The Impact of Bacterial–Fungal Interactions on Childhood Caries Pathogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Prospective Cohort Management
2.3. Oral Examination and Saliva Collection
2.4. DNA Extraction, Amplification, and Illumina Novaseq Sequencing
2.5. Sequencing Data Quality Control
2.6. Bioinformatics and Data Analysis
3. Results
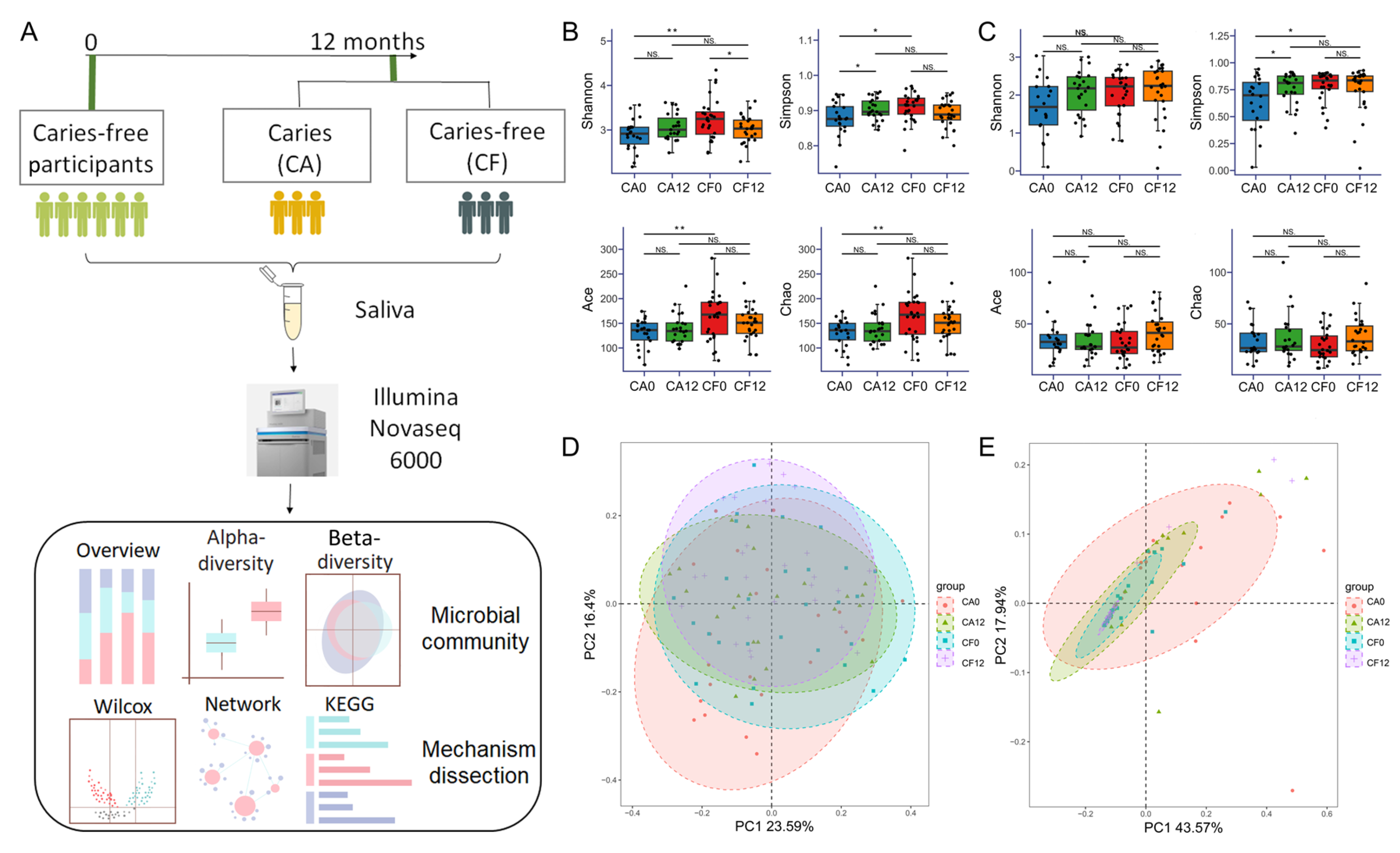
3.1. Participants and General Sequencing Information
3.2. Variation in the Salivary Bacterial Community and Fungal Community During Caries Development
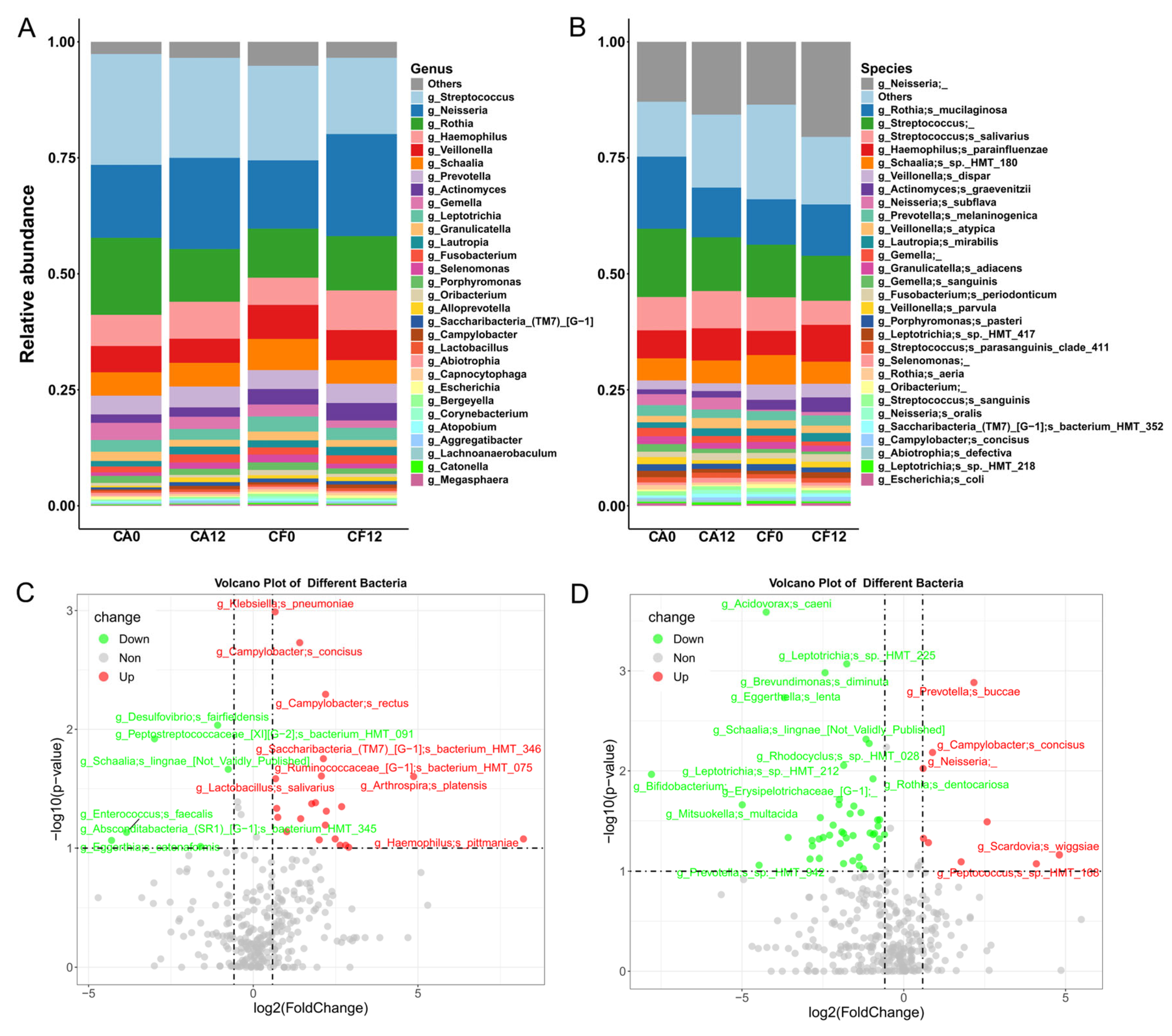
3.2.1. Shift in Salivary Bacterial Community Composition During Caries Development
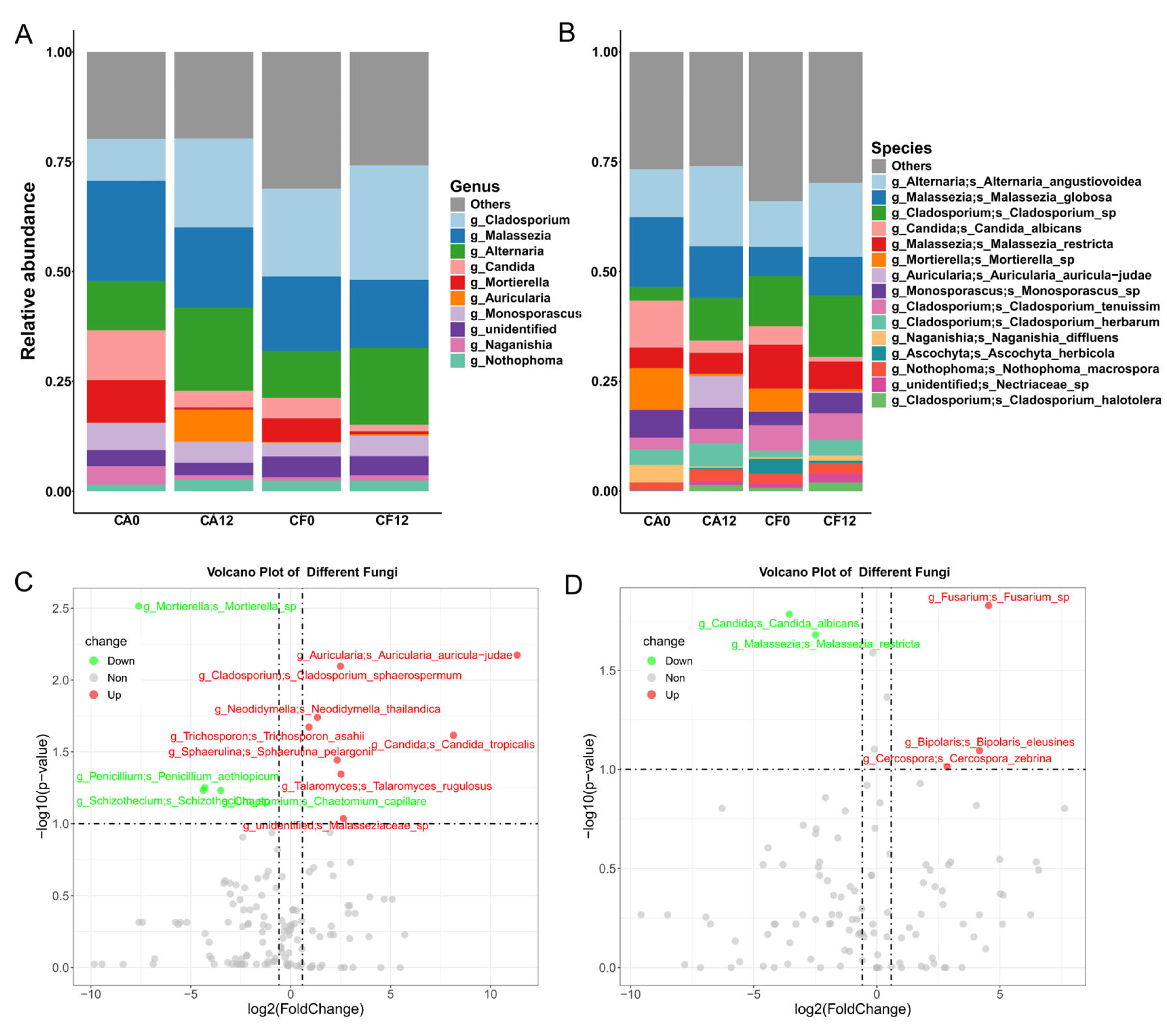
3.2.2. Shift in Salivary Fungal Composition During Caries Development
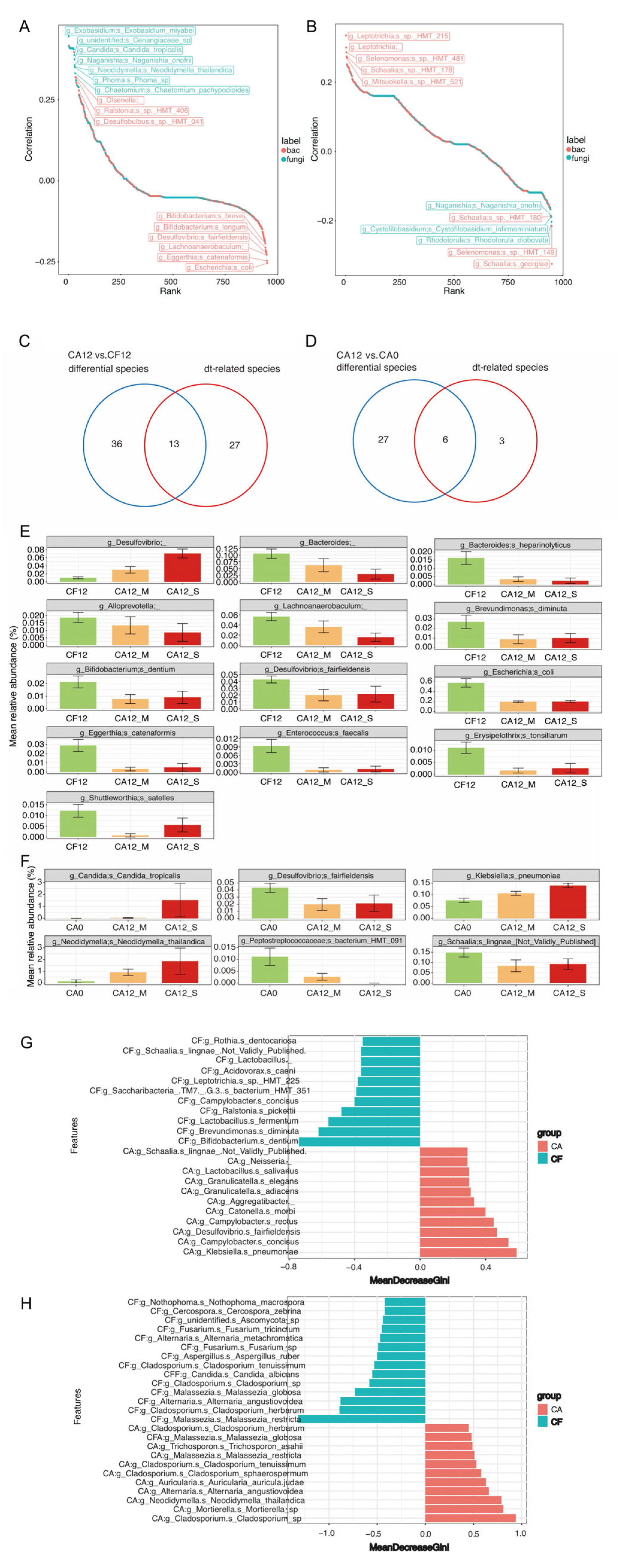
3.3. Salivary Bacterial and Fungal Species Associated with Caries Clinical Feature
3.4. Caries-Discriminatory Bacteria and Fungi in Saliva
3.5. Network Analysis of the Intra-Kingdom and Interkingdom Interactions During Caries Development
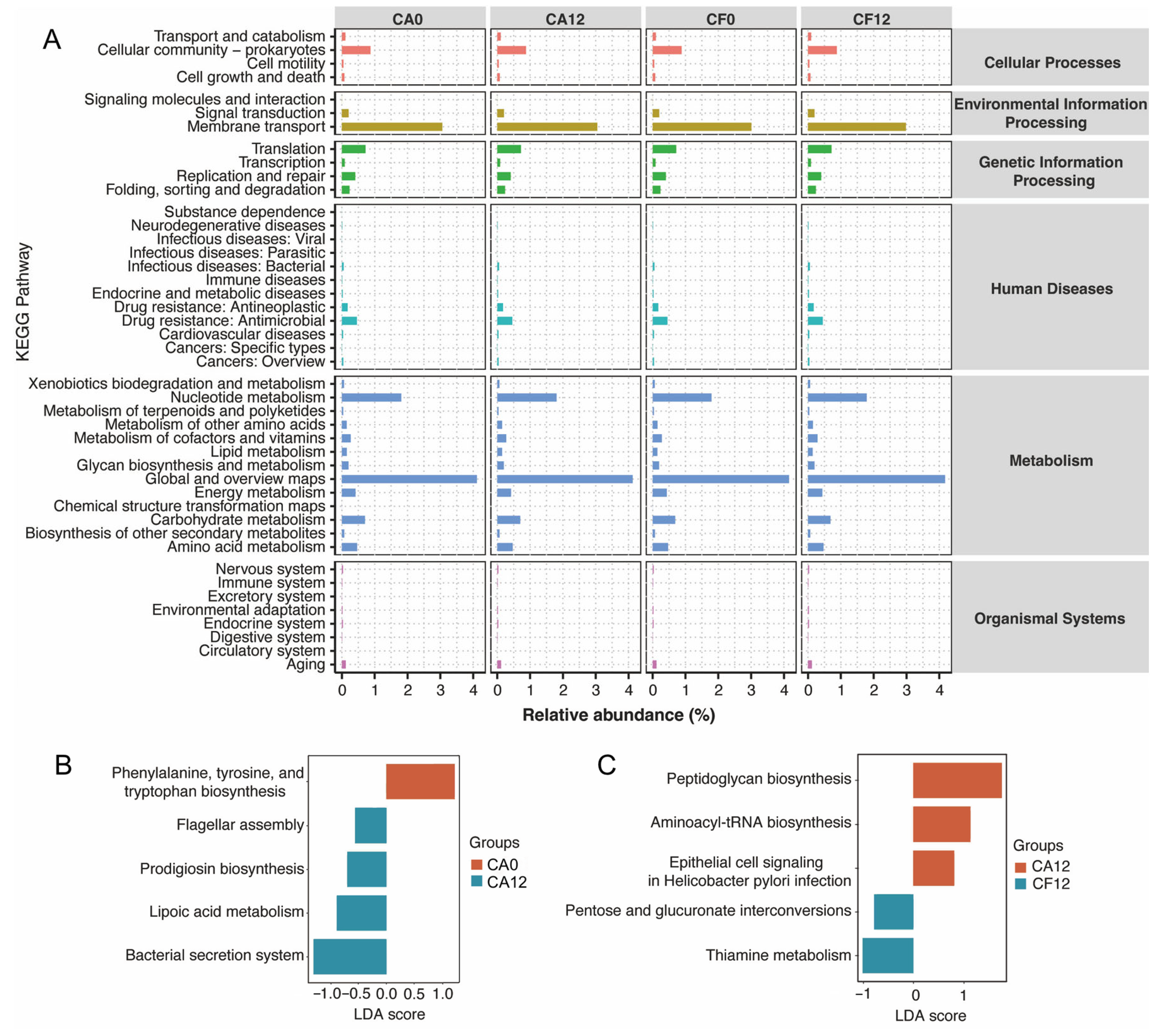
3.6. Prediction of Metabolic Pathways of the Salivary Microbiome in Relation to Childhood Caries
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, M.Q.; Li, Z.; Jiang, H.; Wang, X.; Feng, X.P.; Hu, Y.; Lin, H.C.; Wang, B.; Si, Y.; Wang, C.X.; et al. Dental Caries Status and its Associated Factors among 3- to 5-year-old Children in China: A National Survey. Chin. J. Dent. Res. 2018, 21, 167–179. [Google Scholar] [CrossRef]
- Anil, S.; Anand, P.S. Early Childhood Caries: Prevalence; Risk Factors; and Prevention. Front. Pediatr. 2017, 5, 157. [Google Scholar] [CrossRef]
- Meyer, F.; Enax, J. Early Childhood Caries: Epidemiology, Aetiology, and Prevention. Int. J. Dent. 2018, 2018, 1415873. [Google Scholar] [CrossRef]
- Struzycka, I. The oral microbiome in dental caries. Pol. J. Microbiol. 2014, 63, 127–135. [Google Scholar] [CrossRef]
