Carriage Rate of Enterobacterales Resistant to Extended-Spectrum Cephalosporins in the Tunisian Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Study Design, Bacterial Isolation and Identification
2.3. Antibiotic Susceptibility Testing
2.4. Molecular Typing of the Isolates
2.5. Short-Read Whole-Genome Sequencing and Genomic Analyses
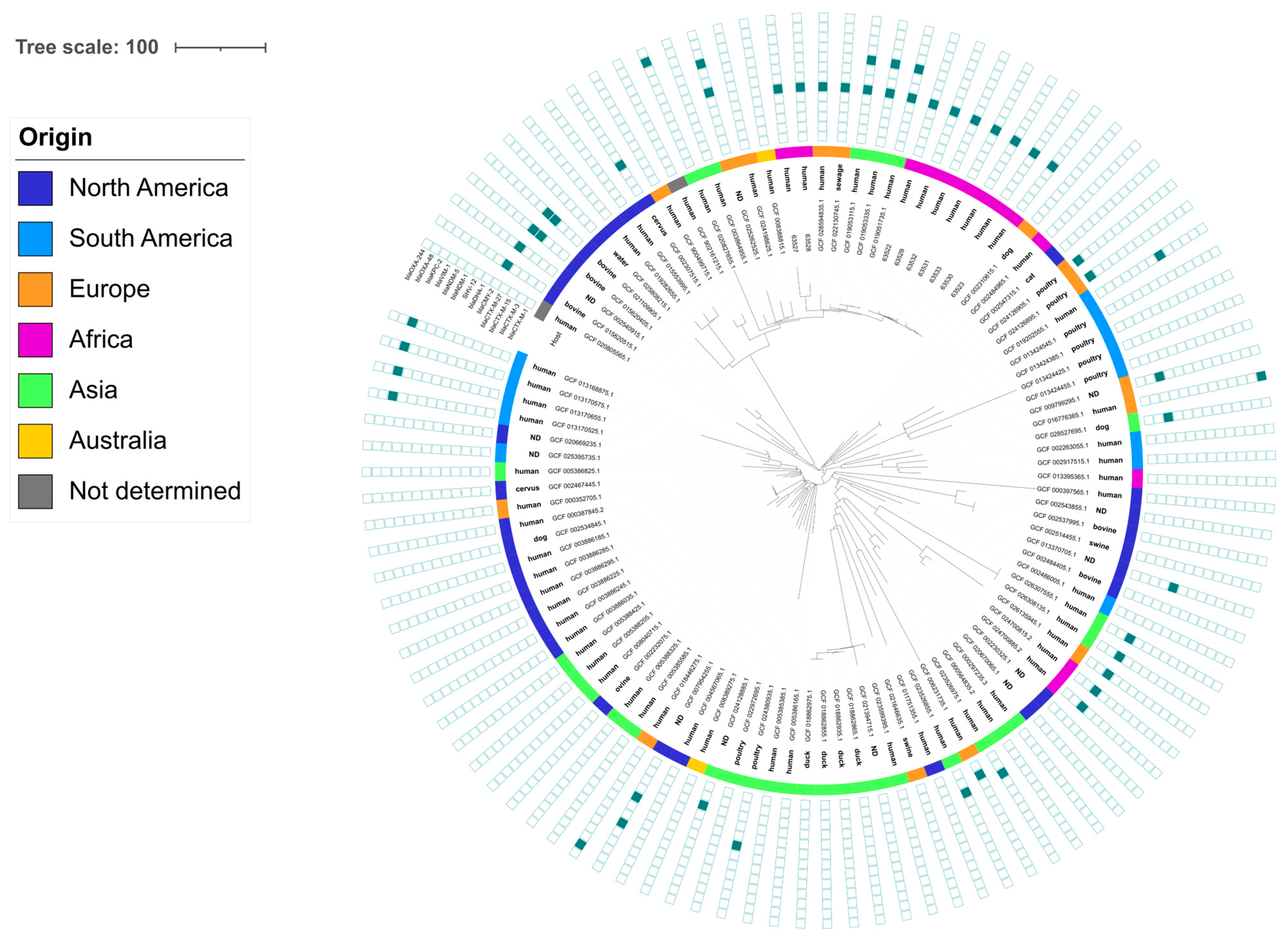
2.6. Phylogenetic Analysis
2.7. Long-Read Sequencing
2.8. Data Availability
3. Results
3.1. Characterization of Healthy Volunteers and Carriage Rate of ESC-Resistant Enterobacterales
3.2. Resistance Phenotypes and Genotypes
3.3. Characterization of ESC-Resistant Enterobacterales
3.4. Characterization of the Genetic Determinants Carrying ESBL/AmpC Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Luchen, C.C.; Chibuye, M.; Spijker, R.; Simuyandi, M.; Chisenga, C.; Bosomprah, S.; Chilengi, R.; Schultsz, C.; Mende, D.R.; Harris, V.C. Impact of antibiotics on gut microbiome composition and resistome in the first years of life in low- to middle-income countries: A systematic review. PLoS Med. 2023, 20, e1004235. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.; Guarner, F.; Bustos Fernandez, L.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as Major Disruptors of Gut Microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 572912. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Raguideau, S.; Sirén, K.; Asnicar, F.; Cumbo, F.; Hildebrand, F.; Segata, N.; Cha, C.-J.; Quince, C. Population-level impacts of antibiotic usage on the human gut microbiome. Nat. Commun. 2023, 14, 1191. [Google Scholar] [CrossRef] [PubMed]
- Birgand, G.; Armand-Lefevre, L.; Lolom, I.; Ruppe, E.; Andremont, A.; Lucet, J.C. Duration of colonization by extended-spectrum beta-lactamase-producing Enterobacteriaceae after hospital discharge. Am. J. Infect. Control 2013, 41, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Alsterlund, R.; Axelsson, C.; Olsson-Liljequist, B. Long-term carriage of extended-spectrum beta-lactamase-producing Escherichia coli. Scand. J. Infect. Dis. 2012, 44, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Al-Mir, H.; Osman, M.; Drapeau, A.; Hamze, M.; Madec, J.Y.; Haenni, M. Spread of ESC-, carbapenem- and colistin-resistant Escherichia coli clones and plasmids within and between food workers in Lebanon. J. Antimicrob. Chemother. 2021, 76, 3135–3143. [Google Scholar] [CrossRef] [PubMed]
- Bezabih, Y.M.; Sabiiti, W.; Alamneh, E.; Bezabih, A.; Peterson, G.M.; Bezabhe, W.M.; Roujeinikova, A. The global prevalence and trend of human intestinal carriage of ESBL-producing Escherichia coli in the community. J. Antimicrob. Chemother. 2021, 76, 22–29. [Google Scholar] [CrossRef]
- Ben Sallem, R.; Ben Slama, K.; Estepa, V.; Jouini, A.; Gharsa, H.; Klibi, N.; Saenz, Y.; Ruiz-Larrea, F.; Boudabous, A.; Torres, C. Prevalence and characterisation of extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli isolates in healthy volunteers in Tunisia. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1511–1516. [Google Scholar] [CrossRef]
