Use of Matrix-Assisted and Laser Desorption/Ionization Time-of-Flight Technology in the Identification of Aeromonas Strains Isolated from Retail Sushi and Sashimi
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Plan
2.2. Preparation of the Bacterial Colonies for Step 1 and Step 2
2.3. Evaluation of SARAMIS Software—V4.12 in Aeromonas Identification
2.4. Implementation of SARAMIS Database for Aeromonas Identification and Evaluation of Its Efficiency
2.5. Evaluation of Aeromonas spp. in Retail Sushi and Sashimi Boxes
2.5.1. Sampling of Retail Sushi and Sashimi Boxes
2.5.2. Microbiological Analysis: Aeromonas Detection and Enumeration
2.6. Data Analysis
3. Results
3.1. Preliminary Assessment of SARAMIS V4.12 Performance in Aeromonas Identification
3.2. Evaluation of the SARAMIS Version Implemented in Aeromonas Identification
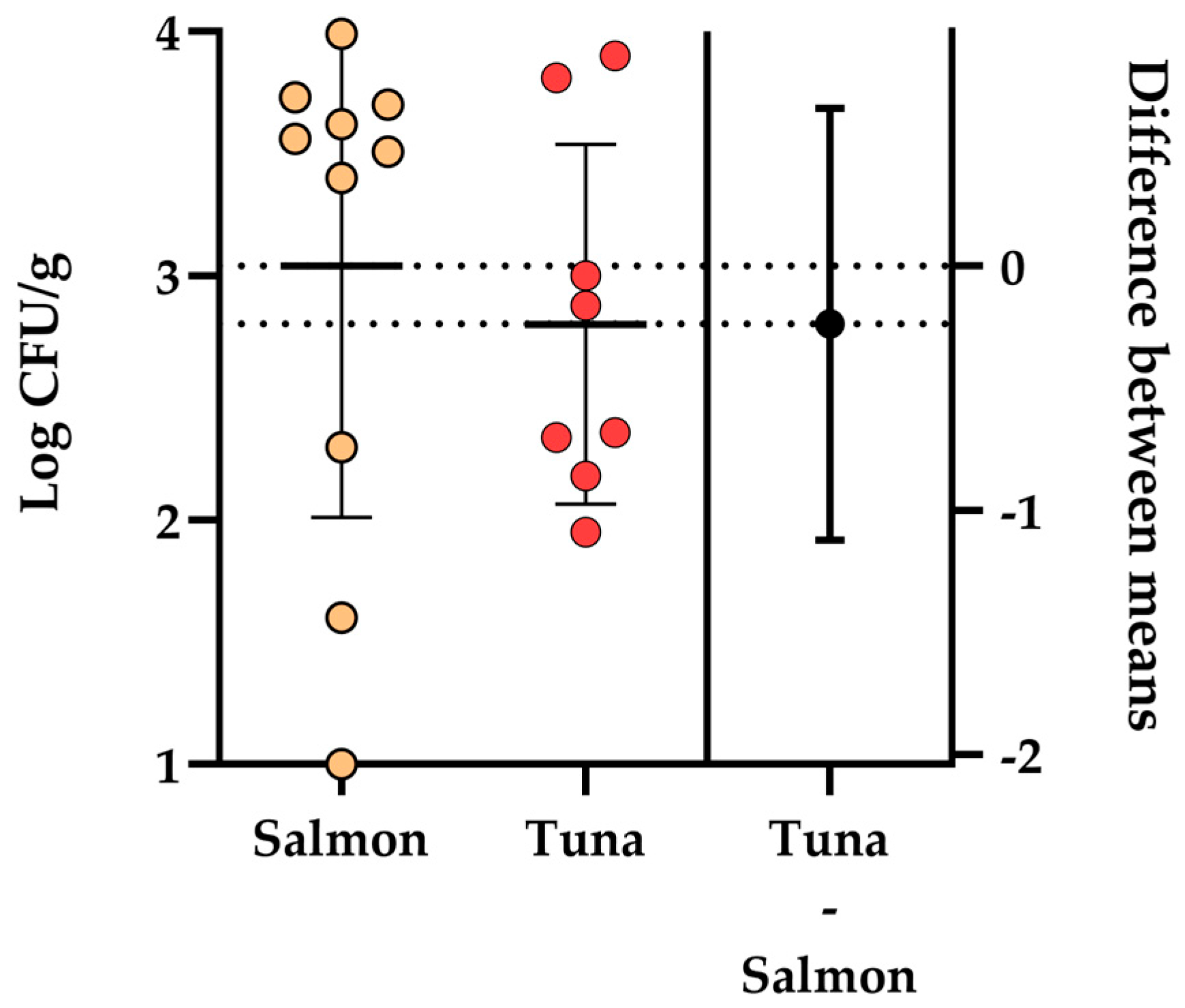
3.3. Evaluation of Aeromonas spp. in Retail Sushi and Sashimi Boxes
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kitagawa, D.; Suzuki, Y.; Abe, N.; Ui, K.; Suzuki, K.; Yamashita, T.; Sakaguchi, A.; Suzuki, S.; Masuo, K.; Nakano, A.; et al. Comparison of MALDI-TOF mass spectrometry and rpoB gene sequencing for the identification of clinical isolates of Aeromonas spp. Heliyon 2022, 8, e11585. [Google Scholar] [CrossRef] [PubMed]
- Botero, L.; Galeano, L.; Montoya, L.J.; Machado, A.; Byrne, J.A.; Fernandez-Ibañez, P.; Hincapié, M. Aeromonas hydrophila in surface water and their removal using a POU technology for drinking in rural communities. Environ. Adv. 2023, 13, 100425. [Google Scholar] [CrossRef]