- O’Connell, L.M.; Santos, R.; Springer, G.; Burne, R.A.; Nascimento, M.M.; Richards, V.P. Site-Specific Profiling of the Dental Mycobiome Reveals Strong Taxonomic Shifts during Progression of Early-Childhood Caries. Appl. Environ. Microbiol. 2020, 86, e02825-19. [Google Scholar] [CrossRef]
- Hurley, E.; Barrett, M.P.J.; Kinirons, M.; Whelton, H.; Ryan, C.A.; Stanton, C.; Harris, H.M.B.; O’Toole, P.W. Comparison of the salivary and dentinal microbiome of children with severe-early childhood caries to the salivary microbiome of caries-free children. BMC Oral Health 2019, 19, 13. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, S.; Koh, D.; Hsu, C.Y. Salivary biomarkers for dental caries. Periodontol 2000 2016, 70, 128–141. [Google Scholar] [CrossRef]
- Baker, J.L.; Bor, B.; Agnello, M.; Shi, W.; He, X. Ecology of the Oral Microbiome: Beyond Bacteria. Trends Microbiol. 2017, 25, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Agnello, M.; Marques, J.; Cen, L.; Mittermuller, B.; Huang, A.; Chaichanasakul Tran, N.; Shi, W.; He, X.; Schroth, R.J. Microbiome Associated with Severe Caries in Canadian First Nations Children. J. Dent. Res. 2017, 96, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Shi, W.; Xu, H.; Wang, G.; He, X.; Chen, F.; Qin, M. Differences in Sole Carbon Source Utilization of the Dental Plaque Microbiota Between Caries-Free and Caries-Affected Children. Front. Microbiol. 2020, 11, 458. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Z.; Cai, T.; Jiang, D.; Luo, J.; Zhou, Z. Untargeted metabolomics of saliva in caries-active and caries-free children in the mixed dentition. Front. Cell Infect. Microbiol. 2023, 13, 1104295. [Google Scholar] [CrossRef]
- Bhaumik, D.; Salzman, E.; Davis, E.; Blostein, F.; Li, G.; Neiswanger, K.; Weyant, R.J.; Crout, R.; McNeil, D.W.; Marazita, M.L.; et al. Plaque Microbiome in Caries-Active and Caries-Free Teeth by Dentition. JDR Clin. Trans. Res. 2024, 9, 61–71. [Google Scholar] [CrossRef]
- WHO. Oral Health Surveys: Basic Methods, 5th ed.; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Rai, A.; Sundas, S.; Dhakal, N.; Khapung, A. Assessment of dental caries based on ICDAS and WHO criteria: A comparative study. Int. J. Paediatr. Dent. 2024, 34, 77–84. [Google Scholar] [CrossRef]
