Genomic Characterization of a Carbapenem-Resistant Acinetobacter pittii Strain Harboring Chromosome-Borne blaNDM-1 from China
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Characterization of the Strain AP8900
2.2. Whole-Genome Sequencing, Assembly, and Annotation
2.3. Bioinformatics Analysis Towards the Genome of Strain AP8900
2.4. Identification of the Bacteria Harboring Chromosome-Borne blaNDM Available in GenBank Database
3. Results
3.1. Antibiotic Resistance Profiles of the Carbapenem-Resistant and Multidrug-Resistant A. pittii Strain AP8900
3.2. Genomic Characteristics of the Carbapenem-Resistant A. pittii Strain AP8900
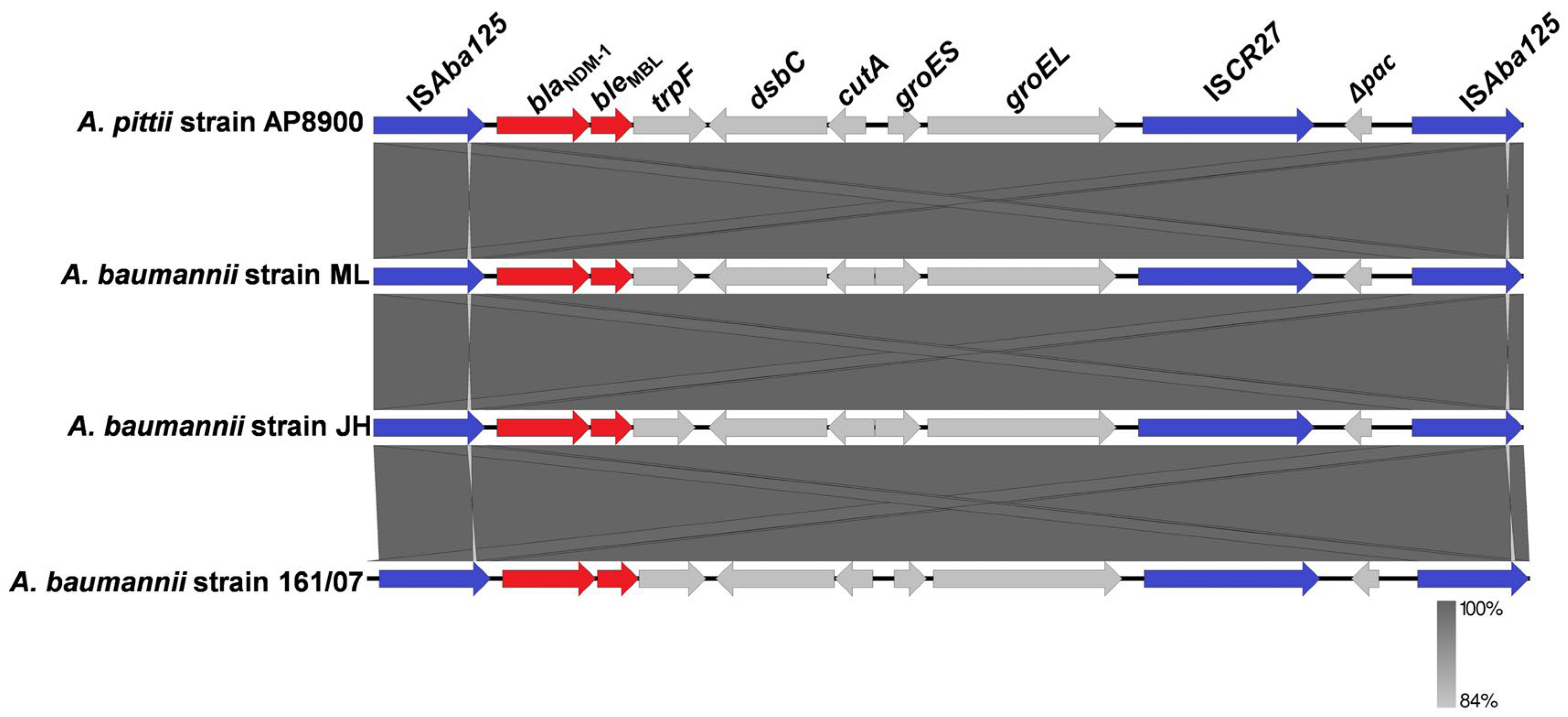
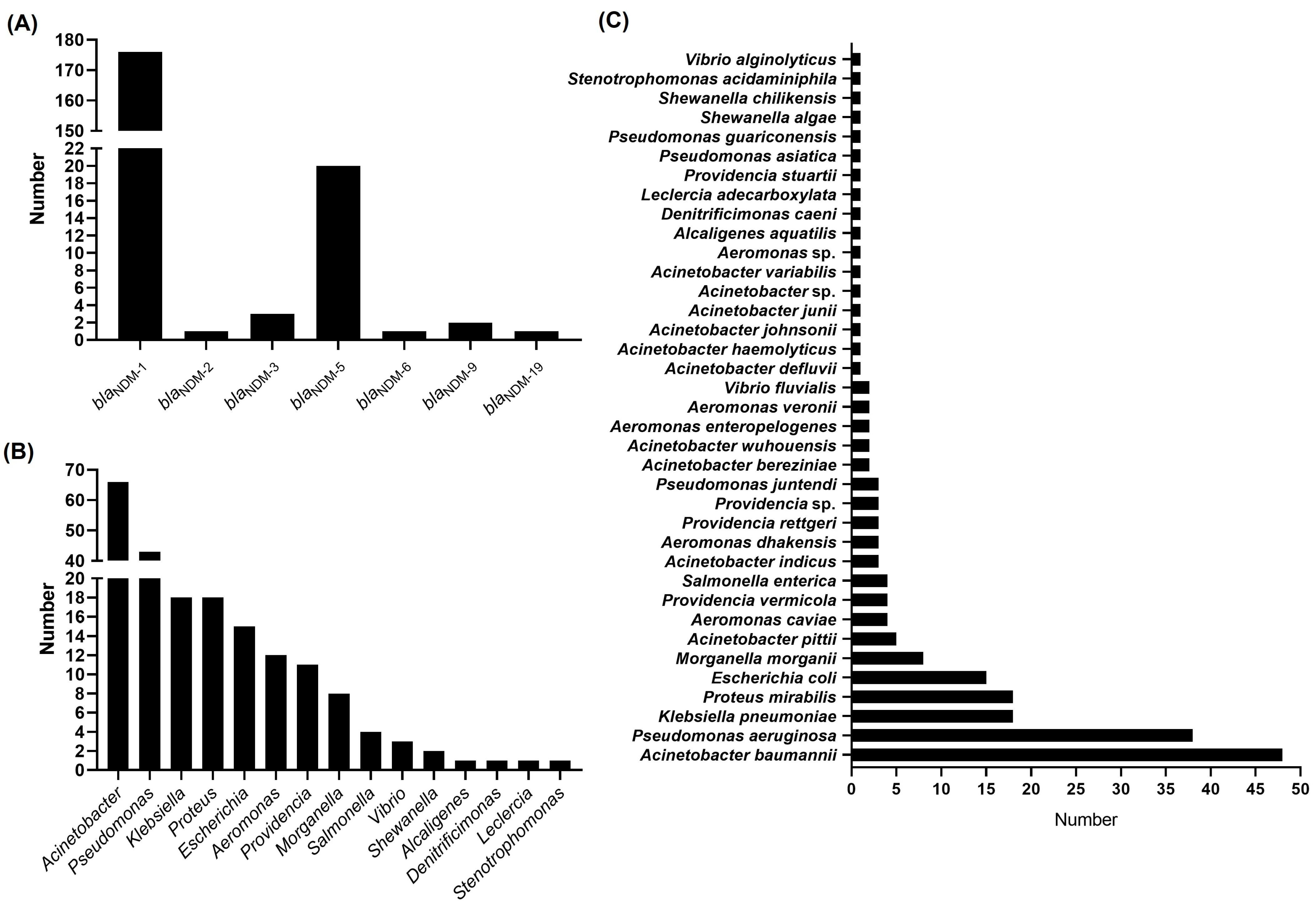
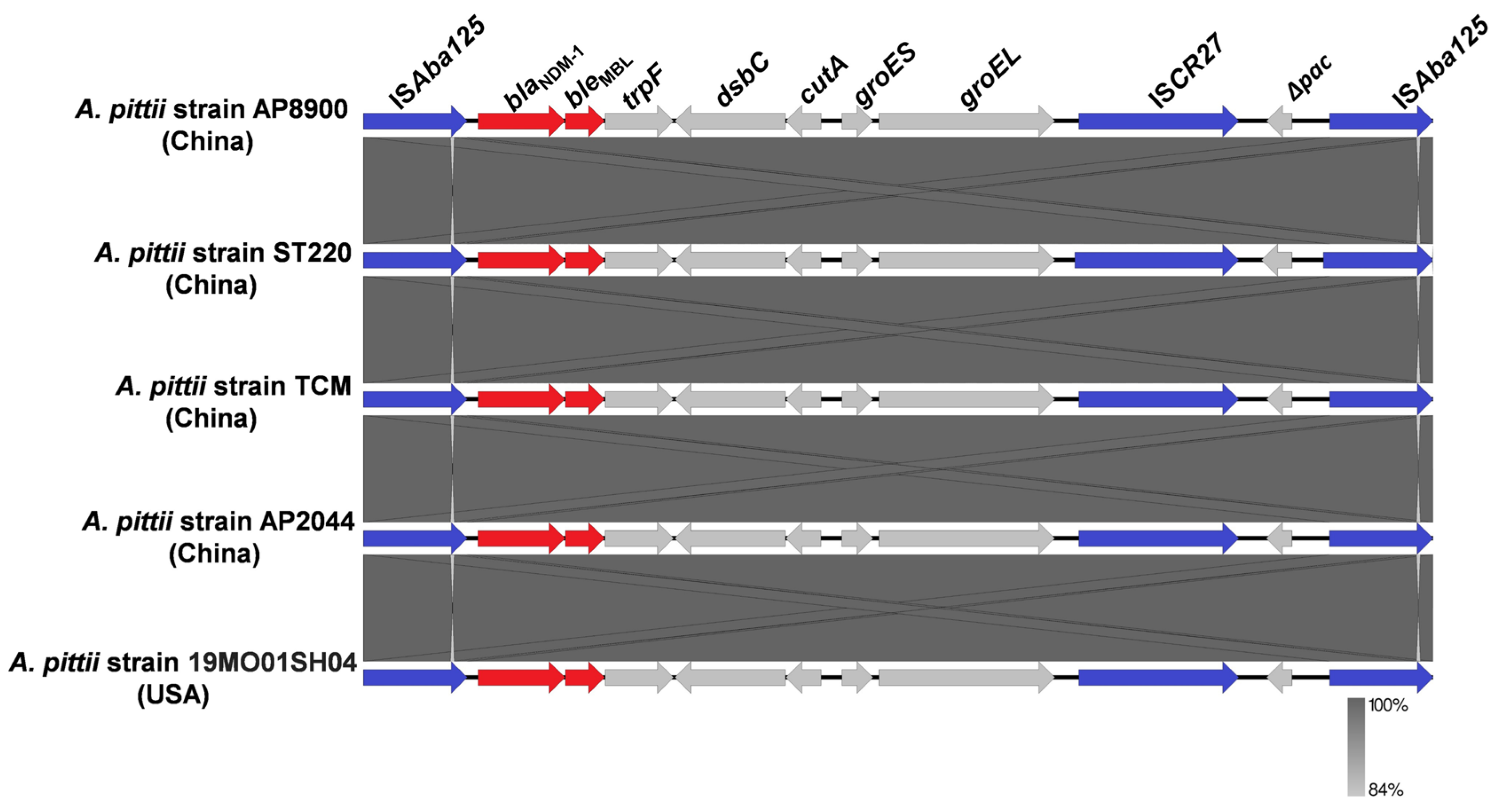
3.3. Bacteria Harboring Chromosome-Borne blaNDM Available in GenBank Database
3.4. Genomic Analysis of the Antibiotic-Resistant Plasmids Carried by A. pittii Strain AP8900
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NDM | New Delhi metallo-beta-lactamase |
| ARGs | Directory of open access journals antibiotic resistance genes |
References
- Hall, B.G.; Barlow, M. Revised Ambler classification of β-lactamases. J. Antimicrob. Chemother. 2005, 55, 1050–1051. [Google Scholar] [CrossRef]
- Wang, T.; Xu, K.; Zhao, L.; Tong, R.; Xiong, L.; Shi, J. Recent research and development of NDM-1 inhibitors. Eur. J. Med. Chem. 2021, 223, 113667. [Google Scholar] [CrossRef]
- Yong, D.; Toleman, M.A.; Giske, C.G.; Cho, H.S.; Sundman, K.; Lee, K.; Walsh, T.R. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 2009, 53, 5046–5054. [Google Scholar] [CrossRef]
- Wu, W.; Feng, Y.; Tang, G.; Qiao, F.; McNally, A.; Zong, Z. NDM Metallo-β-Lactamases and Their Bacterial Producers in Health Care Settings. Clin. Microbiol. Rev. 2019, 32, e00115–e00118. [Google Scholar] [CrossRef] [PubMed]
- Naas, T.; Oueslati, S.; Bonnin, R.A.; Dabos, M.L.; Zavala, A.; Dortet, L.; Retailleau, P.; Iorga, B.I. Beta-lactamase database (BLDB)–structure and function. J. Enzym. Inhib. Med. Chem. 2017, 32, 917–919. [Google Scholar] [CrossRef]
