Human Papillomavirus: An Old New History
Abstract
1. Introduction
2. Materials and Methods
3. Epidemiology
4. Clinical Manifestations and Co-Infection with STI
5. Extragenital HPV-Associated Cancers
6. Testing and Screening
7. Prevention
8. Educational Programs
9. Future Directions
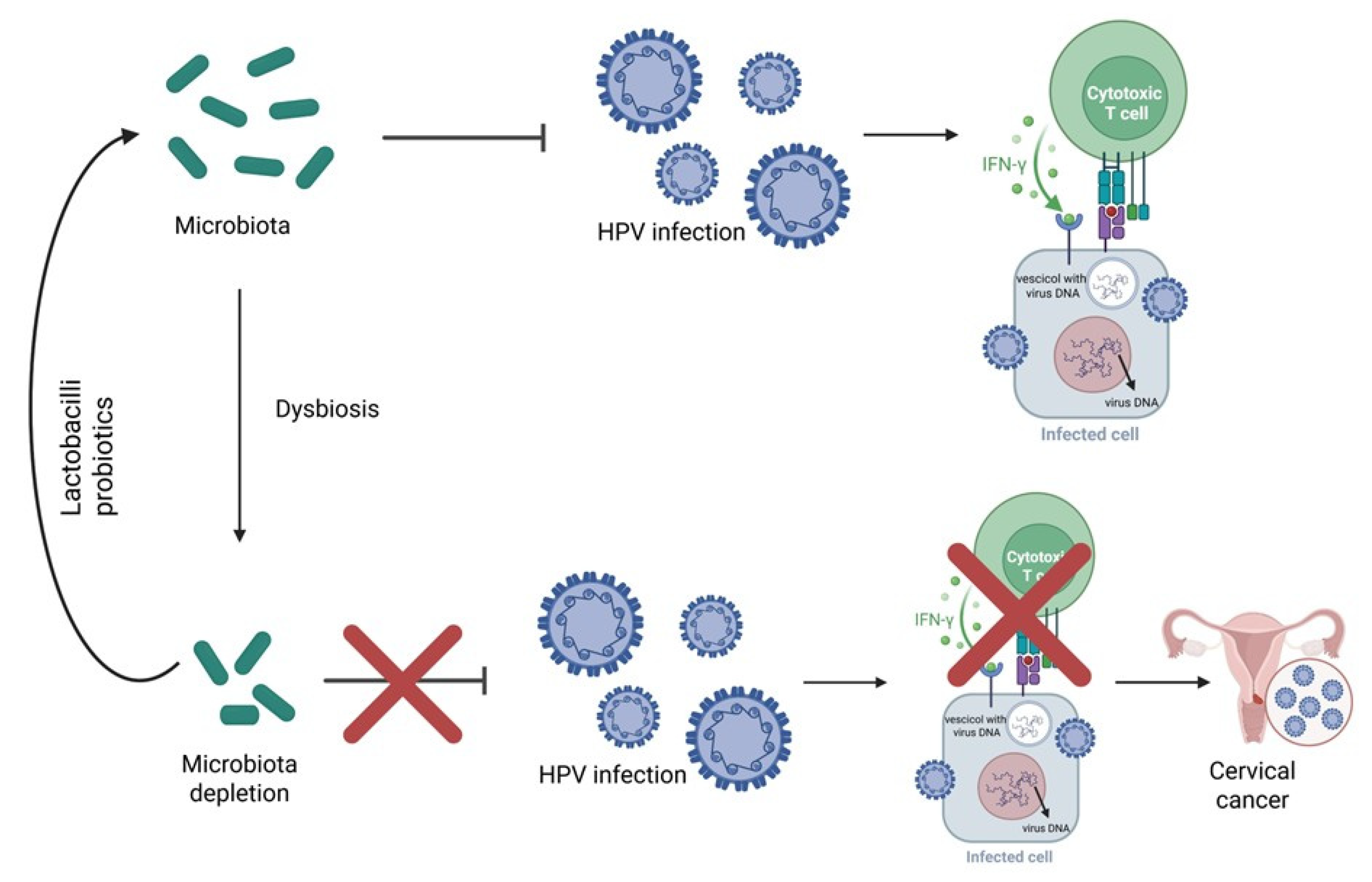
9.1. Microbiome
9.2. Therapeutic Vaccine
10. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- zur Hausen, H. Papillomaviruses and cancer: From basic studies to clinical application. Nat. Rev. Cancer 2002, 2, 342–350. [Google Scholar] [CrossRef]
- Xu, M.; Cao, C.; Wu, P.; Huang, X.; Ma, D. Advances in cervical cancer: Current insights and future directions. Cancer Commun. 2025, 45, 77–109. [Google Scholar] [CrossRef]
- Burd, E.M. Human papillomavirus and cervical cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef]
- Araujo, M.; Bouassaly, J.; Farshadi, F.; Hier, M.; Mascarella, M.; Mlynarek, A.; Alaoui-Jamali, M.; da Silva, S.D. Current status of circulating tumor DNA and circulating cell alterations in HPV-associated head and neck cancer. Oral. Oncol. 2025, 167, 107417. [Google Scholar] [CrossRef]
- Wolf, J.; Kist, L.F.; Pereira, S.B.; Quessada, M.A.; Petek, H.; Pille, A.; Maccari, J.G.; Mutlaq, M.P.; Nasi, L.A. Human papillomavirus infection: Epidemiology, biology, host interactions, cancer development, prevention, and therapeutics. Rev. Med. Virol. 2024, 34, e2537. [Google Scholar] [CrossRef]
- Jain, M.; Yadav, D.; Jarouliya, U.; Chavda, V.; Yadav, A.K.; Chaurasia, B.; Song, M. Epidemiology, molecular pathogenesis, immuno-pathogenesis, immune escape mechanisms and vaccine evaluation for HPV-associated carcinogenesis. Pathogens 2023, 12, 1380. [Google Scholar] [CrossRef]
- Bernard, H.U.; Burk, R.D.; Chen, Z.; van Doorslaer, K.; zur Hausen, H.; de Villiers, E.M. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 2010, 401, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Halec, G.; Alemany, L.; Lloveras, B.; Schmitt, M.; Alejo, M.; Bosch, F.X.; Tous, S.; Klaustermeier, J.E.; Guimerà, N.; Grabe, N.; et al. Pathogenic role of the eight probably/possibly carcinogenic HPV types 26, 53, 66, 67, 68, 70, 73 and 82 in cervical cancer. J. Pathol. 2014, 234, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Insinga, R.P.; Dasbach, E.J.; Elbasha, E.H.; Liaw, K.L.; Barr, E. Incidence and duration of cervical human papillomavirus 6, 11, 16, and 18 infections in young women: An evaluation from multiple analytic perspectives. Cancer Epidemiol. Biomark. Prev. 2007, 16, 709–715. [Google Scholar] [CrossRef]