- Ferjani, S.; Saidani, M.; Hamzaoui, Z.; Alonso, C.A.; Torres, C.; Maamar, E.; Slim, A.F.; Boutiba, B.B.I. Community fecal carriage of broad-spectrum cephalosporin-resistant Escherichia coli in Tunisian children. Diagn. Microbiol. Infect. Dis. 2017, 87, 188–192. [Google Scholar] [CrossRef]
- Ben Sallem, R.; Laribi, B.; Arfaoui, A.; Ben Khelifa Melki, S.; Ouzari, H.I.; Ben Slama, K.; Naas, T.; Klibi, N. Co-occurrence of genes encoding carbapenemase, ESBL, pAmpC and non-β-Lactam resistance among Klebsiella pneumoniae and E. coli clinical isolates in Tunisia. Lett. Appl. Microbiol. 2022, 74, 729–740. [Google Scholar] [CrossRef]
- Harbaoui, S.; Ferjani, S.; Abbassi, M.S.; Saidani, M.; Gargueh, T.; Ferjani, M.; Hammi, Y.; Boutiba-Ben Boubaker, I. Genetic heterogeneity and predominance of blaCTX-M-15 in cefotaxime-resistant Enterobacteriaceae isolates colonizing hospitalized children in Tunisia. Lett. Appl. Microbiol. 2022, 75, 1460–1474. [Google Scholar] [CrossRef] [PubMed]
- Mnif, B.; Ktari, S.; Rhimi, F.M.; Hammami, A. Extensive dissemination of CTX-M-1- and CMY-2-producing Escherichia coli in poultry farms in Tunisia. Lett. Appl. Microbiol. 2012, 55, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Ben Slama, K.; Jouini, A.; Sallem, R.B.; Somalo, S.; Saenz, Y.; Estepa, V.; Boudabous, A.; Torres, C. Prevalence of broad-spectrum cephalosporin-resistant Escherichia coli isolates in food samples in Tunisia, and characterization of integrons and antimicrobial resistance mechanisms implicated. Int. J. Food Microbiol. 2010, 137, 281–286. [Google Scholar] [CrossRef]
- Saidani, M.; Messadi, L.; Chaouechi, A.; Tabib, I.; Saras, E.; Soudani, A.; Daaloul-Jedidi, M.; Mamlouk, A.; Ben Chehida, F.; Chakroun, C.; et al. High genetic diversity of Enterobacteriaceae clones and plasmids disseminating resistance to extended-spectrum cephalosporins and colistin in healthy chicken in Tunisia. Microb. Drug Resist. 2019, 25, 1507–1513. [Google Scholar] [CrossRef]
- Sola, M.; Mani, Y.; Saras, E.; Drapeau, A.; Grami, R.; Aouni, M.; Madec, J.Y.; Haenni, M.; Mansour, W. Prevalence and characterization of extended-spectrum β-lactamase- and carbapenemase-producing Enterobacterales from Tunisian seafood. Microorganisms 2022, 10, 1364. [Google Scholar] [CrossRef] [PubMed]
- Saidani, M.; Messadi, L.; Sahmin, E.; Zouaoui, S.; Soudani, A.; Daaloul-Jedidi, M.; Mamlouk, A.; Chehida, F.B.; Madec, J.-Y.; Haenni, M. ESBL- and mcr-1-producing Escherichia coli in veal calves in Tunisia. J. Glob. Antimicrob. Resist. 2019, 19, 104–105. [Google Scholar] [CrossRef]
- EUCAST. Recommendations for MIC Determination of Colistin (Polymyxin E) as Recommended by the Joint CLSI-EUCAST Polymyxin Breakpoints Working Group. 2016. Available online: https://www.eucast.org/eucastguidancedocuments (accessed on 1 July 2024).
- Doumith, M.; Day, M.J.; Hope, R.; Wain, J.; Woodford, N. Improved multiplex PCR strategy for rapid assignment of the four major Escherichia coli phylogenetic groups. J. Clin. Microbiol. 2012, 50, 3108–3110. [Google Scholar] [CrossRef]
