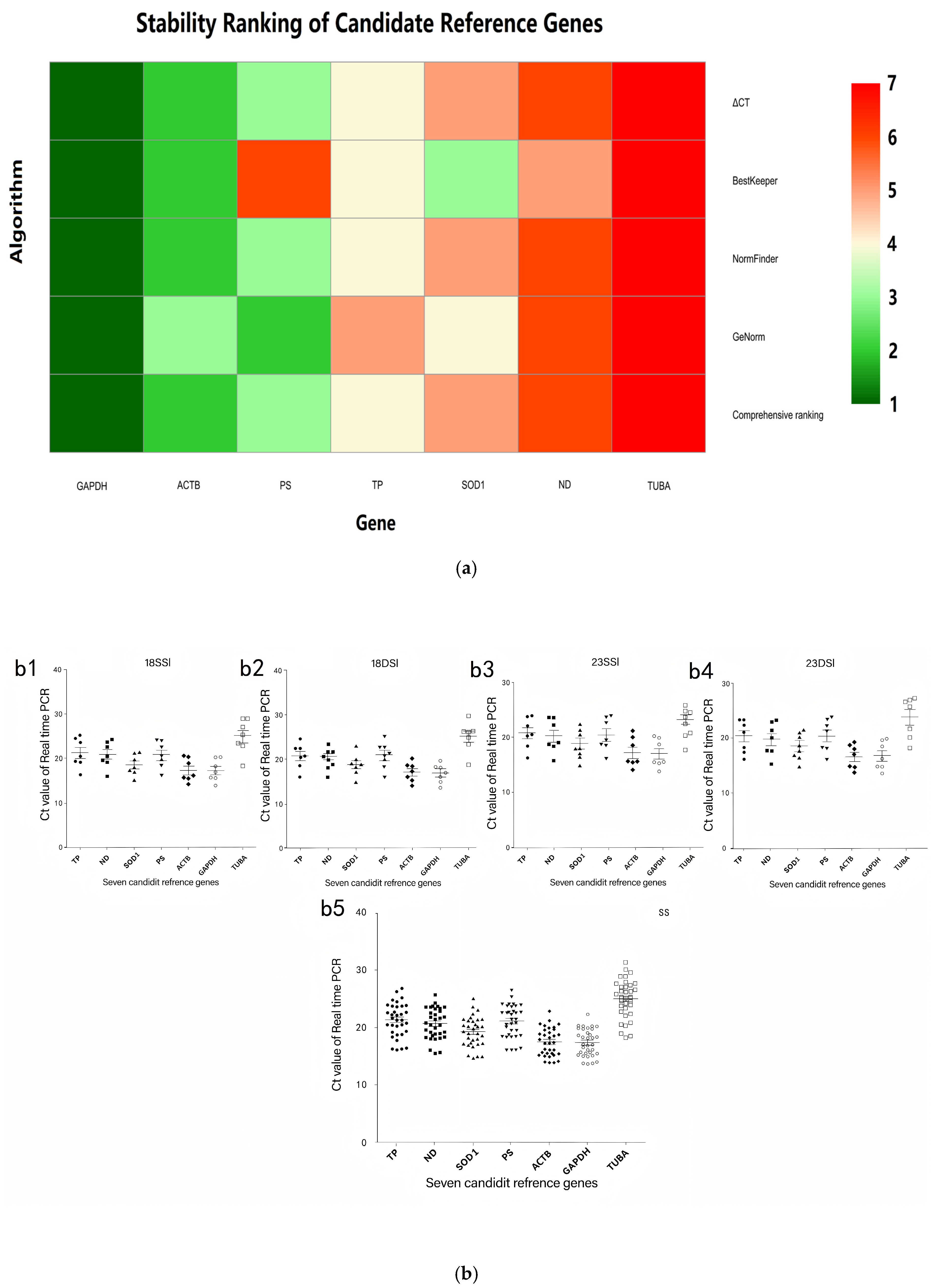
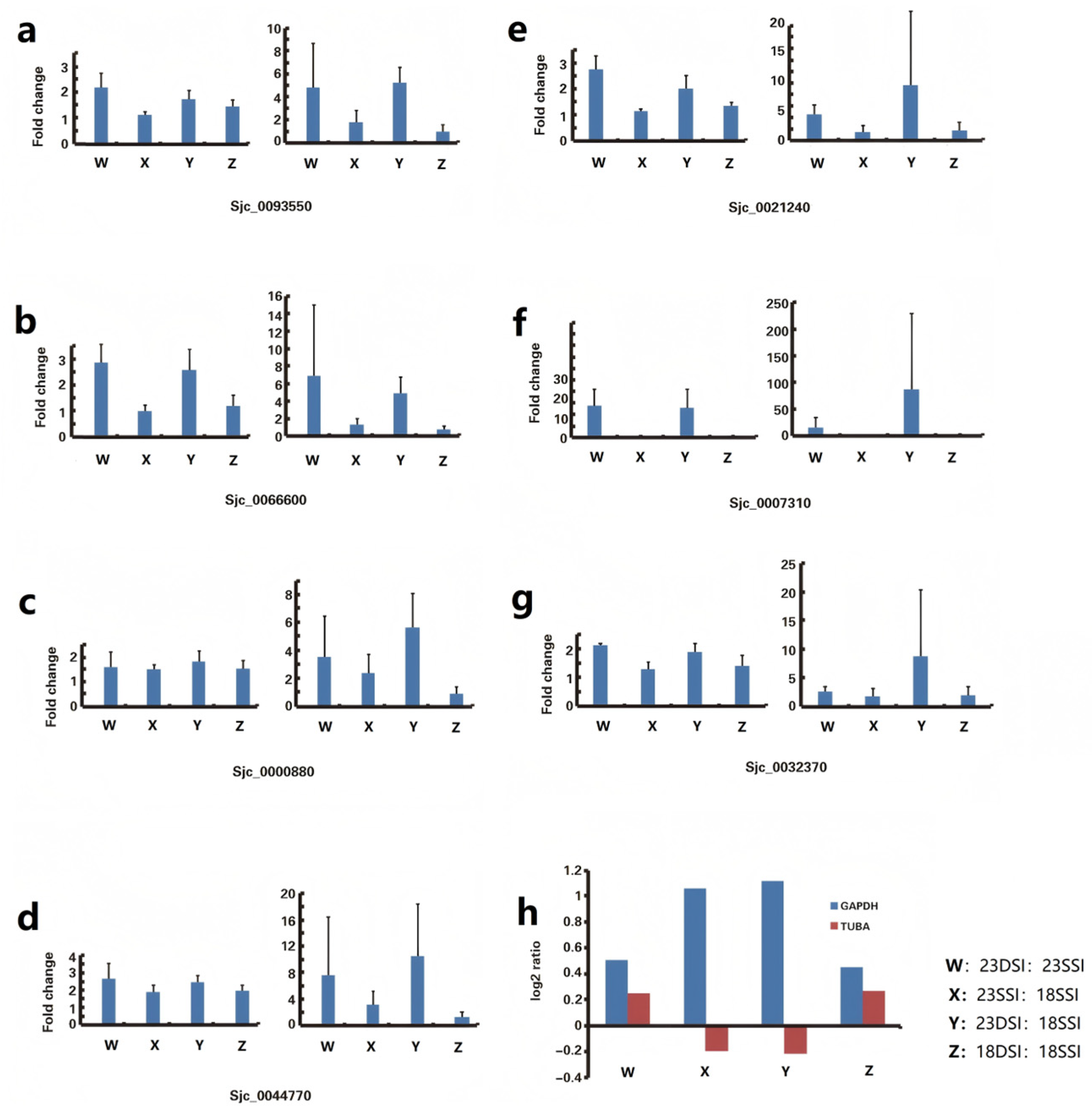

| Gene Symbol | Gene Production Name | Function | GenBank Accession Number | Primer Sequence (5′–3′) Forward/Reverse | Amplicon Length (bp) | Amplification Efficiency (%) | R2 |
|---|---|---|---|---|---|---|---|
| ACTB | Beta-actin | Cytoskeletal structural protein | FN315418 | TGGTAGCACAATGTATCCTGG/GCCTCAGGACAACGGAACC | 115 | 95 | 0.98 |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | Oxidoreductase in glycolysis and gluconeogenesis | FN324551 | ATGGAACAAGGATGGTGCTGAG/CAACAAACATGGGTGCGTCT | 143 | 95 | 0.96 |
| NDUFV2 | NADH dehydrogenase (ubiquinone) flavoprotein 2 | Electron transport in respiratory chain | FN320220 | CGAGGACCTAACAGCAGAGG/TCCGAACGAACTTTGAATCC | 174 | 95 | 0.99 |
| PSMD4 | 26S proteasome non-ATPase regulatory subunit 4 | Binds and presumably selects ubiquitin conjugates for destruction | FN320595 | CCTCACCAACAATTTCCACATCT/GATCACTTATAGCCTTGCGAACAT | 129 | 95 | 0.97 |
| TPC2L | Trafficking protein particle complex subunit 2-like protein | May play a role in vesicular transport from endoplasmic reticulum to Golgi apparatus | FN319821 | CTCAAGCAGCCCAGTTCAGT/AATCGGTGTGCCAGGTTTAT | 137 | 95 | 0.95 |