- Huang, S.; Tian, W.; Tian, J.; Tang, H.; Qin, M.; Zhao, B.; Wang, J.; Chen, Y.; Xu, H. Deep Saliva Proteomics Elucidating the Pathogenesis of Early Childhood Caries and Identifying Biomarkers for Early Prediction. J. Proteome Res. 2025, 24, 750–761. [Google Scholar] [CrossRef]
- Chen, M.; Wu, B.L.; Chen, T.; Liu, Z.; Deng, Z.L.; Peng, L. The impact of different DNA extraction methods on the analysis of microbial diversity of oral saliva from healthy youths by polymerase chain reaction-denaturing gradient gel electrophoresis. J. Dent. Sci. 2016, 11, 54–58. [Google Scholar] [CrossRef][Green Version]
- Yang, C.; Liu, N.; Zhang, Y. Soil aggregates regulate the impact of soil bacterial and fungal communities on soil respiration. Geoderma 2019, 337, 444–452. [Google Scholar] [CrossRef]
- Zhang, J.; Kobert, K.; Flouri, T.; Stamatakis, A. PEAR: A fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 2014, 30, 614–620. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Oberauner, L.; Zachow, C.; Lackner, S.; Högenauer, C.; Smolle, K.H.; Berg, G. The ignored diversity: Complex bacterial communities in intensive care units revealed by 16S pyrosequencing. Sci. Rep. 2013, 3, 1413. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.r-project.org (accessed on 20 November 2023).
- Xu, H.; Tian, J.; Hao, W.; Zhang, Q.; Zhou, Q.; Shi, W.; Qin, M.; He, X.; Chen, F. Oral Microbiome Shifts from Caries-Free to Caries-Affected Status in 3-Year-Old Chinese Children: A Longitudinal Study. Front. Microbiol. 2018, 9, 2009. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Hussain, A.; Siqueira, W.L. Mass spectrometry-based proteomic approaches for salivary protein biomarkers discovery and dental caries diagnosis: A critical review. Mass. Spectrom. Rev. 2024, 43, 826–856. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.L.; Mark Welch, J.L.; Kauffman, K.M.; McLean, J.S.; He, X. The oral microbiome: Diversity, biogeography and human health. Nat. Rev. Microbiol. 2024, 22, 89–104. [Google Scholar] [CrossRef]