- Souguir, M.; Châtre, P.; Drapeau, A.; Azaiez, S.; Hmidi, I.; Ncir, S.; Lupo, A.; Madec, J.-Y.; Haenni, M.; Mansour, W. CTX-M-15/27-positive Escherichia coli and VIM-2-producing Pseudomonas putida in free-living pigeons (Columba livia) in Tunisia. J. Glob. Antimicrob. Resist. 2024, 36, 70–75. [Google Scholar] [CrossRef]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, X.; Lu, N.; Zhu, B. The abundance of antibiotic resistance genes in human guts has correlation to the consumption of antibiotics in animal. Gut Microbes 2014, 5, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Schröder, W.; Sommer, H.; Gladstone, B.P.; Foschi, F.; Hellman, J.; Evengard, B.; Tacconelli, E. Gender differences in antibiotic prescribing in the community: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2016, 71, 1800–1806. [Google Scholar] [CrossRef] [PubMed]
- Rizzetto, L.; Fava, F.; Tuohy, K.M.; Selmi, C. Connecting the immune system, systemic chronic inflammation and the gut microbiome: The role of sex. J. Autoimmun. 2018, 92, 12–34. [Google Scholar] [CrossRef]
- Sarmiento, M.R.A.; de Paula, T.O.; Borges, F.M.; Ferreira-Machado, A.B.; Resende, J.A.; Moreira, A.P.B.; Dutra Luquetti, S.C.P.; Cesar, D.E.; da Silva, V.L.; Diniz, C.G. Obesity, xenobiotic intake and antimicrobial-resistance genes in the human gastrointestinal tract: A comparative study of eutrophic, overweight and obese Individuals. Genes 2019, 10, 349. [Google Scholar] [CrossRef]
- Narayanan, N.; Lin, T.; Vinarov, D.; Bucek, T.; Johnson, L.; Mathew, C.; Chaudhry, S.; Brunetti, L. Relationship between multidrug-resistant Enterobacterales and obesity in older adults. Infect. Drug Resist. 2021, 14, 2527–2532. [Google Scholar] [CrossRef] [PubMed]
- Tenaillon, O.; Skurnik, D.; Picard, B.; Denamur, E. The population genetics of commensal Escherichia coli. Nat. Rev. Microbiol. 2010, 8, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Huang, Z.; Xiao, Y.; Gao, H.; Bai, X.; Wang, D. Global spread characteristics of CTX-M-type extended-spectrum β-lactamases: A genomic epidemiology analysis. Drug Resist. Updates 2024, 73, 101036. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, C.T.; Lipsitch, M.; Levin, B.R. Natural selection, infectious transfer and the existence conditions for bacterial plasmids. Genetics 2000, 155, 1505–1519. [Google Scholar] [CrossRef] [PubMed]
- Haenni, M.; Saras, E.; Metayer, V.; Doublet, B.; Cloeckaert, A.; Madec, J.Y. Spread of the blaTEM-52 gene is mainly ensured by IncI1/ST36 plasmids in Escherichia coli isolated from cattle in France. J. Antimicrob. Chemother. 2012, 67, 2774–2776. [Google Scholar] [CrossRef]
- Hordijk, J.; Wagenaar, J.A.; Kant, A.; van Essen-Zandbergen, A.; Dierikx, C.; Veldman, K.; Wit, B.; Mevius, D. Cross-sectional study on prevalence and molecular characteristics of plasmid mediated ESBL/AmpC-producing Escherichia coli isolated from veal calves at slaughter. PLoS ONE 2013, 8, e65681. [Google Scholar] [CrossRef]