| TUBA | Tubulin alpha | The major constituent of microtubules | AY815746 | TGGAACATTCTGATTGTGCCT/CGATGCCGTAATAGAACTAACA | 140 | 95 | 0.97 |
| SOD1 | Superoxide dismutase 1 | Destroys free superoxide radicals | FN315362.1 | CTGATGACGGAAAGGGAG/CTATGACACCACAAGCTACA | 462 | 95 | 0.98 |
| Phase | Temperature (°C) | Duration | Function |
|---|---|---|---|
| 1 | 95 °C | 30 sec | Enzyme activation and Denaturation |
| 2 | 95 °C → 60 °C | 40 cycles | PCR Cycles |
| 3 | 60 °C → 95 °C | Melting Curve | Specificity verification |
| Step | Temperature (°C) | Time (s) | Number of Cycles |
|---|---|---|---|
| Pre-denaturation | 95 | 30 | 1 |
| Denaturation | 95 | 5 | 40 |
| Annealing | 60 | 34 | 40 |
| Extension | 60 | 34 | 40 |
| Hold | −20 | Hold | - |
| Method | Ranking Order | ||||||
|---|---|---|---|---|---|---|---|
| ← Most Stable Genes | Least Stable Genes → | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| ΔCT | GAPDH | ACTB | PS | TP | SOD1 | ND | TUBA |
| BestKeeper | GAPDH | ACTB | SOD1 | TP | ND | PS | TUBA |
| NormFinder | GAPDH | ACTB | PS | TP | SOD1 | ND | TUBA |
| GeNorm | GAPDH/PS | - | ACTB | SOD1 | TP | ND | TUBA |
