Cytokine Profile and Oxidative Patterns in Murine Models of Disseminated Infection by Mucorales Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains
2.2. Animals
2.3. Mouse Mucormycosis Models
2.4. Fungal Tissue Burden
2.5. Cytokine Profiling
2.6. Oxidative Stress Biomarkers
2.7. Statistics
3. Results
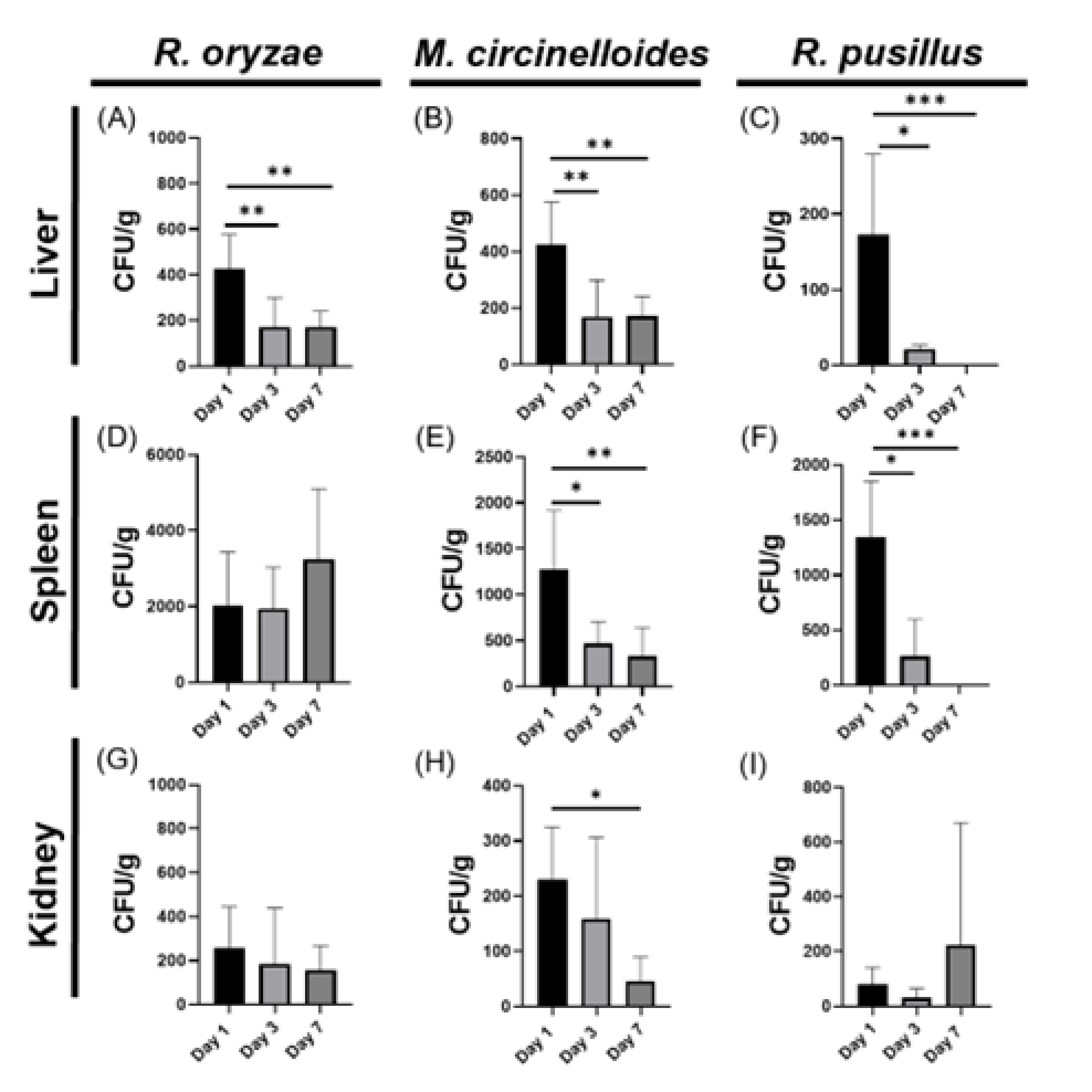
3.1. Organ-Specific Fungal Loading During Systemic Mucormycosis in Immunocompetent Models Reveals Splenic Predominance
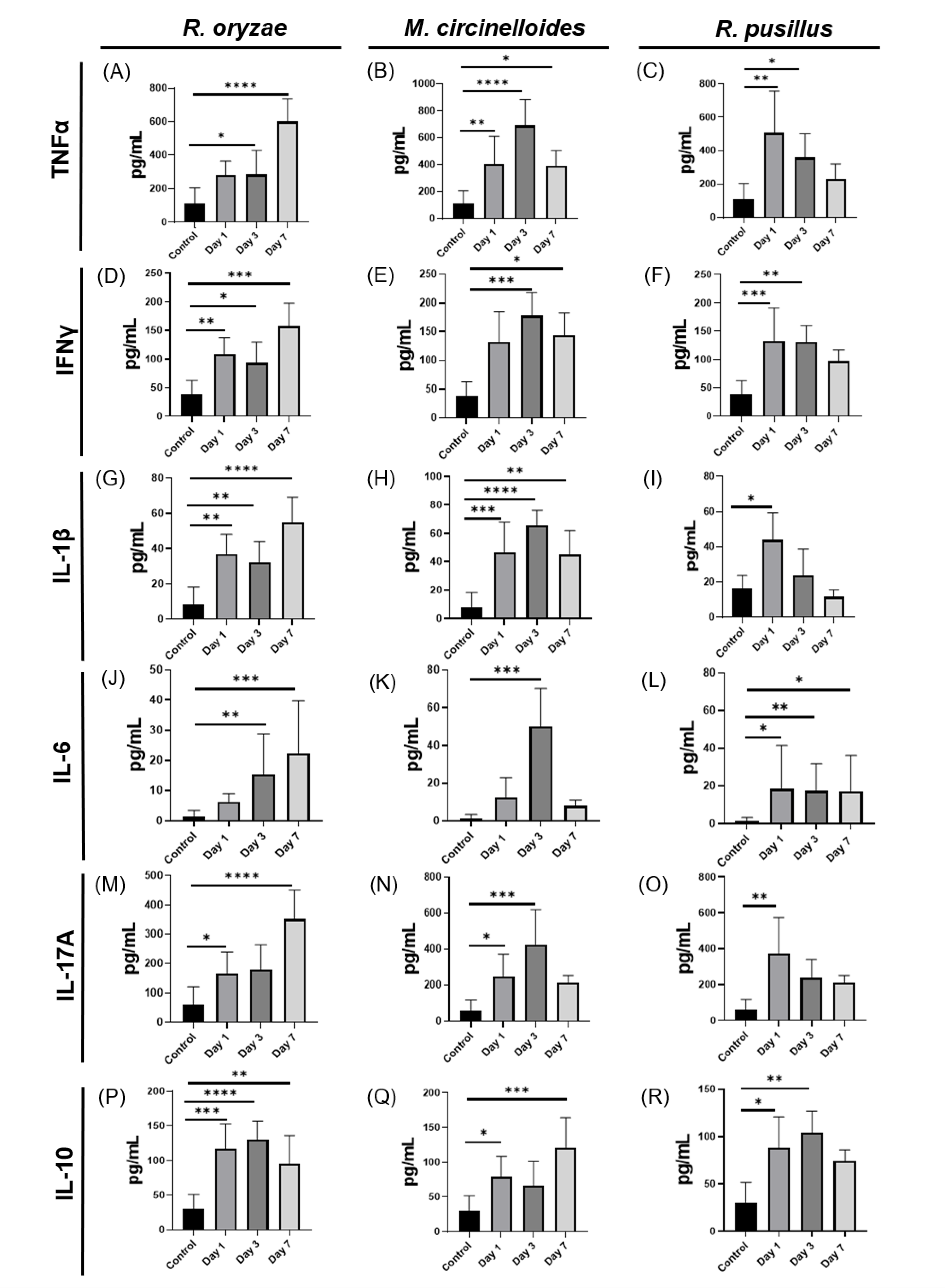
3.2. Specific Dynamics of the Immune Response to Mucoralean Infections with Variations According to Fungus and Postinfection Time
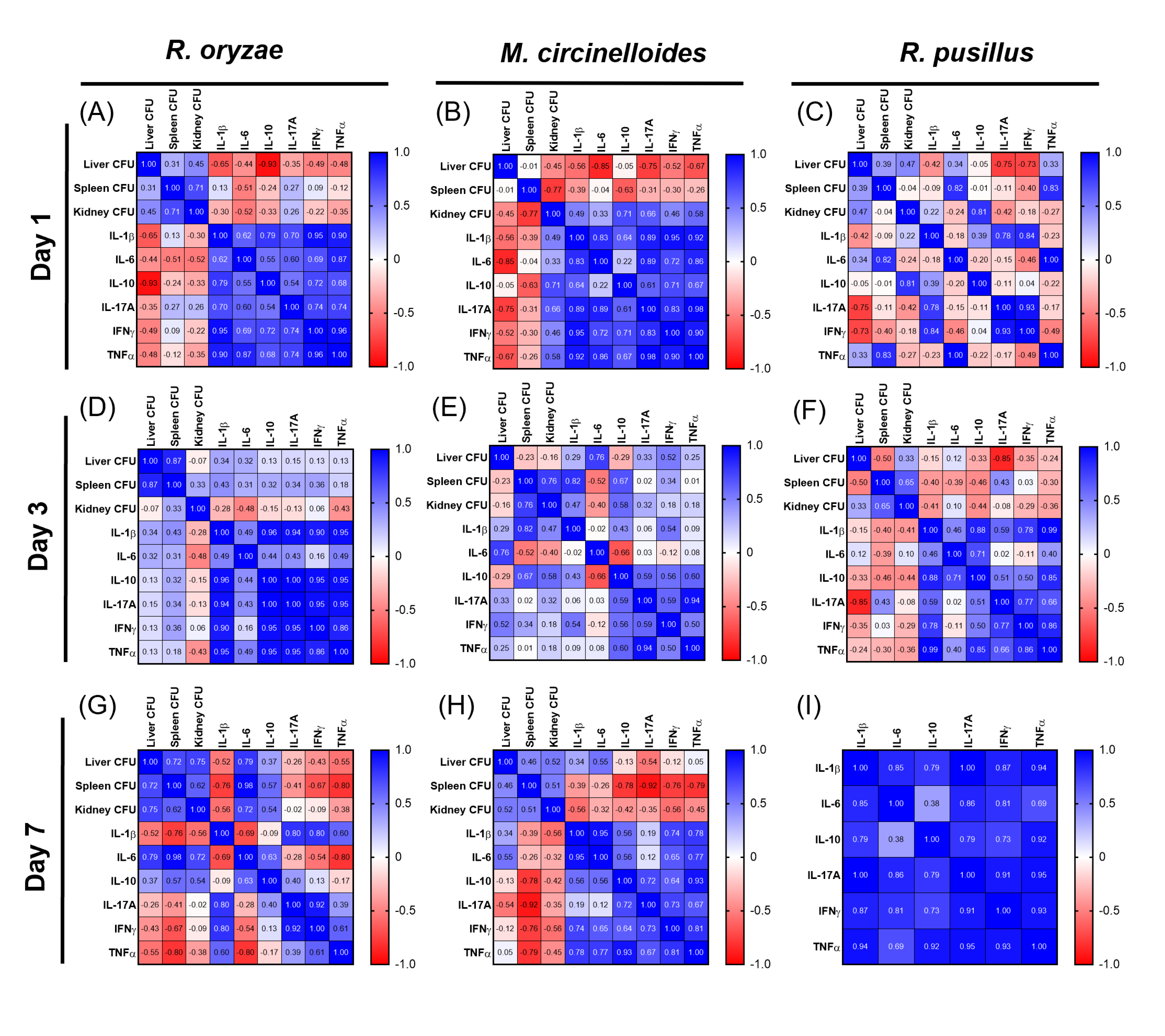
3.3. Correlations Between Fungal Load and Cytokines Reveal Organ- and Fungus-Specific Immune Patterns During Mucormycosis in Immunocompetent Mice
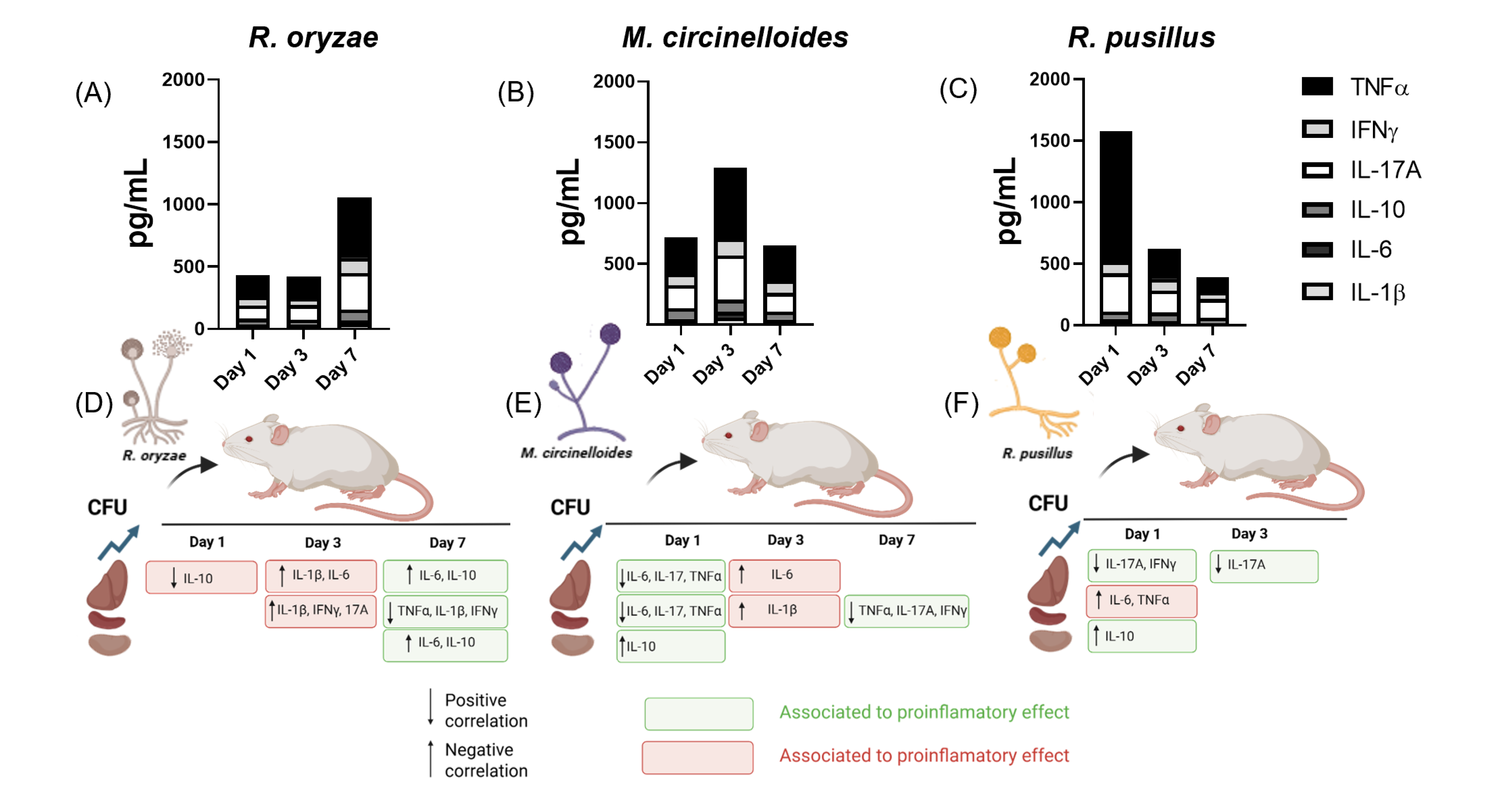
3.4. Differential Regulation of Inflammatory Cytokines and Inflammation Patterns Modulated by Fungal Burden and Infection Time Across Three Mucoralean Species
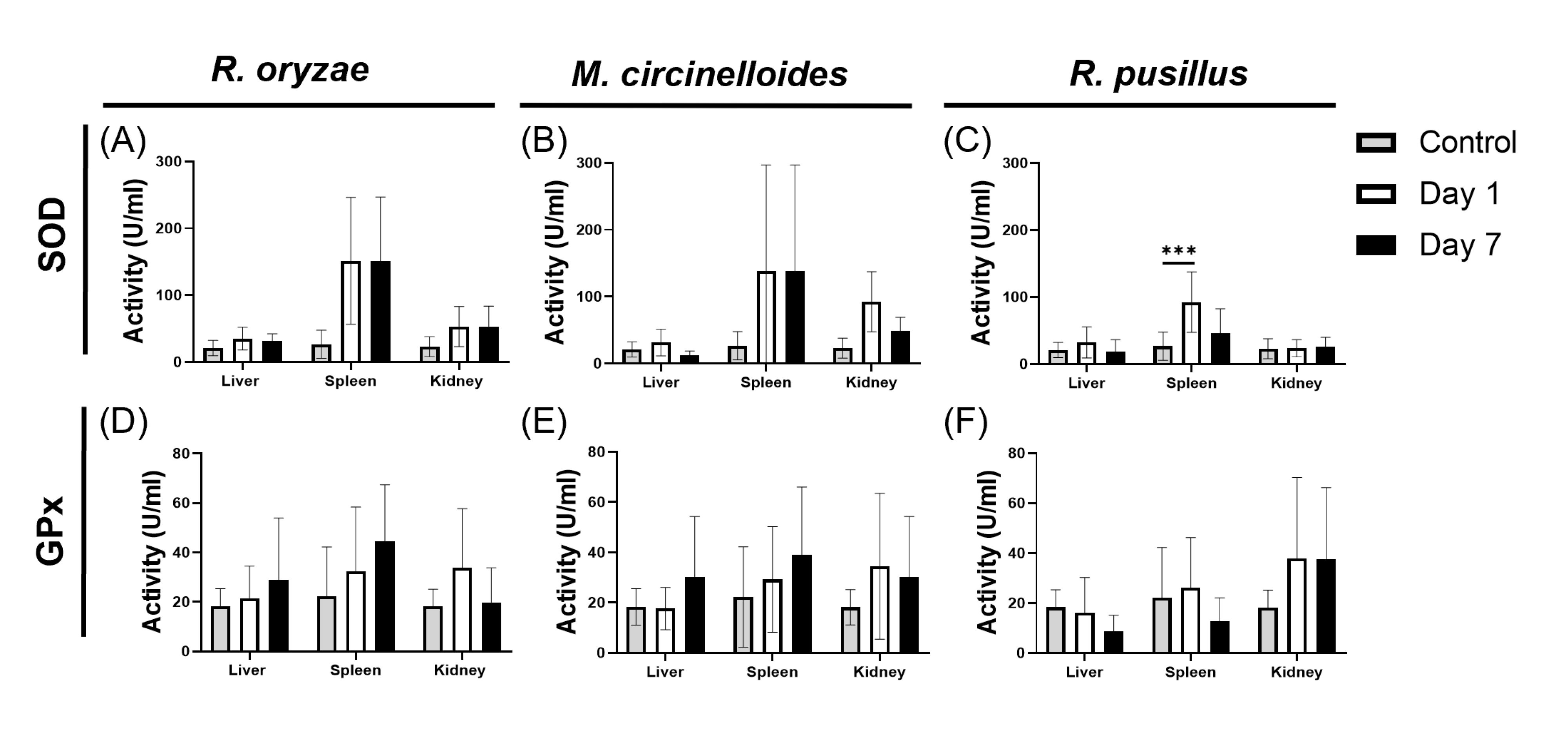
3.5. Comparative Response to Oxidative Stress Induced by Mucoralean Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SOD | Superoxide dismutase |
| GPx | Glutathione peroxidase |
| PBS | Phosphate buffered saline |
| ITS | Internal transcribed spacer |
| D1/D2 | 26S ribosomal DNA region |
| PDA | Potato dextrose agar |
| RPMI | Roswell Park Memorial Institute |
| PKA | Protein kinase A |
| ROS | Reactive oxygen species |
| CotH | Coat protein homolog |
| CFU | Colony-forming units |
References
- Dannaoui, E. Antifungal Resistance in Mucorales. Int. J. Antimicrob. Agents 2017, 50, 617–621. [Google Scholar] [CrossRef]
- Pérez, M.A.; Martínez, L.; Bravo, J.; Rodríguez, B.; Quintero, P.; Moncada, P. Infección Por Aspergillus Flavus y Rhizopus Oryzae Complex En Paciente Con Diabetes Mellitus. Biomédica 2023, 43, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.-L.; Lee, Y.-M.; Kim, T.; Lee, J.-Y.; Chung, Y.-S.; Kim, M.-N.; Kim, S.-H.; Choi, S.-H.; Kim, Y.S.; Woo, J.H.; et al. Risk Factors for Mortality in Patients with Invasive Mucormycosis. Infect. Chemother. 2013, 45, 292. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.S.; Spellberg, B.; Walsh, T.J.; Kontoyiannis, D.P. Pathogenesis of Mucormycosis. Clin. Infect. Dis. 2012, 54, S16–S22. [Google Scholar] [CrossRef] [PubMed]