- Albiger, B.; Glasner, C.; Struelens, M.J.; Grundmann, H.; Monnet, D.L. Carbapenemase-producing Enterobacteriaceae in Europe: Assessment by national experts from 38 countries, May 2015. Eurosurveillance 2015, 20, 17–34. [Google Scholar] [CrossRef]
- Walsh, T.R.; Weeks, J.; Livermore, D.M.; Toleman, M.A. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: An environmental point prevalence study. Lancet Infect. Dis. 2011, 11, 355–362. [Google Scholar] [CrossRef]
- Nordmann, P.; Poirel, L.; Walsh, T.R.; Livermore, D.M. The emerging NDM carbapenemases. Trends Microbiol. 2011, 19, 588–595. [Google Scholar] [CrossRef]
- Wong, D.; Nielsen, T.B.; Bonomo, R.A.; Pantapalangkoor, P.; Luna, B.; Spellberg, B. Clinical and Pathophysiological Overview of Acinetobacter Infections: A Century of Challenges. Clin. Microbiol. Rev. 2017, 30, 409–447. [Google Scholar] [CrossRef] [PubMed]
- Carvalheira, A.; Silva, J.; Teixeira, P. Acinetobacter spp. in food and drinking water—A review. Food Microbiol. 2021, 95, 103675. [Google Scholar] [CrossRef] [PubMed]
- Richards, G.A.; Perovic, O.; Brink, A.J. The challenges of difficult-to-treat Acinetobacter infections. Clin. Microbiol. Rev. 2024, 37, e0009324. [Google Scholar] [CrossRef]
- Kornelsen, V.; Kumar, A. Update on Multidrug Resistance Efflux Pumps in Acinetobacter spp. Antimicrob. Agents Chemother. 2021, 65, e0051421. [Google Scholar] [CrossRef] [PubMed]
- Tierney, B.T.; Singh, N.K.; Simpson, A.C.; Hujer, A.M.; Bonomo, R.A.; Mason, C.E.; Venkateswaran, K. Multidrug-resistant Acinetobacter pittii is adapting to and exhibiting potential succession aboard the International Space Station. Microbiome 2022, 10, 210. [Google Scholar] [CrossRef]
- Bello-López, E.; Escobedo-Muñoz, A.S.; Guerrero, G.; Cruz-Córdova, A.; Garza-González, E.; Hernández-Castro, R.; Zarain, P.L.; Morfín-Otero, R.; Volkow, P.; Xicohtencatl-Cortes, J.; et al. Acinetobacter pittii: The emergence of a hospital-acquired pathogen analyzed from the genomic perspective. Front. Microbiol. 2024, 15, 1412775. [Google Scholar] [CrossRef]
