Sodium Metabisulfite Inhibits Acanthamoeba Trophozoite Growth through Thiamine Depletion
Abstract
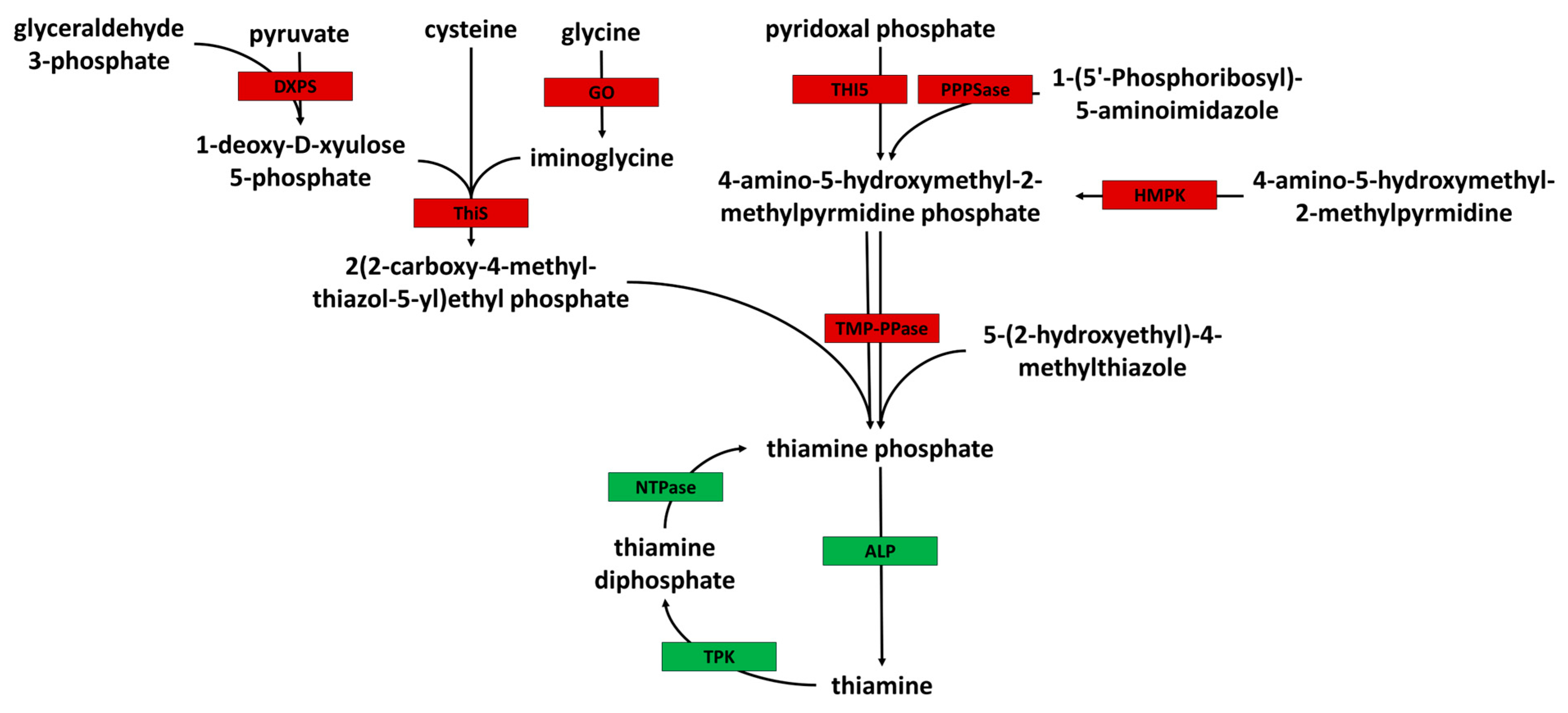
1. Introduction
2. Materials and Methods
2.1. Acanthamoeba Species
2.2. Determination of Minimum Thiamine Concentration for A. castellanii Growth
2.3. Sodium Metabisulfite Acanthamoeba Inhibition Assay
2.4. Thiamine Chromatography
2.5. In Silico Analysis
2.6. Statistical Analysis
3. Results
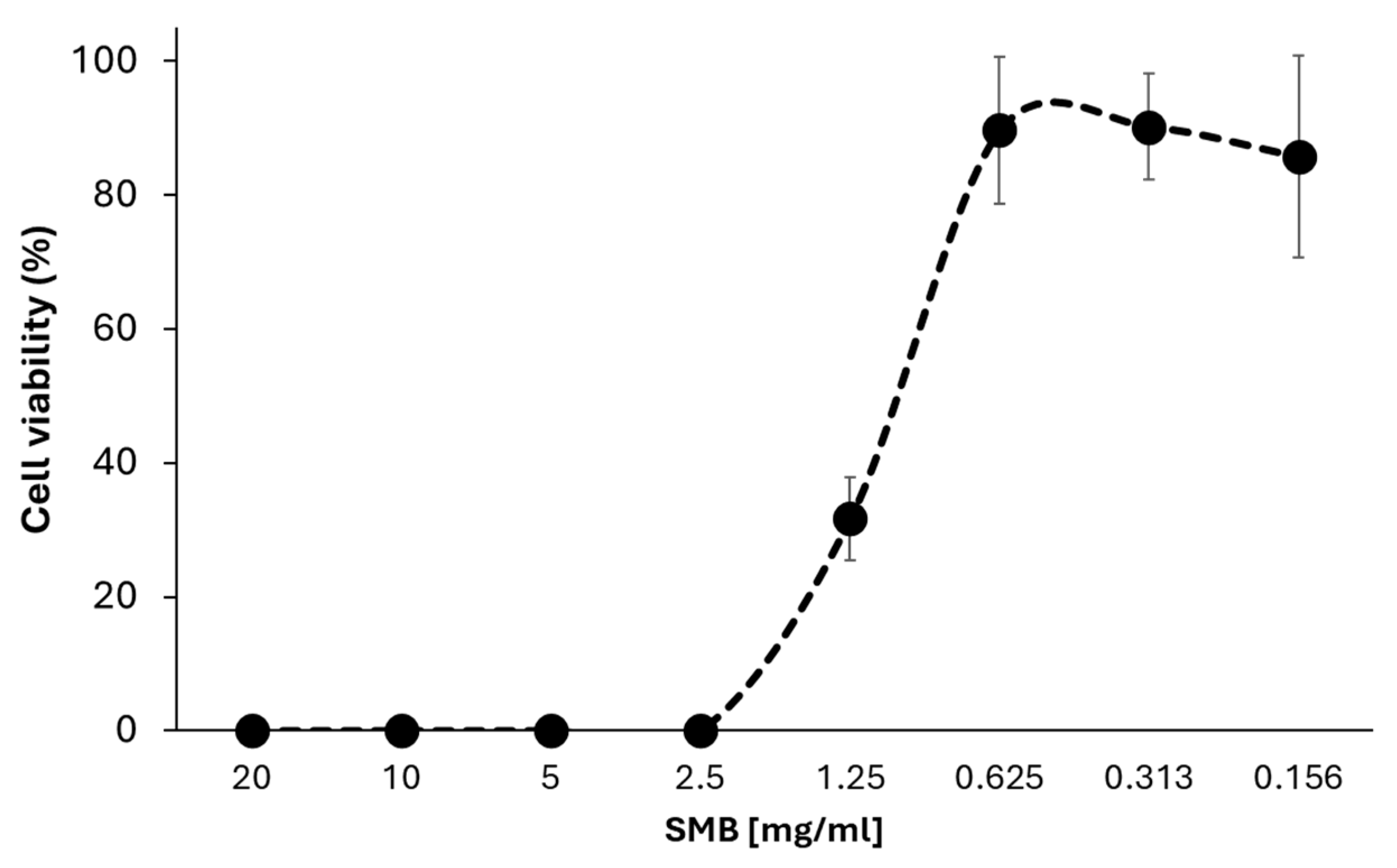
3.1. Sodium Metabisulfite Inhibits the Growth of A. castellanii Trophozoites
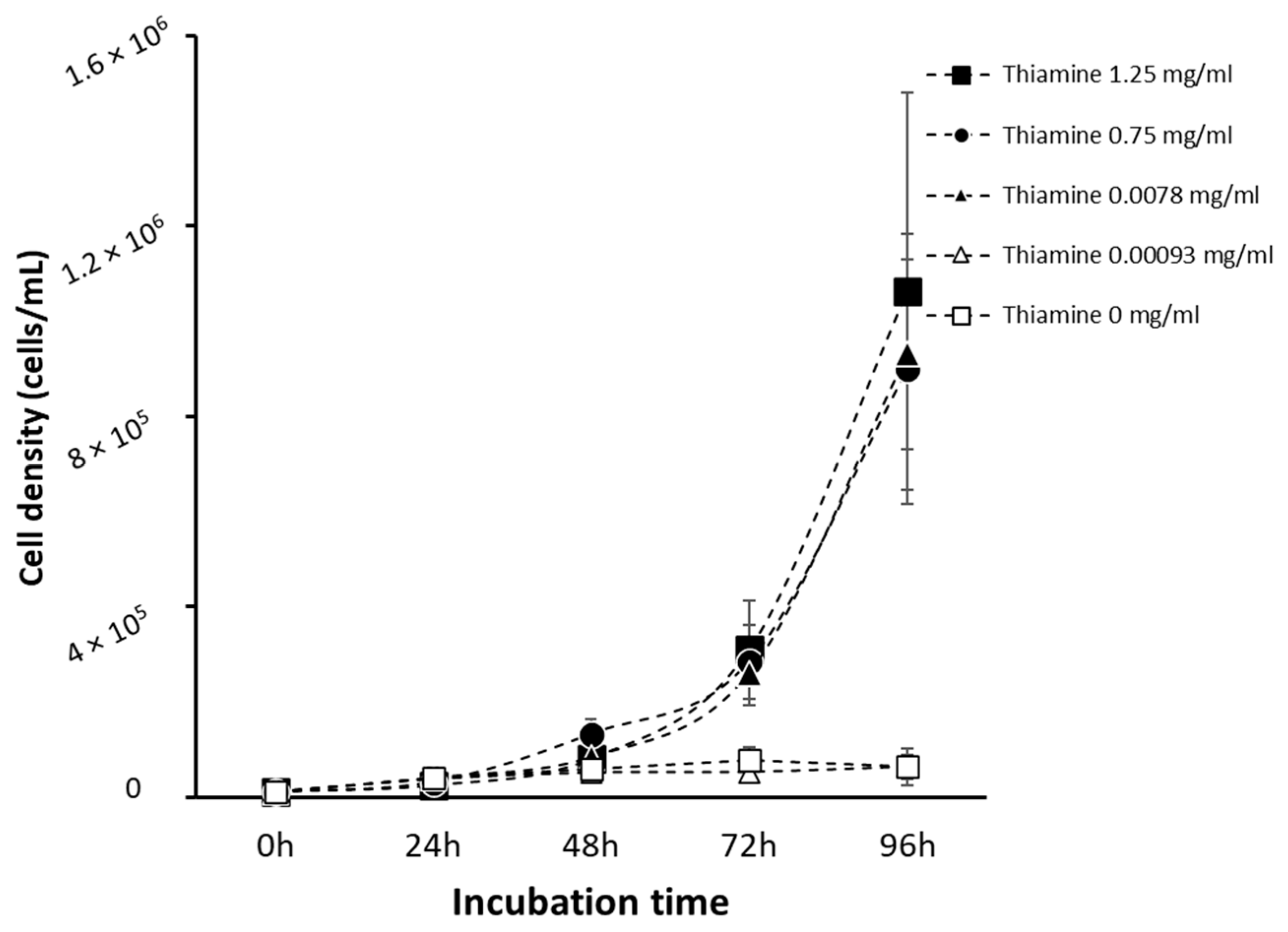
3.2. Acanthamoeba Trophozoites Require Thiamine for Growth
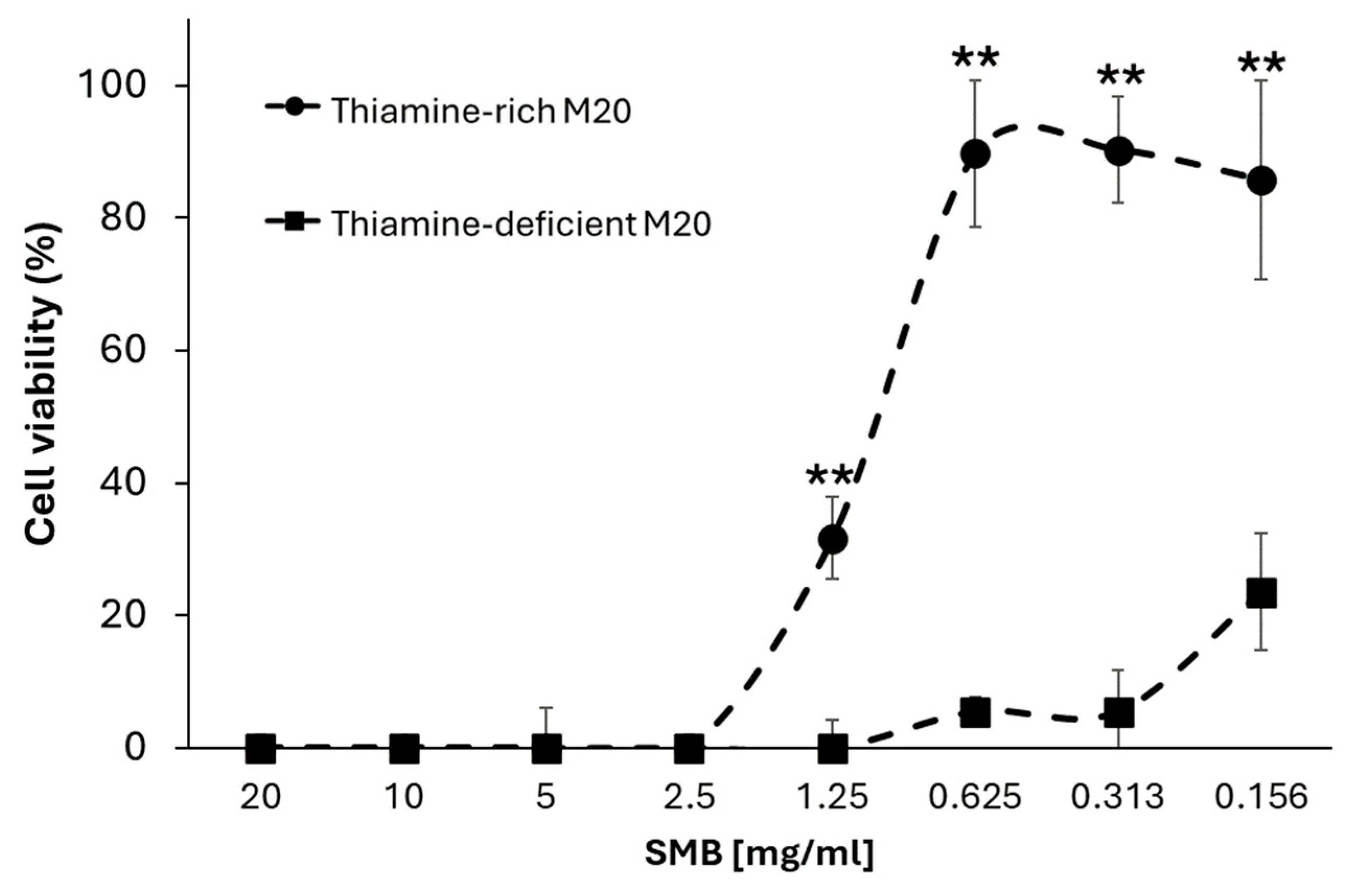
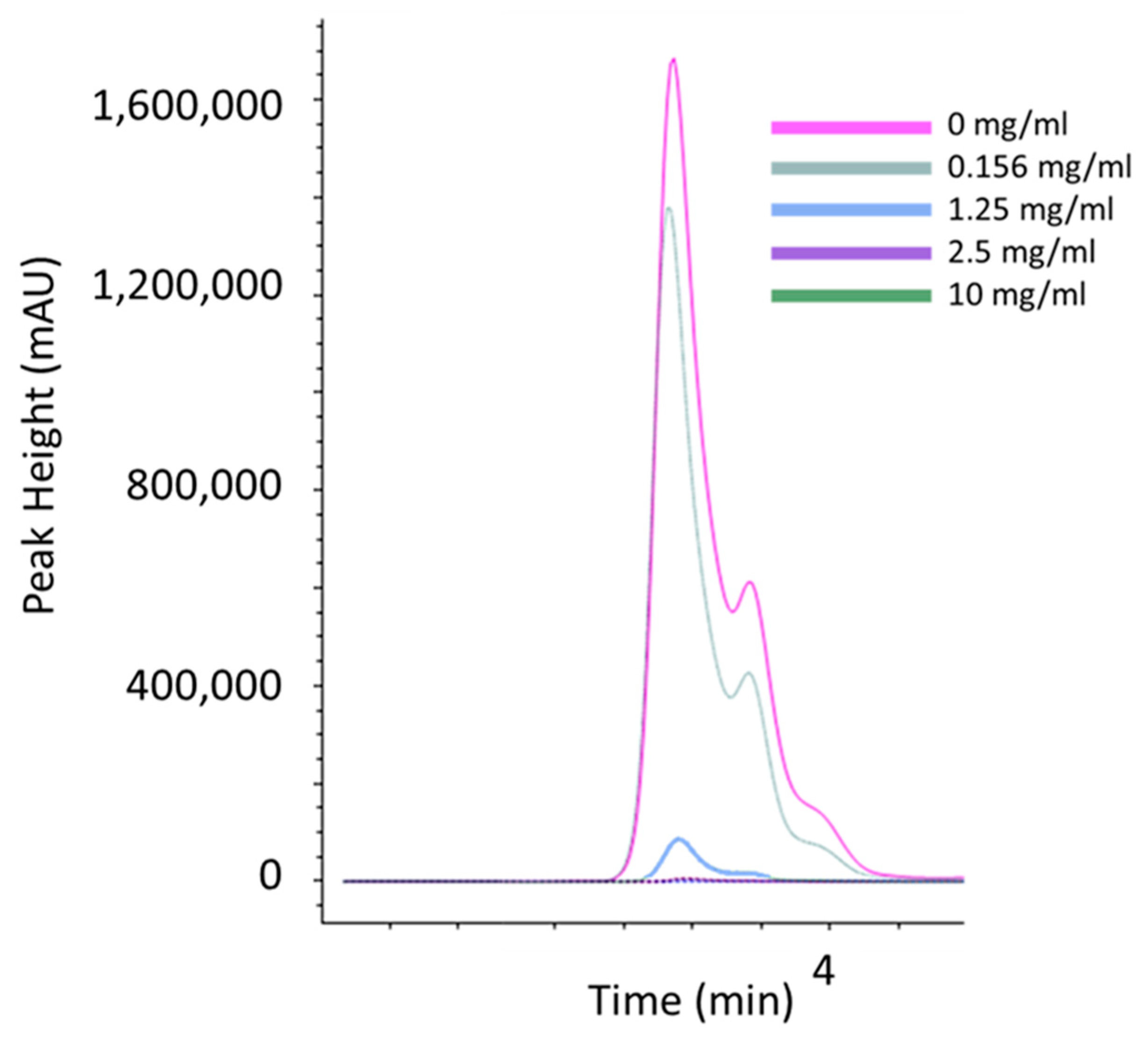
3.3. Sodium Metabisulfite Inhibits Acanthamoeba Growth through Degradation of Thiamine
3.4. Acanthamoeba Is a Thiamine Auxotroph
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Somani, S.N.; Ronquillo, Y.; Moshirfar, M. Acanthamoeba Keratitis; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Scruggs, B.A.; Quist, T.S.; Salinas, J.L.; Greiner, M.A. Notes from the Field: Acanthamoeba Keratitis Cases—Iowa, 2002–2017. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 448–449. [Google Scholar] [CrossRef]
