Serological Diagnosis of Brucella Infection in Cetaceans by Rapid Serum Agglutination Test and Competitive ELISA with Brucella abortus and Brucella ceti as Antigens
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Panel of Samples and Control Sera
2.3. Production of Antigens for Rose Bengal Test and ELISA
2.4. Rose Bengal Test
2.5. Competitive ELISA
2.6. Data Analysis
3. Results
3.1. Rose Bengal Test
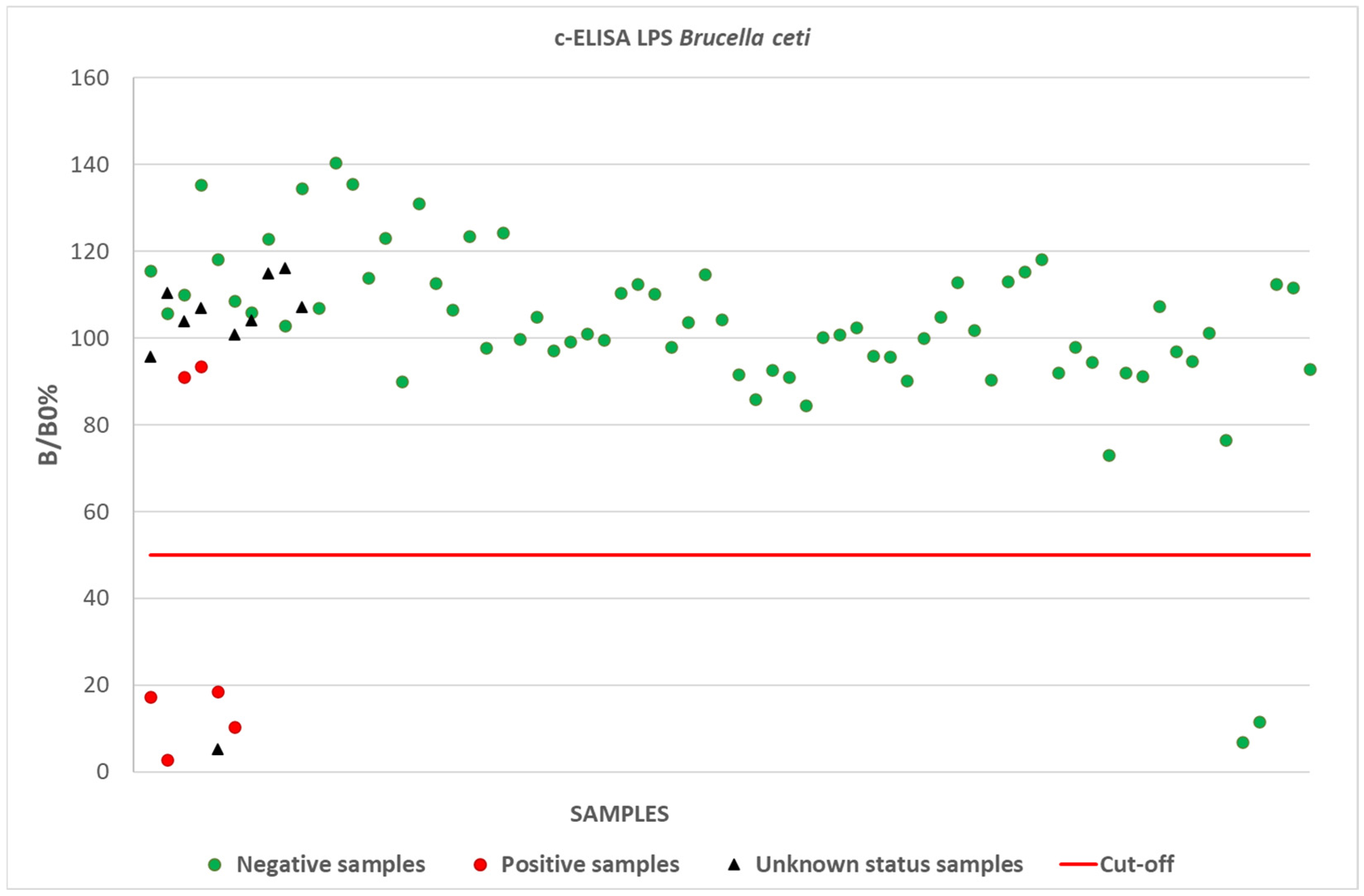
3.2. Competitive ELISA
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ross, H.M.; Foster, G.; Reid, R.J.; Jahans, K.L.; MacMillan, A.P. Brucella species infection in sea-mammals. Vet. Rec. 1994, 134, 359. [Google Scholar] [CrossRef] [PubMed]
- Ewalt, D.R.; Payeur, J.B.; Martin, B.M.; Cummins, D.R.; Miller, W.G. Characteristics of a Brucella species from a bottlenose dolphin (Tursiops truncatus). J. Vet. Diagn. Investig. 1994, 6, 448–452. [Google Scholar] [CrossRef]
- Foster, G.; Osterman, B.S.; Godfroid, J.; Jacques, I.; Cloeckaert, A. Brucella ceti sp. nov. and Brucella pinnipedialis sp. nov. for Brucella strains with cetaceans and seals as their preferred hosts. Int. J. Syst. Evol. Microbiol. 2007, 57 Pt 11, 2688–2693. [Google Scholar] [CrossRef] [PubMed]
- Alba, P.; Terracciano, G.; Franco, A.; Lorenzetti, S.; Cocumelli, C.; Fichi, G.; Eleni, C.; Zygmunt, M.S.; Cloeckaert, A.; Battisti, A. The presence of Brucella ceti ST26 in a striped dolphin (Stenella coeruleoalba) with meningoencephalitis from the Mediterranean Sea. Vet. Microbiol. 2013, 164, 158–163. [Google Scholar] [CrossRef]
- Garofolo, G.; Zilli, K.; Troiano, P.; Petrella, A.; Marotta, F.; Di Serafino, G.; Ancora, M.; Di Giannatale, E. Brucella ceti from two striped dolphins stranded on the Apulia coastline, Italy. J. Med. Microbiol. 2014, 63 Pt 2, 325–329. [Google Scholar] [CrossRef]
- Cvetnić, Ž.; Duvnjak, S.; Đuras, M.; Gomerčić, T.; Zdelar-Tuk, M.; Reil, I.; Habrun, B.; Špičić, S. Brucellosis in marine mammals, with special emphasis on the Republic of Croatia. Med. Sci. 2017, 44, 9–24. [Google Scholar]