- Pérez-Sancho, M.; Cerdá, I.; Fernández-Bravo, A.; Domínguez, L.; Figueras, M.J.; Fernández-Garayzábal, J.F.; Vela, A.I. Limited performance of MALDI-TOF for identification of fish Aeromonas isolates at species level. J. Fish Dis. 2018, 41, 1485–1493. [Google Scholar] [CrossRef] [PubMed]
- Attia, A.S.; Khedr, M.H.; Zaki, M.S. Occurrence of potentially pathogenic Aeromonas species isolated from raw and ready-to-eat fish marketed in Sharkia Governorate, Egypt. Zagazig Vet. J. 2018, 46, 154–159. [Google Scholar] [CrossRef]
- Hafez, A.E.E.; Darwish, W.S.; Elbayomi, R.M.; Hussein, M.A.; El Nahal, S.M. Prevalence, antibiogram and molecular characterization of Aeromonas hydrophila isolated from frozen fish marketed in Egypt. Slov. Vet. Res. 2018, 55, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Hoel, S.; Vadstein, O.; Jakobsen, A.N. The significance of mesophilic Aeromonas spp. in minimally processed ready-to-eat seafood. Microorganisms 2019, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Hoel, S.; Lunestad, B.T.; Lerfall, J.; Jakobsen, A.N. Aeromonas spp. isolated from ready-to-eat seafood on the Norwegian market: Prevalence, putative virulence factors and antimicrobial resistance. J. Appl. Microb. 2021, 130, 1380–1393. [Google Scholar] [CrossRef] [PubMed]
- Lee, H. Mesophilic Aeromonas spp. Isolated from Ready-to-Eat Seafood: Insight into Antimicrobial Resistance, Virulence Factors, and Growth Kinetics in Food. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, September 2023. Available online: https://hdl.handle.net/11250/3090841 (accessed on 1 January 2024).