- Höllhumer, R.; Keay, L.; Watson, S.L. Acanthamoeba Keratitis in Australia: Demographics, Associated Factors, Presentation and Outcomes: A 15-Year Case Review. Eye 2020, 34, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Randag, A.C.; van Rooij, J.; van Goor, A.T.; Verkerk, S.; Wisse, R.P.L.; Saelens, I.E.Y.; Stoutenbeek, R.; van Dooren, B.T.H.; Cheng, Y.Y.Y.; Eggink, C.A. The Rising Incidence of Acanthamoeba Keratitis: A 7-Year Nationwide Survey and Clinical Assessment of Risk Factors and Functional Outcomes. PLoS ONE 2019, 14, e0222092. [Google Scholar] [CrossRef]
- Carnt, N.; Hoffman, J.J.; Verma, S.; Hau, S.; Radford, C.F.; Minassian, D.C.; Dart, J.K.G. Acanthamoeba Keratitis: Confirmation of the UK Outbreak and a Prospective Case-Control Study Identifying Contributing Risk Factors. Br. J. Ophthalmol. 2018, 102, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Morales, J.; Khan, N.A.; Walochnik, J. An Update on Acanthamoeba Keratitis: Diagnosis, Pathogenesis and Treatment. Parasite 2015, 22, 10. [Google Scholar] [CrossRef] [PubMed]
- Szentmáry, N.; Daas, L.; Shi, L.; Laurik, K.L.; Lepper, S.; Milioti, G.; Seitz, B. Acanthamoeba Keratitis—Clinical Signs, Differential Diagnosis and Treatment. J. Curr. Ophthalmol. 2019, 31, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Fanselow, N.; Sirajuddin, N.; Yin, X.-T.; Huang, A.J.W.; Stuart, P.M. Acanthamoeba Keratitis, Pathology, Diagnosis and Treatment. Pathogens 2021, 10, 323. [Google Scholar] [CrossRef] [PubMed]
- de Lacerda, A.G.; Lira, M. Acanthamoeba Keratitis: A Review of Biology, Pathophysiology and Epidemiology. Ophthalmic Physiol. Opt. 2021, 41, 116–135. [Google Scholar] [CrossRef] [PubMed]
