Abstract
Medical devices such as venous catheters (VCs) and urinary catheters (UCs) are widely used in the hospital setting. However, the implantation of these devices is often accompanied by complications. About 60 to 70% of nosocomial infections (NIs) are linked to biofilms. The main complication is the ability of microorganisms to adhere to surfaces and form biofilms which protect them and help them to persist in the host. Indeed, by crossing the skin barrier, the insertion of VC inevitably allows skin flora or accidental environmental contaminants to access the underlying tissues and cause fatal complications like bloodstream infections (BSIs). In fact, 80,000 central venous catheters—BSIs (CVC-BSIs)—mainly occur in intensive care units (ICUs) with a death rate of 12 to 25%. Similarly, catheter-associated urinary tract infections (CA-UTIs) are the most commonlyhospital-acquired infections (HAIs) worldwide.These infections represent up to 40% of NIs.In this review, we present a summary of biofilm formation steps. We provide an overview of two main and important infections in clinical settings linked to medical devices, namely the catheter-asociated bloodstream infections (CA-BSIs) and catheter-associated urinary tract infections (CA-UTIs), and highlight also the most multidrug resistant bacteria implicated in these infections. Furthermore, we draw attention toseveral useful prevention strategies, and advanced antimicrobial and antifouling approaches developed to reduce bacterial colonization on catheter surfaces and the incidence of the catheter-related infections.
1. Introduction
Every year, millions of catheters are implanted by health services to improve the management of acute and chronic diseases in adults and pediatric patients [1,2,3]. Unfortunately, their use inevitably allows patient’s own flora or accidental environmental contaminants to access the underlying tissues and cause fatal complications [4,5,6]. About 60 to 70% of nosocomial infections (NI) are linked to medical devices [7]. The main complication results from the ability of microorganisms to adhere to surfaces and form biofilms [8], which protects them and helps them to persist in the host [9].Indeed, biofilms act as a protection barrier against antimicrobial agents thereby leading to therapeutic failure and increased mortality and morbidity rates [3,10,11]. Moreover, in the biofilm, there is a small subpopulation called persister cells which are characterized by increased tolerance to antimicrobials.Once the antibiotic is removed, surviving persisters are able to re-grow causing infections [12]. Several studies demonstrated that persister cells are strongly involved in chronic infections and their recalcitrance in clinical, making the antibiotic treatment innefective and biofilm eradication impossible [13,14,15,16].
In fact, catheter associated bloodstream infections (CA-BSIs) are an important cause of hospital-acquired infections originating from an intravenous catheter and associated with morbidity, mortality, and hospital cost [17]. Central venous catheters (CVCs) are among the most widely used medical devices in critically ill patients. However, central line-associated bloodstream infections (CLA-BSIs) are the most common complications which are usually associated with the use of these CVCs [4], causing an increase in the rate of morbidity and mortality in health establishments as well as the length and costs of the stay [18]. In fact, 80,000 CVC-associated bloodstream infections (CVC-BSIs) mainly occur in intensive care units (ICUs) with a death rate of 12 to 25% [19]. Concerning peripheral venous catheters (PVCs), it has been estimated that 30 to 80% of hospitalized patients have a PVC in place during their hospitalization [20] and more than a billion PVCs are used each year around the world [21]. Among the side effects observed when a PVC is used are the following: phlebitis, partial dislodgement, accidental removal, occlusion, infiltration (fluid moving into surrounding tissue), and rarely, infections [22,23]. The incidence of bloodstream infections associated with peripheral venous catheters (PVC-BSIs) is generally low, with a rate of 0.1% of short catheters inserted (0.5 episodes per 1000 days of intravascular catheter) [24], unlike the incidence of CVC-BSIs which is 2.7 episodes per 1000 days of intravascular catheter [20]. Moreover, VC can be easily colonized by pathogenic microorganisms which lead to the formation of biofilms, other potential sources of BSIs [25]. Biofilms formed on CVCs were first described in 1982, during an epidemic of Staphylococcus epidermidis BSI [26]. Since that day, several studies have confirmed the involvement of biofilm in the pathogenesis of CVC-related infections and their importance [27]. Additionally, 81% of all vascular catheters that were placed in situ for 1–14 days were reported to be colonized by bacteria in the biofilm [28]. Biofilm colonization of intravenous catheters continues to affect healthcare settings [29]. Several factors increase the risk of catheter infections such as patient immunodeficiency, length of prolonged catheterization, catheter material, anatomical site of catheter insertion, poor hygiene, poor catheter insertion, and handling methods [30]. It has been reported that the incidence of bacteremia associated with PVCs is lower than that of bacteremia associated with CVCs. However, the duration of PVC insertion is 15 times longer than that of CVC insertion; for this, the number of PVC-BSIs is high due to the high number of patients who have a PVC [20,31].
Similarly, catheter-associated urinary tract infections (CA-UTIs) are the most commonly hospital-acquired infections worldwide [32]. These infections represent up to 40% of nosocomial infections. Also, 70% of UTIs are associated with urinary catheters (UC) and approximately 20% of hospitalized patients have a UC, especially those in ICUs [33]. Despite the high risk of acquiring infections with multidrug-resistant (MDR) opportunistic pathogens, most cases of catheter-associated bacteriuria are asymptomatic. However, when an episode of CA-UTI becomes symptomatic, the resulting sequelae can range from mild (fever, urethritis, and cystitis) to severe (catheter encrustation, bladder stones, pyelonephritis, endotoxic shock, and bacteremia). Left untreated, these infections can lead to urosepsis and death [34,35]. Indeed, for each day that a urinary catheter is in situ, there is a 3–8% incidence of bacteriuria, and in the majority of cases, long-term catheterization results in continued bacteriuria and symptomatic CA-UTI [36]. For an infection to be classified as a CA-UTI, a patient must have the following: (i) a urinary catheter implantated for more than 48 h; (ii) a symptom such as fever, pain, suprapubic tenderness, urinary frequency or urgency or dysuria; and (iii) urine culture with ≥105 CFU/mL of a bacterial species [37]. However, there is much controversy over the CFU/mL cut-off in samples taken from a urinary catheter and several authorities consider that a number (greater than or equal to) ≥103 CFU/mL is indicative of a true CA-UTI [38,39,40]. Moreover, other host factors such as female gender, older age (i.e., age > 50 years old), diabetes mellitus, faecal incontinence, immunocompromised status; healthcare factors such as lack of systemic antibiotics, catheter insertion outside the operating room, prolonging the duration of catheterization, and poor quality of catheter care [40,41,42] increase the risk of CA-UTIs. CA-UTI is linked with biofilm formation along the surface of the catheter [36]. Indeed, the presence of a UC facilitates bacterial colonization due to the development of a conditioning film of host proteins which provides bacteria with an ideal substrate for fixation [43].
In view of all this, there is an urgent need to develop novel strategies to fight medical device-associated biofilms. Despite several studies having been conducted in this field, many challenges still remain. This review provides an overview on the medical device-associated biofilm infections (mainly venous catheter-associated bloodstream infections and catheter associated-urinary tract infections), the biofilm development process on these devices, and the most MDR-bacteria implicated in these infections with their virulence factors. Furthermore, the current review highlights the different prevention strategies and the most effective approaches using antimicrobial coating and antifouling methods, to reduce medical device colonization and the incidence of their related infections.
2. Hospital-Acquired Infections
Hospital-acquired infections (HAIs) or nosocomial infections (NI) are defined as infections which were neither present nor incubating during the patient’s hospitalization and were acquired after 48 h of hospitalization. These infections increase patient morbidity and mortality, prolong their hospital stays, and represent a massive additional financial burden for health structures [44]. The severity of infection and its incidence is much higher in patients in burn units, intensive care units, organ transplant receivers, and with newborns due to their immunological status [45]. In addition to the problems associated with nosocomial infections, antibiotic resistance and the emergence of MDR-bacteria is a serious global problem, due to the uncontrolled administration of drugs [46]. These HAIs are often the result of the use of invasive procedures such as the location of temporary indwelling devices (VCs, UCs, endotracheal tubes, and wound drains) or are associated to the placement of cardiovascular or orthopedic implants during a surgical intervention [47]. They include a wide range of infections such as catheter related infections (CRIs), CA-UTIs, and ventilator associated pneumonia (VAP) [48]. These infections are generally designed as “Medical Device-Associated Biofilm Infections” [3]. Several Gram-negative (Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, and Acinetobacter baumannii) and Gram-positive (Staphylococcus aureus, Staphylococcus epidermidis, and Enterococcus faecalis) bacteria are involved in the onset of NIs [49,50]. Their ability to form a biofilm makes the treatment of these infections more complicated [51].
In fact, it is well known that biofilms have a significant impact in medicine through the development of HAIs [52] and it is estimated that bacterial biofilms are involved in 65% of NIs and in more than 80% of chronic infections [53]. Treatment of these infections requires administration of high dose antibiotics and/or replacement of the device, which are both ineffective due to the antibiotic resistant strains and the high risk of re-infection on the new device [51].
3. Biofilm Formation on Medical Devices
Bacteria have always been studied in the laboratory as planktonic microorganisms. However, most bacteria live in multicellular communities called biofilms [54]. The biofilm was observed for the first time in the 17th century by Anthony van Leeuwenhoek through his microscope in his own mouth where he observed aggregated microorganisms on his teeth and tongue [55]. A biofilm is a highly structured bacterial community attached to a surface and protected by a self-produced extracellular polymeric matrix [56,57,58]. This matrix is mainly composed of proteins, polysaccharides, and extracellular DNA (eDNA). Furthermore, the biofilm matrix is highly hydrated and contains up to 97% water, mainly responsible for nutrient transport within the biofilm [53,59]. Bacterial adhesion occurs on a pre-conditioning film formed on the surface after deposition of organic, inorganic, and cellular components (e.g., fibronectin, fibrinogen, laminin, collagen, polysaccharides) found in the environment surrounding the medical device, constituting a base on which the biofilm will develop [51,57]. The bacteria interact with the components of this surface through appendages, attractive forces, or adhesins [57]. The biofilm formation on a medical device proceeds as follows: (i)Transport of bacterial cells to the surface—the bacteria can be transported to the medical device either by diffusion (Brownian motion), convective flow or active movement (motile bacteria) [60]. Bacterial transport can also be induced by chemotaxis due to the presence of diffusible chemical gradients which form from various chemical stimuli or the degradation of components (eg. aspartate, glucose, galactose) [60,61]. However, if the surface is unsuitable for bacterial adhesion, the bacteria return to the planktonic state [11]. (ii) Reversible attachment—this first step of the bacterial adhesion process to the medical device is the transport of cells to the device [61]. The initial attraction mainly involves non-specific physical interactions such as Van der Waals attractive forces, electrostatic forces (attractive or repulsive), hydrophobic interactions, Brownian motion, and gravitational forces [62]. Furthermore, due to the negative charge of their cell membrane, bacteria are subjected to repulsive electrostatic and repulsive hydrodynamic forces when they are near the medical device. In order to overcome these two repulsive barriers, bacteria typically use cellular appendages, such as flagella or pili [63,64]. This initial binding of bacteria to the medical device is important to make irreversible adhesion possible [60]. (iii) Irreversible attachment—this step is characterized by stronger induced cell–surface interactions and shorter distances which allow adhesins exposed to the cell surface to form a bond with the biomaterial [65]. Gene expression which encodes for bacterial surface structures including fimbriae, pili, lipopolysaccharides, and slime will also begin to strengthen adhesion promoting biofilm formation [65,66]. (iv) Cell proliferation and formation of microcolonies:—once the bacteria have become attached to the surface of the medical device and stabilized, the cells will proliferate rapidly and produce intercellular adhesins to form microcolonies. In this step, the QS “quorum sensing” communication system is activated when the bacterial density reaches a threshold [11,57,67]. The gene expression of components required for a biofilm matrix such as polysaccharides, proteins, eDNA, and lipids is also activated [68]. (v) Device surface colonization—the cells continue to proliferate and additional surrounding planktonic cells are also incorporated into the biofilm [69]. (vi) Biofilm maturation—the multilayers continue to form, inducing an increase in thickness, thereby allowing the transition from a two-dimensional arrangement to a three-dimensional arrangement (mature biofilm) [67] also called “mushroom” structure [70]. Channels filled with water are formed in the biofilm allowing the transport of nutrients, signaling molecules, and elimination of waste [53,71]. (viii) Biofilm dispersion—once the biofilm is mature, the planktonic bacteria detach from the biomaterial due to hydrolase enzymes then migrate and colonize new surfaces, spreading the infection [11,12]. The main steps are illustrated in Figure 1 below.
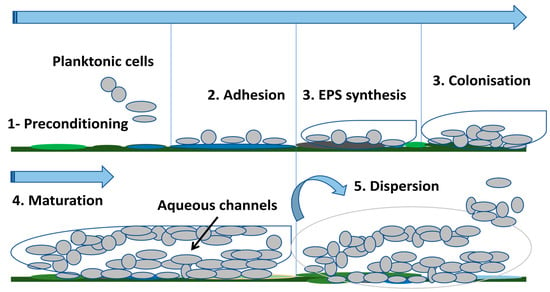
Figure 1.
Biofilm formation steps on medical devices.