- Praveen, P.K.; Debnath, C.; Shekhar, S.; Dalai, N.; Ganguly, S. Incidence of Aeromonas spp. infection in fish and chicken meat and its related public health hazards: A review. Vet. World 2016, 9, 6. [Google Scholar] [PubMed]
- Teodoro, J.R.; Carvalho, G.G.; Queiroz, M.M.; Levy, C.E.; Kabuki, D.Y. Incidence, evaluation of detection and identification methods, and antimicrobial resistance of Aeromonas spp. in ready-to-eat foods. Int. J. Food Microbiol. 2022, 379, 109862. [Google Scholar] [CrossRef] [PubMed]
- Abbott, S.L.; Cheung, W.K.; Janda, J.M. The genus Aeromonas: Biochemical characteristics, atypical reactions, and phenotypic identification schemes. J. Clin. Microbiol. 2003, 41, 2348–2357. [Google Scholar] [CrossRef] [PubMed]
- Janda, J.M.; Abbott, S.L. The genus Aeromonas: Taxonomy, pathogenicity, and infection. Clin. Microbiol. Rev. 2010, 23, 35–73. [Google Scholar] [CrossRef]
- Pessoa, R.B.G.; Oliveira, W.F.D.; Correia, M.T.D.S.; Fontes, A.; Coelho, L.C.B.B. Aeromonas and human health disorders: Clinical approaches. Front. Microbiol. 2022, 13, 868890. [Google Scholar] [CrossRef] [PubMed]
- Giarratana, F. Aeromonas spp. In Igiene e Tecnologie Degli Alimenti, 1st ed.; Colavita, G., Ed.; Point Vètèrinaire Italie: Milan, Italy, 2023; pp. 127–131. [Google Scholar]
- Fernández-Bravo, A.; Figueras, M.J. An update on the genus Aeromonas: Taxonomy, epidemiology, and pathogenicity. Microorganisms 2020, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Teunis, P.; Figueras, M.J. Reassessment of the enteropathogenicity of mesophilic Aeromonas species. Front. Microbiol. 2016, 7, 1395. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, R.B.G.; de Oliveira, W.F.; Marques, D.S.C.; dos Santos Correia, M.T.; de Carvalho, E.V.M.M.; Coelho, L.C.B.B. The genus Aeromonas: A general approach. Microb. Pathog. 2019, 130, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Popović, N.T.; Kazazić, S.P.; Strunjak-Perović, I.; Čož-Rakovac, R. Differentiation of environmental aquatic bacterial isolates by MALDI-TOF MS. Environ. Res. 2017, 152, 7–16. [Google Scholar] [CrossRef]
- Nhung, P.H.; Hata, H.; Ohkusu, K.; Noda, M.; Shah, M.M.; Goto, K.; Ezaki, T. Use of the novel phylogenetic marker dnaJ and DNA–DNA hybridization to clarify interrelationships within the genus Aeromonas. Int. J. Syst. Evol. Microbiol. 2007, 57, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Vávrová, A.; Balážová, T.; Sedláček, I.; Tvrzová, L.; Šedo, O. Evaluation of the MALDI-TOF MS profiling for identification of newly described Aeromonas spp. Folia Microbiol. 2015, 60, 375–383. [Google Scholar] [CrossRef]
- Ashfaq, M.Y.; Da’na, D.A.; Al-Ghouti, M.A. Application of MALDI-TOF MS for identification of environmental bacteria: A review. J. Environ. Manag. 2022, 305, 114359. [Google Scholar] [CrossRef] [PubMed]
- Rychert, J.; Creely, D.; Mayo-Smith, L.M.; Calderwood, S.B.; Ivers, L.C.; Ryan, E.T.; Harris, J.B. Evaluation of matrix-assisted laser desorption ionization–time of flight mass spectrometry for identification of Vibrio cholerae. J. Clin. Microbiol. 2015, 53, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Seng, P.; Rolain, J.M.; Fournier, P.E.; La Scola, B.; Drancourt, M.; Raoult, D. MALDI-TOF-mass spectrometry applications in clinical microbiology. Future Microbiol. 2010, 5, 1733–1754. [Google Scholar] [CrossRef] [PubMed]
- Ziino, G.; Marotta, S.M.; Giarratana, F.; Giuffrida, A.; Panebianco, F. Reliability evaluation of MALDI-TOF MS associated with SARAMIS software in rapid identification of thermophilic Campylobacter isolated from food. Food Anal. Methods 2019, 12, 1128–1132. [Google Scholar] [CrossRef]
- Martiny, D.; Busson, L.; Wybo, I.; El Haj, R.A.; Dediste, A.; Vandenberg, O. Comparison of the Microflex LT and Vitek MS systems for routine identification of bacteria by matrix-assisted laser desorption ionization–time of flight mass spectrometry. J. Clin. Microbiol. 2012, 50, 1313–1325. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.L.; Lee, T.F.; Wu, C.J.; Teng, S.H.; Teng, L.J.; Ko, W.C.; Hsueh, P.R. Matrix-assisted laser desorption ionization–time of flight mass spectrometry can accurately differentiate Aeromonas dhakensis from A. hydrophila, A. caviae, and A. veronii. J. Clin. Microbiol. 2014, 52, 2625–2628. [Google Scholar] [CrossRef] [PubMed]
- SARAMIS Manual; Streit, I.; Welker, M. SARAMISTM Manual, AnagnosTec-Release 3.3.2. 2010. Available online: http://www.anagnostec.eu (accessed on 19 March 2024).