- Nielsen, O.; Stewart, R.E.; Nielsen, K.; Measures, L.; Duignan, P. Serologic survey of Brucella spp. antibodies in some marine mammals of North America. J. Wildl. Dis. 2001, 37, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Foster, G.; MacMillan, A.P.; Godfroid, J.; Howie, F.; Ross, H.M.; Cloeckaert, A.; Reid, R.J.; Brew, S.; Patterson, I.A.P. A review of Brucella sp. infection of sea mammals with particular emphasis on isolates from Scotland. Vet. Microbiol. 2002, 90, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Mora, G.; Manire, C.A.; González-Barrientos, R.; Barquero-Calvo, E.; Guzmán-Verri, C.; Staggs, L.; Thompson, R.; Chaves-Olarte, E.; Moreno, E. Serological diagnosis of Brucella infections in odontocetes. Clin. Vaccine Immunol. 2009, 16, 906–915. [Google Scholar] [CrossRef]
- Guzmán-Verri, C.; González-Barrientos, R.; Hernández-Mora, G.; Morales, J.A.; Baquero-Calvo, E.; Chaves-Olarte, E. Brucella ceti and brucellosis in cetaceans. Front. Cell Infect. Microbiol. 2012, 2, 3. [Google Scholar] [CrossRef]
- Isidoro-Ayza, M.; Ruiz-Villalobos, N.; Pérez, L.; Guzmán-Verri, C.; Muñoz, P.M.; Alegre, F.; Barberán, M.; Chacón-Díaz, C.; Chaves-Olarte, E.; González-Barrientos, R.; et al. Brucella ceti infection in dolphins from the Western Mediterranean sea. BMC Vet. Res. 2014, 10, 206–207. [Google Scholar] [CrossRef]
- Profeta, F.; Di Francesco, C.E.; Marsilio, F.; Mignone, W.; Di Nocera, F.; De Carlo, E.; Lucifora, G.; Pietroluongo, G.; Baffoni, M.; Cocumelli, C.; et al. Retrospective seroepidemiological investigations against Morbillivirus, Toxoplasma gondii and Brucella spp. in cetaceans stranded along the Italian coastline (1998–2014). Res. Vet. Sci. 2015, 101, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Pintore, M.D.; Mignone, W.; Di Guardo, G.; Mazzariol, S.; Ballardini, M.; Florio, C.L.; Goria, M.; Romano, A.; Caracappa, S.; Giorda, F.; et al. Neuropathologic Findings in Cetaceans Stranded in Italy (2002–14). J. Wildl. Dis. 2018, 54, 295–303. [Google Scholar] [CrossRef]
- Spickler, A.R. Brucellosis in Marine Mammals; The Center for Food Security and Public Health: Ames, IA, USA, 2018; pp. 1–10. [Google Scholar]