- Binder, U.; Maurer, E.; Lass-Flörl, C. Mucormycosis—From the Pathogens to the Disease. Clin. Microbiol. Infect. 2014, 20, 60–66. [Google Scholar] [CrossRef]
- Li, Z.; Shi, C.; Huang, Y.; Wang, H.; Li, W.; Cai, L. Phenotypic Analysis and Genome Sequence of Rhizopus Oryzae Strain Y5, the Causal Agent of Tobacco Pole Rot. Front. Microbiol. 2023, 13, 1031023. [Google Scholar] [CrossRef]
- Lee, S.C.; Li, A.; Calo, S.; Heitman, J. Calcineurin Plays Key Roles in the Dimorphic Transition and Virulence of the Human Pathogenic Zygomycete Mucor Circinelloides. PLoS Pathog. 2013, 9, e1003625. [Google Scholar] [CrossRef]
- Ribes, J.A.; Vanover-Sams, C.L.; Baker, D.J. Zygomycetes in Human Disease. Clin. Microbiol. Rev. 2000, 13, 236–301. [Google Scholar] [CrossRef]
- Binder, U.; Navarro-Mendoza, M.I.; Naschberger, V.; Bauer, I.; Nicolas, F.E.; Pallua, J.D.; Lass-Flörl, C.; Garre, V. Generation of A Mucor Circinelloides Reporter Strain—A Promising New Tool to Study Antifungal Drug Efficacy and Mucormycosis. Genes 2018, 9, 613. [Google Scholar] [CrossRef] [PubMed]
- Mueller, K.D.; Zhang, H.; Serrano, C.R.; Billmyre, R.B.; Huh, E.Y.; Wiemann, P.; Keller, N.P.; Wang, Y.; Heitman, J.; Lee, S.C. Gastrointestinal Microbiota Alteration Induced by Mucor Circinelloides in a Murine Model. J. Microbiol. 2019, 57, 509–520. [Google Scholar] [CrossRef]
- López-Fernández, L.; Sanchis, M.; Navarro-Rodríguez, P.; Nicolás, F.E.; Silva-Franco, F.; Guarro, J.; Garre, V.; Navarro-Mendoza, M.I.; Pérez-Arques, C.; Capilla, J. Understanding Mucor circinelloides Pathogenesis by Comparative Genomics and Phenotypical Studies. Virulence 2018, 9, 707–720. [Google Scholar] [CrossRef]
- Roden, M.M.; Zaoutis, T.E.; Buchanan, W.L.; Knudsen, T.A.; Sarkisova, T.A.; Schaufele, R.L.; Sein, M.; Sein, T.; Chiou, C.C.; Chu, J.H.; et al. Epidemiology and Outcome of Zygomycosis: A Review of 929 Reported Cases. Clin. Infect. Dis. 2005, 41, 634–653. [Google Scholar] [CrossRef] [PubMed]
- Wanger, A.; Chavez, V.; Huang, R.S.P.; Wahed, A.; Actor, J.K.; Dasgupta, A. Overview of Fungal Infections. In Microbiology and Molecular Diagnosis in Pathology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 173–189. [Google Scholar]