- Onorato, D.J. Ophthalmic Medications That Contain Sulfites. Arch. Ophthalmol. 1985, 103, 1274–1276. [Google Scholar] [CrossRef]
- Shaver, R.L.; Warshaw, E.M. Contact Allergens in Prescription Topical Ophthalmic Medications. Dermatitis 2022, 33, 135–143. [Google Scholar] [CrossRef]
- Cakmak, H.B.; Cagil, N.; Dal, D.; Simavli, H.; Arifoglu, H.B.; Simsek, S. Effects of Intracameral Use of Adrenalin Solution with Preservative on Corneal Endothelium. Cutan. Ocul. Toxicol. 2010, 29, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Veramme, J.; de Zaeytijd, J.; Lambert, J.; Lapeere, H. Contact Dermatitis in Patients Undergoing Serial Intravitreal Injections. Contact Dermat. 2016, 74, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Behndig, A.; Korobelnik, J.-F. Mydriatic Insert and Intracameral Injections Compared with Mydriatic Eyedrops in Cataract Surgery: Controlled Studies. J. Cataract. Refract. Surg. 2015, 41, 1503–1519. [Google Scholar] [CrossRef] [PubMed]
- García-Gavín, J.; Parente, J.; Goossens, A. Allergic Contact Dermatitis Caused by Sodium Metabisulfite: A Challenging Allergen. A Case Series and Literature Review. Contact Dermat. 2012, 67, 260–269. [Google Scholar] [CrossRef]
- Frank, K.L.; Patel, R. Activity of Sodium Metabisulfite against Planktonic and Biofilm Staphylococcus Species. Diagn. Microbiol. Infect. Dis. 2007, 57, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, G.H.; Yoon, K.S.; Shankar, S.; Rhim, J.-W. Comparative Antibacterial and Antifungal Activities of Sulfur Nanoparticles Capped with Chitosan. Microb. Pathog. 2020, 144, 104178. [Google Scholar] [CrossRef] [PubMed]