- Weng, L.; Cui, Y.; Jian, W.; Zhang, Y.; Pang, L.; Cao, Y.; Zhou, Y.; Liu, W.; Lin, H.; Tao, Y. Inter-kingdom interactions and environmental influences on the oral microbiome in severe early childhood caries. Microbiol. Spectr. 2025, 13, e0251824. [Google Scholar] [CrossRef]
- Man, V.C.W.; Manchanda, S.; Yiu, C.K. Oral Candida-biome and Early Childhood Caries: A Systematic Review and Meta-Analysis. Int. Dent. J. 2025, 75, 1246–1260. [Google Scholar] [CrossRef]
- Tu, Y.; Zhou, Z.; Shu, C.; Zhou, Y.; Zhou, X. The Crosstalk Between Saliva Bacteria and Fungi in Early Childhood Caries. Front. Cell Infect. Microbiol. 2022, 12, 845738. [Google Scholar] [CrossRef]
- Li, K.; Wang, J.; Du, N.; Sun, Y.; Sun, Q.; Yin, W.; Li, H.; Meng, L.; Liu, X. Salivary microbiome and metabolome analysis of severe early childhood caries. BMC Oral Health 2023, 23, 30. [Google Scholar] [CrossRef]
- Gopalakrishnan, U.; Murthy, R.T.; Felicita, A.S.; Alshehri, A.; Awadh, W.; Almalki, A.; Vinothkumar, T.S.; Baeshen, H.A.; Bhandi, S.; Kathir, A.; et al. Sulfate-Reducing Bacteria in Patients Undergoing Fixed Orthodontic Treatment. Int. Dent. J. 2023, 73, 274–279. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wang, Z.; Tang, J.; Cao, D.X.; Qian, Y.; Xie, Y.H.; Chen, H.Y.; Chen, Y.X.; Chen, Z.F.; et al. A clinical nomogram incorporating salivary Desulfovibrio desulfuricans level and oral hygiene index for predicting colorectal cancer. Ann. Transl. Med. 2021, 9, 754. [Google Scholar] [CrossRef]
- Kaur, H.; Chakrabarti, A. Strategies to Reduce Mortality in Adult and Neonatal Candidemia in Developing Countries. J. Fungi 2017, 3, 41. [Google Scholar] [CrossRef] [PubMed]
- de Souza, C.M.; Dos Santos, M.M.; Furlaneto-Maia, L.; Furlaneto, M.C. Adhesion and biofilm formation by the opportunistic pathogen Candida tropicalis: What do we know? Can. J. Microbiol. 2023, 69, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.K.; Chen, Y.C.; Hou, C.J.; Deng, F.S.; Liang, S.H.; Hoo, S.Y.; Hsu, C.C.; Ke, C.L.; Lin, C.H. Evaluation of Biofilm Formation in Candida tropicalis Using a Silicone-Based Platform with Synthetic Urine Medium. Microorganisms 2020, 8, 660. [Google Scholar] [CrossRef]