- Singh, P.; Paul, S.; Shivaprakash, M.R.; Chakrabarti, A.; Ghosh, A.K. Stress Response in Medically Important Mucorales. Mycoses 2016, 59, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Qiu, M.; Xu, H.; Guo, P.; Peng, Y. Application of Metagenomics Next-Generation Sequencing on Diagnosis of Disseminated Infection Caused by Rhizomucor Pusillus in an Acute Lymphoblastic Leukemia Patient. Infect. Drug Resist. 2024, 17, 5707–5713. [Google Scholar] [CrossRef] [PubMed]
- Walther, G.; Wagner, L.; Kurzai, O. Updates on the Taxonomy of Mucorales with an Emphasis on Clinically Important Taxa. J. Fungi 2019, 5, 106. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Joosten, L.A.B.; Shaw, P.J.; Smeekens, S.P.; Malireddi, R.K.S.; van der Meer, J.W.M.; Kullberg, B.; Netea, M.G.; Kanneganti, T. The Inflammasome Drives Protective Th1 and Th17 Cellular Responses in Disseminated Candidiasis. Eur. J. Immunol. 2011, 41, 2260–2268. [Google Scholar] [CrossRef]
- Shankar, J.; Cerqueira, G.C.; Wortman, J.R.; Clemons, K.V.; Stevens, D.A. RNA-Seq Profile Reveals Th-1 and Th-17-Type of Immune Responses in Mice Infected Systemically with Aspergillus Fumigatus. Mycopathologia 2018, 183, 645–658. [Google Scholar] [CrossRef]
- Nicolás, F.E.; Murcia, L.; Navarro, E.; Navarro-Mendoza, M.I.; Pérez-Arques, C.; Garre, V. Mucorales Species and Macrophages. J. Fungi 2020, 6, 94. [Google Scholar] [CrossRef]
- Sun, L.; Wan, Z.; Li, R.; Yu, J. Impairment of Th Cell Response in Card9 Knockout Mice with Cutaneous Mucormycosis Caused by Rhizopus Arrhizus. Exp. Dermatol. 2019, 28, 1244–1251. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, S.; Wan, Z.; Li, R.; Yu, J. In Vivo and In Vitro Impairments in T Helper Cell and Neutrophil Responses against Mucor Irregularis in Card9 Knockout Mice. Infect. Immun. 2021, 89, 5. [Google Scholar] [CrossRef]
- Dantas, A.; Day, A.; Ikeh, M.; Kos, I.; Achan, B.; Quinn, J. Oxidative Stress Responses in the Human Fungal Pathogen, Candida Albicans. Biomolecules 2015, 5, 142–165. [Google Scholar] [CrossRef]