4. Most Common Pathogenic Bacteria Involved in Medical Device-Associated Biofilm Infections
4.1. Escherichia coli
Escherichia coli is responsible for a wide variety of community and HAIs such as UTIs and BSIs with increasing antimicrobial resistance rates [72,73]. Indeed, in addition to the global emergence of resistance to carbapenems, the significant increase in the prevalence of quinolones-resistant E. coli strains has also been reported in several countries limiting the treatment choice for these bacterial infections, thereby constituting a real problem for public health [74,75]. Uropathogenic E. coli (UPEC) is the bacterium mostly involved in 80 to 90% of UTI cases [76,77] and in 40% of hospital acquired UTIs [78]. These strains have become more resistant to antibiotics with the increasing prevalence of extended-spectrum β-lactamases (ESBLs) [74,79]. E. coli is one of the bacteria most implicated in biofilm-related infections, particularly in CA-UTIs [80,81,82,83,84] and CVC-BSIs [85,86,87,88]. In fact, biofilm formation is the major cause of these infections in catheterized patients, making them hard to eradicate [89,90]. Indeed, their ability to form a biofilm is associated with the persistence and chronicity of inflammations leading to complicated and/or recurrent infections [91,92]. E. coli strains have an arsenal of virulence factors which contribute to adhesion, colonization, and persistence allowing the different defense mechanisms of the host to be overcome [93]. Among them, we distinguish adhesins, fimbriae, toxins, siderophores, etc. Table 1 summarizes the virulence factors implicated in E. coli pathogenesis and especially adhesion and biofilm formation. The role of virulence genes of E. coli in its adhesion to catheter surfaces has been reported in several studies [82,83,89,90,92,94,95,96,97,98,99,100,101,102]. For example, Reisner et al. [94] reported that 73% of E. coli strains isolated from catheterized patients expressed type 1 fimbriae. In a recent study of Zou et al. [90], they showed that biofilm associated genes such as iron transport systems (ferric citrate) and antigen 43 may be involved in the pathogenic CA-UTI strains. Another recent study demonstrated that the knockout of luxS, fimH, and bolA genes decreased EPS matrix production, which is very important in E. coli biofilm-associated UTIs [83].

Table 1.
Main medical device-associated bacteria and their virulence factors.
4.2. Klebsiella pneumoniae
Klebsiella pneumoniae is considered to be one of the most important opportunistic pathogens responsible for NIs including sepsis, soft tissue infections, pneumonia [168], and UTIs, which are the most common worldwide [169]. K. pneumoniae is implicated in 6–17% of opportunistic UTI cases mainly linked to bacterial adhesion in the inner and outer surfaces of the urinary catheter [170,171]. Moreover, it is the second pathogen involved in BSIs, after E. coli [172]. The prevalence of NIs due to K. pneumoniae has been reported to be approximately 10% worldwide [173]. Several studies revealed the implication of K. pneumoniae in HAIs, especially in catheter-related infections (CRIs) [18,85,172,174,175,176,177,178,179]. Since this bacterium is commensal in humans, gastrointestinal colonization represents the major source of transmission and the development of infections towards other sites [180]. The global antibiotic resistance rate of K. pneumoniae is approximately 70% with mortality rates ranging from 40% to 70% [181] and the emergence and spread of MDR-strains of K. pneumoniae have becomea real global problem [182]. In fact, this bacterium is able to acquire resistance genes such as ESBLs or carbapenemases (resistance to cephalosporins or third-generation carbapenems), limiting treatment options for infections [168], causing serious or even fatal infections, while also increasing the length of hospitalization and the costs of processing [183]. Moreover, carbapenem-resistant K. pneumoniae are the major cause of BSIs with high rates of mortality and morbidity in the world [179]. Another group of K. pneumoniae strains called “hypervirulent”, able to express acquired virulence factors, has also emerged causing serious community-acquired infections [184]. Many K. pneumoniae isolates are able to form biofilms, resulting in increased impermeability to antibiotics causing treatment failure [185]. For this, the World Health Organization (WHO) classifies this bacterium among the high priority species and encourages the development of new antimicrobial molecules in order to counter its antibiotic resistance [169]. K. pneumoniae has several virulence factors, including types 1 and 3 fimbriae, capsule polysaccharides, LPS, quorum-sensing, and PGA [118] used to escape host immune defenses, biofilm formation, and to persist during infection [186].The adhesins of K. pneumoniae allow the establishment of strong biofilms [116,187,188,189,190,191,192,193,194].An in vitro study reported the improvement of biofilm formation on UCs by the presence of types 1 and 3 fimbriae in a bladder model [116]. In a recent study, the results showed that 87.5%, 46.4%, and 53.6% of strains harbored fimH, mrkA, and mrkD fimbrial genes, respectively [193]. Other genes have been reported to be involved in biofilm formation [119,187,191,194,195,196,197]. The main virulence factors of K. pneumoniae are summarized in Table 1.
4.3. Proteus mirabilis
Proteus mirabilis is an opportunistic pathogen that causes infections in immunocompromised individuals but also NIs, including wound infections, blood infections, and mainly UTIs [50]. It is recognized as the main cause of CA-UTIs, and in the USA, 3% of all HAIs and 44% of CA-UTIs are linked to this bacterium [126]. In fact, P. mirabilis is considered the third most common cause of UTIs and the second most common cause of CA-UTIs in long-term catheterized patients [198]. Furthermore, UTIs, particularly CA-UTIs, caused by P. mirabilis generate serious complications such as the formation of bladder and kidney stones, permanent kidney damage, or even bacteremia/sepsis which can be fatal for patients [199]. In addition, trauma to the urethra and bladder mucosa may also occur during catheter removal [124]. All these complications are due to the capacity of P. mirabilis to form crystalline biofilms, leading to an obstruction [126]. P. mirabilis was reported as a common agent causing UTIs and CA-UTIs in several studies [122,126,198,200,201,202,203]. This commensally bacterium colonizing the perianal area of patients, easily penetrates into the bladder after implantation of the catheter, and thus adheres to it [204]. In addition, in recent years, P. mirabilis strains have become increasingly resistant to drugs, especially, the isolates producing ESBLs, leading to therapeutic failure, thereby constituting a worldwide problem for public health [123,205,206,207].
Proteus mirabilis has a panel of virulence factors (Table 1); however, its pathogenicity is exacerbated by biofilm formation, making the infection worse [208]. In fact, this uropathogen has been shown to have a great ability to form biofilm and is involved in the encrustation and blockage of UCs—a common complication in patients with long-term indwelling urinary catheterization—and is a major cause of morbidity and mortality in CA-UTIs [124,129]. Numerous adhesins, including MR/PM fimbriae, MR/KH, PM fimbriae, urethroepithelial adhesin (UCA), and ambient-temperature fimbriae (ATF) have been associated to P. mirabilis adhesion onto UCs and biofilm formation during CA-UTIs [126,203,209,210,211].
4.4. Pseudomonas aeruginosa
Pseudomonas aeruginosa is one of the six bacterial pathogens of the ESKAPE group (Enterococcus faecium, Staphylococcus aureus, K. pneumoniae, Acinetobacter baumannii, P. aeruginosa, and Enterobacter species) known for their high antibiotic resistance and increased virulence, which pose big challenges to treatment worldwide [136,212,213]. In fact, the resistance of this pathogen to many antibiotics, particularly resistance to carbapenems, constitutes a serious threat to global public health and increases morbidity and mortality rates, particularly in ICUs [137,214]. This is why the WHO designated this MDR-bacterium as one of the priority antibiotic-resistant pathogens for which new antibiotics are urgently needed [136]. P. aeruginosa is one of the most opportunistic pathogens causing fatal acute or chronic infections in immunocompromised hosts. Indeed, it is one of the most common pathogens found in hospitals and is responsible for more than 50% of NIs [213]. The most HAIs caused by P. aeruginosa are VAP, UTIs, CA-BSIs, burn wound infections, skin and soft tissue infections, surgical site infections, and ocular infections [131,215]. Moreover, P. aeruginosa has the ability to form biofilms during infections causing increased resistance to antibiotics and the persistence of NIs, which are considered fatal for patients [216]. It is estimated that P. aeruginosa is responsible for 28% of device-related infections [217]. A rate of 11.5% of P. aeruginosa strains was recovered from UCs and all of them were MDR [217]. The implication of P. aeruginosa in CA-UTIs [218,219,220,221] and in CL-BSIs [85,87,88,175,222] has been widely reported. Importantly, a recent international study on infections in ICU-patients demonstrated that P. aeruginosa was responsible for 23% of all infections acquired in ICUs, with the respiratory source being its main site [223]. These bacteria have an impressive arsenal of virulence factors which contributes to their pathogenesis (Table 1). Flagella and pili are virulence factors involved in motility as well as bacterial adhesion, which is the starting point of infections [214,224,225,226,227]. Also, the EPS Psl and Pel, major components of the biofilm matrix of P. aeruginosa, significantly contribute in bacterial adhesion to the catheter surface, cell–cell aggregation, and stability of the biofilm structure [217]. Several reports showed the importance of these EPS [219,228,229].
4.5. Acinetobacter baumannii
Acinetobacter baumannii is a nosocomial pathogen that is responsible for a large number of infections in humans, including endocarditis, UTIs, meningitis, pneumonia (in mechanically ventilated patients), and sepsis [141]. The incidence rate of A. baumannii infections is estimated to be approximately one million cases per year in the world, with high mortality rates, especially in critically ill patients [230]. The high prevalence of MDR-A. baumannii has become a serious situation in a hospital setting. One of the major factors responsible for the chronicity and persistence of infections and resistance to antibiotics of A. baumannii is its ability to colonize and form a biofilm on biotic and abiotic surfaces (e.g., vascular catheters, cerebrospinal fluid shunts, or Foleys catheter) [231,232]. In addition to MDR-A. baumannii, extremely-drug resistant (XDR) and pan-drug resistant (PDR) isolates have also been reported worldwide [230]. Most A. baumannii infections occur in ICU-patients and account for up to 20% of ICU-infections worldwide [141,233], with an increase in mortality rates (30% to 75%) [234]. Moreover, their ability to acquire resistance to antibiotics and persistence in the environment are the main factors contributing to their survival in the hospital environment [235]. CRIs associated with MDR-A. baumannii biofilms have been widely reported [236,237,238,239,240,241,242,243]. A. baumannii possess several adhesins (Table 1), which contribute to biofilm formation and bacterial colonization on medical devices [230]. Several reports have shown the presence of ompA (detection from 81 to 100%), csuE (detection from 80 to 100%), and bap (detection from 43 to 91.4%) in clinical A. baumannii strains producing strong biofilms [232,244,245,246,247,248]. In a recent study, Kasperski et al. [249] reported that 72% of strong biofilm producer A. baumannii strains harbored four genes associated with biofilm formation (bap, bfmS, csuE, and ompA), showing their implication in bacterial adhesion on surfaces.
4.6. Staphylococcus aureus
Staphylococcus aureus is a clinical pathogen that causes infections in both humans and animals, ranging from mild infections to severe invasive life-threatening infections [250]. This organism is also the main agent involved in NIs due to its incredible ability to adhere to the surface of medical devices and form a biofilm, which often leads to chronic infections such as osteomyelitis, endocarditis, CF, catheters infections, prostheses infections, and other medical device-associated infections [251,252,253]. S. aureus is one of the major pathogens responsible for nosocomial blood infections with an incidence estimated between 10–30 cases/100,000 persons/year and associated with mortality rates ranging between 15 and 40% [254]. In addition to this, the global emergence of methicillin-resistant S. aureus (MRSA) is a major public health concern given its high virulence and therefore, treatment failure is unavoidable [155], increasing mortality and morbidity in patients [251]. It is important to note that the mecA gene is the genetic determinant of methicillin resistance in MRSA, which is located on the mobile genetic element called staphylococcal cassette chromosome mec (SCCmec) [255]. It has a remarkable arsenal of virulence factors (Table 1) including toxins, proteases, nucleases, but also many proteins anchored in the cell wall which important factors are allowing it to adhere to tissues, to surfaces, to form a biofilm, and to escape the host’s immune defense [256,257]. It has been reported that the mortality rates associated with MRSA bacteremia were higher than those associated with methicillin-sensitive S. aureus (MSSA) bacteremia [258]. CR-BSIs due to S. aureus are considered to be the most fearful of infections. Indeed, Mandolfo et al. [259] identified 113 CR-BSIs caused by MRSA (47.5%) and MSSA (52.5%) in hemodialysis patients. In the study of Bonnal et al. [260], 56% of S. aureus in PVC-BSIs and 34% of S. aureus in CVC-BSIs were identified. Recently, Pinto et al. [261] identified S. aureus as the main causative agent of CR-BSIs with a rate of 24.1%. Other studies [85,175,222,258,262,263,264,265,266] also reported implication of S. aureus in CR-BSIs. S. aureus, especially MRSA, also constitutes a serious problematic pathogen in CA-UTIs [267]. In fact, S. aureus can colonize the urinary tract via urinary catheterization causing ascending UTIs. The presence of MRSA complicates the situation, extending the length of hospital stay [268]. This pathogen causes approximately 0.2–4% of UTIs and is more often found in patients in long-term care and with long-term catheters [269]. Several studies have shown the implication of S. aureus in CA-UTIs [267,270,271].
Various reports showed the implication of virulence genes in S. aureus healthcare infections including CRIs. The major factor in staphylococcal biofilm formation is the polysaccharide intercellular adhesin (PIA) or poly-N-acetyl β-1-6 glucosamine (PNAG), encoded by the icaADBC operon [272,273,274]. This polysaccharide plays an important role in colonization, biofilm formation and biofilm-related infections, antimicrobial resistance, immune evasion, and phagocytosis [146]. Several studies reported a positive correlation between the presence of icaAD genes and the ability of Staphylococcus strains to produce a biofilm [275,276,277]. However, Pinto et al. [261] showed no relationship between the presence/absence of the ica operon and biofilm formation on CVCs. Another recent study showed that 4/6 S. aureus strains, that did not carry ica genes, were strong biofilm producers [278]. This indicates that other genes may be involved in biofilm formation. Indeed, S. aureus contains also a range of proteins categorised as “microbial surface component recognising adhesive matrix molecules (MSCRAMM)” [146,149] such as fibronectin-binding proteins (FnBPA and FnBPB), clumping factors (ClfA and ClfB), collagen adhesin (Can), elastin binding protein (EbpS), fibrinogen binding protein (Fib), laminin-binding protein (Eno), and serine aspartate repeat proteins C, D, and E (SdrC, SdrD, SdrE) [279,280], which have been implicated in binding host matrix components (fibronectin, fibrinogen, collagen) to initiate cell attachment and/or biofilm formation [150]. The association between the MSCRAMMs adhesins ClfA/B, FnbA/B, and Cna with bacteremia and catheter-related bacteremia has also been reported [281,282]. Similarly, Walker et al. [267] reported that the clumping factor ClfB, interacting with fibrinogen, facilitates colonization of the UC and bladder leading to infection in mice and humans in a CA-UTI model.The expression of these virulence factors and biofilm formation are regulated by global regulatory systems such as accessory gene regulator (agr), staphylococcal accessory element (sae), and also by staphylococcal accessory regulator A (sarA) [155]. Pérez-Montarelo et al. [283] reported that more than half of MRSA-related bloodstream isolates belong to the accessory gene regulator (agr) group II.