- Pateraki, M.; Dekanea, A.; Mitchell, D.; Lydakis, D.; Magan, N. Influence of Sulphur Dioxide, Controlled Atmospheres and Water Availability on in Vitro Germination, Growth and Ochratoxin A Production by Strains of Aspergillus carbonarius Isolated from Grapes. Postharvest Biol. Technol. 2007, 44, 141–149. [Google Scholar] [CrossRef]
- Turkkan, M.; Erper, I. Evaluation of Antifungal Activity of Sodium Salts against Onion Basal Rot Caused by Fusarium oxysporum f.sp. cepae. Plant Prot. Sci. 2014, 50, 19–25. [Google Scholar] [CrossRef]
- Natskoulis, P.I.; Lappa, I.K.; Panagou, E.Z. Evaluating the Efficacy of Turbimetric Measurements as a Rapid Screening Technique to Assess Fungal Susceptibility to Antimicrobial Compounds as Exemplified by the Use of Sodium Metabisulfite. Food Res. Int. 2018, 106, 1037–1041. [Google Scholar] [CrossRef]
- Askarne, L.; Boubaker, H.; Boudyach, E.H.; Aoumar, A.A.B. Use of Food Additives to Control Postharvest Citrus Blue Mold Disease. Atlas J. Biol. 2013, 2, 147–153. [Google Scholar] [CrossRef]
- Scheiner, J.M.; Araujo, M.M.; DeRitter, E. Thiamine Destruction by Sodium Bisulfite in Infusion Solutions. Am. J. Health Pharm. 1981, 38, 1911–1913. [Google Scholar] [CrossRef]
- Singleton, C.; Martin, P. Molecular Mechanisms of Thiamine Utilization. Curr. Mol. Med. 2001, 1, 197–207. [Google Scholar] [CrossRef]