- Porte, L.; Garcia, P.; Braun, S.; Ulloa, M.T.; Lafourcade, M.; Montaña, A.; Weitzel, T. Head-to-head comparison of Microflex LT and Vitek MS systems for routine identification of microorganisms by MALDI-TOF mass spectrometry in Chile. PLoS ONE 2017, 12, e0177929. [Google Scholar] [CrossRef]
- Shin, H.B.; Yoon, J.; Lee, Y.; Kim, M.S.; Lee, K. Comparison of MALDI-TOF MS, housekeeping gene sequencing, and 16S rRNA gene sequencing for identification of Aeromonas clinical isolates. Yonsei Med. J. 2015, 56, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Soler, L.; Yanez, M.A.; Chacon, M.R.; Aguilera-Arreola, M.G.; Catalán, V.; Figueras, M.J.; Martínez-Murcia, A.J. Phylogenetic analysis of the genus Aeromonas based on two housekeeping genes. Int. J. Syst. Evol. Microbiol. 2004, 54, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.D.; Terio, V.; Pinto, P.D.; Tantillo, G. Detection of potentially pathogenic Aeromonas isolates from ready-to-eat seafood products by PCR analysis. Int. J. Food Sci. Tech. 2012, 47, 269–273. [Google Scholar] [CrossRef]
- Hoel, S.; Mehli, L.; Bruheim, T.; Vadstein, O.; Jakobsen, A.N. Assessment of microbiological quality of retail fresh sushi from selected sources in Norway. J. Food Prot. 2015, 78, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shi, G.Q.; Tang, G.P.; Zou, Z.T.; Yao, G.H.; Zeng, G. A foodborne outbreak of Aeromonas hydrophila in a college, Xingyi City, Guizhou, China, 2012. West Pac. Surveill. Resp. J. 2012, 3, 39. [Google Scholar] [CrossRef]
- Hoel, S.; Vadstein, O.; Jakobsen, A.N. Species distribution and prevalence of putative virulence factors in mesophilic Aeromonas spp. isolated from fresh retail sushi. Front. Microbiol. 2017, 8, 265738. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Han, J.E.; Kwon, H.; Park, S.C.; Kim, J.H. Recent insights into Aeromonas salmonicida and its bacteriophages in aquaculture: A comprehensive review. J. Microbiol. Biotechnol. 2020, 30, 1443. [Google Scholar] [CrossRef] [PubMed]
- Kumru, S.; Tekedar, H.C.; Griffin, M.J.; Waldbieser, G.C.; Liles, M.R.; Sonstegard, T.; Schroeder, S.G.; Lawrence, M.L.; Karsi, A. Draft Genome Sequence of Fish Pathogen Aeromonas bestiarum GA97-22. Genome Announc. 2018, 6, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Carusi, J.; Kabuki, D.Y.; de Seixas Pereira, P.M.; Cabral, L. Aeromonas spp. in drinking water and food: Occurrence, virulence potential and antimicrobial resistance. Food Res. Int. 2023, 175, 113710. [Google Scholar] [CrossRef] [PubMed]
- Bandeira Junior, G.; Baldisserotto, B. Fish infections associated with the genus Aeromonas: A review of the effects on oxidative status. J. Appl. Microbiol. 2021, 131, 1083–1101. [Google Scholar] [CrossRef] [PubMed]
- Muscolino, D.; Giarratana, F.; Beninati, C.; Tornambene, A.; Panebianco, A.; Ziino, G. Hygienic-sanitary evaluation of sushi and sashimi sold in Messina and Catania, Italy. Ital. J. Food Saf. 2014, 3, 1701. [Google Scholar] [CrossRef] [PubMed]
- Ørmen, Ø. The Regulation of the Aerolysin Production from Aeromonas spp.; The Norwegian School of Veterinary Science: Oslo, Norway, 2000. [Google Scholar]
- Sherif, A.H.; Kassab, A.S. Multidrug-resistant Aeromonas bacteria prevalence in Nile tilapia broodstock. BMC Microbiol. 2023, 23, 80. [Google Scholar] [CrossRef] [PubMed]