4.7. Staphylococcus epidermidis
Staphylococcus epidermidis is one of the most ubiquitous opportunistic pathogens [284] that causes serious infections in immunocompromised patients, especially those associated with the presence of invasive medical devices (e.g., VC and artificial heart valves) [156]. However, this bacterium rarely causes CA-UTIs and rarely pyelonephritis (without an indwelling urinary device) [285]. It is estimated that 30% of CA-BSIs are due to S. epidermidis strains [286]. S. epidermidis isolates were implicated in several CA-BSIs. A recent report revealed that this species was the most causative agent in CA-BSIs (13.3%) in the emergency department [287]. A prospective observational study conducted by Pinto et al. [261] showed that S. epidermidis was the most etiological agent of CR-BSI. A similar retrospective study on CA-BSI in coronavirus disease 2019 ICU showed also the prevalence of S. epidermidis [288]. Other prevalence rates have been also reported: 31.37% [289], 31% [290], 28% [291], 18.1% [292], 12.33% [286], 8.3% [293], and 7.7% [261]. The European Center for Disease Prevention and Control reported that S. epidermidis caused 23.6% of CA-BSI cases in ICUs [261]. It has also been reported that children are very susceptible to infection associated-methicillin-resistant S. epidermidis strains in perinatal units [294]. In addition, in recent years, the situation has become complicated with the capacity of S. epidermidis strains to form biofilms on medical devices and their increased resistance to antibiotics, mainly methillin resistance, leading to significantly high mortality and morbidity rates and medical costs [295,296]. It has been reported that more than 70% of S. epidermidis strains are resistant to methicillin, which is encoded by mecA gene, making the treatment of infections ineffective but above all that this resistance to methicillin can be quickly disseminated to other Gram-positive strains via horizontal gene transfer [297]. S. epidermidis strains produce a variety of virulence factors contributing to their pathogenicity (Table 1). However, unlike S. aureus, S. epidermidis isolates are weakly virulent, do not produce aggressive toxins, and are commonly non-hemolytic [159]. The main virulence factor is its ability to adhere to the surface of medical devices and form a biofilm [298,299]. During accumulation, S. epidermidis produces a major component of the biofilm matrix which is PIA [156]. As for S. aureus, PIA, encoded by ica opeon, is an essential factor in S. epidermidis biofilms allowing their adhesion to surfaces [300]. Cherifi et al. [301] found that the ica operon was significantly more present in CR-BSI isolates than in commensal isolates. However, François et al. [274] reviewed that the expression of ica operon is not essential in the colonization of a surface, and the presence of other virulence genes such as atlE, fbe, and embp are involved in catheter-related infections linked to S. epidermidis biofilms [286] The pathogenesis of S. epidermidis is regulated by two key systems, the agr and the sar regulators, which allow the expression or repression of the virulence genes in a coordinated manner during infection [160]. An early study showed that the agr QS system played an important role in the long-term development of S. epidermidis biofilm during medical device-associated infections [302].
4.8. Enterococcus spp.
Enterococci are Gram-positive, non-motile, lactic acid-producing bacteria widely found in the gut microbiota of humans and animals [303]. Enterococci are facultative anaerobes that tolerate a variety of environmental conditions such as extreme pH, salinity, and a wide temperature range (10 to >45 °C) [163]. Enterococci are tenacious microorganisms characterized by increased tolerance to desiccation and starvation, making them resistant to environmental stresses [304]. The emergence of Enterococcus strains as HAIs agents [305] can be explained by the following: (i) these bacteria are intrinsically resistant to several classes of antibiotics (cephalosporins, macrolides, clindamycin, and trimethoprim–sulfamethoxazole) and they acquired resistance to ampicillin, ciprofloxacin, high-level aminoglycosides, and vancomycin [306], causing a serious problem for the treatment of these infections given the limited choice of available antibiotics (linezolid, daptomycin, quinupristin/dalfopristin, and vancomycin) [307]; (ii) they have an impressive capacity to acquire new resistance genes due to the plasticity of their genome [308].
Enterococcus spp., particulary En. faecalis and En. faecium, are the most opportunistic pathogens causing several infections, including medical device-associated infections, UTIs, wound infections, and BSIs [308]. En. faecalis is responsible for 80 to 90% of cases of Enterococci-associated NIs, followed by En. faecium (5 to 10% of infections) [309,310]. Putta et al. [311] revealed that En. faecalis was the most causative agent of CA-UTIs in ICU-patients. In another study, 13.11% of En. faecium was isolated from CA-UTIs [312]. Different rates were obtained in other studies: 91% [313], 29% [84], 22.9% [314], 19% [315], and 7.1% [316]. The presence of these pathogens in CL-BSIs was also reported [317,318]. En. faecalis was the 5th most frequently isolated bacteria from CA-UTIs and the 3rd from CLA-BSIs, unlike En. faecium, which was the 11th and 5th in CA-UTIs and CLA-BSIs, respectively [303]. However, in recent years, the prevalence of infections due to En. faecium has increased, overtaking the prevalence of En. Faecalis, and this is due to the emergence of antibiotic resistance, especially vancomycin-resistant En. faecium (VREfm), which is responsible for the most vancomycin-resistant Enterococcus (VRE) infections in the world [319,320,321], thus leading the WHO to include VREfm in the list of high priority pathogens [321].Of note, glycopeptides resistance in Enterococci is attributed to the acquisition of different clusters of genes (e.g., vanA, vanB, vanD, vanE, vanG, and vanL) which confer resistance [322,323]. In addition to antibiotic resistance, the ability of Enterococcus strains to form biofilms is one of the primary factors involved in their virulence and pathogenicity notably on medical devices [324,325]. Several investigations have been conducted on Enterococci virulence factors involved in biofilm formation. Soares et al. [326] found that En. faecalis possessed esp, gelE, and asa1 genes. Kafil and Mobarez [327] reported the presence of esp, ebpA, and ebpB genes found in high biofilm producers. Similar results were found in the study of Khalil et al. [325]. It has been reported also that the endocarditis and biofilm-associated (Ebp) pilus is involved in biofilm formation on UC leading to CA-UTI [327,328]. However, in presence of urine, Ebp is not capable to initiate En. faecalis adhesion on UC. This fact was explained by the release of fibrinogen covering the catheter following an inflammatory response caused by the catheter itself [327]. In another study, the analysis of mutations affecting two proteases, secreted by En. faecalis (GelE, SprE), revealed that loss of both factors resulted in decreased CA-UTI and defective biofilm establishment in a murine CA-UTI model, whereas the loss of either had no effect. They revealed also that the high expression of these proteases depends on the fsr QS system [329]. Another study reported the importance of Ace and Esp adhesins in the bacterial attachment on the catheter surface, and biofilm accumulation [330].Given that En. faecalis is the most identified species in biofilm-associated infections [331,332], the virulence factors cited in Table 1 only concern this bacterial species.
5. Pathogenesis of Venous Catheter Contamination and Catheter-Associated Bloodstream Infections (CA-BSIs)
After insertion of a VC, the surface of the device is immediately covered by a conditioning film composed of organic molecules such as fibronectin and fibrinogen, collagen, elastin, and laminin [27,333]. There are several pathways involved in catheter contamination: colonization of the surface of the catheter tip by skin microorganisms originating from health care worker’s hands, contaminated disinfectant, which migrates to the insertion site in the skin pathway of the catheter and along the catheter surface (extraluminal) which is the most common route of infection for short-term catheters (inserted for ≤14 days), colonization of the inner surface of the catheter by contaminated infusion product and catheter hub (intraluminal), and finally, by hematogenous seeding (rare route) [17,334]. In contact with blood, microorganisms interact with fibrin to produce an adherent biofilm which will promote bacterial colonization and the spread of these microorganisms [334].
Colonization and biofilm formation on the catheter surface occurs 24 h after device insertion [335]. These pioneer bacteria allow the attachment of other pathogens by providing more diverse adhesion sites. After multiplication, the bacteria produce an extracellular matrix which maintains the biofilm, leading to irreversible adhesion to the surface of the catheter [27]. Once the biofilm is mature, bacteria can disperse, cause catheter associated bloodstream infections (CA-BSIs), and colonize other sites in the body [336].The formation of fibrin sheaths is also observed. Once the catheter is implanted, the fibrinogen, albumin, lipoproteins and coagulation factors, released due to the lesion of the blood vessels, begin to deposit on the surface of the catheter within 24 h forming a fibrin sheath which covers the surface of the catheter within days or even weeks. This fibrin sheath is responsible for the late stage catheter dysfunction, which usually occurs about three months after the catheter placement [333,337].
6. Pathogenesis of Urinary Catheter Colonization and Catheter-Associated Urinary Tract Infections (CA-UTIs)
Transurethral ascension of microorganisms is the most common mechanism for the development of UTIs, which explains the increased risk of infection after catheterization [338]. Bacteria can colonize UCs either by the endoluminal route which involves exogenous flora originating from colonization of the collecting sac or from a breach of the closed system during manipulations of the urinary catheter, or by the exoluminal route which involves the endogenous flora of the urinary meatus and occurs early during catheter placement or later following colonization of the urinary meatus by the digestive flora [6]. After insertion of the catheter, the bacteria overcome first the electrostatic repulsion observed between bacterial cell and catheter surface to allow intimate interactions to occur, then adhere to a conditioning film of urine components and host proteins, such as Tamm–Horsfall protein, magnesium and calcium ions, which form along the catheter surface. It is also reported that the urinary catheter elicits an inflammatory response resulting in the release of the host protein fibrinogen into the lumen of the bladder which will cover the surface of the catheter [330].Pathogens such as S. aureus and Enterococcus faecalis possess adhesins such as the fibronectin-binding protein A (FnBPA) and the endocarditis and biofilm-associated pilus (EbpA), respectively, which bind to the host fibrinogen in order to disrupt blood clotting, initiate biofilm formation, as well as immune evasion [339,340]. Once irreversibly fixed to the surface of the catheter via adhesins and pili, bacteria begin to change their phenotype, producing exopolysaccharides which protect them and form a biofilm [106,341]. The presence of biofilms promotes the appearance of epithelial lesions due to the proteases and bacterial toxins produced. The uropathogenic bacteria can then ascend to the kidneys, attaching again to the renal epithelium, causing kidney infections. Left untreated, these infections can progress to bacteremia by crossing the tubular epithelial cell barrier into the bloodstream [341].
7. Prevention of CA-BSIs
7.1. Education, Training and Surveillance
The lack of knowledge and skills is one of the main obstacles to medical practice. Indeed, compliance with guidelines for the use of intravascular catheters is very important in order to decrease the incidence of CA-BSIs and their associated health costs. Educating healthcare personnel regarding techniques for using intravascular catheters, the procedures for inserting and maintaining intravascular catheters, periodically assessing their knowledge and ensuring appropriate levels of nursing staff in intensive care units are a first line of prevention [342,343]. In addition, another effective measure to reduce CA-BSIs is to avoid unnecessary catheterization of patients as well as the rapid removal of venous catheters which are no longer necessary, particularly long-term catheters [344].
7.2. Aseptic Techniques
Hand hygiene before handling catheters, disinfecting catheter sites, catheter hubs or injection ports with an appropriate agent before accessing the catheter are essential for the prevention of CA-BSIs [345]. The use of 2% chlorhexidine–alcohol as an antiseptic agent before insertion of a VC and during dressing changes is recommended to prevent the development of CA-BSIs. The incidence of CA-BSIs was shown to be five times lower using 2% chlorhexidine–alcohol solution, compared to 5% polyvidone iodine–alcohol [346]. The catheter tip is also a major source of contamination. For this, its disinfection with appropriate antiseptic or antimicrobial ointments is recommended. Use of a povidone–iodine antiseptic ointment or bacitracin/gramicidin/polymyxin ointment at the exit site after catheter insertion is recommended [335]. The use of sterile gloves, a sterile long-sleeved gown, mask, and large sterile sheath sheet during insertion of a CVC are essential for the prevention of CA-BSIs. A checklist should also be used to improve adherence to procedures at the time of insertion [347]. After insertion of the catheter, the risk of infection should decrease with the use of aseptic techniques. However, insertion and maintenance of VCs by inexperienced personnel could increase the risk of catheter colonization and the development of infection. Having an experienced infusion therapy team in place to insert and maintain catheters decreases CA-BSI levels up to eight times [27].
7.3. Catheter Insertion Site
The catheter insertion site is an important parameter whose choice should be based on both the benefits and risks of the procedure (infection, thrombosis, and mechanical complications). The subclavian site is the ideal insertion site for CVCs, which helps reduce infectious complications [347]. This is probably explained by the fact that the subclavian route has the longest subcutaneous distance between the skin and the entrance to the vessel [348]. In addition, according to previous studies, subclavian catheterization was associated with a lower risk of infectious and thrombotic complications than femoral and jugular catheterization [349,350,351].
7.4. Catheter Lock Solutions
Another approach that shows promise for the prevention of CA-BSIs is antimicrobial lock therapy. It involves instilling a highly concentrated antimicrobial solution into the lumen of the catheter when not in use [352,353] to remove the blood so that the occlusion and bacterial growth are minimized [5] and also preventing biofilm formation [354]. This technique is useful especially in cases of uncomplicated long-term CA-BSIs caused by pathogens [47]. A variety of antimicrobial agents can be used such as heparin (anti-occlusion) [355,356], vancomycin, gentamicin (antibiotics) [357,358], citrate, ethanol, and taurolidine (antimicrobials) [357,359,360,361]. Antibiotics are generally used for therapeutic measures once a CA-BSI has been diagnosed, while heparin, citrate, ethanol, and taurolidine are used prophylactically [5]. Furthermore, Kumar et al. [362] demonstrated that using S-nitroso-N-acetyl-l-cysteine ethyl ester (SNACET)is very effective; this is able to generate nitric oxide with antimicrobial properties as a catheter locking solution. Indeed, a significant reduction of 99% in the adhesion of S. aureus and E. coli on catheters was observed.