- Xu, H.; Sobue, T.; Bertolini, M.; Thompson, A.; Vickerman, M.; Nobile, C.J.; Dongari-Bagtzoglou, A.S. oralis activates the Efg1 filamentation pathway in C. albicans to promote cross-kingdom interactions and mucosal biofilms. Virulence 2017, 8, 1602–1617. [Google Scholar] [CrossRef] [PubMed]
- Koopman, J.E.; Roling, W.F.; Buijs, M.J.; Sissons, C.H.; ten Cate, J.M.; Keijser, B.J.; Crielaard, W.; Zaura, E. Stability and resilience of oral microcosms toward acidification and Candida outgrowth by arginine supplementation. Microb. Ecol. 2015, 69, 422–433. [Google Scholar] [CrossRef]
- Kraneveld, E.A.; Buijs, M.J.; Bonder, M.J.; Visser, M.; Keijser, B.J.; Crielaard, W.; Zaura, E. The relation between oral Candida load and bacterial microbiome profiles in Dutch older adults. PLoS ONE 2012, 7, e42770. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Yang, F.; Huang, S.; Bo, C.; Xu, Z.Z.; Amir, A.; Knight, R.; Ling, J.; Xu, J. Prediction of Early Childhood Caries via Spatial-Temporal Variations of Oral Microbiota. Cell Host Microbe 2015, 18, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Wu, C.; Chen, X.; Duan, Z.; Xu, Q.; Jiang, W.; Xu, L.; Wang, T.; Su, L.; et al. Oral Microbiome Alterations Associated with Early Childhood Caries Highlight the Importance of Carbohydrate Metabolic Activities. mSystems 2019, 4, e00450-19. [Google Scholar] [CrossRef]
- Colombo, A.P.; Boches, S.K.; Cotton, S.L.; Goodson, J.M.; Kent, R.; Haffajee, A.D.; Socransky, S.S.; Hasturk, H.; Van Dyke, T.E.; Dewhirst, F.; et al. Comparisons of subgingival microbial profiles of refractory periodontitis; severe periodontitis; and periodontal health using the human oral microbe identification microarray. J. Periodontol. 2009, 80, 1421–1432. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Malcangi, G.; Semjonova, A.; Inchingolo, A.M.; Patano, A.; Coloccia, G.; Ceci, S.; Marinelli, G.; Di Pede, C.; Ciocia, A.M.; et al. Oralbiotica/Oralbiotics: The Impact of Oral Microbiota on Dental Health and Demineralization: A Systematic Review of the Literature. Children 2022, 9, 1014. [Google Scholar] [CrossRef]