- Saoulidis, S.; Simitsopoulou, M.; Dalakiouridou, M.; Walsh, T.J.; Wheat, L.J.; Papaioannidou, P.; Roilides, E. Antifungal Activity of Posaconazole and Granulocyte Colony-stimulating Factor in the Treatment of Disseminated Zygomycosis (Mucormycosis) in a Neutropaenic Murine Model. Mycoses 2011, 54, e486–e492. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Bowman, J.C.; Avanessian, V.; Brown, K.; Spellberg, B.; Edwards, J.E.; Douglas, C.M. Caspofungin Inhibits Rhizopus Oryzae 1,3-β-d-Glucan Synthase, Lowers Burden in Brain Measured by Quantitative PCR, and Improves Survival at a Low but Not a High Dose during Murine Disseminated Zygomycosis. Antimicrob. Agents Chemother. 2005, 49, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Heitman, J. Drug-Resistant Epimutants Exhibit Organ-Specific Stability and Induction during Murine Infections Caused by the Human Fungal Pathogen Mucor Circinelloides. mBio 2019, 10, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Gillrie, M.; Chow, B.; Griener, T.; Johnson, A.; Church, D. Hepatosplenic Mucormycosis Due to Rhizomucor pusillus Identified by Panfungal PCR/Sequencing of Ribosomal ITS2 and LSU Regions in a Patient with Acute Myelogenous Leukemia: A Case Report. J. Assoc. Med. Microbiol. Infect. Dis. Can. 2023, 8, 105–110. [Google Scholar] [CrossRef]
- Samdavid Thanapaul, R.J.R.S.; Alamneh, Y.A.; Finnegan, D.K.; Antonic, V.; Abu-Taleb, R.; Czintos, C.; Boone, D.; Su, W.; Sajja, V.S.; Getnet, D.; et al. Development of a Combat-Relevant Murine Model of Wound Mucormycosis: A Platform for the Pre-Clinical Investigation of Novel Therapeutics for Wound-Invasive Fungal Diseases. J. Fungi 2024, 10, 364. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, X.; Yan, X.; Liu, Y.; Liu, Y.; Cao, H.; Su, K.; Wang, C.; Gao, S.; Liu, Q. Aggressive Disseminated Rhizomucor Pusillus Infection in a Ph-like Acute Lymphoblastic Leukemia Patient: Early Detection by Cell-Free DNA next-Generation Sequencing. J. Infect. Chemother. 2022, 28, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Stuehler, C.; Khanna, N.; Bozza, S.; Zelante, T.; Moretti, S.; Kruhm, M.; Lurati, S.; Conrad, B.; Worschech, E.; Stevanović, S.; et al. Cross-Protective TH1 Immunity against Aspergillus Fumigatus and Candida Albicans. Blood 2011, 117, 5881–5891. [Google Scholar] [CrossRef]