- Djahanschiri, B.; Di Venanzio, G.; Distel, J.S.; Breisch, J.; Dieckmann, M.A.; Goesmann, A.; Averhoff, B.; Göttig, S.; Wilharm, G.; Feldman, M.F.; et al. Evolutionarily stable gene clusters shed light on the common grounds of pathogenicity in the Acinetobacter calcoaceticus-baumannii complex. PLoS Genet. 2022, 18, e1010020. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Y.; Wang, Y.; Tang, K.; Wang, D.; Hong, J.; Wang, P.; Ye, S.; Yan, J.; Li, S.; et al. Genetic landscape and evolution of Acinetobacter pittii, an underestimated emerging nosocomial pathogen. Commun. Biol. 2025, 8, 738. [Google Scholar] [CrossRef]
- Kumari, S.; Narendrakumar, L.; Chawla, M.; Das, S.; Koley, H.; Das, B. Investigating the molecular transmission dynamics of bla(NDM) in antibiotic-selective environments. J. Bacteriol. 2025, 207, e0013325. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, H.; Li, X.; Li, W.; Yang, G.; Ni, W.; Zhan, M.; Lu, L.; Zhang, Z.; Li, X.; et al. Genetic Diversity and Characteristics of bla (NDM)-Positive Plasmids in Escherichia coli. Front. Microbiol. 2021, 12, 729952. [Google Scholar] [CrossRef]
- Yehya, A.; Ezzeddine, Z.; Chakkour, M.; Dhaini, Z.; Bou Saba, M.S.; Bou Saba, A.S.; Nohra, L.; Nassar, N.B.; Yassine, M.; Bahmad, H.F.; et al. The intricacies of Acinetobacter baumannii: A multifaceted comprehensive review of a multidrug-resistant pathogen and its clinical significance and implications. Front. Microbiol. 2025, 16, 1565965. [Google Scholar] [CrossRef] [PubMed]
- Acman, M.; Wang, R.; van Dorp, L.; Shaw, L.P.; Wang, Q.; Luhmann, N.; Yin, Y.; Sun, S.; Chen, H.; Wang, H.; et al. Role of mobile genetic elements in the global dissemination of the carbapenem resistance gene bla(NDM). Nat. Commun. 2022, 13, 1131. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, Y.; Wilharm, G.; Zander, E.; Wichelhaus, T.A.; Göttig, S.; Hunfeld, K.P.; Seifert, H.; Witte, W.; Higgins, P.G. Molecular characterization of blaNDM-1 in an Acinetobacter baumannii strain isolated in Germany in 2007. J. Antimicrob. Chemother. 2011, 66, 1998–2001. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Bonnin, R.A.; Boulanger, A.; Schrenzel, J.; Kaase, M.; Nordmann, P. Tn125-related acquisition of blaNDM-like genes in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2012, 56, 1087–1089. [Google Scholar] [CrossRef]