7.5. Dressing
To prevent complications for patients, a dressing is often placed where the integrity of the skin is compromised. Several materials are used as dressing for VCs [362,363]. A gauze dressing is often used when blood seeps from the catheter insertion site. However, their use increases the risk of bacterial contamination and infection [347,364]. Transparent semi-permeable dressings are widely used and allow continuous observation of the skin insertion site and reduce the risk of extrinsic colonization. They should be changed immediately if they become wet, loose, or soiled [347]. The risk of CA-BSI increases more than 3-fold after rupture of the second dressing and more than 12-fold if the final dressing is ruptured [365,366]. The chlorhexidine-impregnated dressing, an innovative strategy, shows promising results in the prevention of infections linked to CVCs, with a reduction in colonization (6.5% versus 13.2%) [367] and infection (1.51/1000 versus 5.87/1000 catheter-days) compared to traditional dressings [368]. Moreover, Puig-Asensio et al. [369] reported that chlorhexidine dressings reduced the risk of CA-BSIs in patients with short-term CVCs, including those with an onco-hematological disease. There is also other evidence that shows that dressings impregnated with chlorhexidine may reduce the risk of CVC-BSI, compared to standard polyurethane dressings, and other types of non-impregnated dressings (gauze and tape dressing) [369,370,371,372,373]. A recent study conducted by Hou et al. [374] found that the chlorhexidine gluconate gel dressings used more effectively reduced the risk of CVC-BSI in patients unlike the chlorhexidine gluconate sponge dressings.
7.6. Antimicrobial Agents Release
Several strategies using coating or impregnating catheters with antibiotics/antimicrobials, peptides, metals, nitric oxide, or other compounds have been developed to prevent biofilm formation and constitute a promising alternative to reduce infection rates and CA-BSIs [375,376]. The use of antimicrobial agents as a coating is the most popular approach due to their ability to target microorganisms in different ways [377]. Inhibition of bacterial adhesion on catheter surfaces could be prevented by releasing the antimicrobial agent [378]. This approach aims to attach the antimicrobial agent to the catheters by adsorption, which will diffuse after exposure to body fluids [379]. This approach allows the release of high doses of the antimicrobial agent without exceeding the toxic threshold, reducing the development of resistance. However, the drawback with this technique is that the release is uncontrolled and lacks long-term properties [380,381].
Catheters coated with chlorhexidine–silver sulfadiazine, minocycline–rifampicin and miconazole and rifampin are the most commonly studied and are associated with a decreased prevalence of catheter colonization and CA-BSIs [382,383,384,385]. In fact, it has been reported that these impregnated catheters had the potential to reduce the risk of colonization of these devices and the incidence rates of CA-BSIs per 1000 catheter days [5]. Among catheters based on antimicrobial agent release which have been approved and commercialized, there is ARROWg+ard® (chlorhexidine and silver sulfadiazine coating), Spectrum® (minocycline and rifampin coating) and Chlorag+ard® (chlorhexidine coating) [5]. Other agents are able to reduce bacterial colonization on venous catheter surfaces. Table 2 summarizes most research studies which tested the antimicrobial agent coating/imprenated and surface modifications approaches for the prevention of bacterial colonization and CA-BSI.

Table 2.
The effectiveness of different antimicrobial agent coating and surface modification approaches.
7.7. Contact Kill Systems
Contact destruction of bacteria relies on the use of antimicrobials grafted onto the surface of catheters to form a lethal barrier for these pathogens [5]. Indeed, these antimicrobial molecules are mainly cationic or enzymes that bind covalently to the surface of the catheter via hydrophobic polymer chains, and kill these bacteria on contact via membrane interactions.Additionally, this strategy exhibits longer antimicrobial activity and low toxicity [379] and does not output biocides in body fluids [397]. Several compounds such as quaternary ammonium compounds, peptides, graphene derivated (Table 2) have been evaluated as promising contact killing agents. However, the major concern with this strategy is that the bioactive surface can be inactivated when coated with proteins from body fluids [379]. For that, further research studies are needed to improve the strategy.
7.8. Antifouling Approaches
Surface modifications, such as hydrophilic polymeric surface coatings, work also by reducing microbial adhesion to the catheter surface, thereby minimizing infection [375]. Indeed, surface hydration is an important parameter of antifouling coatings due to the water layer formed on the surface of the polymer, which acts as a barrier preventing bacterial adhesion and proteins adsorption [376].The most hydrophilic polymers used in the antifouling approach are poly(ethylene glycol) (PEG) (most commonly used), poly-2-hydroxyethyl methacrylate, poly(2-hydroxypropyl acrylamide), dextran, and zwitterionic polymers [400]. The immobilization of zwitterionic compounds or PEG provides promising results for CR-BSI prevention [398,401]. A recent technology which is the fluoro-passivation of catheters has emerged as an effective approach which consists of coating the catheter with fluoropolymer to increase its biocompatibility and reduce infection [337]. Among the coated catheters commercialized are the AngioDynamics BioFlo PICC catheter (endexo) and the CerebroFlo extraventricular drain catheter (endexo) [337]. Furthermore, another prevention way is the use of materials characterized by low energy, such as hydrophobic polymers (PTFE) [5].A few studies on surface modifications of venous catheters are cited in Table 2. Figure 2 illustrates the main prevention strategies of CA-BSIs.
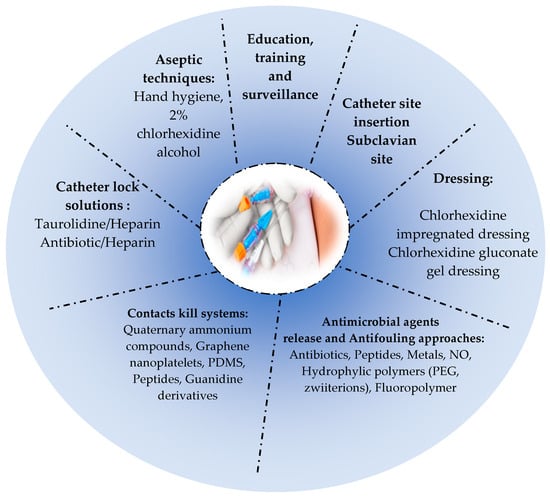
Figure 2.
Main prevention strategies of CA-BSIs.
8. Prevention of CA-UTIs
CA-UTI is one of the most common device-related infections in which preventive measures should be taken [402]. These precautions to prevent the transmission of MDR-bacteria must be scrupulously observed in catheterized patients and also limit the uncontrolled use of antibiotics [403].
8.1. Avoidance of Urinary Catheter Use
The main CA-UTI prevention strategy is to avoid or reduce the use of catheters. Overall, UCs are overused and placed for inappropriate indications in 21–50% of catheterized patients [403]. Accepted indications for the use of a catheter are considered the first step in limiting their uses. Among these limitations are the following: urological surgery, monitoring of urine flow in seriously ill patients, management of acute urinary retention and urinary obstruction, or for end-of-life care to improve patient comfort [404]. Limiting the duration of catheterization is also very important. Indeed, when a catheter is placed, it must be removed quickly once it is no longer needed [404]. Healthcare providers should be aware of the existence of the UC. Therefore, catheter remainder interventions that include a verbal/written reminder, a sticker reminder on the patient’s chart or an electronic reminder that indicates that a urinary catheter is still in place is a good prevention strategy. Another type of intervention called a “stop order” that requires the clinician (nurse or doctor) to remove the catheter after a period of catheterization or a condition has occurred, unless the catheter remains clinically appropriate, can be followed also in case the reminders are ignored [405]. Institutional policies should also reduce the use of peri-operative catheters by encouraging early removal of post-operative catheters and monitoring bladder volume using ultrasound bladder scanners [404].
8.2. Alternatives to Indwelling UC
Studies have shown decreased UTIs or deaths in patients who used condom catheters. In addition, this type of catheter seems to be less painful and more comfortable than indwelling catheters. Therefore, condom catheters may be an alternative for patients with retained or obstructed bladder. It has also been reported that the use of intermittent catheterization may be beneficial in long-term catheterized patients with neurogenic bladder or after hip surgery has reduced the risk of bacteriuria thereby minimizing the need for an indwelling catheter [403].
8.3. Education and Training
Health care personnel and others who handle urinary catheters should be trained in the procedures for inserting, maintaining, and removing urinary catheters. Education should also be offered on catheter associated urinary tract infections, complications of urinary catheterization, and alternatives to indwelling catheters [406].
8.4. Aseptic Techniques for Insertion and Maintenance of UCs
When indwelling catheterization is required, aseptic catheter insertion and maintenance are recommended to prevent CA-UTIs. For this, UCs must be placed by a qualified healthcare professional [403]. Among these recommendations are the following: hand washing with soap and water should be carried out immediately before and after handling a urinary catheter; the surface of the urethral meatus must also be clean before insertion of the urinary catheter; the catheter must be attached to the patient’s thigh to avoid lesions of the urethral meatus; in case of any skin irritation, the catheter should be changed immediately; sterile and closed urine drainage should be used to reduce the risk of infections; finally, irrigation of the bladder with normal saline or a solution containing antibiotics is not recommended, except in cases of obstruction [407].
8.5. Antimicrobial Coatings
Although improved hygiene procedures, replacement of UCs, and the use of prophylactic antibiotics have helped to reduce the incidence of CA-UTIs, it has not been avoided sufficiently. One of the most promising approaches is the use of antimicrobial coatings on UC surfaces to prevent CA-UTIs but more specifically to prevent adhesion, biofilm formation and encrustation of catheters [32,408,409,410,411]. Such strategies reduce the viability of pathogens by inhibiting the metabolic pathways necessary for their survival such as inhibiting the synthesis of nucleic acids and proteins involved in cell wall synthesis [410]. However, the development of these devices with antimicrobial surfaces must meet certain requirements, including easy and reproducible production, resistance to mechanical stresses, biocompatible and non-toxic, antimicrobial efficacy for a long time, and finally avoiding the development of resistance [411]. Among the antimicrobial agents used for UC coating are the following: metal (silver, nanoparticles), antibiotics, nitric oxide, antimicrobial peptides, bacteriophages [410]. Table 3 summarizes most research studies which tested the antimicrobial agent coating for the prevention of bacterial colonization and CA-UTIs.

Table 3.
The effectiveness of different antimicrobial agent coating and surface modifications approaches used in CA-UTIs prevention.
Metals or composite nanoparticles represent suitable alternatives for CA-UTI prevention and biofilm-related infections [434]. Several studies [409,435,436,437,438,439] have reported that UCs coated with thin-films of silver alloy could reduce bacterial adhesion but also the incidence of asymptomatic bacteriuria and CA-UTI. Other metals have been tested as coatings such as copper (Cu) [412].
Recently, novel advance, namely nanoparticles, constitutes a promising approach in biomedical devices [47]. Several studies reported the efficiency of a silver nanoparticle coating method in colonization prevention of several pathogens such as E. coli, P. mirabilis, P. aeruginosa, Stapholococcus spp., and Enterococcus spp. [413,429,440,441,442,443]. Throughout the years, other nanoparticles have been studied as coating, including green–silver nanoparticles [444,445], gold nanoparticles [446], copper nanoparticles [447] and zinc-doped(Zn) copper oxide (CuO) nanoparticles [414,448].
Antibiotics have been extensively studied over the years and despite the emergence of MDR-pathogenic bacteria, several studies have shown the effectiveness of many antibiotics on infections caused by Gram-negative and Gram-positive bacteria [449]. Moreover, antibiotics are often used for CA-UTI treatment [378]. However, due to their high cost and conflicting results between in vitro studies and clinical trials, their use is questionable [377].Antibiotics that have been commonly studied are such as nitrofurazone. Nitrofurazone-coated UCs was tested against several pathogen biofilms (E. coli, P. aeruginosa, S. epidermidis, En. faecalis) with promising results [450,451,452]. However, their carcinogenic potential in animal models induced their removed from the market and prohibition by the FDA [410]. Other antibiotics such as gentamicin [453], chlorhexidine [454], ciprofloxacin [455], norfloxacin [456], triclosan [457], and sparfloxacin [418] have been tested as a coating agent. Although giving promising results, the antibiotic-based approach favors the apparition of bacterial resistance for long-term catheters (e.g., triclosan), leading to more serious infections [434,458].
For that, the antimicrobial peptides (AMPs) are considered as the most promising strategies to conventional antibiotics [420].AMPs are host defense peptides widely used in the treatment of biofilms associated with several clinical pathogens and kill them by membrane permeabilization [459,460]. Recently, these peptides (e.g., RK1, RK2, CWR11, Bmap-28, E6, Chain 201D) were tested as coating agents for UCs to prevent biofilm formation and CA-UTIs [419,461,462,463].
In other studies, nitric oxide (NO), a natural gas molecule, with a short half-life, has been demonstrated as being able to protect the host against several pathogens [464]. Its mechanism of action is that it binds covalently to DNA, proteins, and lipids to inhibit or kill the pathogen [377]. The approach based on NO appears to be a promising alternative to combat bacterial infections and the formation of biofilms [465,466].
Bacteriphages have also been suggested as a new strategy to combat bacterial biofilms. They specifically infect bacteria and disrupt their metabolism to self-replicate and then, kill them [128,467]. In addition to their specificity and self replication as advantages, they are able to degrade biofilm matrix and prevent resistance development, while the treatment is improved when phages cocktail is used [378,410,468]. Phage therapy has been used to treat wide bacterial infections with little or no side effects constituting a promising technology in clinical application [469]. Until now, several phage-based coating UCs have been developed and tested for uropathogens including E. coli, K. pneumoniae, P. mirabilis, P. aeruginosa, and Staphylococcus spp. [468,470,471,472,473].