| Comprehensive ranking | GAPDH | ACTB | PS | TP | SOD1 | ND | TUBA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Feng, L.; Sun, J. Evaluation of Suitable Reference Gene During the Development of Paired or Unpaired Female Schistosoma japonicum on the 18th and the 23rd Days Post Infection. Pathogens 2025, 14, 1066. https://doi.org/10.3390/pathogens14101066
Wang S, Feng L, Sun J. Evaluation of Suitable Reference Gene During the Development of Paired or Unpaired Female Schistosoma japonicum on the 18th and the 23rd Days Post Infection. Pathogens. 2025; 14(10):1066. https://doi.org/10.3390/pathogens14101066
Chicago/Turabian StyleWang, Suwen, Liang Feng, and Jun Sun. 2025. "Evaluation of Suitable Reference Gene During the Development of Paired or Unpaired Female Schistosoma japonicum on the 18th and the 23rd Days Post Infection" Pathogens 14, no. 10: 1066. https://doi.org/10.3390/pathogens14101066
APA StyleWang, S., Feng, L., & Sun, J. (2025). Evaluation of Suitable Reference Gene During the Development of Paired or Unpaired Female Schistosoma japonicum on the 18th and the 23rd Days Post Infection. Pathogens, 14(10), 1066. https://doi.org/10.3390/pathogens14101066