- Chagla, A.H.; Griffiths, A.J. Growth and Encystation of Acanthamoeba castellanii. J. Gen. Microbiol. 1974, 85, 139–145. [Google Scholar] [CrossRef]
- Shukla, O.P.; Kaul, S.M.; Mehlotra, R.K. Nutritional Studies on Acanthamoeba culbertsoni and Development of Chemically Defined Medium. J. Protozool. 1990, 37, 237–242. [Google Scholar] [CrossRef]
- McBride, J.; Ingram, P.R.; Henriquez, F.L.; Roberts, C.W. Development of Colorimetric Microtiter Plate Assay for Assessment of Antimicrobials against Acanthamoeba. J. Clin. Microbiol. 2005, 43, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Troshin, P.V.; Procter, J.B.; Barton, G.J. Java Bioinformatics Analysis Web Services for Multiple Sequence Alignment—JABAWS:MSA. Bioinformatics 2011, 27, 2001–2002. [Google Scholar] [CrossRef]
- Troshin, P.V.; Procter, J.B.; Sherstnev, A.; Barton, D.L.; Madeira, F.; Barton, G.J. JABAWS 2.2 Distributed Web Services for Bioinformatics: Protein Disorder, Conservation and RNA Secondary Structure. Bioinformatics 2018, 34, 1939–1940. [Google Scholar] [CrossRef] [PubMed]
- Coquille, S.; Roux, C.; Mehta, A.; Begley, T.P.; Fitzpatrick, T.B.; Thore, S. High-Resolution Crystal Structure of the Eukaryotic HMP-P Synthase (THIC) from Arabidopsis thaliana. J. Struct. Biol. 2013, 184, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Nair, B.; Elmore, A.R. Final Report on the Safety Assessment of Sodium Sulfite, Potassium Sulfite, Ammonium Sulfite, Sodium Bisulfite, Ammonium Bisulfite, Sodium Metabisulfite and Potassium Metabisulfite. Int. J. Toxicol. 2003, 22, 63–88. [Google Scholar] [CrossRef]