8.6. Antifouling Approaches
Antifouling approaches can also prevent bacterial adhesion on UCs and biofilm formation by repelling them without harming them [377]. The principle of these strategies is the acquisition of anti-adhesive properties by physicochemical modifications of catheter surfaces in order to prevent bacterial adhesion in addition to a good antibacterial activity and low toxicity [376,434]. Moreover, this approach provides the advantage of low risk of drug resistance emergence [474]. Additionally, the hydration layer increases patient comfort due to low friction during UC placement [434]. There are wide antifouling approaches, especially hydrogels which are the most popular due to their hydrophilic structure which reduces bacterial growth [475]. Poly(tetrafluoroethylene) (PTFE), poly(ethylene glycol) (PEG), polyzwitterion, and enzyme coating were also studied [377] to prevent the development of biofilms on UC surfaces and CA-UTI prevention. These polymers repel foulants due to the formation of a hydration layer on the surface [449]. Among the hydrophilic coatings, some of them have been already commercialized including HydroPlus™ from Boston Scientific, AQ® from Cook Urological, heparin-based coating Endo-Sof™ Radiance™ from Cook Urological, and SL-6 from Applied Medical [434].
Hydrogels are hydrophilic polymers widely studied as coatings due to their excellent hydropilicity, high hydration, and porous structures [476]. Several studies reported the efficiency of this approach [477,478,479]. However, it was reported that this approach caused the encrustation of catheters which is contradictory to other results [480]. Further studies are needed to provide more information about long-term prevention of CA-UTIs and biofilm formation and validate their effectiveness.
Another polymer used for coating is poly(tetrafluoroethylene) (PTFE), called also teflon which is characterized by high non-stick properties and resistance to bacterial adhesion, making it an excellent option for biofilm prevention [378,409]. The teflon-coated catheters are commercially available from Bard Medical [377]. Various early studies have been also conducted for the same purpose [429,481,482].
Similary, polyethylene glycol (PEG) possesses nonimmunogenic, nonantigenic, and protein repellent properties thus appearing to be a good antifouling agent [377].
Polyzwitterions that contain both cationic and anionic ions constitute also promising antifouling agents for the coating of UCs due to their superhydrophilicity [483]. Researchers designed different polyzwitterion silicone catheter surfaces and studied their effectiveness including sulfobetaine methacrylate (SBMA) [428], copolymer-coated Ti6Al4V (Ti6Al4V@DMA-MPC) [484], polysulfobetaine (PSB) [485], poly(sulfobetaine methacrylate) (pSBMA), and poly(carboxybetaine methacrylate) (pCBMA) [486].
In the recent past, the effectiveness of the enzymes was evaluated toward bacterial adhesion [377]. Furthermore, the enzymes are natural, safe, and non-toxic to other than their target cells which is an advantage. They were recently studied in the UC-coatings field [449,487]. Among the enzymes already tested are the following: acylase, cellobiase dehydrogenase, α-chymotrypsin, and glycoside hydrolases [377]. Table 3 summarizes most of the studies showing the effectiveness of different antifouling approaches. Figure 3 illustrates the main prevention strategies of CA-UTIs.
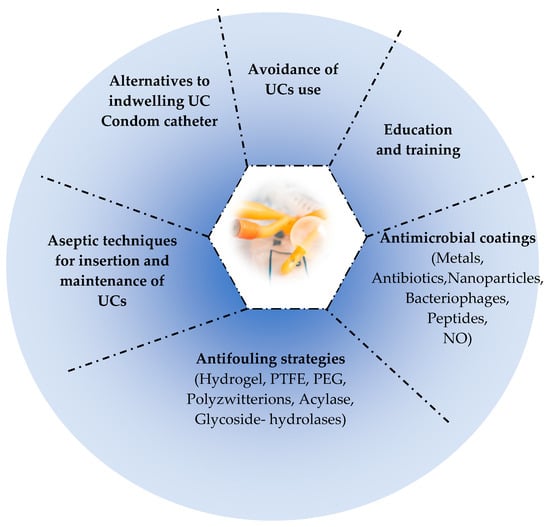
Figure 3.
Main prevention strategies of CA-UTIs.
9. Conclusions
Medical device-associated biofilm infections, mainly catheter associated bloodstream infections and catheter associated urinary tract infections, which are the most common infections in healthcare, constitute a real problem in hospitals. In addition to the global emergence of multidrug resistance, the biofilm formation on these devices, especially the presence of persistent cells, makes these infections worse, causing the recalcitrance of infections and therapeutic failure, thereby increasing the rate of morbidity, mortality, healthcare cost, and length of hospitalization.
However, the risk of these infections could be reduced by respecting the prevention guidelines, including educating healthcare personnel, hygiene, limiting use, choice of catheter insertion site, and the antimicrobial lock therapy. In recent years, novel and effective advanced strategies have been developed as contact kill systems—antimicrobial-coated catheters with metals, nanoparticles, phages, antibiotics, antimicrobial peptides and other compounds—and have helped to reduce bacterial adhesion, biofilm formation, catheter encrustation, cytotoxicity, and complications for patients. Antifouling approaches also constitute promising alternatives to prevent medical device associated infections. Despite all the in vitro and in vivo studies that have been conducted in this area, no ideal strategy has been found until now due to the divergence of the results obtained. Further, additional research studies, notably clinical trials, are still needed to develop biocompatible strategies and fully validate their efficiency in order to prevent and fight medical device-associated biofilm infections, especially for long-term catheters.
Author Contributions
Conceptualization, F.B.; validation, F.B. and P.H.N.; resources, F.B.; data curation, F.B.; writing—original draft preparation, N.B.; writing—review and editing, F.B.; visualization, P.H.N.; supervision, F.B.; project administration, F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ullman, A.J.; Marsh, N.; Mihala, G.; Cooke, M.; Rickard, C.M. Complications of central venous access devices: A systematic review. Pediatrics 2015, 136, e1331–e1344. [Google Scholar] [CrossRef]
- Santos, F.K.Y.; Flumignan, R.L.G.; Areias, L.L.; Sarpe, A.K.P.; Amaral, F.C.F.; Ávila, R.B.; Vasconcelos, V.T.; Guedes Neto, H.J.; Amorim, J.E.; Nakano, L.C.U. Peripherally inserted central catheter versus central venous catheter for intravenous access: A protocol for systematic review and meta-analysis. Medicine 2020, 99, e20352. [Google Scholar] [CrossRef]
- Zhang, K.; Li, X.; Yu, C.; Wang, Y. Promising therapeutic strategies against microbial biofilm challenges. Front. Cell. Infect. Microbiol. 2020, 10, 359. [Google Scholar] [CrossRef]
- Guenezan, J.; Drugeon, B.; Marjanovic, N.; Mimoz, O. Treatment of central line-associated bloodstream infections. Crit. Care 2018, 22, 303. [Google Scholar] [CrossRef]
- Casimero, C.; Ruddock, T.; Hegarty, C.; Barber, R.; Devine, A.; Davis, J. Minimising blood stream infection: Developing new materials for intravascular catheters. Medicines 2020, 7, 49. [Google Scholar] [CrossRef]
- Aumeran, C.; Mottet-Auselo, B.; Forestier, C.C.; Nana, P.A.; Hennequin, C.; Robin, F.; Souweine, B.; Traoré, O.; Lautrette, A. A prospective study on the pathogenesis of catheter-associated bacteriuria in critically ill patients. BMC Microbiol. 2021, 21, 86. [Google Scholar] [CrossRef]
- Yasir, M.; Willcox, M.D.P.; Dutta, D. Action of antimicrobial peptides against bacterial biofilms. Materials 2018, 11, 2468. [Google Scholar] [CrossRef]
- Wi, Y.M.; Patel, R. Understanding biofilms and novel approaches to the diagnosis, prevention, and treatment of medical devicea-Associated infections. Infect. Dis. Clin. N. Am. 2018, 32, 915–929. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Speziale, P.; Montanaro, L.; Costerton, J.W. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials 2012, 33, 5967–5982. [Google Scholar] [CrossRef]
- Kannappan, A.; Sivaranjani, M.; Srinivasan, R.; Rathna, J.; Pandian, S.K.; Ravi, A.V. Inhibitory efficacy of geraniol on biofilm formation and development of adaptive resistance in Staphylococcus epidermidis RP62A. J. Med. Microbiol. 2017, 66, 1506–1515. [Google Scholar] [CrossRef]
- Li, P.; Yin, R.; Cheng, J.; Lin, J. Bacterial biofilm formation on biomaterials and approaches to its treatment and prevention. Int. J. Mol. Sci. 2023, 24, 11680. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Breslawec, A.P.; Liang, T.; Deng, Z.; Kuperman, L.L.; Yu, Q. Strategy to combat biofilms: A focus on biofilm dispersal enzymes. NPJ Biofilms Microbiomes 2023, 9, 63. [Google Scholar] [CrossRef]
- Yan, J.; Bassler, B.L. Surviving as a community: Antibiotic tolerance and persistence in bacterial biofilms. Cell Host Microbe 2019, 26, 15–21. [Google Scholar] [CrossRef]
- Wainwright, J.; Hobbs, G.; Nakouti, I. Persister cells: Formation, resuscitation and combative therapies. Arch. Microbiol. 2021, 203, 5899–5906. [Google Scholar] [CrossRef]
- Stojowska-Swędrzyńska, K.; Kuczyńska-Wiśnik, D.; Laskowska, E. New strategies to kill metabolically-dormant cells directly bypassing the need for active cellular processes. Antibiotics 2023, 12, 1044. [Google Scholar] [CrossRef]
- Theis, T.J.; Daubert, T.A.; Kluthe, K.E.; Brodd, K.L.; Nuxoll, A.S. Staphylococcus aureus persisters are associated with reduced clearance in a catheter-associated biofilm infection. Front. Cell. Infect. Microbiol. 2023, 13, 1178526. [Google Scholar] [CrossRef]
- Gahlot, R.; Nigam, C.; Kumar, V.; Yadav, G.; Anupurba, S. Catheter-related bloodstream infections. Int. J. Crit. Illn. Inj. Sci. 2014, 4, 162–167. [Google Scholar] [CrossRef]
- Pitiriga, V.; Kanellopoulos, P.; Bakalis, I.; Kampos, E.; Sagris, I.; Saroglou, G.; Tsakris, A. Central venous catheter-related bloodstream infection and colonization: The impact of insertion site and distribution of multidrug-resistant pathogens. Antimicrob. Resist. Infect. Control 2020, 9, 189. [Google Scholar] [CrossRef]
- Zander, Z.K.; Becker, M.L. Antimicrobial and antifouling strategies for polymeric medical devices. ACS Macro. Lett. 2017, 7, 16–25. [Google Scholar] [CrossRef]
- Sato, A.; Nakamura, I.; Fujita, H.; Tsukimori, A.; Kobayashi, T.; Fukushima, S.; Fujii, T.; Matsumoto, T. Peripheral venous catheter-related bloodstream infection is associated with severe complications and potential death: A retrospective observational study. BMC Infect. Dis. 2017, 17, 434. [Google Scholar] [CrossRef]
- Beecham, G.B.; Tackling, G. Peripheral Line Placement. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar] [PubMed]
- Miliani, K.; Taravella, R.; Thillard, D.; Chauvin, V.; Martin, E.; Edouard, S.; Astagneau, P.; CATHEVAL Study Group. Peripheral venous catheter-related adverse events: Evaluation from a multicentre epidemiological study in France (the CATHEVAL Project). PLoS ONE 2017, 12, e0168637. [Google Scholar] [CrossRef]
- Chen, Y.M.; Fan, X.W.; Liu, M.H.; Wang, J.; Yang, Y.Q.; Su, Y.F. Risk factors for peripheral venous catheter failure: A prospective cohort study of 5345 patients. J. Vasc. Access 2021, 13, 11297298211015035. [Google Scholar] [CrossRef]
- Bertoglio, S.; van Boxtel, T.; Goossens, G.A.; Dougherty, L.; Furtwangler, R.; Lennan, E.; Pittiruti, M.; Sjovall, K.; Stas, M. Improving outcomes of short peripheral vascular access in oncology and chemotherapy administration. J. Vasc. Access 2017, 18, 89–96. [Google Scholar] [CrossRef]
- Chauhan, A.; Bernardin, A.; Mussard, W.; Kriegel, I.; Estève, M.; Ghigo, J.M.; Beloin, C.; Semetey, V. Preventing biofilm formation and associated occlusion by biomimetic glycocalyxlike polymer in central venous catheters. J. Infect. Dis. 2014, 210, 1347–1356. [Google Scholar] [CrossRef]
- Christensen, G.D.; Simpson, W.A.; Bisno, A.L.; Beachey, E.H. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect. Immun. 1982, 37, 318–326. [Google Scholar] [CrossRef]
- Gominet, M.; Compain, F.; Beloin, C.; Lebeaux, D. Central venous catheters and biofilms: Where do we stand in 2017? APMIS 2017, 125, 365–375. [Google Scholar] [CrossRef]
- Richards, G.A.; Brink, A.J.; McIntosh, R.; Steel, H.C.; Cockeran, R. Investigation of biofilm formation on a charged intravenous catheter relative to that on a similar but uncharged catheter. Med. Devices 2014, 7, 219–224. [Google Scholar] [CrossRef]
- Donlan, R.M. A new approach to mitigate biofilm formation on totally implantable venous access ports. J. Infect. Dis. 2014, 210, 1345–1346. [Google Scholar] [CrossRef]
- Lutwick, L.; Al-Maani, A.S.; Mehtar, S.; Memish, Z.; Rosenthal, V.D.; Dramowski, A.; Lui, G.; Osman, T.; Bulabula, A.; Bearman, G. Managing and preventing vascular catheter infections: A position paper of the international society for infectious diseases. Int. J. Infect. Dis. 2019, 84, 22–29. [Google Scholar] [CrossRef]