- Gazzetta, S.; Valent, F.; Sala, A.; Driul, L.; Brunelli, L. Sexually transmitted infections and the HPV-related burden: Evolution of Italian epidemiology and policy. Front. Public Health 2024, 12, 1336250. [Google Scholar] [CrossRef] [PubMed]
- Bruni, L.; Albero, G.; Rowley, J.; Alemany, L.; Arbyn, M.; Giuliano, A.R.; Markowitz, L.E.; Broutet, N.; Taylor, M. Global and regional estimates of genital human papillomavirus prevalence among men: A systematic review and meta-analysis. Lancet Glob. Health 2023, 11, e1345–e1362. [Google Scholar] [CrossRef]
- Akbari, E.; Milani, A.; Seyedinkhorasani, M.; Bolhassani, A. HPV co-infections with other pathogens in cancer development: A comprehensive review. J. Med. Virol. 2023, 95, e29236. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Human Papillomavirus Infection: Recommended Vaccinations. Available online: https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=38&SelectedCountryIdByDisease=-1 (accessed on 28 August 2025).
- Italian Ministry of Health. National Vaccination Prevention Plan 2016–2018. 2015. Available online: https://extranet.who.int/countryplanningcycles/sites/default/files/planning_cycle_repository/italy/allegato1955037.pdf (accessed on 28 August 2025).
- Sundaram, N.; Voo, T.C.; Tam, C.C. Adolescent HPV vaccination: Empowerment, equity and ethics. Hum. Vaccin. Immunother. 2020, 16, 1835–1840. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Cancers Associated with Human Papillomavirus. Available online: https://www.cdc.gov/united-states-cancer-statistics/publications/hpv-associated-cancers.html (accessed on 28 August 2025).
- Bruni, L.; Albero, G.; Serrano, B.; Mena, M.; Collado, J.J.; Muñoz, J.; Gómez, D.; Bosch, F.X.; de Sanjosé, S. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human papillomavirus and Related Diseases in Europe. Summary Report. 10 March 2023. Available online: www.hpvcentre.net (accessed on 28 August 2025).
- International Agency for Research on Cancer Monographs on the Evaluation of Carcinogenic Risks to Humans. Biological agents. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100, 1–363. [Google Scholar]
- World Health Organization. Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem; Seventy-Third World Health Assembly. 2020. Available online: https://www.who.int/publications/i/item/9789240014107 (accessed on 28 August 2025).