- Ed-Dra, A.; Filali, F.R.; Presti, V.L.; Zekkori, B.; Nalbone, L.; Elsharkawy, E.R.; Giuffrida, A.; Giarratana, F. Effectiveness of essential oil from the Artemisia herba-alba aerial parts against multidrug-resistant bacteria isolated from food and hospitalized patients. Biodiversitas J. Biolog. Divers. 2021, 22, 2995–3005. [Google Scholar] [CrossRef]
- Yoon, E.J.; Jeong, S.H. MALDI-TOF mass spectrometry technology as a tool for the rapid diagnosis of antimicrobial resistance in bacteria. Antibiotics 2021, 10, 982. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Chen, Q.; Zhang, J. Elucidating Resistance Mechanisms in Staphylococcus epidermidis: A High-Performing MALDI-TOF MS-Based Proteomic Approach for Predictive Modeling. bioRxiv 2024, 1–24. [Google Scholar] [CrossRef]
| Species | Sample Name | Source |
|---|---|---|
| A. allosaccharophila | CECT 4199T | Eel (Anguilla anguilla) |
| A. allosaccharophila | CECT 4220 | Feces from patient with diarrhea |
| A. allosaccharophila | CECT 4911 | Feces from patient with diarrhea |
| A. allosaccharophila | CECT 4912 | Feces from patient with diarrhea |
| A. bestiarum | NCIMB 1134 | Rainbow trout |
| A. bestiarum | DSM 13956T | Infected fish |
| A. bestiarum | CECT 5233 | Feces from patient with diarrhea |
| A. caviae | NCIMB 882 | Goldfish (Crassius auratus) |
| A. caviae | CECT 838T | Epizootic of young guinea pigs |
| A. caviae | CECT 5237 | Feces from patient with diarrhea |
| A. caviae | CECT 5241 | Feces from patient with diarrhea |
| A. encheleia | DSM 11577T | Healthy eel in fresh water |
| A. enteropelogenes | CECT 4255T | Human feces |
| A. enteropelogenes | CECT 4487T | Human feces |
| A. enteropelogenes | CECT 4936 | Feces from patient with diarrhea |
| A. enteropelogenes | CECT 4937 | Feces from patient with diarrhea |
| A. eucrenophila | DSM 17534T | Fresh water fish |
| A. hydrophila | ATCC 7966T | Milk |
| A. hydrophila | CECT 398 | Human feces of a child with diarrhea |
| A. hydrophila sub. dhakensis | CECT 5743 | Feces from patient with diarrhea |
| A. hydrophila sub. dhakensis | CECT 5744 | Feces from patient with diarrhea |
| A. hydrophila sub. dhakensis | CECT 5745 | Feces from patient with diarrhea |
| A. jandaei | CECT 4228T | Feces from patient with diarrhea |
| A. jandaei | CECT 4813 | Feces from patient with diarrhea |
| A. jandaei | CECT 4815 | Feces from patient with diarrhea |
| A. media | DSM 4881T | Fish farm effluent |
| A. molluscorum | CECT 5864 | Wedge shells (Donax trunculus) |
| A. popoffii | DSM 19604T | Drinking water |
| A. salmonicida | NCIMB 1102T | Atlantic salmon |
| A. sanarellii | CECT 7402 | Human wound |
| A. schubertii | CECT 4240T | Forehead abscess |
| A. sobria | NCIMB 75 | Diseased freshwater fish |
| A. sobria | CECT 4245T | Fish |
| A. taiwanensis | CECT 7403 | Human wound |
| A. tecta | CECT 7083 | Feces from patient with diarrhea |
| A. veronii | CECT 4258 | Feces from patient with diarrhea |
| A. veronii | CECT 4259 | Feces from patient with diarrhea |
| A. veronii | CECT 4904 | Feces from patient with diarrhea |
| A. veronii | CECT 4906 | Feces from patient with diarrhea |
| A. veronii | CECT 4907 | Feces from patient with diarrhea |
| A. veronii | CECT 4908 | Feces from patient with diarrhea |