- Maki, D.G.; Kluger, D.M.; Crnich, C.J. The risk of bloodstream infection in adults with different intravascular devices: A systematic review of 200 published prospective studies. Mayo. Clin. Proc. 2006, 81, 1159–1171. [Google Scholar] [CrossRef]
- Cortese, Y.J.; Wagner, V.E.; Tierney, M.; Devine, D.; Fogarty, A. Review of catheter-associated urinary tract infections and in vitro urinary tract models. J. Healthc. Eng. 2018, 2018, 2986742. [Google Scholar] [CrossRef] [PubMed]
- Melo, L.D.R.; Veiga, P.; Cerca, N.; Kropinski, A.M.; Almeida, C.; Azeredo, J.; Sillankorva, S. Development of a phage cocktail to control Proteus mirabilis catheter-associated urinary tract infections. Front. Microbiol. 2016, 7, 1024. [Google Scholar] [CrossRef] [PubMed]
- Jordan, R.P.C.; Malic, S.; Waters, M.G.; Stickler, D.J.; Williams, D.W. Development of an antimicrobial urinary catheter to inhibit urinary catheter encrustation. Microbiol. Discov. 2015, 3, 1. [Google Scholar] [CrossRef][Green Version]
- Adegun, P.T.; Odimayo, M.S.; Olaogun, J.G.; Emmanuel, E.E. Comparison of uropathogens and antibiotic susceptibility patterns in catheterized ambulant middle-aged and elderly Nigerian patients with bladder outlet obstruction. Turk. J. Urol. 2019, 45, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Gaston, J.R.; Andersen, M.J.; Johnson, A.O.; Bair, K.L.; Sullivan, C.M.; Guterman, L.B.; White, A.N.; Brauer, A.L.; Learman, B.S.; Flores-Mireles, A.L.; et al. Enterococcus faecalis polymicrobial interactions facilitate biofilm formation, antibiotic recalcitrance, and persistent colonization of the catheterized urinary tract. Pathogens 2020, 9, 835. [Google Scholar] [CrossRef] [PubMed]
- Letica-Kriegel, A.S.; Salmasian, H.; Vawdrey, D.K.; Youngerman, B.E.; Green, R.A.; Furuya, E.Y.; Calfee, D.P.; Perotte, R. Identifying the risk factors for catheter-associated urinary tract infections: A large cross-sectional study of six hospitals. BMJ Open 2019, 9, e022137. [Google Scholar] [CrossRef] [PubMed]
- Maki, D.G.; Tambyah, P.A. Engineering out the risk for infection with urinary catheters. Emerg. Infect. Dis. 2001, 7, 342–347. [Google Scholar] [CrossRef]
- Schmiemann, G.; Kniehl, E.; Gebhardt, K.; Matejczyk, M.M.; Hummers-Pradier, E. The diagnosis of urinary tract infection: A systematic review. Dtsch. Arztebl. Int. 2010, 107, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Crader, M.F.; Leslie, S.W. Bacteruria. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar] [PubMed]
- Haque, M.; Sartelli, M.; McKimm, J.; Abu Bakar, M. Health care-associated infections- an overview. Infect. Drug. Resist. 2018, 11, 2321–2333. [Google Scholar] [CrossRef]
- Anggi, A.; Wijaya, D.W.; Ramayani, O.R. Risk factors for catheter-associated urinary tract infection and uropathogen bacterial profile in the intensive care unit in hospitals in Medan, Indonesia. Open Access Maced. J. Med. Sci. 2019, 7, 3488–3492. [Google Scholar] [CrossRef]
- Loose, M.; Naber, K.G.; Purcell, L.; Wirth, M.P.; Wagenlehner, F. Anti-biofilm effect of octenidine and polyhexanide on uropathogenic biofilm-producing bacteria. Urol. Int. 2021, 105, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Tassew, S.G.; Alebachew Woldu, M.; Amogne Degu, W.; Shibeshi, W. Management of hospital-acquired infections among patients hospitalized at Zewditu memorial hospital, Addis Ababa, Ethiopia: A prospective cross-sectional study. PLoS ONE 2020, 15, e0231949. [Google Scholar] [CrossRef] [PubMed]
- Avershina, E.; Shapovalova, V.; Shipulin, G. Fighting antibiotic resistance in hospital-acquired infections: Current state and emerging technologies in disease prevention, diagnostics and therapy. Front. Microbiol. 2021, 12, 707330. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Guo, R.; Xie, B.; Lai, Q.; Xu, J.; Hu, N.; Wan, L.; Dai, M.; Zhang, B. Drug resistance of pathogens causing nosocomial infection in orthopedics from 2012 to 2017: A 6-year retrospective study. J. Orthop. Surg. Res. 2021, 16, 100. [Google Scholar] [CrossRef] [PubMed]
- Dadi, N.C.T.; Radochová, B.; Vargová, J.; Bujdáková, H. Impact of healthcare-associated infections connected to medical devices- an Update. Microorganisms 2021, 9, 2332. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-associated infections, medical devices and biofilms: Risk, toleranceand control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Vergalito, F.; Pietrangelo, L.; Petronio, G.; Colitto, F.; Alfio Cutuli, M.; Magnifico, I.; Venditti, N.; Guerra, G.; Di Marco, R. Vitamin E for Prevention of Biofilm-caused healthcare-associated infections. Open Med. 2019, 15, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Gmiter, D.; Kaca, W. Into the understanding the multicellular lifestyle of Proteus mirabilis on solid surfaces. Front. Cell. Infect. Microbiol. 2022, 12, 864305. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef]
- Olivares, E.; Badel-Berchoux, S.; Provot, C.; Prévost, G.; Bernardi, T.; Jehl, F. Clinical impact of antibiotics for the treatment of Pseudomonas aeruginosa biofilm infections. Front. Microbiol. 2020, 10, 2894. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Perry, E.K.; Tan, M.W. Bacterial biofilms in the human body: Prevalence and impacts on health and disease. Front. Cell. Infect. Microbiol. 2023, 13, 1237164. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N. A personal history of research on microbial biofilms and biofilm infections. Pathog. Dis. 2014, 70, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Kreve, S.; Reis, A.C.D. Bacterial adhesion to biomaterials: What regulates this attachment? A review. Jpn. Dent. Sci. Rev. 2021, 57, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.E.; He, L.; Heo, J.; Hwang, G. Implication of surface properties, bacterial motility, and hydrodynamic conditions on bacterial surface sensing and their initial adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular components in structured microbial communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Feng, G.; Moraru, C.I. Micro- and nanotopography sensitive bacterial attachment mechanisms: A review. Front. Microbiol. 2019, 10, 191. [Google Scholar] [CrossRef]
- Nakanishi, E.Y.; Palacios, J.H.; Godbout, S.; Fournel, S. Interaction between biofilm formation, surface material and cleanability considering different materials used in pig facilities—An Overview. Sustainability 2021, 13, 5836. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, C.; Chen, Z.; Allan, E.; Van Der Mei, H.C.; Busscher, H.J. Emergent heterogeneous microenvironments in biofilms: Substratum surface heterogeneity and bacterial adhesion force-sensing. FEMS Microbiol. Rev. 2018, 42, 259–272. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Berne, C.; Ducret, A.; Hardy, G.G.; Brun, Y.V. Adhesins involved in attachment to abiotic surfaces by gram-negative bacteria. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Hao, Y.; Liu, Y.; Dong, Z.; Li, K. Biological and physiochemical methods of biofilm adhesion resistance control of medical-context surface. Int. J. Biol. Sci. 2021, 17, 1769–1781. [Google Scholar] [CrossRef]
- Hug, I.; Deshpande, S.; Sprecher, K.S.; Pfohl, T.; Jenal, U. Second messenger-mediated tactile response by a bacterial rotary motor. Science 2017, 358, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Soto, I.; McTiernan, C.; Gonzalez-Gomez, M.; Ross, A.; Gupta, K.; Suuronen, E.J.; Mah, T.F.; Griffith, M.; Alarcon, E.I. Mimicking biofilm formation and development: Recent progress in in vitro and in vivo biofilm models. iScience 2021, 24, 102443. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A.; Mitterer, F.; Pombo, J.P.; Schild, S. Biofilms by bacterial human pathogens: Clinical relevance-development, composition and regulation—Therapeutical strategies. Microb. Cell 2021, 8, 28–56. [Google Scholar] [CrossRef] [PubMed]
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmølle, M.; Stewart, P.S.; Bjarnsholt, T. The biofilm life cycle: Expanding the conceptual model of biofilm formation. Nat. Rev. Microbiol. 2022, 20, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond risk: Bacterial biofilms and their regulating approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Preda, V.G.; Săndulescu, O. Communication is the key: Biofilms, quorum sensing, formation and prevention. Discoveries 2019, 7, e100. [Google Scholar] [CrossRef]
- Cergole-Novella, M.C.; Pignatari, A.C.; Guth, B.E. Adhesion, biofilm and genotypic characteristics of antimicrobial resistant Escherichia coli isolates. Braz. J. Microbiol. 2015, 46, 167–171. [Google Scholar] [CrossRef][Green Version]
- Ludden, C.; Coll, F.; Gouliouris, T.; Restif, O.; Blane, B.; Blackwell, G.A.; Kumar, N.; Naydenova, P.; Crawley, C.; Brown, N.M.; et al. Defining nosocomial transmission of Escherichia coli and antimicrobial resistance genes: A genomic surveillance study. Lancet Microbe 2021, 2, e472–e480. [Google Scholar]
- D’Incau, S.; Atkinson, A.; Leitner, L.; Kronenberg, A.; Kessler, T.M.; Marschall, J. Bacterial species and antimicrobial resistance differ between catheter and non-catheter-associated urinary tract infections: Data from a national surveillance network. Antimicrob. Steward. Healthc. Epidemiol. 2023, 3, e55. [Google Scholar] [CrossRef]
- Bunduki, G.K.; Heinz, E.; Phiri, V.S.; Noah, P.; Feasey, N.; Musaya, J. Virulence factors and antimicrobial resistance of uropathogenic Escherichia coli (UPEC) isolated from urinary tract infections: A systematic review and meta-analysis. BMC Infect. Dis. 2021, 21, 753. [Google Scholar] [CrossRef]
- Terlizzi, M.E.; Gribaudo, G.; Maffei, M.E. UroPathogenic Escherichia coli (UPEC) infections: Virulence factors, bladder responses, antibiotic, and non-antibiotic antimicrobial strategies. Front. Microbiol. 2017, 8, 1566. [Google Scholar] [CrossRef]
- Valiatti, T.B.; Santos, F.F.; Santos, A.C.M.; Nascimento, J.A.S.; Silva, R.M.; Carvalho, E.; Sinigaglia, R.; Gomes, T.A.T. Genetic and virulence characteristics of a hybrid atypical enteropathogenic and uropathogenic Escherichia coli (aEPEC/UPEC) strain. Front. Cell. Infect. Microbiol. 2020, 10, 492. [Google Scholar] [CrossRef]
- Kumar, M.N.; Bhat, S.; Bhat, K.A.; Saralaya, V.; Shenoy Mulki, S. Characterization of virulence factors and antibiotic resistance pattern of uropathogenic Escherichia coli strains in a tertiary care center. F1000Research 2022, 11, 1163. [Google Scholar] [CrossRef]
- Cantas, L.; Suer, K.; Guler, E.; Imir, T. High emergence of ESBL-producing E. coli cystitis: Time to get smarter in Cyprus. Front. Microbiol. 2016, 6, 1446. [Google Scholar] [CrossRef]
- Weiner, L.; Webb, A.; Limbago, B.; Dudeck, M.; Patel, J.; Kallen, A.; Edwards, J.R.; Sievert, D. Antimicrobial-resistant pathogens associated with healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect. Control Hosp. Epidemiol. 2016, 37, 1288–1301. [Google Scholar] [CrossRef]
- Musinguzi, B.; Kabajulizi, I.; Mpeirwe, M.; Turugurwa, J.; Kabanda, T. Incidence and etiology of catheter associated urinary tract infection among admitted patients at kabale regional referral hospital, south western Uganda. Adv. Infect. Dis. 2019, 9, 183–196. [Google Scholar] [CrossRef]
- Ballén, V.; Cepas, V.; Ratia, C.; Gabasa, Y.; Soto, S.M. Clinical Escherichia coli: From biofilm formation to new antibiofilm strategies. Microorganisms 2022, 10, 1103. [Google Scholar] [CrossRef]
- Alshammari, M.; Ahmad, A.; AlKhulaifi, M.; Al Farraj, D.; Alsudir, S.; Alarawi, M.; Takashi, G.; Alyamani, E. Reduction of biofilm formation of Escherichia coli by targeting quorum sensing and adhesion genes using the CRISPR/Cas9-HDR approach, and its clinical application on urinary catheter. J. Infect. Public Health 2023, 16, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Nye, T.M.; Zou, Z.; Obernuefemann, C.L.P.; Pinkner, J.S.; Lowry, E.; Kleinschmidt, K.; Bergeron, K.; Klim, A.; Dodson, K.W.; Flores-Mireles, A.L.; et al. Microbial co-occurrences on catheters from long-term catheterized patients. Nat. Commun. 2024, 15, 61. [Google Scholar] [CrossRef] [PubMed]
- Ripa, M.; Morata, L.; Rodríguez-Núñez, O.; Cardozo, C.; Puerta-Alcalde, P.; Hernández-Meneses, M.; Ambrosioni, J.; Linares, L.; Bodro, M.; Valcárcel, A.; et al. Short-term peripheral venous catheter-related bloodstream infections: Evidence for increasing prevalence of Gram-negative microorganisms from a 25-Year prospective observational study. Antimicrob. Agents Chemother. 2018, 62, e00892-18. [Google Scholar] [CrossRef]