- Wang, W.V.; Kothari, S.; Skufca, J.; Giuliano, A.R.; Sundström, K.; Nygård, M.; Koro, C.; Baay, M.; Verstraeten, T.; Luxembourg, A.; et al. Real-world impact and effectiveness of the quadrivalent HPV vaccine: An updated systematic literature review. Expert. Rev. Vaccines 2022, 21, 1799–1817. [Google Scholar] [CrossRef]
- Jach, R.; Basta, A.; Kotarski, J.; Markowska, J.; Paszkowski, T.; Dębski, R.; Rokita, W.; Kędzia, W.; Kiszka, K. Ten years of anti-HPV vaccinations: What do we know? Prz. Menopauzalny 2016, 15, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Ploner, A.; Astorga Alsina, A.M.; Deng, Y.; Ask Schollin, L.; Lei, J. Effectiveness of quadrivalent human papillomavirus vaccination against high-grade cervical lesions by age and doses: A population-based cohort study. Lancet Reg. Health Eur. 2025, 49, 101178. [Google Scholar] [CrossRef]
- Kreimer, A.R.; Watson-Jones, D.; Kim, J.J.; Dull, P. Single-dose human papillomavirus vaccination: An update. J. Natl. Cancer Inst. Monogr. 2024, 2024, 313–316. [Google Scholar] [CrossRef]
- Harper, D.M.; Navarro-Alonso, J.A.; Bosch, F.X.; Paavonen, J.; Stanley, M.; Sasieni, P.; Yébenes, M.; Martínez-Martínez, N.; Rodriguez, Á.; García, A.; et al. Impact of human papillomavirus vaccines in the reduction of infection, precursor lesions, and cervical cancer: A systematic literature review. Hum. Vaccin. Immunother. 2025, 21, 2497608. [Google Scholar] [CrossRef] [PubMed]
- Fappani, C.; Bianchi, S.; Panatto, D.; Petrelli, F.; Colzani, D.; Scuri, S.; Gori, M.; Amendola, A.; Grappasonni, I.; Tanzi, E.; et al. HPV type-specific prevalence a decade after the implementation of the vaccination program: Results from a pilot study. Vaccines 2021, 9, 336. [Google Scholar] [CrossRef]
- Nabi, S.; Mimba, B.R.; Akunne, O. Eliminating cervical cancer: The impact of screening and human papilloma virus vaccination. Prev. Chronic Dis. 2025, 22, E46. [Google Scholar] [CrossRef]
- Flores, E.R.; Allen-Hoffmann, B.L.; Lee, D.; Sattler, C.A.; Lambert, P.F. Establishment of the human papillomavirus type 16 (HPV-16) life cycle in an immortalized human foreskin keratinocyte cell line. Virology 1999, 262, 344–354. [Google Scholar] [CrossRef]
- Flores, E.R.; Allen-Hoffmann, B.L.; Lee, D.; Lambert, P.F. The human papillomavirus type 16 E7 oncogene is required for the productive stage of the viral life cycle. J. Virol. 2000, 74, 6622–6631. [Google Scholar] [CrossRef]
- Thomas, J.T.; Hubert, W.G.; Ruesch, M.N.; Laimins, L.A. Human papillomavirus type 31 oncoproteins e6 and e7 are required for the maintenance of episomes during the viral life cycle in normal human keratinocytes. Proc. Natl. Acad. Sci. USA 1999, 96, 8449–8454. [Google Scholar] [CrossRef]
- Yim, E.K.; Park, J.S. The role of HPV e6 and e7 oncoproteins in HPV-associated cervical carcinogenesis. Cancer Res. Treat. 2005, 37, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Dediol, I.; Buljan, M.; Vurnek-A Ivkoviä, M.; Bulat, V.; A Itum, M.; A Ubriloviä, A. Psychological burden of anogenital warts. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 1035–1038. [Google Scholar] [CrossRef]
- Fortes, H.R.; von Ranke, F.M.; Escuissato, D.L.; Araujo Neto, C.A.; Zanetti, G.; Hochhegger, B.; Souza, C.A.; Marchiori, E. Recurrent respiratory papillomatosis: A state-of-the-art review. Respir. Med. 2017, 126, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Bourcier, M.; Bhatia, N.; Lynde, C.; Vender, R. Managing external genital warts: Practical aspects of treatment and prevention. J. Cutan. Med. Surg. 2013, 17 (Suppl. S2), S68–S75. [Google Scholar]
- Workowski, K.A.; Bolan, G.A.; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR. Recomm. Rep. 2015, 64, 1–137. [Google Scholar] [PubMed]
- El Moussaoui, S.; Fernández-Campos, F.; Alonso, C.; Limón, D.; Halbaut, L.; Garduño-Ramirez, M.L.; Calpena, A.C.; Mallandrich, M. Topical mucoadhesive alginate-based hydrogel loading ketorolac for pain management after pharmacotherapy, ablation, or surgical removal in condyloma acuminata. Gels 2021, 7, 8. [Google Scholar] [CrossRef]