- Kaur, R.; Gilbert, S.C.; Sheehy, E.C.; Beighton, D. Salivary levels of Bifidobacteria in caries-free and caries-active children. Int. J. Paediatr. Dent. 2013, 23, 32–38. [Google Scholar] [CrossRef]
- Manome, A.; Abiko, Y.; Kawashima, J.; Washio, J.; Fukumoto, S.; Takahashi, N. Acidogenic Potential of Oral Bifidobacterium and Its High Fluoride Tolerance. Front. Microbiol. 2019, 10, 1099. [Google Scholar] [CrossRef]
- Wu, G.; Zhao, H.; Li, C.; Rajapakse, M.P.; Wong, W.C.; Xu, J.; Saunders, C.W.; Reeder, N.L.; Reilman, R.A.; Scheynius, A.; et al. Genus-Wide Comparative Genomics of Malassezia Delineates Its Phylogeny; Physiology; and Niche Adaptation on Human Skin. PLoS Genet. 2015, 11, e1005614. [Google Scholar] [CrossRef]
- Fechney, J.M.; Browne, G.V.; Prabhu, N.; Irinyi, L.; Meyer, W.; Hughes, T.; Bockmann, M.; Townsend, G.; Salehi, H.; Adler, C.J. Preliminary Study of the Oral Mycobiome of Children with and Without Dental Caries. J. Oral Microbiol. 2019, 11, 1536182. [Google Scholar] [CrossRef] [PubMed]
- Baraniya, D.; Chen, T.; Nahar, A.; Alakwaa, F.; Hill, J.; Tellez, M.; Ismail, A.; Puri, S.; Al-Hebshi, N.N. Supragingival Mycobiome and Inter-Kingdom Interactions in Dental Caries. J. Oral Microbiol. 2020, 12, 1729305. [Google Scholar] [CrossRef] [PubMed]
- de Jesus, V.C.; Shikder, R.; Oryniak, D.; Mann, K.; Alamri, A.; Mittermuller, B.; Duan, K.; Hu, P.; Schroth, R.J.; Chelikani, P. Sex-Based Diverse Plaque Microbiota in Children with Severe Caries. J. Dent. Res. 2020, 99, 703–712. [Google Scholar] [CrossRef]
- Hong, B.Y.; Hoare, A.; Cardenas, A.; Dupuy, A.K.; Choquette, L.; Salner, A.L.; Schauer, P.K.; Hegde, U.; Peterson, D.E.; Dongari-Bagtzoglou, A.; et al. The Salivary Mycobiome Contains 2 Ecologically Distinct Mycotypes. J. Dent. Res. 2020, 99, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Alkhars, N.; Zeng, Y.; Alomeir, N.; Al Jallad, N.; Wu, T.T.; Aboelmagd, S.; Youssef, M.; Jang, H.; Fogarty, C.; Xiao, J. Oral Candida Predicts Streptococcus mutans Emergence in Underserved US Infants. J. Dent. Res. 2022, 101, 54–62. [Google Scholar] [CrossRef]