| A. veronii | CECT 4910 | Feces from patient with diarrhea |
| A. veronii biovar veronii | CECT 4257T | Sputum of drowning victim |
| n. | Reference Spectra | n. | Super Spectra |
|---|---|---|---|
| 1 | Aeromonas bestiarum | 1 | Aeromonas bestiarum |
| 2 | Aeromonas eucrenophila | 2 | Aeromonas encheleia |
| 3 | Aeromonas hydrophila | 3 | Aeromonas eucrenophila |
| 4 | Aeromonas hydrophila ssp. hydrophila | 4 | Aeromonas hydrophila |
| 5 | Aeromonas hydrophila/caviae | 5 | Aeromonas media |
| 6 | Aeromonas punctata ssp. caviae | 6 | Aeromonas molluscorum |
| 7 | Aeromonas punctata ssp. punctata | 7 | Aeromonas popoffi |
| 8 | Aeromonas salmonicida ssp. masoucida | 8 | Aeromonas punctata |
| 9 | Aeromonas salmonicida ssp. salmonicida | 9 | Aeromonas punctata ssp. caviae |
| 10 | Aeromonas sobria | 10 | Aeromonas punctata ssp. caviae/punctata |
| 11 | Aeromonas spp. | 11 | Aeromonas salmonicida |
| 12 | Aeromonas tecta | 12 | Aeromonas schubertii |
| 13 | Aeromonas veronii | 13 | Aeromonas sharmana |
| 14 | Aeromonas veronii biovar sobria | 14 | Aeromonas simiae |
| 15 | Aeromonas veronii biovar veronii | 15 | Aeromonas sobria |
| 16 | Aeromonas tecta | ||
| 17 | Aeromonas trota | ||
| 18 | Aeromonas veronii |
| Species | Identification | |||||
|---|---|---|---|---|---|---|
| TSA | TSAS | |||||
| C 1 | C 2 | C 3 | C 1 | C 2 | C 3 | |
| A. allosaccharophila (CECT 4199T) | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| A. allosaccharophila (CECT 4220) | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| A. allosaccharophila (CECT 4911) | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| A. allosaccharophila (CECT 4912) | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| A. bestiarum (NCIMB 1134) | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| A. bestiarum (DSM 13956T) | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| A. bestiarum (CECT 5233) | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| A. caviae (NCIMB 882) | Aeromonas sp. 77.8% | Aeromonas sp. 80.0% | Aeromonas sp. 79.3% | Aeromonas sp. 78.1% | Aeromonas sp. 78.1% | Aeromonas sp. 78.1% |
| A. caviae (CECT 838T) | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| A. caviae (CECT 5237) | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| A. caviae (CECT 5241) | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| A. encheleia (DSM 11577T) | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| A. enteropelogenes (CECT 4255T) | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| A. enteropelogenes (CECT 4487T) | n.i. | Aeromonas sp. 79.1% | Aeromonas sp. 78.9% | n.i. | n.i. | n.i. |
| A. enteropelogenes (CECT 4936) | Aeromonas sp. 76.3% | n.i. | n.i. | n.i. | n.i. | n.i. |
| A. enteropelogenes (CECT 4937) | n.i. | Aeromonas sp. 77.1% | n.i. | n.i. | n.i. | n.i. |
| A. eucrenophila (DSM 17534T) | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| A. hydrophila (ATCC 7966T) | Aeromonas sp. 86.3% | Aeromonas sp. 76.9% | Aeromonas sp. 88.9% | Aeromonas sp. 92.5% | Aeromonas sp. 90.9% | Aeromonas sp. 78.3% |
| A. hydrophila (CECT 398) | Aeromonas sp. 85.4% | Aeromonas sp. 71.7% | Aeromonas sp. 84.4% | Aeromonas sp. 82.5% | Aeromonas sp. 81.0% | n.i. |
| A. hydrophila sub. dhakensis (CECT 5743) | n.i. | Aeromonas sp. 86.2% | n.i. | Aeromonas sp. 85.5% | Aeromonas sp. 86.3% | Aeromonas sp. 86.0% |