- Araldi, R.P.; Sant’Ana, T.A.; Módolo, D.G.; de Melo, T.C.; Spadacci-Morena, D.D.; de Cassia Stocco, R.; Cerutti, J.M.; de Souza, E.B. The human papillomavirus (HPV)-related cancer biology: An overview. Biomed. Pharmacother. 2018, 106, 1537–1556. [Google Scholar] [CrossRef]
- Oyervides-Muñoz, M.A.; Pérez-Maya, A.A.; Rodríguez-Gutiérrez, H.F.; Gómez-Macias, G.S.; Fajardo-Ramírez, O.R.; Treviño, V.; Barrera-Saldaña, H.A.; Garza-Rodríguez, M.L. Understanding the HPV integration and its progression to cervical cancer. Infect. Genet. Evol. 2018, 61, 134–144. [Google Scholar] [CrossRef]
- Bañuelos-Villegas, E.G.; Pérez-yPérez, M.F.; Alvarez-Salas, L.M. Cervical cancer, papillomavirus, and miRNA dysfunction. Front. Mol. Biosci. 2021, 8, 758337. [Google Scholar] [CrossRef] [PubMed]
- Koliopoulos, G.; Nyaga, V.N.; Santesso, N.; Bryant, A.; Martin-Hirsch, P.P.; Mustafa, R.A.; Schünemann, H.; Paraskevaidis, E.; Arbyn, M. Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst. Rev. 2017, 8, CD008587. [Google Scholar] [CrossRef] [PubMed]
- Merz, J.; Bossart, M.; Bamberg, F.; Eisenblaetter, M. Revised figo staging for cervical cancer—A new role for mri. Rofo 2020, 192, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, S.; Odicino, F. Cervical cancer staging. Cancer J. 2003, 9, 390–394. [Google Scholar] [CrossRef]
- Liu, G.; Mugo, N.R.; Brown, E.R.; Mgodi, N.M.; Chirenje, Z.M.; Marrazzo, J.M.; Winer, R.L.; Mansoor, L.; Palanee-Phillips, T.; Siva, S.S.; et al. Prevalent human papillomavirus infection increases the risk of HIV acquisition in African women: Advancing the argument for human papillomavirus immunization. AIDS 2022, 36, 257–265. [Google Scholar] [CrossRef]
- Pérez-González, A.; Cachay, E.; Ocampo, A.; Poveda, E. Update on the epidemiological features and clinical implications of Human Papillomavirus Infection (HPV) and Human Immunodeficiency Virus (HIV) coinfection. Microorganisms 2022, 10, 1047. [Google Scholar] [CrossRef]
- Kumari, S.; Bhor, V.M. A literature review on correlation between HPV coinfection with C. Trachomatis and cervical neoplasia-coinfection mediated cellular transformation. Microb. Pathog. 2022, 168, 105587. [Google Scholar] [CrossRef]
- Zou, Q.; Wu, Y.; Zhang, S.; Li, S.; Su, Y.; Zhang, L.; Li, Q.; Zou, H.; Zhang, X.; Wang, T.; et al. And HPV16 coinfection may contribute to the development of cervical cancer. Virulence 2024, 15, 2319962. [Google Scholar] [CrossRef]
- Sausen, D.G.; Shechter, O.; Gallo, E.S.; Dahari, H.; Borenstein, R. Herpes simplex virus, human papillomavirus, and cervical cancer: Overview, relationship, and treatment implications. Cancers 2023, 15, 3692. [Google Scholar] [CrossRef]
- Liu, C.; Guo, Y.; Wang, L.; Guo, R.; Lei, D. Association between herpes simplex virus type 2 and high-risk human papillomavirus infections: A population study of the national health and nutrition examination survey, 2009–2016. J. Infect. Dis. 2025, 231, e650–e658. [Google Scholar] [CrossRef]
- Ray, A. Human papillomavirus and other relevant issues in cervical cancer pathogenesis. Int. J. Mol. Sci. 2025, 26, 5549. [Google Scholar] [CrossRef]
- Skinner, G.R. Transformation of primary hamster embryo fibroblasts by type 2 simplex virus: Evidence for a “Hit and run” Mechanism. Br. J. Exp. Pathol. 1976, 57, 361–376. [Google Scholar]
- Hamar, B.; Teutsch, B.; Hoffmann, E.; Hegyi, P.; Váradi, A.; Nyirády, P.; Hunka, Z.; Ács, N.; Lintner, B.; Hermánné, R.J.; et al. Trichomonas vaginalis infection is associated with increased risk of cervical carcinogenesis: A systematic review and meta-analysis of 470,000 patients. Int. J. Gynaecol. Obstet. 2023, 163, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Osafo, K.S.; Lin, W.; Dong, B.; Sun, P. Trichomonas vaginalis and human papillomavirus: Association with the microbiota and burden on the cervix. Gynecol. Obstet. Clin. Med. 2023, 3, 207–212. [Google Scholar] [CrossRef]
- Augustin, J.G.; Lepine, C.; Morini, A.; Brunet, A.; Veyer, D.; Brochard, C.; Mirghani, H.; Péré, H.; Badoual, C. HPV detection in head and neck squamous cell carcinomas: What is the issue? Front. Oncol. 2020, 10, 1751. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.; Liu, J.; Masterson, L.; Fenton, T.R. HPV-associated oropharyngeal cancer: Epidemiology, molecular biology and clinical management. Nat. Rev. Clin. Oncol. 2022, 19, 306–327. [Google Scholar] [CrossRef]