- Bontron, S.; Nordmann, P.; Poirel, L. Transposition of Tn125 Encoding the NDM-1 Carbapenemase in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2016, 60, 7245–7251. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: Pluses, perils, and pitfalls. J. Clin. Microbiol. 2007, 45, 2761–2764. [Google Scholar] [CrossRef]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef] [PubMed]
- Sayers, E.W.; Cavanaugh, M.; Clark, K.; Ostell, J.; Pruitt, K.D.; Karsch-Mizrachi, I. GenBank. Nucleic Acids Res. 2020, 48, D84–D86. [Google Scholar] [CrossRef]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef]
- Siguier, P.; Perochon, J.; Lestrade, L.; Mahillon, J.; Chandler, M. ISfinder: The reference centre for bacterial insertion sequences. Nucleic Acids Res. 2006, 34, D32–D36. [Google Scholar] [CrossRef] [PubMed]
- Morgulis, A.; Coulouris, G.; Raytselis, Y.; Madden, T.L.; Agarwala, R.; Schäffer, A.A. Database indexing for production MegaBLAST searches. Bioinformatics 2008, 24, 1757–1764. [Google Scholar] [CrossRef]
- Sullivan, M.J.; Petty, N.K.; Beatson, S.A. Easyfig: A genome comparison visualizer. Bioinformatics 2011, 27, 1009–1010. [Google Scholar] [CrossRef]
- Alikhan, N.F.; Petty, N.K.; Ben Zakour, N.L.; Beatson, S.A. BLAST Ring Image Generator (BRIG): Simple prokaryote genome comparisons. BMC Genom. 2011, 12, 402. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Thirunarayan, M.A.; Krishnan, P. Coexistence of blaOXA-23 with blaNDM-1 and armA in clinical isolates of Acinetobacter baumannii from India. J. Antimicrob. Chemother. 2010, 65, 2253–2254. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Z.; Jiang, Y.; Yu, Y. Emergence of NDM-1-producing Acinetobacter baumannii in China. J. Antimicrob. Chemother. 2011, 66, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Hu, Y.; Pan, Y.; Liang, H.; Wang, H.; Wang, X.; Hao, Q.; Yang, X.; Yang, X.; Xiao, X.; et al. Novel plasmid and its variant harboring both a bla(NDM-1) gene and type IV secretion system in clinical isolates of Acinetobacter lwoffii. Antimicrob. Agents Chemother. 2012, 56, 1698–1702. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y.; Jia, X.; Luo, Y.; Song, Q.; Zhao, W.; Wang, Y.; Liu, H.; Zheng, D.; Xia, Y.; et al. Dissemination and characterization of NDM-1-producing Acinetobacter pittii in an intensive care unit in China. Clin. Microbiol. Infect. 2012, 18, E506–E513. [Google Scholar] [CrossRef]