- Dahmen, S.; Haenni, M.; Madec, J.Y. IncI1/ST3 plasmids contribute to the dissemination of the blaCTX-M-1 gene in Escherichia coli from several animal species in France. J. Antimicrob. Chemother. 2012, 67, 3011–3012. [Google Scholar] [CrossRef]
- Ferreira, J.C.; Penha Filho, R.A.; Andrade, L.N.; Berchieri, A., Jr.; Darini, A.L. IncI1/ST113 and IncI1/ST114 conjugative plasmids carrying blaCTX-M-8 in Escherichia coli isolated from poultry in Brazil. Diagn. Microbiol. Infect. Dis. 2014, 80, 304–306. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.A.; Dierikx, C.M.; van Essen-Zandbergen, A.; van Roermund, H.J.; Mevius, D.J.; Stegeman, A.; Klinkenberg, D. The IncI1 plasmid carrying the blaCTX-M-1 gene persists in in vitro culture of a Escherichia coli strain from broilers. BMC Microbiol. 2014, 14, 77. [Google Scholar] [CrossRef]
- Zurfluh, K.; Wang, J.; Klumpp, J.; Nuesch-Inderbinen, M.; Fanning, S.; Stephan, R. Vertical transmission of highly similar blaCTX-M-1-harboring IncI1 plasmids in Escherichia coli with different MLST types in the poultry production pyramid. Front. Microbiol. 2014, 5, 519. [Google Scholar] [CrossRef]
- Cloeckaert, A.; Praud, K.; Lefevre, M.; Doublet, B.; Pardos, M.; Granier, S.A.; Brisabois, A.; Weill, F.X. IncI1 plasmid carrying extended-spectrum beta-lactamase gene blaCTX-M-1 in Salmonella enterica isolates from poultry and humans in France, 2003 to 2008. Antimicrob. Agents Chemother. 2010, 54, 4484–4486. [Google Scholar] [CrossRef]
- Madec, J.Y.; Haenni, M.; Metayer, V.; Saras, E.; Nicolas-Chanoine, M.H. High prevalence of the animal-associated blaCTX-M-1 IncI1/ST3 plasmid in human Escherichia coli isolates. Antimicrob. Agents Chemother. 2015, 59, 5860–5861. [Google Scholar] [CrossRef]
- Ben Sallem, R.; Ben Slama, K.; Rojo-Bezares, B.; Porres-Osante, N.; Jouini, A.; Klibi, N.; Boudabous, A.; Saenz, Y.; Torres, C. IncI1 plasmids carrying blaCTX-M-1 or blaCMY-2 genes in Escherichia coli from healthy humans and animals in Tunisia. Microb. Drug Resist. 2014, 20, 495–500. [Google Scholar] [CrossRef]
- Du, P.; Zhang, P.; Wang, J.; Li, R.; Fanning, S.; Bai, L. Molecular characterization of two novel NDM-1-producing atypical enteroaggregative Escherichia coli isolates from patients. Plasmid 2021, 115, 102568. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, A.P.A.; Landman, F.; de Haan, A.; Witteveen, S.; van Santen-Verheuvel, M.G.; Schouls, L.M.; Dutch Cpe Surveillance Study Group. blaOXA-48-like genome architecture among carbapenemase-producing Escherichia coli and Klebsiella pneumoniae in the Netherlands. Microb. Genom. 2021, 7, 000512. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Ngan, B.T.; Huong, B.T.; Hirai, I.; Tuyen, L.D.; Yamamoto, Y. Limited transmission of blaCTX-M-9-type-positive Escherichia coli between humans and poultry in Vietnam. Antimicrob. Agents Chemother. 2015, 59, 3574–3577. [Google Scholar] [CrossRef]
- Grevskott, D.H.; Ghavidel, F.Z.; Svanevik, C.S.; Marathe, N.P. Resistance profiles and diversity of β-lactamases in Escherichia coli strains isolated from city-scale sewage surveillance in Bergen, Norway mimic clinical prevalence. Ecotoxicol. Environ. Saf. 2021, 226, 112788. [Google Scholar] [CrossRef] [PubMed]