- Baba, S.K.; Alblooshi, S.S.E.; Yaqoob, R.; Behl, S.; Al Saleem, M.; Rakha, E.A.; Malik, F.; Singh, M.; Macha, M.A.; Akhtar, M.K.; et al. Human papilloma virus (HPV) mediated cancers: An insightful update. J. Transl. Med. 2025, 23, 483. [Google Scholar] [CrossRef]
- Malagón, T.; Franco, E.L.; Tejada, R.; Vaccarella, S. Epidemiology of HPV-associated cancers past, present and future: Towards prevention and elimination. Nat. Rev. Clin. Oncol. 2024, 21, 522–538. [Google Scholar] [CrossRef]
- Silva, L.L.D.; Teles, A.M.; Santos, J.M.O.; Souza de Andrade, M.; Medeiros, R.; Faustino-Rocha, A.I.; Oliveira, P.A.; Dos Santos, A.P.A.; Ferreira Lopes, F.; Braz, G.; et al. Malignancy associated with low-risk hpv6 and hpv11: A systematic review and implications for cancer prevention. Cancers 2023, 15, 4068. [Google Scholar] [CrossRef]
- Hathaway, J.K. HPV: Diagnosis, prevention, and treatment. Clin. Obstet. Gynecol. 2012, 55, 671–680. [Google Scholar] [CrossRef]
- Bartosik, M.; Moranova, L.; Izadi, N.; Strmiskova, J.; Sebuyoya, R.; Holcakova, J.; Hrstka, R. Advanced technologies towards improved HPV diagnostics. J. Med. Virol. 2024, 96, e29409. [Google Scholar] [CrossRef]
- Fontham, E.T.H.; Wolf, A.M.D.; Church, T.R.; Etzioni, R.; Flowers, C.R.; Herzig, A.; Guerra, C.E.; Oeffinger, K.C.; Shih, Y.T.; Walter, L.C.; et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the american cancer society. CA Cancer J. Clin. 2020, 70, 321–346. [Google Scholar] [CrossRef] [PubMed]
- Bruni, L.; Serrano, B.; Roura, E.; Alemany, L.; Cowan, M.; Herrero, R.; Poljak, M.; Murillo, R.; Broutet, N.; Riley, L.M.; et al. Cervical cancer screening programmes and age-specific coverage estimates for 202 countries and territories worldwide: A review and synthetic analysis. Lancet Glob. Health 2022, 10, e1115–e1127. [Google Scholar] [CrossRef]
- Fashedemi, O.; Ozoemena, O.C.; Peteni, S.; Haruna, A.B.; Shai, L.J.; Chen, A.; Rawson, F.; Cruickshank, M.E.; Grant, D.; Ola, O.; et al. Advances in human papillomavirus detection for cervical cancer screening and diagnosis: Challenges of conventional methods and opportunities for emergent tools. Anal. Methods 2025, 17, 1428–1450. [Google Scholar] [CrossRef] [PubMed]
- Çubuk, F.; Yıldız, M.; Balcı, F.; İrgin, A.; Aşkın, S.; Sağtaş, E.; Kaygusuz, S. A new approach for detecting HPV DNA in cervical swabs: Comparison of nucleic acid extraction with direct PCR. Virol. J. 2025, 22, 8. [Google Scholar] [CrossRef] [PubMed]
- Serrano, B.; Ibáñez, R.; Robles, C.; Peremiquel-Trillas, P.; de Sanjosé, S.; Bruni, L. Worldwide use of HPV self-sampling for cervical cancer screening. Prev. Med. 2022, 154, 106900. [Google Scholar] [CrossRef]
- He, Z.; Zheng, X.; Liu, R.; Zhao, K.; Mao, D.; Zhang, L.; Wan, R.; Zhang, H.; Wang, X. Recent advances in HPV detection: From traditional methods to nanotechnology and the application of quantum dots. Int. J. Nanomed. 2025, 20, 6333–6356. [Google Scholar] [CrossRef]
- Daponte, N.; Valasoulis, G.; Michail, G.; Magaliou, I.; Daponte, A.I.; Garas, A.; Grivea, I.; Bogdanos, D.P.; Daponte, A. HPV-based self-sampling in cervical cancer screening: An updated review of the current evidence in the literature. Cancers 2023, 15, 1669. [Google Scholar] [CrossRef]
- Włoszek, E.; Krupa, K.; Skrok, E.; Budzik, M.P.; Deptała, A.; Badowska-Kozakiewicz, A. HPV and cervical cancer-biology, prevention, and treatment updates. Curr. Oncol. 2025, 32, 122. [Google Scholar] [CrossRef]
- Salta, S.; Lobo, J.; Magalhães, B.; Henrique, R.; Jerónimo, C. DNA methylation as a triage marker for colposcopy referral in HPV-based cervical cancer screening: A systematic review and meta-analysis. Clin. Epigenetics 2023, 15, 125. [Google Scholar] [CrossRef]
- Wang, M.; Ma, Y.; Zhang, N.; Li, L.; Fu, Y.; Zhai, Q. Methylation testing versus cervical cytology for triage of HPV-positive women: A comparative study. Diagn. Microbiol. Infect. Dis. 2025, 113, 116917. [Google Scholar] [CrossRef]
- Burdier, F.R.; Waheed, D.E.; Nedjai, B.; Steenbergen, R.D.M.; Poljak, M.; Baay, M.; Vorsters, A.; Van Keer, S. DNA methylation as a triage tool for cervical cancer screening—A meeting report. Prev. Med. Rep. 2024, 41, 102678. [Google Scholar] [CrossRef]