- Lomeli-Martinez, S.M.; Valentin-Goméz, E.; Varela-Hernández, J.J.; Alvarez-Zavala, M.; Sanchez-Reyes, K.; Ramos-Solano, M.; Cabrera-Silva, R.I.; Ramirez-Anguiano, V.M.; Lomeli-Martinez, M.A.; Martinez-Salazar, S.Y.; et al. Candida Spp. Determination and Th1/Th2 Mixed Cytokine Profile in Oral Samples From HIV+ Patients With Chronic Periodontitis. Front. Immunol. 2019, 10, 1465. [Google Scholar] [CrossRef]
- Butcher, M.J.; Zhu, J. Recent Advances in Understanding the Th1/Th2 Effector Choice. Fac. Rev. 2021, 10, 30. [Google Scholar] [CrossRef]
- Jang, D.; Lee, A.-H.; Shin, H.-Y.; Song, H.-R.; Park, J.-H.; Kang, T.-B.; Lee, S.-R.; Yang, S.-H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Maddur, M.S.; Miossec, P.; Kaveri, S.V.; Bayry, J. Th17 Cells: Biology, Pathogenesis of Autoimmune and Inflammatory Diseases, and Therapeutic Strategies. Am. J. Pathol. 2012, 181, 8–18. [Google Scholar] [CrossRef]
- García-Juárez, M.; Camacho-Morales, A. Defining the Role of Anti- and Pro-Inflammatory Outcomes of Interleukin-6 in Mental Health. Neuroscience 2022, 492, 32–46. [Google Scholar] [CrossRef]
- Mirkov, I.; Stojanovic, I.; Stosic-Grujicic, S.; Glamoclija, J.; Zolotarevski, L.; Kataranovski, D.; Kataranovski, M. Splenic and Lung Response to Nonlethal Systemic Aspergillus fumigatus Infection in C57BL/6 Mice. Med. Mycol. 2010, 48, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Dam, P.; Cardoso, M.H.; Mandal, S.; Franco, O.L.; Sağıroğlu, P.; Polat, O.A.; Kokoglu, K.; Mondal, R.; Mandal, A.K.; Ocsoy, I. Surge of Mucormycosis during the COVID-19 Pandemic. Travel. Med. Infect. Dis. 2023, 52, 102557. [Google Scholar] [CrossRef]
- Alqarihi, A.; Gebremariam, T.; Gu, Y.; Swidergall, M.; Alkhazraji, S.; Soliman, S.S.M.; Bruno, V.M.; Edwards, J.E.; Filler, S.G.; Uppuluri, P.; et al. GRP78 and Integrins Play Different Roles in Host Cell Invasion during Mucormycosis. mBio 2020, 11, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Baldin, C.; Ibrahim, A.S. Molecular Mechanisms of Mucormycosis—The Bitter and the Sweet. PLoS Pathog. 2017, 13, e1006408. [Google Scholar] [CrossRef]
- Liu, M.; Spellberg, B.; Phan, Q.T.; Fu, Y.; Fu, Y.; Lee, A.S.; Edwards, J.E.; Filler, S.G.; Ibrahim, A.S. The Endothelial Cell Receptor GRP78 Is Required for Mucormycosis Pathogenesis in Diabetic Mice. J. Clin. Investig. 2010, 120, 1914–1924. [Google Scholar] [CrossRef]