- Haverkate, M.R.; Platteel, T.N.; Fluit, A.C.; Cohen Stuart, J.W.; Leverstein-van Hall, M.A.; Thijsen, S.F.T.; Scharringa, J.; Kloosterman, R.C.; Bonten, M.J.M.; Bootsma, M.C.J. Quantifying within-household transmission of extended-spectrum β-lactamase-producing bacteria. Clin. Microbiol. Infect. 2017, 23, 46.e1–46.e7. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Baño, J.; López-Cerero, L.; Navarro, M.D.; de Alba, P.D.; Pascual, A. Faecal carriage of extended-spectrum β-lactamase-producing Escherichia coli: Prevalence, risk factors and molecular epidemiology. J. Antimicrob. Chemother. 2008, 62, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Mughini-Gras, L.; Dorado-García, A.; van Duijkeren, E.; van den Bunt, G.; Dierikx, C.M.; Bonten, M.J.M.; Bootsma, M.C.J.; Schmitt, H.; Hald, T.; Evers, E.G.; et al. Attributable sources of community-acquired carriage of Escherichia coli containing β-lactam antibiotic resistance genes: A population-based modelling study. Lancet Planet. Health 2019, 3, e357–e369. [Google Scholar] [CrossRef] [PubMed]


| E. coli (n = 24) | ||
|---|---|---|
| No. of Strains | % of Resistance | |
| Kanamycin | 2 | 8.3 |
| Tobramycin | 1 | 4.2 |
| Gentamicin | 1 | 4.2 |
| Apramycin | 0 | 0.0 |
| Streptomycin | 14 | 58.3 |
| Amikacin | 0 | 0.0 |
| Netilmicin | 0 | 0.0 |
| Tetracycline | 18 | 75.0 |
| Chloramphenicol | 2 | 8.3 |
| Florfenicol | 2 | 8.3 |
| Colistin | 0 | 0.0 |
| Nalidixic acid | 2 | 8.3 |
| Ciprofloxacin | 1 | 4.2 |
| Trimethoprim | 18 | 75.0 |
| Sulfonamides | 18 | 75.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahjoub Khachroub, A.; Souguir, M.; Châtre, P.; Elhouda Bouhlel, N.; Jaidane, N.; Drapeau, A.; El Kantaoui, M.; Azaiez, S.; Madec, J.-Y.; Mansour, W.; et al. Carriage Rate of Enterobacterales Resistant to Extended-Spectrum Cephalosporins in the Tunisian Population. Pathogens 2024, 13, 624. https://doi.org/10.3390/pathogens13080624
Mahjoub Khachroub A, Souguir M, Châtre P, Elhouda Bouhlel N, Jaidane N, Drapeau A, El Kantaoui M, Azaiez S, Madec J-Y, Mansour W, et al. Carriage Rate of Enterobacterales Resistant to Extended-Spectrum Cephalosporins in the Tunisian Population. Pathogens. 2024; 13(8):624. https://doi.org/10.3390/pathogens13080624
Chicago/Turabian StyleMahjoub Khachroub, Ahlem, Meriem Souguir, Pierre Châtre, Nour Elhouda Bouhlel, Nadia Jaidane, Antoine Drapeau, Marah El Kantaoui, Sana Azaiez, Jean-Yves Madec, Wejdene Mansour, and et al. 2024. "Carriage Rate of Enterobacterales Resistant to Extended-Spectrum Cephalosporins in the Tunisian Population" Pathogens 13, no. 8: 624. https://doi.org/10.3390/pathogens13080624
APA StyleMahjoub Khachroub, A., Souguir, M., Châtre, P., Elhouda Bouhlel, N., Jaidane, N., Drapeau, A., El Kantaoui, M., Azaiez, S., Madec, J.-Y., Mansour, W., & Haenni, M. (2024). Carriage Rate of Enterobacterales Resistant to Extended-Spectrum Cephalosporins in the Tunisian Population. Pathogens, 13(8), 624. https://doi.org/10.3390/pathogens13080624