- Tsuboi, M.; Hayakawa, K.; Mezaki, K.; Katanami, Y.; Yamamoto, K.; Kutsuna, S.; Takeshita, N.; Ohmagari, N. Comparison of the epidemiology and microbiology of peripheral line-and central line associated bloodstream infections. Am. J. Infect. Control 2019, 47, 8–10. [Google Scholar] [CrossRef]
- Surapat, B.; Montakantikul, P.; Malathum, K.; Kiertiburanakul, S.; Santanirand, P.; Chindavijak, B. Microbial epidemiology and risk factors for relapse in Gram-negative bacteria catheter-related bloodstream infection with a pilot prospective study in patients with catheter removal receiving short-duration of antibiotic therapy. BMC. Infect. Dis. 2020, 20, 604. [Google Scholar] [CrossRef] [PubMed]
- Lendak, D.; Puerta-Alcalde, P.; Moreno-García, E.; Chumbita, M.; García-Pouton, N.; Cardozo, C.; Morata, L.; Suárez-Lledó, M.; Hernández-Meneses, M.; Ghiglione, L.; et al. Changing epidemiology of catheter-related bloodstream infections in neutropenic oncohematological patients. PLoS ONE 2021, 16, e0251010. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Sharma, S.; Sharma, P.; Chandola, D.; Dang, S.; Gupta, S.; Gabrani, R. Escherichia coli biofilm: Development and therapeutic strategies. J. Appl. Microbiol. 2016, 121, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Potter, R.F.; McCoy, W.H.; Wildenthal, J.A.; Katumba, G.L.; Mucha, P.J.; Dantas, G.; Henderson, J.P.E. E. coli catheter-associated urinary tract infections are associated with distinctive virulence and biofilm gene determinants. JCI Insight 2023, 8, e161461. [Google Scholar] [CrossRef] [PubMed]
- Delcaru, C.; Alexandru, I.; Podgoreanu, P.; Grosu, M.; Stavropoulos, E.; Chifiriuc, M.; Lazar, V. Microbial biofilms in urinary tract infections and prostatitis: Etiology, pathogenicity, and combating strategies. Pathogens 2016, 5, 65. [Google Scholar] [CrossRef] [PubMed]
- Katongole, P.; Nalubega, F.; Florence, N.C.; Asiimwe, B.; Andia, I. Biofilm formation, antimicrobial susceptibility and virulence genes of uropathogenic Escherichia coli isolated from clinical isolates in Uganda. BMC Infect. Dis. 2020, 20, 453. [Google Scholar] [CrossRef]
- Dadi, B.R.; Abebe, T.; Zhang, L.; Mihret, A.; Abebe, W.; Amogne, W. Distribution of virulence genes and phylogenetics of uropathogenic Escherichia coli among urinary tract infection patients in Addis Ababa, Ethiopia. BMC Infect. Dis. 2020, 20, 108. [Google Scholar] [CrossRef] [PubMed]
- Reisner, A.; Maierl, M.; Jörger, M.; Krause, R.; Berger, D.; Haid, A.; Tesic, D.; Zechner, E.L. Type 1 fimbriae contribute to catheter-associated urinary tract infections caused by Escherichia coli. J. Bacteriol. 2014, 196, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Hojati, Z.; Zamanzad, B.; Hashemzadeh, M.; Molaie, R.; Gholipour, A. The FimH gene in uropathogenic Escherichia coli strains isolated from patients with urinary tract infection. Jundishapur. J. Microbiol. 2015, 8, e17520. [Google Scholar] [CrossRef] [PubMed]
- Inegol, P.E.; Turkyilmaz, S. Investigation of P fimbriae presence in Escherichia coli strains isolated from urine samples in human, and their antibacterial resistance. Jundishapur. J. Microbiol. 2018, 11, e66119. [Google Scholar] [CrossRef]
- Kallas, P.; Haugen, H.J.; Gadegaard, N.; Stormonth-Darling, J.; Hulander, M.; Andersson, M.; Valen, H. Adhesion of Escherichia Coli to nanostructured surfaces and the role of type 1 fimbriae. Nanomaterials 2020, 10, 2247. [Google Scholar] [CrossRef] [PubMed]
- Sarshar, M.; Behzadi, P.; Ambrosi, C.; Zagaglia, C.; Palamara, A.T.; Scribano, D. FimH and anti-adhesive therapeutics: A disarming strategy against uropathogens. Antibiotics 2020, 9, 397. [Google Scholar] [CrossRef] [PubMed]
- Carere-Sigl, A.; Nowakowska, J.; Hevey, R.; Khanna, N.; Ernst, B. FimH antagonists prevent biofilm formation on catheter surfaces. In Carbohydrate Chemistry: Chemical and Biological Approaches; Rauter, A.P., Lindhorst, T.K., Queneau, Y., Eds.; Royal Society of Chemistry: London, UK, 2021; Volume 45, pp. 519–536. [Google Scholar]
- McLellan, L.K.; McAllaster, M.R.; Kim, A.S.; Tóthová, L.; Olson, P.D.; Pinkner, J.S.; Daugherty, A.L.; Hreha, T.N.; Janetka, J.W.; Fremont, D.H.; et al. A host receptor enables type 1 pilus-mediated pathogenesis of Escherichia coli pyelonephritis. PLoS Pathog. 2021, 17, e1009314. [Google Scholar] [CrossRef] [PubMed]
- Mousa, W.O.N.; Riad, O.; Omran, M. Expression of bla-CTX-M and fimH genes in uropathogenic Escherichia coli isolated from egyptian catheterized patients. Azhar Int. J. Pharm. Med. Sci. 2022, 2, 94–104. [Google Scholar] [CrossRef]
- Ramírez Castillo, F.Y.; Guerrero Barrera, A.L.; Harel, J.; Avelar González, F.J.; Vogeleer, P.; Arreola Guerra, J.M.; González Gámez, M. Biofilm formation by Escherichia coli isolated from urinary tract infections from Aguascalientes, Mexico. Microorganisms 2023, 11, 2858. [Google Scholar] [CrossRef]
- Ghavidel, M.; Gholamhosseini-Moghadam, T.; Nourian, K.; Ghazvini, K. Virulence factors analysis and antibiotic resistance of uropathogenic Escherichia coli isolated from patients in northeast of Iran. Iran. J. Microbiol. 2020, 12, 223–230. [Google Scholar] [CrossRef]
- Stærk, K.; Khandige, S.; Kolmos, H.J.; Møller-Jensen, J.; Andersen, T.E. Uropathogenic Escherichia coli express type 1 fimbriae only in surface adherent populations under physiological growth conditions. J. Infect. Dis. 2016, 213, 386–394. [Google Scholar] [CrossRef][Green Version]
- Zuberi, A.; Ahmad, N.; Khan, A.U. CRISPRi induced suppression of fimbriae gene (fmH) of a uropathogenic Escherichia coli: An approach to inhibit microbial bioflms. Front. Immunol. 2017, 8, 1552. [Google Scholar]
- Jacobsen, S.M.; Stickler, D.J.; Mobley, H.L.; Shirtliff, M.E. Complicated catheter-associated urinary tract infections due to Esche-richia coli and Proteus mirabilis. Clin. Microbiol. Rev. 2008, 21, 26–59. [Google Scholar] [CrossRef]
- Gupta, S.; Kumar, P.; Rathi, B.; Verma, V.; Dhanda, R.S.; Devi, P.; Yadav, M. Targeting of Uropathogenic Escherichia coli papG gene using CRISPR-dot nanocomplex reduced virulence of UPEC. Sci. Rep. 2021, 11, 17801. [Google Scholar] [CrossRef]
- Nhu, N.T.K.; Phan, M.D.; Peters, K.M.; Lo, A.W.; Forde, B.M.; Min Chong, T.; Yin, W.F.; Chan, K.G.; Chromek, M.; Brauner, A.; et al. Discovery of new genes involved in curli production by a uropathogenic Escherichia coli strain from the highly virulent O45:K1:H7 lineage. mBio 2018, 9, e01462-18. [Google Scholar] [CrossRef]
- Luna-Pineda, V.M.; Moreno-Fierros, L.; Cázares-Domínguez, V.; Ilhuicatzi-Alvarado, D.; Ochoa, S.A.; Cruz-Córdova, A.; Va-lencia-Mayoral, P.; Rodríguez-Leviz, A.; Xicohtencatl-Cortes, J. Curli of uropathogenic Escherichia coli enhanceurinary tract col-onization as a fitness factor. Front. Microbiol. 2019, 10, 2063. [Google Scholar] [CrossRef]
- Murase, K.; Martin, P.; Porcheron, G.; Houle, S.; Helloin, E.; Pénary, M.; Nougayrède, J.P.; Dozois, C.M.; Hayashi, T.; Oswald, E. HlyF Produced by Extraintestinal Pathogenic Escherichia coli is a virulence factor that regulates outer membrane vesicle biogenesis. J. Infect. Dis. 2016, 213, 856–865. [Google Scholar] [CrossRef]
- van der Westhuizen, W.A.; Theron, C.W.; Boucher, C.E.; Bragg, R.R. Regulation of outer-membrane proteins (OMPs) A and F, during hlyF-induced outer-membrane vesicle (OMV) biosynthesis. Heliyon 2019, 5, e02014. [Google Scholar] [CrossRef]
- Raheel, I.; Hassan, W.H.; Salem, S.; Salam, H. Biofilm forming potentiality of Escherichia coli isolated from bovine endometritis and their antibiotic resistance profiles. J. Adv. Vet. Anim. Res. 2020, 7, 442–451. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Teng, D.; Mao, R.; Hao, Y.; Yang, N.; Wang, X.; Li, Z.; Wang, X.; Wang, J. Development of chimeric peptides to facilitate the neutralisation of lipopolysaccharides during bactericidal targeting of multidrug-resistant Escherichia coli. Commun. Biol. 2020, 3, 41. [Google Scholar] [CrossRef]
- Sachdeva, S.; Palur, R.V.; Sudhakar, K.U.; Rathinavelan, T.E. coli group 1 capsular polysaccharide exportation nanomachinary as a plausible antivirulence target in the perspective of emerging antimicrobial resistance. Front. Microbiol. 2017, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Muciño, E.; Arenas-Hernández, M.M.P.; Luna-Guevara, M.L. Mechanisms of Inhibition of Quorum Sensing as an Alternative for the Control of E. coli and Salmonella. Microorganisms 2022, 10, 884. [Google Scholar] [CrossRef] [PubMed]
- Stahlhut, S.G.; Struve, C.; Krogfelt, K.A.; Reisner, A. Biofilm formation of Klebsiella pneumoniae on urethral catheters requires either type 1 or type 3 fimbriae. FEMS Immunol. Med. Microbiol. 2012, 65, 350–359. [Google Scholar] [CrossRef]
- Gomes, A.É.I.; Pacheco, T.; Santos, C.d.S.d.; Pereira, J.A.; Ribeiro, M.L.; Darrieux, M.; Ferraz, L.F.C. Functional insights from KpfR, a new transcriptional regulator of fimbrial expression that is crucial for Klebsiella pneumoniae pathogenicity. Front. Microbiol. 2021, 11, 601921. [Google Scholar] [CrossRef] [PubMed]
- Vuotto, C.; Longo, F.; Pascolini, C.; Donelli, G.; Balice, M.P.; Libori, M.F.; Tiracchia, V.; Salvia, A.; Varaldo, P.E. Biofilm formation and antibiotic resistance in Klebsiella pneumoniae urinary strains. J. Appl. Microbiol. 2017, 123, 1003–1018. [Google Scholar] [CrossRef] [PubMed]
- Vargas, J.M.; Moreno Mochi, M.P.; Nuñez, J.M.; Cáceres, M.; Mochi, S.; Del Campo Moreno, R.; Jure, M.A. Virulence factors and clinical patterns of multiple-clone hypermucoviscous KPC-2 producing K. pneumoniae. Heliyon 2019, 5, e01829. [Google Scholar] [CrossRef]
- Riwu, K.H.P.; Effendi, M.H.; Rantam, F.A.; Khairullah, A.R.; Widodo, A. A review: Virulence factors of Klebsiella pneumonia as emerging infection on the food chain. Vet. World 2022, 15, 2172–2179. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wilksch, J.J.; Liu, H.; Zhang, X.; Torres, V.V.L.; Bi, W.; Mandela, E.; Cao, J.; Li, J.; Lithgow, T.; et al. Investigation of LuxS-mediated quorum sensing in Klebsiella pneumoniae. J. Med. Microbiol. 2020, 69, 402–413. [Google Scholar] [CrossRef]
- Armbruster, C.E.; Mobley, H.L.T.; Pearson, M.M. Pathogenesis of Proteus mirabilis Infection. EcoSal Plus 2018, 8. [Google Scholar] [CrossRef]
- Filipiak, A.; Chrapek, M.; Literacka, E.; Wawszczak, M.; Głuszek, S.; Majchrzak, M.; Wróbel, G.; Łysek-Gładysińska, M.; Gniadkowski, M.; Adamus-Białek, W. Pathogenic factors correlate with antimicrobial resistance among clinical Proteus mirabilis strains. Front. Microbiol. 2020, 11, 579389. [Google Scholar] [CrossRef]
- Wasfi, R.; Hamed, S.M.; Amer, M.A.; Fahmy, L.I. Proteus mirabilis Biofilm: Development and therapeutic strategies. Front. Cell. Infect. Microbiol. 2020, 10, 414. [Google Scholar] [CrossRef]
- Elhoshi, M.; El-Sherbiny, E.; Elsheredy, A.; Aboulela, A.G. A correlation study between virulence factors and multidrug resistance among clinical isolates of Proteus mirabilis. Braz. J. Microbiol. 2023, 54, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Huang, Z.; Yang, T.; Wang, G.; Li, P.; Yang, B.; Li, J. Pathogenesis of Proteus mirabilis in catheter-associated urinary tract infections. Urol. Int. 2021, 105, 354–361. [Google Scholar] [CrossRef]
- Hamilton, A.L.; Kamm, M.A.; Ng, S.C.; Morrison, M. Proteus spp. as putative gastrointestinal pathogens. Clin. Microbiol. Rev. 2018, 31, e00085-17. [Google Scholar] [CrossRef]
- Milo, S.; Nzakizwanayo, J.; Hathaway, H.J.; Jones, B.V.; Jenkins, A.T.A. Emerging medical and engineering strategies for the prevention of long-term indwelling catheter blockage. Proc. Inst. Mech. Eng. H 2019, 233, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Milo, S.; Heylen, R.A.; Glancy, J.; Williams, G.T.; Patenall, B.L.; Hathaway, H.J.; Thet, N.T.; Allinson, S.L.; Laabei, M.; Jenkins, A.T.A. A small-molecular inhibitor against Proteus mirabilis urease to treat catheter-associated urinary tract infections. Sci Rep. 2021, 11, 3726. [Google Scholar] [CrossRef]
- Diggle, S.P.; Whiteley, M. Microbe profile: Pseudomonas aeruginosa: Opportunistic pathogen and lab rat. Microbiology 2020, 166, 30–33. [Google Scholar] [CrossRef]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef]
- Huszczynski, S.M.; Lam, J.S.; Khursigara, C.M. The role of Pseudomonas aeruginosa lipopolysaccharide in bacterial pathogenesis and physiology. Pathogens 2019, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H.A. Pseudomonas aeruginosa biofilms. Int. J. Mol. Sci. 2020, 21, 8671. [Google Scholar] [CrossRef]