- Chennareddy, S.; Chen, S.; Levinson, C.; Genden, E.M.; Posner, M.R.; Roof, S.A. Circulating tumor DNA in human papillomavirus-associated oropharyngeal cancer management: A systematic review. Oral. Oncol. 2025, 164, 107262. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.Y.; Chen, L.; Wong, W.; Morris, L.G.T.; Yacoub, I.; Lee, N.Y.; Ma, J. The role of circulating tumor DNA (ctDNA) in the treatment of human papilloma virus-positive (hpv+) oropharyngeal squamous cell carcinoma. Cancer J. 2025, 31, e0769. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, M.; Tanaka, H.; Takenaka, Y.; Suzuki, M.; Fukusumi, T.; Eguchi, H.; Watabe, T.; Kato, H.; Yachida, S.; Inohara, H.; et al. Association of circulating tumor HPV16DNA levels and quantitative pet parameters in patients with HPV-positive head and neck squamous cell carcinoma. Sci. Rep. 2024, 14, 3278. [Google Scholar] [CrossRef]
- Kore, V.B.; Anjankar, A. A comprehensive review of treatment approaches for cutaneous and genital warts. Cureus 2023, 15, e47685. [Google Scholar] [CrossRef]
- Bryan, J.T. Developing an HPV vaccine to prevent cervical cancer and genital warts. Vaccine 2007, 25, 3001–3006. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Ebihara, K.; Tanaka, Y.; Noda, K. Efficacy of quadrivalent human papillomavirus (types 6, 11, 16 and 18) vaccine (gardasil) in japanese women aged 18-26 years. Cancer Sci. 2013, 104, 465–472. [Google Scholar] [CrossRef]
- Paavonen, J.; Jenkins, D.; Bosch, F.X.; Naud, P.; Salmerón, J.; Wheeler, C.M.; Chow, S.N.; Apter, D.L.; Kitchener, H.C.; Castellsague, X.; et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: An interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007, 369, 2161–2170. [Google Scholar] [CrossRef] [PubMed]
- Paavonen, J.; Naud, P.; Salmerón, J.; Wheeler, C.M.; Chow, S.N.; Apter, D.; Kitchener, H.; Castellsague, X.; Teixeira, J.C.; Skinner, S.R.; et al. Efficacy of human papillomavirus (HPV)-16/18 as04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (patricia): Final analysis of a double-blind, randomised study in young women. Lancet 2009, 374, 301–314. [Google Scholar] [CrossRef]
- Lehtinen, M.; Paavonen, J.; Wheeler, C.M.; Jaisamrarn, U.; Garland, S.M.; Castellsagué, X.; Skinner, S.R.; Apter, D.; Naud, P.; Salmerón, J.; et al. Overall efficacy of HPV-16/18 as04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind patricia trial. Lancet Oncol. 2012, 13, 89–99. [Google Scholar] [CrossRef]
- Hildesheim, A.; Wacholder, S.; Catteau, G.; Struyf, F.; Dubin, G.; Herrero, R.; Group, C. Efficacy of the HPV-16/18 vaccine: Final according to protocol results from the blinded phase of the randomized costa rica HPV-16/18 vaccine trial. Vaccine 2014, 32, 5087–5097. [Google Scholar] [CrossRef]
- Wang, R.; Pan, W.; Jin, L.; Huang, W.; Li, Y.; Wu, D.; Gao, C.; Ma, D.; Liao, S. Human papillomavirus vaccine against cervical cancer: Opportunity and challenge. Cancer Lett. 2020, 471, 88–102. [Google Scholar] [CrossRef]
- Pils, S.; Joura, E.A. From the monovalent to the nine-valent HPV vaccine. Clin. Microbiol. Infect. 2015, 21, 827–833. [Google Scholar] [CrossRef]
- Hu, Y.M.; Huang, S.J.; Chu, K.; Wu, T.; Wang, Z.Z.; Yang, C.L.; Cai, J.P.; Jiang, H.M.; Wang, Y.J.; Guo, M.; et al. Safety of an escherichia coli-expressed bivalent human papillomavirus (types 16 and 18) L1 virus-like particle vaccine: An open-label phase I clinical trial. Hum. Vaccin. Immunother. 2014, 10, 469–475. [Google Scholar] [CrossRef]
- World Health Organization. HPV Vaccine Global Market Study April 2022. Available online: https://www.who.int/publications/m/item/who-hpv-vaccine-global-market-study-april-2022 (accessed on 24 April 2022).
- World Health Organization. Human Papillomavirus Vaccines: WHO Position Paper, 2022 Update. Available online: https://www.who.int/publications/i/item/who-wer9750-645-672 (accessed on 27 August 2025).
- Simone, B.; Guidance, E. Introduction of HPV Vaccines in European Union Countries—An Update. 2012. Available online: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/20120905_GUI_HPV_vaccine_update.pdf (accessed on 28 August 2025).