- Hervieux, V.; Yaganza, E.S.; Arul, J.; Tweddell, R.J. Effect of Organic and Inorganic Salts on the Development of Helminthosporium solani, the Causal Agent of Potato Silver Scurf. Plant Dis. 2002, 86, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.A.S.; Platt, H.W.; Hurta, R.A.R. Effect of Salt Compounds on Mycelial Growth, Sporulation and Spore Germination of Various Potato Pathogens. Postharvest Biol. Technol. 2004, 34, 341–350. [Google Scholar] [CrossRef]
- Mecteau, M.R.; Arul, J.; Tweddell, R.J. Effect of Organic and Inorganic Salts on the Growth and Development of Fusarium sambucinum, a Causal Agent of Potato Dry Rot. Mycol. Res. 2002, 106, 688–696. [Google Scholar] [CrossRef]
- Mecteau, M.R.; Arul, J.; Tweddell, R.J. Effect of Different Salts on the Development of Fusarium solani Var. coeruleum, a Causal Agent of Potato Dry Rot. Phytoprotection 2009, 89, 1–6. [Google Scholar] [CrossRef]
- Al-Jaza, D.; Medina, A.; Magan, N. Efficacy of Sodium Metabisulphite for Control of Aspergillus flavus and Aflatoxin B1 Contamination in Vitro and in Chilli Powder and Whole Red Chillies. Food Control 2022, 135, 108786. [Google Scholar] [CrossRef]