- Zhang, C.; Qiu, S.; Wang, Y.; Qi, L.; Hao, R.; Liu, X.; Shi, Y.; Hu, X.; An, D.; Li, Z.; et al. Higher isolation of NDM-1 producing Acinetobacter baumannii from the sewage of the hospitals in Beijing. PLoS ONE 2014, 8, e64857. [Google Scholar] [CrossRef]
- Jean, S.S.; Harnod, D.; Hsueh, P.R. Global Threat of Carbapenem-Resistant Gram-Negative Bacteria. Front. Cell. Infect. Microbiol. 2022, 12, 823684. [Google Scholar] [CrossRef] [PubMed]
- Paudel, R.; Shrestha, E.; Chapagain, B.; Tiwari, B.R. Carbapenemase producing Gram negative bacteria: Review of resistance and detection methods. Diagn. Microbiol. Infect. Dis. 2024, 110, 116370. [Google Scholar] [CrossRef] [PubMed]
- Khoshdel, N.; Noursalehigarakani, M.; Seghatoleslami, Z.S.; Hadavand, F.; Eghbal, E.; Nasiri, M.J.; Simula, E.; Ahmed, P.; Sechi, L.A. Efficacy of Ceftazidime-avibactam in treating Gram-negative infections: A systematic review and meta-analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2025, 44, 767–778. [Google Scholar] [CrossRef]
- Tillotson, G.S. Trojan Horse Antibiotics-A Novel Way to Circumvent Gram-Negative Bacterial Resistance? Infect. Dis. 2016, 9, 45–52. [Google Scholar] [CrossRef]
- Ezzeddine, Z.; Ghssein, G. Towards new antibiotics classes targeting bacterial metallophores. Microb. Pathog. 2023, 182, 106221. [Google Scholar] [CrossRef]
- Mussi, M.A.; Limansky, A.S.; Viale, A.M. Acquisition of resistance to carbapenems in multidrug-resistant clinical strains of Acinetobacter baumannii: Natural insertional inactivation of a gene encoding a member of a novel family of beta-barrel outer membrane proteins. Antimicrob. Agents Chemother. 2005, 49, 1432–1440. [Google Scholar] [CrossRef]
- Krishnaraju, M.; Kamatchi, C.; Jha, A.K.; Devasena, N.; Vennila, R.; Sumathi, G.; Vaidyanathan, R. Complete sequencing of an IncX3 plasmid carrying blaNDM-5 allele reveals an early stage in the dissemination of the blaNDM gene. Indian J. Med. Microbiol. 2015, 33, 30–38. [Google Scholar] [CrossRef]