- Phillips, M.; Morais, E.; Kothari, S.; Tantri, A.; Parellada, C.I.; Cashat, M.; Walia, A.; Shrestha, A.; Perez Amaya, G.E. Evolution of gender-neutral HPV vaccination in national immunization programs around the world. In Proceedings of the 32nd International Papillomavirus Conference, Sydney, Australia, 2–6 October 2018; p. 545. [Google Scholar]
- Wheeler, C.M.; Kjaer, S.K.; Sigurdsson, K.; Iversen, O.E.; Hernandez-Avila, M.; Perez, G.; Brown, D.R.; Koutsky, L.A.; Tay, E.H.; García, P.; et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in sexually active women aged 16–26 years. J. Infect. Dis. 2009, 199, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Aldakak, L.; Huber, V.M.; Rühli, F.; Bender, N. Sex difference in the immunogenicity of the quadrivalent human papilloma virus vaccine: Systematic review and meta-analysis. Vaccine 2021, 39, 1680–1686. [Google Scholar] [CrossRef] [PubMed]
- Bruni, L.; Saura-Lázaro, A.; Montoliu, A.; Brotons, M.; Alemany, L.; Diallo, M.S.; Afsar, O.Z.; LaMontagne, D.S.; Mosina, L.; Contreras, M.; et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev. Med. 2021, 144, 106399. [Google Scholar] [CrossRef]
- Brunelli, L.; Valent, F.; Comar, M.; Suligoi, B.; Salfa, M.C.; Gianfrilli, D.; Sesti, F.; Restivo, V.; Casuccio, A.; Group, E.S.C. Study protocol for a pre/post study on knowledge, attitudes and behaviors regarding stis and in particular HPV among Italian adolescents, teachers, and parents in secondary schools. Front. Public Health 2024, 12, 1414631. [Google Scholar] [CrossRef]
- Kesic, V.; Carcopino, X.; Preti, M.; Vieira-Baptista, P.; Bevilacqua, F.; Bornstein, J.; Chargari, C.; Cruickshank, M.; Erzeneoglu, E.; Gallio, N.; et al. The european society of gynaecological oncology (esgo), the international society for the study of vulvovaginal disease (issvd), the european college for the study of vulval disease (ecsvd), and the european federation for colposcopy (efc) consensus statement on the management of vaginal intraepithelial neoplasia. Int. J. Gynecol. Cancer 2023, 33, 446–461. [Google Scholar] [CrossRef]
- Davies, C.; Stoney, T.; Hutton, H.; Parrella, A.; Kang, M.; Macartney, K.; Leask, J.; McCaffery, K.; Zimet, G.; Brotherton, J.M.L.; et al. School-based HPV vaccination positively impacts parents’ attitudes toward adolescent vaccination. Vaccine 2021, 39, 4190–4198. [Google Scholar] [CrossRef]
- Thompson, A.D.; Spratling, R. Adolescent self-consent for the HPV vaccine and the effects on vaccine rates. J. Pediatr. Health Care 2024, 38, 932–935. [Google Scholar] [CrossRef]
- Brunelli, L.; Bravo, G.; Romanese, F.; Righini, M.; Lesa, L.; De Odorico, A.; Bastiani, E.; Pascut, S.; Miceli, S.; Brusaferro, S. Beliefs about HPV vaccination and awareness of vaccination status: Gender differences among Northern Italy adolescents. Prev. Med. Rep. 2021, 24, 101570. [Google Scholar] [CrossRef]
- Lehtinen, M.; Bruni, L.; Elfström, M.; Gray, P.; Logel, M.; Mariz, F.C.; Baussano, I.; Vänskä, S.; Franco, E.L.; Dillner, J. Scientific approaches toward improving cervical cancer elimination strategies. Int. J. Cancer 2024, 154, 1537–1548. [Google Scholar] [CrossRef] [PubMed]
- Seraceni, S.; Campisciano, G.; Contini, C.; Comar, M. HPV genotypes distribution in chlamydia trachomatis co-infection in a large cohort of women from north-east Italy. J. Med. Microbiol. 2016, 65, 406–413. [Google Scholar] [CrossRef]
- Zhou, Z.W.; Long, H.Z.; Cheng, Y.; Luo, H.Y.; Wen, D.D.; Gao, L.C. From microbiome to inflammation: The key drivers of cervical cancer. Front. Microbiol. 2021, 12, 767931. [Google Scholar] [CrossRef]
- Huang, R.; Liu, Z.; Sun, T.; Zhu, L. Cervicovaginal microbiome, high-risk HPV infection and cervical cancer: Mechanisms and therapeutic potential. Microbiol. Res. 2024, 287, 127857. [Google Scholar] [CrossRef] [PubMed]
- Ntuli, L.; Mtshali, A.; Mzobe, G.; Liebenberg, L.J.; Ngcapu, S. Role of immunity and vaginal microbiome in clearance and persistence of human papillomavirus infection. Front. Cell Infect. Microbiol. 2022, 12, 927131. [Google Scholar] [CrossRef] [PubMed]
- Campisciano, G.; Iebba, V.; Zito, G.; Luppi, S.; Martinelli, M.; Fischer, L.; De Seta, F.; Basile, G.; Ricci, G.; Comar, M. Lactobacillus iners and gasseri, Prevotella bivia and HPV Belong to the Microbiological Signature Negatively Affecting Human Reproduction. Microorganisms. Microorganisms 2020, 9, 39. [Google Scholar] [CrossRef]