- Palmieri, F.; Monné, M.; Fiermonte, G.; Palmieri, L. Mitochondrial Transport and Metabolism of the Vitamin B-derived Cofactors Thiamine Pyrophosphate, Coenzyme A, FAD and NAD+, and Related Diseases: A Review. IUBMB Life 2022, 74, 592–617. [Google Scholar] [CrossRef]
- Kato, M.; Wynn, R.M.; Chuang, J.L.; Tso, S.-C.; Machius, M.; Li, J.; Chuang, D.T. Structural Basis for Inactivation of the Human Pyruvate Dehydrogenase Complex by Phosphorylation: Role of Disordered Phosphorylation Loops. Structure 2008, 16, 1849–1859. [Google Scholar] [CrossRef]
- Armstrong, C.T.; Anderson, J.L.R.; Denton, R.M. Studies on the Regulation of the Human E1 Subunit of the 2-Oxoglutarate Dehydrogenase Complex, Including the Identification of a Novel Calcium-Binding Site. Biochem. J. 2014, 459, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Seki, N.; Kihara, A. Phytosphingosine Degradation Pathway Includes Fatty Acid α-Oxidation Reactions in the Endoplasmic Reticulum. Proc. Natl. Acad. Sci. USA 2017, 114, E2616–E2623. [Google Scholar] [CrossRef]
- Foulon, V.; Antonenkov, V.D.; Croes, K.; Waelkens, E.; Mannaerts, G.P.; Van Veldhoven, P.P.; Casteels, M. Purification, Molecular Cloning, and Expression of 2-Hydroxyphytanoyl-CoA Lyase, a Peroxisomal Thiamine Pyrophosphate-Dependent Enzyme That Catalyzes the Carbon–Carbon Bond Cleavage during α-Oxidation of 3-Methyl-Branched Fatty Acids. Proc. Natl. Acad. Sci. USA 1999, 96, 10039–10044. [Google Scholar] [CrossRef]