- Ohishi, K.; Amano, M.; Nakamatsu, K.; Miyazaki, N.; Tajima, Y.; Yamada, T.K.; Matsuda, A.; Ochiai, M.; Matsuishi, T.F.; Taru, H.; et al. Serologic survey of Brucella infection in cetaceans inhabiting along the coast of Japan. J. Vet. Med. Sci. 2020, 82, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Garofolo, G.; Petrella, A.; Lucifora, G.; Di Francesco, G.; Di Guardo, G.; Pautasso, A.; Iulini, B.; Varello, K.; Giorda, F.; Goria, M.; et al. Occurrence of Brucella ceti in striped dolphins from Italian Seas. PLoS ONE 2020, 15, e0240178. [Google Scholar] [CrossRef] [PubMed]
- Martino, L.; Cuvertoret-Sanz, M.; Wilkinson, S.; Allepuz, A.; Perlas, A.; Ganges, L.; Pérez, L.; Domingo, M. Serological Investigation for Brucella ceti in Cetaceans from the Northwestern Mediterranean Sea. Animals 2024, 14, 2417. [Google Scholar] [CrossRef]
- World Organisation for Animal Health (WOAH). Chapter 3.1.4. Brucellosis (Brucella abortus, B. melitensis and B. suis) (Infection with B. abortus, B. melitensis and B. suis). In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; World Organisation for Animal Health (WOAH): Paris, France, 2024; pp. 1–47. [Google Scholar]
- McDonald, W.L.; Jamaludin, R.; Mackereth, G.; Hansen, M.; Humphrey, S.; Short, P.; Taylor, T.; Swingler, J.; Dawson, C.E.; Whatmore, A.M.; et al. Characterization of a Brucella sp. strain as a marine-mammal type despite isolation from a patient with spinal osteomyelitis in New Zealand. J. Clin. Microbiol. 2006, 44, 4363–4370. [Google Scholar] [CrossRef] [PubMed]
- Whatmore, A.M.; Dawson, C.; Groussaud, P.; Koylass, M.S.; King, A.; Shankster, S.J.; Sohn, A.H.; Probert, W.S.; McDonald, W.L. Marine mammal Brucella genotype associated with zoonotic infection. Emerg. Infect. Dis. 2008, 14, 517–518. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.D.; Vaid, R.K.; Panda, A.K.; Saini, Y. Marine mammal brucellosis: A new dimension to an old zoonosis. Curr. Sci. 2012, 103, 902–910. [Google Scholar]
- Brucellosis European Reference Laboratory (ANSES). Brucellosis. Specifications for Validation of ELISA Kits (i-ELISA and c-ELISA); ANSES: Paris, France, 2021.
- Brucellosis European Reference, Laboratory (ANSES). Brucellosis. Specifications for Validation of Antigen for Rose Bengal Test; ANSES: Paris, France, 2021.