- Poirel, L.; Bonnin, R.A.; Nordmann, P. Analysis of the resistome of a multidrug-resistant NDM-1-producing Escherichia coli strain by high-throughput genome sequencing. Antimicrob. Agents Chemother. 2011, 55, 4224–4229. [Google Scholar] [CrossRef]
- Hamidian, M.; Hancock, D.P.; Hall, R.M. Horizontal transfer of an ISAba125-activated ampC gene between Acinetobacter baumannii strains leading to cephalosporin resistance. J. Antimicrob. Chemother. 2013, 68, 244–245. [Google Scholar] [CrossRef]
- Partridge, S.R.; Iredell, J.R. Genetic contexts of blaNDM-1. Antimicrob. Agents Chemother. 2012, 56, 6065–6067. [Google Scholar] [CrossRef] [PubMed]
- Toleman, M.A.; Bennett, P.M.; Walsh, T.R. ISCR elements: Novel gene-capturing systems of the 21st century? Microbiol. Mol. Biol. Rev. 2006, 70, 296–316. [Google Scholar] [CrossRef] [PubMed]
- Toleman, M.A.; Spencer, J.; Jones, L.; Walsh, T.R. blaNDM-1 is a chimera likely constructed in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2012, 56, 2773–2776. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Zhang, X.; Zhou, Y.; Zhang, Z.; Zheng, X. Whole-genome sequencing of an NDM-1- and OXA-58-producing Acinetobacter towneri isolate from hospital sewage in Sichuan Province, China. J. Glob. Antimicrob. Resist. 2019, 16, 4–5. [Google Scholar] [CrossRef]
- Hamidian, M.; Ambrose, S.J.; Hall, R.M. A large conjugative Acinetobacter baumannii plasmid carrying the sul2 sulphonamide and strAB streptomycin resistance genes. Plasmid 2016, 87–88, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Shen, W.; Zhang, Z.; Zhang, J.; Wang, G.; Xiang, L.; She, J.; Hu, X.; Zou, G.; Zhu, B.; et al. Whole-Genome Analysis of Two Copies of bla (NDM-1) Gene Carrying Acinetobacter johnsonii Strain Acsw19 Isolated from Sichuan, China. Infect. Drug Resist. 2020, 13, 855–865. [Google Scholar] [CrossRef] [PubMed]

| Antibiotics | MIC (μg/mL) | Interpretation | |
|---|---|---|---|
| Categories | Name | ||
| Cephalosporins | Ceftazidime | ≥64 | R |
| Cefepime | ≥32 | R | |