- Potenza, L.; Vallerini, D.; Barozzi, P.; Riva, G.; Forghieri, F.; Zanetti, E.; Quadrelli, C.; Candoni, A.; Maertens, J.; Rossi, G.; et al. Mucorales-Specific T Cells Emerge in the Course of Invasive Mucormycosis and May Be Used as a Surrogate Diagnostic Marker in High-Risk Patients. Blood 2011, 118, 5416–5419. [Google Scholar] [CrossRef] [PubMed]
- Page, L.; Weis, P.; Müller, T.; Dittrich, M.; Lazariotou, M.; Dragan, M.; Waaga-Gasser, A.M.; Helm, J.; Dandekar, T.; Einsele, H.; et al. Evaluation of Aspergillus and Mucorales Specific T-Cells and Peripheral Blood Mononuclear Cell Cytokine Signatures as Biomarkers of Environmental Mold Exposure. Int. J. Med. Microbiol. 2018, 308, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Montaño, D.E.; Voigt, K. Host Immune Defense upon Fungal Infections with Mucorales: Pathogen-Immune Cell Interactions as Drivers of Inflammatory Responses. J. Fungi 2020, 6, 173. [Google Scholar] [CrossRef] [PubMed]
- Chamilos, G.; Lewis, R.E.; Lamaris, G.; Walsh, T.J.; Kontoyiannis, D.P. Zygomycetes Hyphae Trigger an Early, Robust Proinflammatory Response in Human Polymorphonuclear Neutrophils through Toll-Like Receptor 2 Induction but Display Relative Resistance to Oxidative Damage. Antimicrob. Agents Chemother. 2008, 52, 722–724. [Google Scholar] [CrossRef]
- Belic, S.; Page, L.; Lazariotou, M.; Waaga-Gasser, A.M.; Dragan, M.; Springer, J.; Loeffler, J.; Morton, C.O.; Einsele, H.; Ullmann, A.J.; et al. Comparative Analysis of Inflammatory Cytokine Release and Alveolar Epithelial Barrier Invasion in a Transwell® Bilayer Model of Mucormycosis. Front. Microbiol. 2019, 9, 3204. [Google Scholar] [CrossRef]
- Schmidt, S.; Tramsen, L.; Perkhofer, S.; Lass-Flörl, C.; Röger, F.; Schubert, R.; Lehrnbecher, T. Characterization of the Cellular Immune Responses to Rhizopus Oryzae with Potential Impact on Immunotherapeutic Strategies in Hematopoietic Stem Cell Transplantation. J. Infect. Dis. 2012, 206, 135–139. [Google Scholar] [CrossRef]
- dos Santos, A.R.; Fraga-Silva, T.F.; Almeida, D.d.F.; dos Santos, R.F.; Finato, A.C.; Amorim, B.C.; Andrade, M.I.; Soares, C.T.; Lara, V.S.; Almeida, N.L.; et al. Rhizopus -Host Interplay of Disseminated Mucormycosis in Immunocompetent Mice. Future Microbiol. 2020, 15, 739–752. [Google Scholar] [CrossRef]