- Koo, H.; Andes, D.R.; Krysan, D.J. Candida-streptococcal interactions in biofilm-associated oral diseases. PLoS Pathog. 2018, 14, e1007342. [Google Scholar] [CrossRef]
- Khan, F.; Bamunuarachchi, N.I.; Pham, D.T.N.; Tabassum, N.; Khan, M.S.A.; Kim, Y.M. Mixed biofilms of pathogenic Candida-bacteria: Regulation mechanisms and treatment strategies. Crit. Rev. Microbiol. 2021, 47, 699–727. [Google Scholar] [CrossRef]
- Ren, Z.; Jeckel, H.; Simon-Soro, A.; Xiang, Z.; Liu, Y.; Cavalcanti, I.M.; Xiao, J.; Tin, N.N.; Hara, A.; Drescher, K.; et al. Interkingdom assemblages in human saliva display group-level surface mobility and disease-promoting emergent functions. Proc. Natl. Acad. Sci. USA 2022, 119, e2209699119. [Google Scholar] [CrossRef]
- Du, Q.; Ren, B.; He, J.; Peng, X.; Guo, Q.; Zheng, L.; Li, J.; Dai, H.; Chen, V.; Zhang, L.; et al. Candida albicans promotes tooth decay by inducing oral microbial dysbiosis. ISME J. 2021, 15, 894–908. [Google Scholar] [CrossRef]
- Alkhars, N.; Al Jallad, N.; Wu, T.T.; Xiao, J. Multilocus sequence typing of Candida albicans oral isolates reveals high genetic relatedness of mother-child dyads in early life. PLoS ONE 2024, 19, e0290938. [Google Scholar] [CrossRef]
- Yang, T.; Tedersoo, L.; Liu, X.; Gao, G.F.; Dong, K.; Adams, J.M.; Chu, H. Fungi stabilize multi-kingdom community in a high elevation timberline ecosystem. iMeta 2022, 1, e49. [Google Scholar] [CrossRef] [PubMed]
- Choera, T.; Zelante, T.; Romani, L.; Keller, N.P. A Multifaceted Role of Tryptophan Metabolism and Indoleamine 2,3-Dioxygenase Activity in Aspergillus fumigatus-Host Interactions. Front. Immunol. 2017, 8, 1996. [Google Scholar] [CrossRef] [PubMed]
- Costantini, C.; Bellet, M.M.; Renga, G.; Stincardini, C.; Borghi, M.; Pariano, M.; Cellini, B.; Keller, N.; Romani, L.; Zelante, T. Tryptophan Co-Metabolism at the Host-Pathogen Interface. Front. Immunol. 2020, 11, 67. [Google Scholar] [CrossRef] [PubMed]

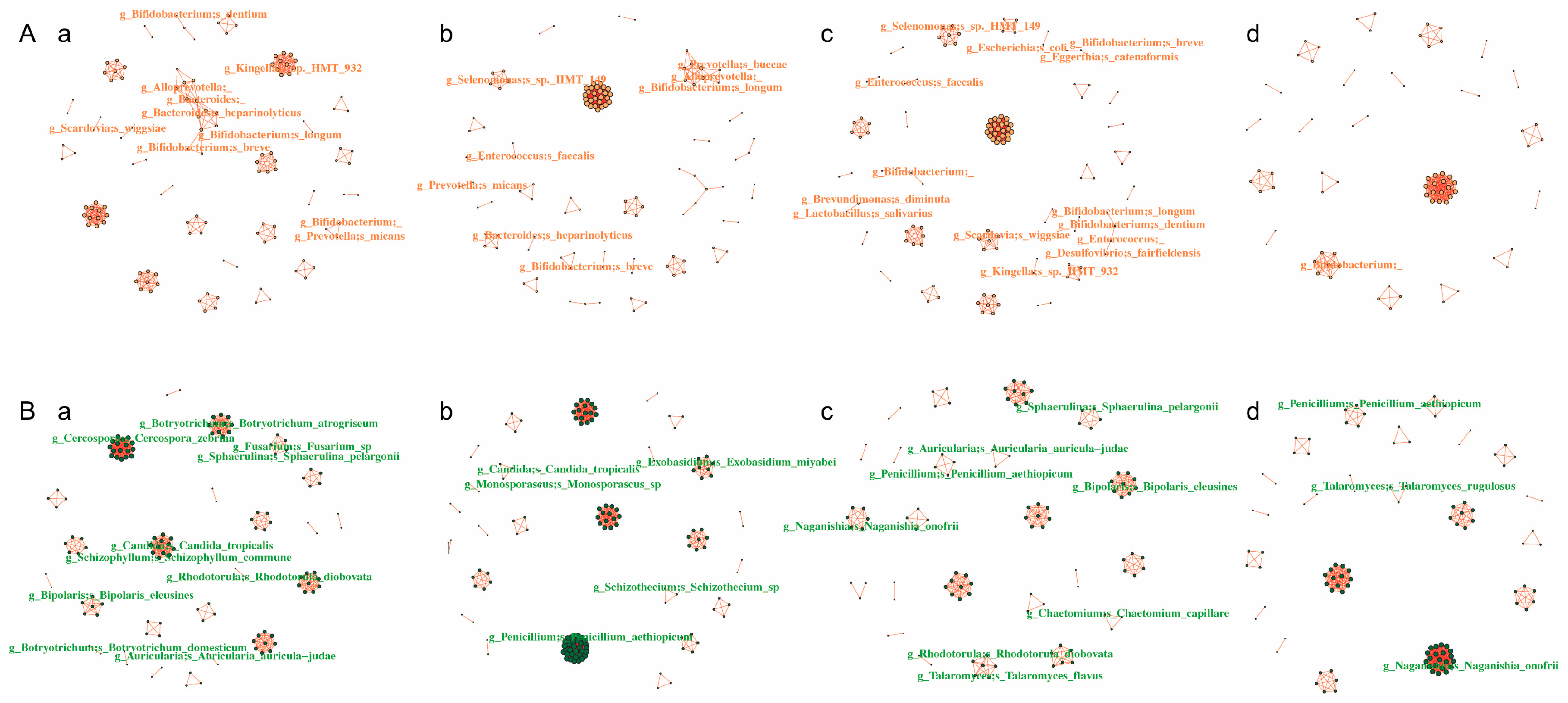