| A. hydrophila sub. dhakensis (CECT 5744) | Aeromonas sp. 76.2% | n.i. | Aeromonas sp. 85.4% | n.i. | n.i. | n.i. |
| A. hydrophila sub. dhakensis (CECT 5745) | Aeromonas sp. 91.8% | Aeromonas sp. 79.3% | n.i. | n.i. | n.i. | n.i. |
| A. jandaei (CECT 4228T) | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| A. jandaei (CECT 4813) | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| A. jandaei (CECT 4815) | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| A. media (DSM 4881T) | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| A. molluscorum (CECT 5864) | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| A. popoffii (DSM 19604T) | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| A. salmonicida (NCIMB 1102T) | n.i. | Aeromonas sp. 78.3% | Aeromonas sp. 79.3% | n.i. | n.i. | n.i. |
| A. sanarellii (CECT 7402) | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| A. schubertii (CECT 4240T) | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| A. sobria (NCIMB 75) | n.i. | A. sobria 89.3% | A. sobria 87.6% | n.i. | A. sobria 77.1% | |
| A. sobria (CECT 4245T) | A. sobria 77.5% | A. sobria 85.6% | n.i. | n.i. | A. sobria 86.9% | A. sobria 87.3% |
| A. taiwanensis (CECT 7403) | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| A. tecta (CECT 7083) | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| A. veronii (CECT 4258) | Aeromonas sp. 79.3% | n.i. | A. sobria 75.6% | n.i. | A. sobria 77.3% | n.i. |
| A. veronii (CECT 4259) | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| A. veronii (CECT 4904) | n.i. | Aeromonas sp. 89.3% | n.i. | n.i. | n.i. | Aeromonas sp. 71.9% |
| A. veronii (CECT 4906) | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| A. veronii (CECT 4907) | n.i. | Aeromonas sp. 77.5% | n.i. | n.i. | Aeromonas sp. 77.4% | n.i. |
| A. veronii (CECT 4908) | Aeromonas sp. 78.2% | Aeromonas sp. 88.3% | n.i. | n.i. | n.i. | n.i. |
| A. veronii (CECT 4910) | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| A. veronii bv veronii (CECT 4257T) | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| STRAINS | SARAMIS V4.12 | SARAMIS V4.12plus |
|---|---|---|
| A. allosaccharophila (CECT 4199T) | n.i. | A. allosaccharophila 85.6% |
| A. allosaccharophila (CECT4220) | n.i. | A. allosaccharophila 86.8% |
| A. allosaccharophila (CECT4911) | n.i. | A. allosaccharophila 87.7% |
| A. allosaccharophila (CECT4912) | n.i. | A. allosaccharophila 85.1% |
| A. bestiarum (NCIMB 1134) | n.i. | A. bestiarum 89.7% |
| A. bestiarum (DSM 13956T) | n.i. | A. bestiarum 85.4% |
| A. bestiarum (CECT5233) | n.i. | A. bestiarum 86.1% |
| A. caviae (NCIMB 882) | Aeromonas sp. 80.0% | A. caviae 94.5% |
| A. caviae (CECT 838T) | n.i. | A. caviae 87.9% |
| A. caviae (CECT5237) | n.i. | A. caviae 87.0% |
| A. caviae (CECT5241) | n.i. | A. caviae 86.3% |
| A. encheleia (DSM 11577T) | n.i. | Aeromonas sp. 85.8% |
| A. enteropelogenes (CECT 4255T) | n.i. | A. enteropelogenes 86.0% |
| A. enteropelogenes (CECT 4487T) | Aeromonas sp. 79.1% | A. enteropelogenes 89.8% |
| A. enteropelogenes (CECT4936) | Aeromonas sp. 76.3% | A. enteropelogenes 92.0% |
| A. enteropelogenes (CECT4937) | Aeromonas sp. 77.1% | A. enteropelogenes 93.4% |
| A. eucrenophila (DSM 17534T) | n.i. | Aeromonas sp. 86.6% |