- Castillo, P.; Wright, K.E.; Kontoyiannis, D.P.; Walsh, T.; Patel, S.; Chorvinsky, E.; Bose, S.; Hazrat, Y.; Omer, B.; Albert, N.; et al. A New Method for Reactivating and Expanding T Cells Specific for Rhizopus Oryzae. Mol. Ther. Methods Clin. Dev. 2018, 9, 305–312. [Google Scholar] [CrossRef]
- Voelz, K.; Gratacap, R.L.; Wheeler, R.T. A Zebrafish Larval Model Reveals Early Tissue-Specific Innate Immune Responses to Mucor Circinelloides. Dis. Model. Mech. 2015, 8, 1375–1388. [Google Scholar] [CrossRef]
- Schober, S.; Cabanillas Stanchi, K.M.; Riecker, A.; Pfeiffer, M.; Tsiflikas, I.; Wiegand, G.; Quintanilla-Martinez, L.; Haen, S.; Ebinger, M.; Lang, P.; et al. Fulminant Rhizomucor Pusillus Mucormycosis during Anti-Leukemic Treatment with Blinatumomab in a Child: A Case Report and Review of the Literature. Med. Mycol. Case Rep. 2021, 32, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Jin, L.; Fu, H.; Shen, Y.; Lu, G.; Mei, H.; Cao, X.; Wang, H.; Liu, W. Interleukin-22 Mediates Early Host Defense against Rhizomucor Pusilluscan Pathogens. PLoS ONE 2013, 8, e65065. [Google Scholar] [CrossRef]
- Deb, S.; Savio, J.; Padaki, P.A.; M., L. P181 Mucor in the Land of the Liver: A Case Report. Med. Mycol. 2022, 60, 119. [Google Scholar] [CrossRef]
- Elsiesy, H.; Saad, M.; Shorman, M.; Amr, S.; Abaalkhail, F.; Hashim, A.; Al Hamoudi, W.; Al Sebayel, M.; Selim, K. Invasive Mucormycosis in a Patient with Liver Cirrhosis: Case Report and Review of the Literature. Hepat. Mon. 2013, 13, e10858. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Tremblay, J.-A.; Chapdelaine, H.; Luong, M.-L.; Carrier, F.M. Pulmonary Mucormycosis in a Patient with Acute Liver Failure: A Case Report and Systematic Review of the Literature. J. Crit. Care 2020, 56, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ahmad, A.; Akhtar, F.; Yousuf, S.; Xess, I.; Khan, L.A.; Manzoor, N. Induction of Oxidative Stress as a Possible Mechanism of the Antifungal Action of Three Phenylpropanoids. FEMS Yeast Res. 2011, 11, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Mahl, C.D.; Behling, C.S.; Hackenhaar, F.S.; de Carvalho e Silva, M.N.; Putti, J.; Salomon, T.B.; Alves, S.H.; Fuentefria, A.; Benfato, M.S. Induction of ROS Generation by Fluconazole in Candida Glabrata: Activation of Antioxidant Enzymes and Oxidative DNA Damage. Diagn. Microbiol. Infect. Dis. 2015, 82, 203–208. [Google Scholar] [CrossRef]