- Geraci Joseph, R.; Lounsbury Valerie, J. Marine Mammals Ashore: A Field Guide for Strandings, 2nd ed.; Texas A&M University Sea Grant College: Galveston, TX, USA, 2005. [Google Scholar]
- Grattarola, C.; Petrella, A.; Lucifora, G.; Di Francesco, G.; Di Nocera, F.; Pintore, A.; Cocumelli, C.; Terracciano, G.; Battisti, A.; Di Renzo, L.; et al. Brucella ceti Infection in Striped Dolphins from Italian Seas: Associated Lesions and Epidemiological Data. Pathogens 2023, 12, 1034. [Google Scholar] [CrossRef] [PubMed]
- Meegan, J.; Field, C.; Sidor, I.; Romano, T.; Casinghino, S.; Smith, C.R.; Kashinsky, L.; Fair, P.A.; Bossart, G.; Wells, R.; et al. Development, validation, and utilization of a competitive enzyme-linked immunosorbent assay for the detection of antibodies against Brucella species in marine mammals. J. Vet. Diagn. Investig. 2010, 22, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Portanti, O.; Tittarelli, M.; Di Febo, T.; Luciani, M.; Mercante, M.T.; Conte, A.; Lelli, R. Development and validation of a competitive ELISA kit for the serological diagnosis of ovine, caprine and bovine brucellosis. J. Vet. Med. B Infect. Dis. Vet. Public Health 2006, 53, 494–498. [Google Scholar] [CrossRef]
- Di Febo, T.; Luciani, M.; Portanti, O.; Bonfini, B.; Lelli, R.; Tittarelli, M. Development and evaluation of diagnostic tests for the serological diagnosis of brucellosis in swine. Vet. Ital. 2012, 48, 133–156. [Google Scholar] [PubMed]
- Di Francesco, G.; Petrini, A.; D’Angelo, A.R.; Di Renzo, L.; Luciani, M.; Di Febo, T.; Ruggieri, E.; Petrella, A.; Grattarola, C.; Iulini, B.; et al. Immunohistochemical investigations on Brucella ceti-infected, neurobrucellosis-affected striped dolphins (Stenella coeruleoalba). Vet. Ital. 2019, 55, 363–367. [Google Scholar] [PubMed]
- World Organisation for Animal Health (WOAH). Chapter 1.1.6. Validation of diagnostic assays for infectious diseases of terrestrial animals. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; World Organisation for Animal Health (WOAH): Paris, France, 2024; pp. 1–27. [Google Scholar]
- Zou, K.H.; O’Malley, A.J.; Mauri, L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation 2007, 115, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Prideaux, M. Conserving Cetaceans: The Convention on Migratory Species and Its Relevant Agreements for Cetacean Conservation; WDCS: Munich, Germany, 2003; pp. 1–24. [Google Scholar]
- Nollens, H.H.; Green, L.G.; Duke, D.; Walsh, M.T.; Chittick, B.; Gearhart, S.; Klein, P.A.; Jacobson, E.R. Development and validation of monoclonal and polyclonal antibodies for the detection of immunoglobulin G of bottlenose dolphins (Tursiops Truncatustruncatus). J. Vet. Diagn. Investig. 2007, 19, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Nollens, H.H.; Ruiz, C.; Walsh, M.T.; Gulland, F.M.; Bossart, G.; Jensen, E.D.; McBain, J.F.; Wellehan, J.F. Cross-reactivity between immunoglobulin G antibodies of whales and dolphins correlates with evolutionary distance. Clin. Vaccine Immunol. 2008, 15, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- World Organisation for Animal Health (WOAH). Chapter 2.2.7. Validation of diagnostic tests for infectious diseases applicable to wildlife. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; World Organisation for Animal Health (WOAH): Paris, France, 2024; pp. 1–7. [Google Scholar]