- Raffone, A.; Travaglino, A.; Angelino, A.; Esposito, R.; Pontillo, M.; Mollo, A.; Santoro, A.; Zannoni, G.F.; Insabato, L.; Sansone, M.; et al. Gardnerella vaginalis and trichomonas vaginalis infections and the risk of persistence or progression of low-grade cervical intraepithelial neoplasia. Pathol. Res. Pr. Pract. 2020, 216, 153234. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, Q.; Zhang, L.; Ma, L.; Wang, D.; Yang, Y.; Jia, P.; Wu, Y.; Wang, F. Human papillomavirus and cervical cancer in the microbial world: Exploring the vaginal microecology. Front. Cell Infect. Microbiol. 2024, 14, 1325500. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of action of probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef]
- Barzegari, A.; Kheyrolahzadeh, K.; Hosseiniyan Khatibi, S.M.; Sharifi, S.; Memar, M.Y.; Zununi Vahed, S. The battle of probiotics and their derivatives against biofilms. Infect. Drug Resist. 2020, 13, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Wang, D.; Liu, H.; Shen, M.; Yu, H. Two strains of probiotic Lactobacillus enhance immune response and promote naive T cell polarization to Th1. Food Agric. Immunol. 2019, 30, 281–295. [Google Scholar] [CrossRef]
- Taghinezhad-S, S.; Keyvani, H.; Bermúdez-Humarán, L.G.; Donders, G.G.G.; Fu, X.; Mohseni, A.H. Twenty years of research on HPV vaccines based on genetically modified lactic acid bacteria: An overview on the gut-vagina axis. Cell Mol. Life Sci. 2021, 78, 1191–1206. [Google Scholar] [CrossRef]
- Mohseni, A.H.; Taghinezhad-S, S.; Keyvani, H.; Razavilar, V. Extracellular overproduction of e7 oncoprotein of iranian human papillomavirus type 16 by genetically engineered lactococcus lactis. BMC Biotechnol. 2019, 19, 8. [Google Scholar] [CrossRef]
- Taghinezhad-S, S.; Mohseni, A.H.; Keyvani, H.; Razavi, M.R. Phase 1 safety and immunogenicity trial of recombinant. Mol. Ther. Methods Clin. Dev. 2019, 15, 40–51. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, M.; Zhu, H.; Du, X.; Wang, J. Mucosal vaccine delivery: A focus on the breakthrough of specific barriers. Acta Pharm. Sin. B 2022, 12, 3456–3474. [Google Scholar] [CrossRef]
- Park, Y.C.; Ouh, Y.T.; Sung, M.H.; Park, H.G.; Kim, T.J.; Cho, C.H.; Park, J.S.; Lee, J.K. A phase 1/2a, dose-escalation, safety and preliminary efficacy study of oral therapeutic vaccine in subjects with cervical intraepithelial neoplasia 3. J. Gynecol. Oncol. 2019, 30, e88. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, L.; Valent, F.; Comar, M.; Suligoi, B.; Salfa, M.C.; Gianfrilli, D.; Sesti, F.; Capra, G.; Casuccio, A.; De Luca, E.; et al. Knowledge about HPV and the HPV vaccine: Observational study on a convenience sample of adolescents from select schools in three regions in Italy. Vaccines 2025, 13, 227. [Google Scholar] [CrossRef] [PubMed]
| Commercial Name | Type of Vaccines (Target Genotypes) | Intended Recipients | Age of Administration | Vaccine Boosters | Vaccine Boosters Beyond Age of Administration |
|---|---|---|---|---|---|
| Cervarix | Bivalent vaccines (HPV-16, HPV-18) | Female and male | 9–14 | 2-dose (5–13 months apart) | 3-dose (1–2.5 and 5–12 months) |
| Cecolin | Bivalent vaccines (HPV-16, HPV-18) | Female | 9–14 | 2-dose (6 months apart) | 3-dose (1–2 and 5–8 months) |
| Walrinvax | Bivalent vaccines (HPV-16, HPV-18) | Female | 9–14 | 2-dose (6 months apart) | 3-dose (2–3 and 6–7 months) |
| Gardasil | Quadrivalent vaccines (HPV-6, HPV-11, HPV-16, HPV-18) | Female and male | 9–13 | 2-dose (6 months apart) | 3-dose (1–2 and 4–6 months) |
| Cervavax | Quadrivalent vaccines (HPV-6, HPV-11, HPV-16, HPV-18) | Female and male | 9–14 | 2-dose (6 months apart) | 3-dose (2 and 6 months) |
| Gardasil9 | Nonavalent vaccines (HPV-6, HPV-11, HPV-16, HPV-18, HPV-31, HPV-33, HPV-45, HPV-52, HPV-58) | Female and male | 9–14 | 2-dose (5–13 months apart) | 3-dose (1–2 and 4–6 months) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
West, N.; Boz, V.; Zanotta, N.; Cason, C.; Campisciano, G.; Casuccio, A.; Gianfrilli, D.; Fasciana, T.M.A.; Capra, G.; Salfa, M.C.; et al. Human Papillomavirus: An Old New History. Pathogens 2025, 14, 1043. https://doi.org/10.3390/pathogens14101043
West N, Boz V, Zanotta N, Cason C, Campisciano G, Casuccio A, Gianfrilli D, Fasciana TMA, Capra G, Salfa MC, et al. Human Papillomavirus: An Old New History. Pathogens. 2025; 14(10):1043. https://doi.org/10.3390/pathogens14101043
Chicago/Turabian StyleWest, Nicole, Valentina Boz, Nunzia Zanotta, Carolina Cason, Giuseppina Campisciano, Alessandra Casuccio, Daniele Gianfrilli, Teresa Maria Assunta Fasciana, Giuseppina Capra, Maria Cristina Salfa, and et al. 2025. "Human Papillomavirus: An Old New History" Pathogens 14, no. 10: 1043. https://doi.org/10.3390/pathogens14101043
APA StyleWest, N., Boz, V., Zanotta, N., Cason, C., Campisciano, G., Casuccio, A., Gianfrilli, D., Fasciana, T. M. A., Capra, G., Salfa, M. C., Sesti, F., Suligoi, B., Valent, F., Brunelli, L., & Comar, M. (2025). Human Papillomavirus: An Old New History. Pathogens, 14(10), 1043. https://doi.org/10.3390/pathogens14101043