| Carbapenems | Imipenem | 8 | R |
| Meropenem | 4 | I | |
| Fluoroquinolones | Ciprofloxacin | ≥4 | R |
| Levofloxacin | ≥8 | R | |
| Sulfonamides | Sulfamethoxazole/trimethoprim | 160 | R |
| β-lactam/β-lactamase inhibitor combinations | Piperacillin/tazobactam | ≥128 | R |
| Ticarcillin/clavulanate | ≤8 | R | |
| Species | Number of Strains Harboring Tn125 | Number of Strains Harboring Chromosome-Borne blaNDM | Proportion * (%) |
|---|---|---|---|
| Acinetobacter baumannii | 31 | 48 | 64.58 |
| Acinetobacter bereziniae | 2 | 2 | 100.00 |
| Acinetobacter defluvii | 1 | 1 | 100.00 |
| Acinetobacter haemolyticus | 1 | 1 | 100.00 |
| Acinetobacter indicus | 3 | 3 | 100.00 |
| Acinetobacter johnsonii | 1 | 1 | 100.00 |
| Acinetobacter junii | 1 | 1 | 100.00 |
| Acinetobacter pittii | 5 | 5 | 100.00 |
| Acinetobacter sp. | 1 | 1 | 100.00 |
| Acinetobacter variabilis | 1 | 1 | 100.00 |
| Acinetobacter wuhouensis | 2 | 2 | 100.00 |
| Klebsiella pneumoniae | 3 | 18 | 16.67 |
| Morganella morganii | 1 | 8 | 12.50 |
| Proteus mirabilis | 4 | 18 | 22.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Wang, H.; Zhu, W.; Li, X.; He, Y.; Su, W. Genomic Characterization of a Carbapenem-Resistant Acinetobacter pittii Strain Harboring Chromosome-Borne blaNDM-1 from China. Pathogens 2025, 14, 1037. https://doi.org/10.3390/pathogens14101037
Liu W, Wang H, Zhu W, Li X, He Y, Su W. Genomic Characterization of a Carbapenem-Resistant Acinetobacter pittii Strain Harboring Chromosome-Borne blaNDM-1 from China. Pathogens. 2025; 14(10):1037. https://doi.org/10.3390/pathogens14101037
Chicago/Turabian StyleLiu, Wenjuan, Haixia Wang, Weijian Zhu, Xiaobin Li, Ying He, and Wen Su. 2025. "Genomic Characterization of a Carbapenem-Resistant Acinetobacter pittii Strain Harboring Chromosome-Borne blaNDM-1 from China" Pathogens 14, no. 10: 1037. https://doi.org/10.3390/pathogens14101037
APA StyleLiu, W., Wang, H., Zhu, W., Li, X., He, Y., & Su, W. (2025). Genomic Characterization of a Carbapenem-Resistant Acinetobacter pittii Strain Harboring Chromosome-Borne blaNDM-1 from China. Pathogens, 14(10), 1037. https://doi.org/10.3390/pathogens14101037