| Network Type | Group | Node | Edge | Average Network Connectivity | Average Path Length | Diameter |
|---|---|---|---|---|---|---|
| Total | CA0 | 394 | 1562 | 7.918934 | 1.966502 | 6.456498 |
| CA12 | 395 | 2116 | 10.71392 | 2.150628 | 11.34136 | |
| CF0 | 420 | 1537 | 7.319048 | 4.250946 | 11.90128 | |
| CF12 | 356 | 1080 | 6.067416 | 2.600149 | 8.836524 | |
| Bacteria to fungi | CA0 | 228 | 545 | 4.780702 | 2.410201 | 9.173526 |
| CA12 | 217 | 495 | 4.562212 | 1.69457 | 2 | |
| CF0 | 225 | 408 | 3.626667 | 1.669015 | 5.684053 | |
| CF12 | 176 | 288 | 3.272727 | 1.563037 | 2.570177 | |
| Bacteria | CA0 | 257 | 783 | 6.093385 | 3.820119 | 9.647465 |
| CA12 | 200 | 414 | 4.14 | 4.362419 | 12.15992 | |
| CF0 | 278 | 551 | 3.964029 | 4.649041 | 14.1364 | |
| CF12 | 259 | 468 | 3.6139 | 4.975864 | 12.69673 | |
| Fungi | CA0 | 149 | 464 | 6.228188 | 1.149706 | 4.021474 |
| CA12 | 173 | 910 | 10.52023 | 1.64026 | 5.713452 | |
| CF0 | 121 | 274 | 4.528962 | 1.095462 | 4.001958 | |
| CF12 | 131 | 426 | 6.5387 | 1.039188 | 2.425624 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Wang, H.; Tian, J.; Qin, M.; Gao, R.; Zhao, B.; Wang, J.; Wu, H.; Xu, H. The Impact of Bacterial–Fungal Interactions on Childhood Caries Pathogenesis. Pathogens 2025, 14, 1033. https://doi.org/10.3390/pathogens14101033
Huang S, Wang H, Tian J, Qin M, Gao R, Zhao B, Wang J, Wu H, Xu H. The Impact of Bacterial–Fungal Interactions on Childhood Caries Pathogenesis. Pathogens. 2025; 14(10):1033. https://doi.org/10.3390/pathogens14101033
Chicago/Turabian StyleHuang, Shiyan, Haojie Wang, Jing Tian, Man Qin, Ruixiang Gao, Bingqian Zhao, Jingyan Wang, Huajun Wu, and He Xu. 2025. "The Impact of Bacterial–Fungal Interactions on Childhood Caries Pathogenesis" Pathogens 14, no. 10: 1033. https://doi.org/10.3390/pathogens14101033
APA StyleHuang, S., Wang, H., Tian, J., Qin, M., Gao, R., Zhao, B., Wang, J., Wu, H., & Xu, H. (2025). The Impact of Bacterial–Fungal Interactions on Childhood Caries Pathogenesis. Pathogens, 14(10), 1033. https://doi.org/10.3390/pathogens14101033