- Martchenko, M.; Alarco, A.-M.; Harcus, D.; Whiteway, M. Superoxide Dismutases in Candida Albicans: Transcriptional Regulation and Functional Characterization of the Hyphal-Induced SOD5 Gene. Mol. Biol. Cell 2004, 15, 456–467. [Google Scholar] [CrossRef]
- Mora-Martínez, A.; Murcia, L.; Rodríguez-Lozano, F.J. Oral Manifestations of Mucormycosis: A Systematic Review. J. Fungi 2023, 9, 935. [Google Scholar] [CrossRef]
- Gu, Y.; Singh, S.; Alqarihi, A.; Alkhazraji, S.; Gebremariam, T.; Youssef, E.G.; Liu, H.; Fan, X.; Jiang, W.-R.; Andes, D.; et al. A Humanized Antibody against Mucormycosis Targets Angioinvasion and Augments the Host Immune Response. Sci. Transl. Med. 2025, 17, eads7369. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Lewis, R.E. How I Treat Mucormycosis. Blood 2011, 118, 1216–1224. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villanueva-Lozano, H.; García-Juárez, M.; Rosas-Taraco, A.G.; Treviño-Rangel, R.d.J.; González, G.M. Cytokine Profile and Oxidative Patterns in Murine Models of Disseminated Infection by Mucorales Species. Pathogens 2025, 14, 1036. https://doi.org/10.3390/pathogens14101036
Villanueva-Lozano H, García-Juárez M, Rosas-Taraco AG, Treviño-Rangel RdJ, González GM. Cytokine Profile and Oxidative Patterns in Murine Models of Disseminated Infection by Mucorales Species. Pathogens. 2025; 14(10):1036. https://doi.org/10.3390/pathogens14101036
Chicago/Turabian StyleVillanueva-Lozano, Hiram, Martín García-Juárez, Adrián G. Rosas-Taraco, Rogelio de J. Treviño-Rangel, and Gloria M. González. 2025. "Cytokine Profile and Oxidative Patterns in Murine Models of Disseminated Infection by Mucorales Species" Pathogens 14, no. 10: 1036. https://doi.org/10.3390/pathogens14101036
APA StyleVillanueva-Lozano, H., García-Juárez, M., Rosas-Taraco, A. G., Treviño-Rangel, R. d. J., & González, G. M. (2025). Cytokine Profile and Oxidative Patterns in Murine Models of Disseminated Infection by Mucorales Species. Pathogens, 14(10), 1036. https://doi.org/10.3390/pathogens14101036