| A. hydrophila (ATCC 7966T) | Aeromonas sp. 92.5% | A. hydrophila 97.1% |
| A. hydrophila (CECT 398) | Aeromonas sp. 85.4% | A. hydrophila 94.7% |
| A. hydrophila sub. dhakensis (CECT5743) | Aeromonas sp. 86.3% | A. hydrophila 93.0% |
| A. hydrophila sub. dhakensis (CECT5744) | Aeromonas sp. 85.4% | A. hydrophila 92.6% |
| A. hydrophila sub. dhakensis (CECT5745) | Aeromonas sp. 91.8% | A. hydrophila 96.3% |
| A. jandaei (CECT 4228T) | n.i. | A. jandaei 89.0% |
| A. jandaei (CECT4813) | n.i. | A. jandaei 87.7% |
| A. jandaei (CECT4815) | n.i. | A. jandaei 86.9% |
| A. media (DSM 4881T) | n.i. | Aeromonas sp. 88.1% |
| A. molluscorum (CECT5864) | n.i. | Aeromonas sp. 89.4% |
| A. popoffii (DSM 19604T) | n.i. | Aeromonas sp. 91.0% |
| A. salmonicida (NCIMB 1102T) | Aeromonas sp. 79.3% | A. salmonicida 94.9% |
| A. sanarellii (CECT7402) | n.i. | Aeromonas sp. 85.2% |
| A. schubertii (CECT 4240T) | n.i. | Aeromonas sp. 85.7% |
| A. sobria (NCIMB 75) | A. sobria 89.3% | A. sobria 99.8% |
| A. sobria (CECT 4245T) | A. sobria 87.3% | A. sobria 99.0% |
| A. taiwanensis (CECT7403) | n.i. | Aeromonas sp. 88.8% |
| A. tecta (CECT7083) | n.i. | Aeromonas sp. 85.7% |
| A. veronii (CECT4258) | A. sobria 79.3% | A. veronii 86.1% |
| A. veronii (CECT4259) | n.i. | A. veronii 89.2% |
| A. veronii (CECT4904) | Aeromonas sp. 89.3% | A. veronii 95.5% |
| A. veronii (CECT4906) | n.i. | A. veronii 87.3% |
| A. veronii (CECT4907) | Aeromonas sp. 77.5% | A. veronii 93.4% |
| A. veronii (CECT4908) | Aeromonas sp. 88.3% | A. veronii 96.6% |
| A. veronii (CECT4910) | n.i. | A. veronii 85.9% |
| A. veronii bv veronii (CECT 4257T) | n.i. | A. veronii 88.9% |
| Identification | Salmon Sashimi | Tuna Sashimi | Total |
|---|---|---|---|
| Aeromonas spp. | 11 (13.92%) | 10 (14.49%) | 21 (14.19%) |
| A. bestiarum | 6 (7.59%) | 18 (26.09%) | 24 (16.22%) |
| A. caviae | 3 (3.80%) | 0 | 3 (2.03%) |
| A. salmonicida | 59 (74.68%) | 41 (59.42%) | 100 (67.57%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nalbone, L.; Forgia, S.; Pirrone, F.; Giarratana, F.; Panebianco, A. Use of Matrix-Assisted and Laser Desorption/Ionization Time-of-Flight Technology in the Identification of Aeromonas Strains Isolated from Retail Sushi and Sashimi. Pathogens 2024, 13, 432. https://doi.org/10.3390/pathogens13060432
Nalbone L, Forgia S, Pirrone F, Giarratana F, Panebianco A. Use of Matrix-Assisted and Laser Desorption/Ionization Time-of-Flight Technology in the Identification of Aeromonas Strains Isolated from Retail Sushi and Sashimi. Pathogens. 2024; 13(6):432. https://doi.org/10.3390/pathogens13060432
Chicago/Turabian StyleNalbone, Luca, Salvatore Forgia, Federico Pirrone, Filippo Giarratana, and Antonio Panebianco. 2024. "Use of Matrix-Assisted and Laser Desorption/Ionization Time-of-Flight Technology in the Identification of Aeromonas Strains Isolated from Retail Sushi and Sashimi" Pathogens 13, no. 6: 432. https://doi.org/10.3390/pathogens13060432
APA StyleNalbone, L., Forgia, S., Pirrone, F., Giarratana, F., & Panebianco, A. (2024). Use of Matrix-Assisted and Laser Desorption/Ionization Time-of-Flight Technology in the Identification of Aeromonas Strains Isolated from Retail Sushi and Sashimi. Pathogens, 13(6), 432. https://doi.org/10.3390/pathogens13060432