- Du, Q.; Wang, H.; Xie, J. Thiamin (Vitamin B1) Biosynthesis and Regulation: A Rich Source of Antimicrobial Drug Targets? Int. J. Biol. Sci. 2011, 7, 41–52. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mooney, R.; Giammarini, E.; Corbett, E.; Thomson, S.; McKinley, K.; Sinisterra Sebastian, P.; Rodgers, K.; O’Donnell, J.; McGinness, C.; Roberts, C.W.; et al. Sodium Metabisulfite Inhibits Acanthamoeba Trophozoite Growth through Thiamine Depletion. Pathogens 2024, 13, 431. https://doi.org/10.3390/pathogens13060431
Mooney R, Giammarini E, Corbett E, Thomson S, McKinley K, Sinisterra Sebastian P, Rodgers K, O’Donnell J, McGinness C, Roberts CW, et al. Sodium Metabisulfite Inhibits Acanthamoeba Trophozoite Growth through Thiamine Depletion. Pathogens. 2024; 13(6):431. https://doi.org/10.3390/pathogens13060431
Chicago/Turabian StyleMooney, Ronnie, Elisa Giammarini, Erin Corbett, Scott Thomson, Kevin McKinley, Paula Sinisterra Sebastian, Kiri Rodgers, Jana O’Donnell, Charles McGinness, Craig W. Roberts, and et al. 2024. "Sodium Metabisulfite Inhibits Acanthamoeba Trophozoite Growth through Thiamine Depletion" Pathogens 13, no. 6: 431. https://doi.org/10.3390/pathogens13060431
APA StyleMooney, R., Giammarini, E., Corbett, E., Thomson, S., McKinley, K., Sinisterra Sebastian, P., Rodgers, K., O’Donnell, J., McGinness, C., Roberts, C. W., Ramaesh, K., & Henriquez, F. L. (2024). Sodium Metabisulfite Inhibits Acanthamoeba Trophozoite Growth through Thiamine Depletion. Pathogens, 13(6), 431. https://doi.org/10.3390/pathogens13060431