- Tryland, M.; Handeland, K.; Bratberg, A.; Solbakk, I.; Oksanen, A. Persistence of antibodies in blood and body fluids in decaying fox carcasses, as exemplified by antibodies against Microsporum canis. Acta Vet. Scand. 2006, 48, 10. [Google Scholar] [CrossRef][Green Version]
- Anderson, T.; DeJardin, A.; Howe, D.K.; Dubey, J.P.; Michalski, M.L. Neospora caninum antibodies detected in Midwestern white-tailed deer (Odocoileus virginianus) by Western blot and ELISA. Vet. Parasitol. 2007, 145, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Chambers, M.A.; Lyashchenko, K.P.; Greenwald, R.; Esfandiari, J.; James, E.; Barker, L.; Jones, J.; Watkins, G.; Rolfe, S. Evaluation of a rapid serological test for the determination of Mycobacterium bovis infection in badgers (Meles meles) found dead. Clin. Vaccine Immunol. 2010, 17, 408–411. [Google Scholar] [CrossRef]
- Jakubek, E.; Mattsson, R.; Mörner, T.; Mattsson, J.G.; Gavier-Widén, D. Potential application of serological tests on fluids from carcasses: Detection of antibodies against Toxoplasma gondii and Sarcoptes scabiei in red foxes (Vulpes vulpes). Acta Vet. Scand. 2012, 54, 13. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, C.E.; Marsilio, F.; Proietto, U.; Mignone, W.; Casalone, C.; Di Guardo, G. Anti-Morbillivirus antibodies in stranded dolphins (Stenella coeruleoalba): Time and temperature dependent fluctuations. Aquat. Mamm. 2010, 36, 294–297. [Google Scholar] [CrossRef]
- Jakubek, E.; Uggla, A. Persistence of Neospora caninum-specific immunoglobulin G antibodies in bovine blood and lung tissue stored at room temperature. J. Vet. Diagn. Investig. 2005, 17, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Feeley, J.C. Somatic O antigen relationship of Brucella and Vibrio cholerae. J. Bacteriol. 1969, 99, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Bonfini, B.; Chiarenza, G.; Paci, V.; Sacchini, F.; Salini, R.; Vesco, G.; Villari, S.; Zilli, K.; Tittarelli, M. Cross-reactivity in serological tests for brucellosis: A comparison of immune response of Escherichia coli O157:H7 and Yersinia enterocolitica O:9 vs Brucella spp. Vet. Ital. 2018, 54, 107–114. [Google Scholar]
- Baucheron, S.; Grayon, M.; Zygmunt, M.S.; Cloeckaert, A. Lipopolysaccharide heterogeneity in Brucella strains isolated from marine mammals. Res. Microbiol. 2002, 153, 277–280. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Scientific opinion on performances of brucellosis diagnostic methods for bovines, sheep and goats. EFSA J. 2006, 432, 1–44. [Google Scholar]
- Di Febo, T.; Di Francesco, G.; Grattarola, C.; Sonsini, L.; Matteucci, O.; Di Renzo, L.; Lucifora, G.; Puleio, R.; Di Francesco, C.E.; Di Guardo, G.; et al. Serological diagnosis of Brucella spp. infection in cetaceans by Rose Bengal test and competitive ELISA with B. abortus S99 and B. ceti as antigens. In Proceedings of the 9th International Conference on Emerging Zoonoses, Palermo, Italy, 9–12 June 2024. [Google Scholar]
| Species 1 | Status (PCR/Isolation) | Matrices 2 |
|---|---|---|
| Stenella coeruleoalba (51) | 6 positive | Serum (6) |
| 35 negative | Serum (25), blood (11), cerebrospinal fluid (4), aqueous humor (3), pericardial fluid (3) | |
| not available for 10 animals | Serum (10) | |
| Tursiops truncatus (16) | 16 negative | Serum (13), blood (4), pericardial fluid (2), aqueous humor (1), tracheal fluid (1) |
| Grampus griseus (1) | 1 negative | Serum (1) |
| Globicephala melas (1) | 1 negative | Blood (1), cerebrospinal fluid (1), pericardial fluid (1) |
| Ziphius cavirostris (2) | 2 negative | Serum (1), blood (1), cerebrospinal fluid (1) |
| Brucella Positive (Isolation—PCR) | Brucella Negative (Isolation—PCR) | Total | ||
|---|---|---|---|---|
| RBT “homologous antigen” | Positives | 4 | 33 | 37 |
| Negatives | 1 | 26 | 27 | |
| Total | 5 | 59 | 64 | |
| Value | LCL (95%) | UCL (95%) | ||
| Diagnostic sensitivity | 80.0% | 35.9% | 95.7% | |
| Diagnostic specificity | 44.1% | 32.1% | 56.8% | |
| Diagnostic accuracy | 46.9% | 35.2% | 59.0% | |
| Predictive positive value | 10.8% | 4.4% | 24.8% | |
| Predictive negative value | 96.3% | 81.7% | 99.1% |
| Brucella Positive (Isolation—PCR) | Brucella Negative (Isolation—PCR) | Total | ||
|---|---|---|---|---|
| RBT “heterologous antigen” | Positives | 4 | 49 | 53 |
| Negatives | 1 | 10 | 11 | |
| Total | 5 | 59 | 64 | |
| Value | LCL (95%) | UCL (95%) | ||
| Diagnostic sensitivity | 80.0% | 35.9% | 95.7% | |
| Diagnostic specificity | 17.0% | 9.5% | 28.5% | |
| Diagnostic accuracy | 21.9% | 13.5% | 33.5% | |
| Predictive positive value | 7.6% | 3.1% | 17.9% | |
| Predictive negative value | 90.9% | 61.5% | 97.9% |
| Brucella Positive (Isolation—PCR) | Brucella Negative (Isolation—PCR) | Total | ||
|---|---|---|---|---|
| c-ELISA | Positives | 4 | 2 | 6 |
| Negatives | 2 | 72 | 74 | |
| Total | 6 | 74 | 80 | |
| Value | LCL (95%) | UCL (95%) | ||
| Diagnostic sensitivity | 66.7% | 29.0% | 90.1% | |
| Diagnostic specificity | 97.3% | 90.7% | 99.2% | |
| Diagnostic accuracy | 95.0% | 87.8% | 98.0% | |
| Predictive positive value | 66.7% | 29.0% | 90.1% | |
| Predictive negative value | 97.3% | 90.7% | 99.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Febo, T.; Di Francesco, G.; Grattarola, C.; Sonsini, L.; Di Renzo, L.; Lucifora, G.; Puleio, R.; Di Francesco, C.E.; Smoglica, C.; Di Guardo, G.; et al. Serological Diagnosis of Brucella Infection in Cetaceans by Rapid Serum Agglutination Test and Competitive ELISA with Brucella abortus and Brucella ceti as Antigens. Pathogens 2025, 14, 26. https://doi.org/10.3390/pathogens14010026
Di Febo T, Di Francesco G, Grattarola C, Sonsini L, Di Renzo L, Lucifora G, Puleio R, Di Francesco CE, Smoglica C, Di Guardo G, et al. Serological Diagnosis of Brucella Infection in Cetaceans by Rapid Serum Agglutination Test and Competitive ELISA with Brucella abortus and Brucella ceti as Antigens. Pathogens. 2025; 14(1):26. https://doi.org/10.3390/pathogens14010026
Chicago/Turabian StyleDi Febo, Tiziana, Gabriella Di Francesco, Carla Grattarola, Luigina Sonsini, Ludovica Di Renzo, Giuseppe Lucifora, Roberto Puleio, Cristina Esmeralda Di Francesco, Camilla Smoglica, Giovanni Di Guardo, and et al. 2025. "Serological Diagnosis of Brucella Infection in Cetaceans by Rapid Serum Agglutination Test and Competitive ELISA with Brucella abortus and Brucella ceti as Antigens" Pathogens 14, no. 1: 26. https://doi.org/10.3390/pathogens14010026
APA StyleDi Febo, T., Di Francesco, G., Grattarola, C., Sonsini, L., Di Renzo, L., Lucifora, G., Puleio, R., Di Francesco, C. E., Smoglica, C., Di Guardo, G., & Tittarelli, M. (2025). Serological Diagnosis of Brucella Infection in Cetaceans by Rapid Serum Agglutination Test and Competitive ELISA with Brucella abortus and Brucella ceti as Antigens. Pathogens, 14(1), 26. https://doi.org/10.3390/pathogens14010026