- Lopes, J.A.; Rghei, A.D.; Thompson, B.; Susta, L.; Khursigara, C.M.; Wootton, S.K. Overcoming barriers to preventing and treating P. aeruginosa infections using AAV vectored immunoprophylaxis. Biomedicines 2022, 10, 3162. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Martín, I.; Sainz-Mejías, M.; McClean, S. Pseudomonas aeruginosa: An audacious pathogen with an adaptable arsenal of virulence factors. Int. J. Mol. Sci. 2021, 22, 3128. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Huang, X.; Wang, Q.; Yao, D.; Lu, W. Virulence factors of Pseudomonas Aeruginosa and antivirulence strategies to combat its drug resistance. Front. Cell. Infect. Microbiol. 2022, 12, 926758. [Google Scholar] [CrossRef]
- Reynolds, D.; Kollef, M. The Epidemiology and pathogenesis and treatment of Pseudomonas aeruginosa infections: An update. Drugs 2021, 81, 2117–2131. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Cheng, J.; Wang, J.; Li, P.; Lin, J. Treatment of Pseudomonas aeruginosa infectious biofilms: Challenges and strategies. Front. Microbiol. 2022, 13, 955286. [Google Scholar] [CrossRef] [PubMed]
- Colquhoun, J.M.; Rather, P.N. Insights into mechanisms of biofilm formation in Acinetobacter baumannii and implications for uropathogenesis. Front. Cell. Infect. Microbiol. 2020, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Shenkutie, A.M.; Yao, M.Z.; Siu, G.K.; Wong, B.K.C.; Leung, P.H. Biofilm-induced antibiotic resistance in clinical Acinetobacter baumannii isolates. Antibiotics 2020, 9, 817. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Lee, J.H.; Park, M.; Park, K.S.; Bae, I.K.; Kim, Y.B.; Cha, C.J.; Jeong, B.C.; Lee, S.H. Biology of Acinetobacter baumannii: Pathogenesis, antibiotic resistance mechanisms, and prospective treatment options. Front. Cell. Infect. Microbiol. 2017, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Sarshar, M.; Behzadi, P.; Scribano, D.; Palamara, A.T.; Ambrosi, C. Acinetobacter baumannii: An ancient commensal with weapons of a pathogen. Pathogens 2021, 10, 387. [Google Scholar] [CrossRef]
- Uppalapati, S.R.; Sett, A.; Pathania, R. The outer membrane proteins OmpA, CarO, and OprD of Acinetobacter baumannii confer a two-pronged defense in facilitating its success as a potent human pathogen. Front. Microbiol. 2020, 11, 589234. [Google Scholar] [CrossRef]
- Pompilio, A.; Scribano, D.; Sarshar, M.; Di Bonaventura, G.; Palamara, A.T.; Ambrosi, C. Gram-negative bacteria holdingtogether in a biofilm: The Acinetobacter baumannii way. Microorganisms 2021, 9, 1353. [Google Scholar] [CrossRef] [PubMed]
- Morris, F.C.; Dexter, C.; Kostoulias, X.; Uddin, M.I.; Peleg, A.Y. The mechanisms of disease caused by Acinetobacter baumannii. Front. Microbiol. 2019, 10, 1601. [Google Scholar] [CrossRef]
- Idrees, M.; Sawant, S.; Karodia, N.; Rahman, A. Staphylococcus aureus biofilm: Morphology, genetics, pathogenesis and treatment strategies. Int. J. Environ. Res. Public Health 2021, 18, 7602. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Ravaioli, S.; Montanaro, L. Polysaccharide intercellular adhesin in biofilm: Structural and regulatory aspects. Front. Cell. Infect. Microbiol. 2015, 5, 7. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Nguyen, T.H.; Otto, M. The staphylococcal exopolysaccharide PIA—Biosynthesis and role in biofilm formation, colonization, and infection. Comput. Struct. Biotechnol. J. 2020, 4, 3324–3334. [Google Scholar] [CrossRef]
- Magro, G.; Biffani, S.; Minozzi, G.; Ehricht, R.; Monecke, S.; Luini, M.; Piccinini, R. Virulence genes of S. aureus from dairy cow mastitis and contagiousness risk. Toxins 2017, 9, E195. [Google Scholar] [CrossRef] [PubMed]
- Moormeier, D.E.; Bayles, K.W. Staphylococcus aureus biofilm: A complex developmental organism. Mol. Microbiol. 2017, 104, 365–376. [Google Scholar] [CrossRef]
- McCarthy, H.; Waters, E.M.; Bose, J.L.; Foster, S.; Bayles, K.W.; O’Neill, E.; Fey, P.D.; O’Gara, J.P. The major autolysin is redundant for Staphylococcus aureus USA300 LAC JE2 virulence in a murine device-related infection model. FEMS Microbiol. Lett. 2016, 363, fnw087. [Google Scholar] [CrossRef]
- Hiltunen, A.K.; Savijoki, K.; Nyman, T.A.; Miettinen, I.; Ihalainen, P.; Peltonen, J.; Fallarero, A. Structural and functional dynamics of Staphylococcus aureus biofilms and biofilm matrix proteins on different clinical materials. Microorganisms 2019, 7, 584. [Google Scholar] [CrossRef]
- Cucarella, C.; Solano, C.; Valle, J.; Amorena, B.; Lasa, I.; Penadés, J.R. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J. Bacteriol. 2001, 183, 2888–2896. [Google Scholar] [CrossRef]
- Valle, J.; Fang, X.; Lasa, I. Revisiting Bap multidomain protein: More than sticking bacteria together. Front. Microbiol. 2020, 11, 613581. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, A.; Jayasree, T.; Valliammai, A.; Pandian, S.K. Myrtenol attenuates MRSA biofilm and virulence by suppressing sarA expression dynamism. Front. Microbiol. 2019, 10, 2027. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.; Rohde, H.; Vilanova, M.; Cerca, N. Fighting Staphylococcus epidermidis biofilm-associated infections: Can iron be the key to success? Front. Cell. Infect. Microbiol. 2021, 11, 798563. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcal biofilms. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J. Surface proteins of Staphylococcus epidermidis. Front. Microbiol. 2020, 11, 1829. [Google Scholar] [CrossRef] [PubMed]
- Joubert, I.A.; Otto, M.; Strunk, T.; Currie, A.J. Look who’s talking: Host and pathogen drivers of Staphylococcus epidermidis virulence in neonatal sepsis. Int. J. Mol. Sci. 2022, 23, 860. [Google Scholar] [CrossRef] [PubMed]
- Michalik, S.; Sundaramoorthy, N.; Murr, A.; Depke, M.; Völker, U.; Bröker, B.M.; Aamot, H.V.; Schmidt, F. Early-Stage Staphylococcus aureus bloodstream infection causes changes in the concentrations of lipoproteins and acute-phase proteins and is associated with low Antibody titers against bacterial virulence factors. mSystems 2020, 5, e00632-19. [Google Scholar] [CrossRef] [PubMed]
- Stępień-Pyśniak, D.; Hauschild, T.; Kosikowska, U.; Dec, M.; Urban-Chmiel, R. Biofilm formation capacity and presence of virulence factors among commensal Enterococcus spp. from wild birds. Sci Rep. 2019, 9, 11204. [Google Scholar] [CrossRef] [PubMed]
- Codelia-Anjum, A.; Lerner, L.B.; Elterman, D.; Zorn, K.C.; Bhojani, N.; Chughtai, B. Enterococcal urinary tract infections: A review of the pathogenicity, epidemiology, and treatment. Antibiotics 2023, 12, 778. [Google Scholar] [CrossRef]
- Ramos, Y.; Morales, D.K. Exopolysaccharide-mediated surface penetration as new virulence trait in Enterococcus faecalis. Commun. Integr. Biol. 2019, 12, 144–147. [Google Scholar] [CrossRef]
- Madsen, K.T.; Skov, M.N.; Gill, S.; Kemp, M. Virulence factors associated with Enterococcus Faecalis infective endocarditis: A mini review. Open Microbiol. J. 2017, 11, 1–11. [Google Scholar] [CrossRef]
- Șchiopu, P.; Toc, D.A.; Colosi, I.A.; Costache, C.; Ruospo, G.; Berar, G.; Gălbău, Ș.G.; Ghilea, A.C.; Botan, A.; Pană, A.G.; et al. An overview of the factors involved in biofilm production by the Enterococcus genus. Int. J. Mol. Sci. 2023, 24, 11577. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, J.; Chajęcka-Wierzchowska, W.; Byczkowska-Rostkowska, Z.; Saki, M. Biofilm formation capacity and presence of virulence determinants among Enterococcus species from milk and raw milk cheeses. Life 2023, 13, 495. [Google Scholar] [CrossRef] [PubMed]
- Hashem, Y.A.; Abdelrahman, K.A.; Aziz, R.K. Phenotype–genotype correlations and distribution of key virulence factors in Enterococcus faecalis isolated from patients with urinary tract infections. Infect. Drug Resist. 2021, 14, 1713–1723. [Google Scholar] [CrossRef]
- Gorrie, C.L.; Mirčeta, M.; Wick, R.R.; Judd, L.M.; Lam, M.M.C.; Gomi, R.; Abbott, I.J.; Thomson, N.R.; Strugnell, R.A.; Pratt, N.F.; et al. Genomic dissection of Klebsiella pneumoniae infections in hospital patients reveals insights into an opportunistic pathogen. Nat. Commun. 2022, 13, 3017. [Google Scholar] [CrossRef]
- Ballén, V.; Gabasa, Y.; Ratia, C.; Ortega, R.; Tejero, M.; Soto, S. Antibiotic resistance and virulence profiles of Klebsiella pneumoniae strains isolated from different clinical sources. Front. Cell. Infect. Microbiol. 2021, 11, 738223. [Google Scholar] [CrossRef]
- Mączyńska, B.; Paleczny, J.; Oleksy-Wawrzyniak, M.; Choroszy-Król, I.; Bartoszewicz, M. In Vitro susceptibility of multi-drug resistant Klebsiella pneumoniae strains causing nosocomial infections to fosfomycin, a comparison of determination methods. Pathogens 2021, 10, 512. [Google Scholar] [CrossRef]
- Kaye, K.S.; Gupta, V.; Mulgirigama, A.; Joshi, A.V.; Ye, G.; Scangarella-Oman, N.E.; Yu, K.; Mitrani-Gold, F.S. Prevalence, regional distribution, and trends of antimicrobial resistance among female outpatients with urine Klebsiella spp. isolates: A multicenter evaluation in the United States between 2011 and 2019. Antimicrob. Resist. Infect. Control 2024, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Hafiz, T.A.; Alanazi, S.; Alghamdi, S.S.; Mubaraki, M.A.; Aljabr, W.; Madkhali, N.; Alharbi, S.R.; Binkhamis, K.; Alotaibi, F. Klebsiella pneumoniae bacteraemia epidemiology: Resistance profiles and clinical outcome of King Fahad Medical City isolates, Riyadh, Saudi Arabia. BMC Infect. Dis. 2023, 23, 579. [Google Scholar] [CrossRef]
- Ibrahim, M.E. Risk factors in acquiring multidrug-resistant Klebsiella pneumoniae infections in a hospital setting in Saudi Arabia. Sci Rep. 2023, 13, 11626. [Google Scholar] [CrossRef]
- Barbadoro, P.; Labricciosa, F.M.; Recanatini, C.; Gori, G.; Tirabassi, F.; Martini, E.; Gioia, M.G.; D’Errico, M.M.; Prospero, E. Catheter-associated urinary tract infection: Role of the setting of catheter insertion. Am. J. Infect. Control 2015, 43, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Silva, A.; Simões, L.; Guimarães, R.A. Incidence of central venous catheter-related bloodstream infections: Evaluation of bundle prevention in two intensive care units in central Brazil. Sci. World J. 2019, 2019, 1025032. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oleksy-Wawrzyniak, M.; Junka, A.; Brożyna, M.; Paweł, M.; Kwiek, B.; Nowak, M.; Mączyńska, B.; Bartoszewicz, M. The in vitro ability of Klebsiella pneumoniae to form biofilm and the potential of various compounds to eradicate it from urinary catheters. Pathogens 2021, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Cortés, P.; Martin-Loeches, I.; Rodríguez, A.; Bou, G.; Cantón, R.; Diaz, E.; De la Fuente, C.; Torre-Cisneros, J.; Nuvials, F.X.; Salavert, M.; et al. Current positioning against severe infections due to Klebsiella pneumoniae in hospitalized adults. Antibiotics 2022, 11, 1160. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Wu, H.; Chen, H.; Jiang, Y. A case report: Intermittent catheterization combined with rehabilitation in the treatment of carbapenem-resistant Klebsiella pneumoniae catheter-associated urinary tract infection. Front. Cell. Infect. Microbiol. 2022, 12, 1027576. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Xu, J.; Chen, X.; Ren, Y.; Zhao, L. Risk factors and molecular epidemiology of bloodstream infections due to carbapenem-resistant Klebsiella pneumoniae. Diagn. Microbiol. Infect. Dis. 2023, 106, 115955. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Sharma, L.; Dela Cruz, C.S.; Zhang, D. Clinical epidemiology, risk factors, and control strategies of Klebsiella pneumoniae infection. Front. Microbiol. 2021, 12, 750662. [Google Scholar] [CrossRef]
- Li, Y.; Kumar, S.; Zhang, L.; Wu, H. Klebsiella pneumonia and its antibiotic resistance: A bibliometric analysis. BioMed Res. Int. 2022, 2022, 1668789. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, G.; Chao, X.; Xie, L.; Wang, H. The Characteristic of virulence, biofilm and antibiotic resistance of Klebsiella pneumoniae. Int. J. Environ. Res. Public Health 2020, 17, 6278. [Google Scholar] [CrossRef]
- Snitkin, E.S.; Won, S.; Pirani, A.; Lapp, Z.; Weinstein, R.A.; Lolans, K.; Hayden, M.K. Integrated genomic and interfacility patient-transfer data reveal the transmission pathways of multidrug-resistant Klebsiella pneumoniae in a regional outbreak. Sci. Transl. Med. 2017, 9, eaan0093. [Google Scholar] [CrossRef]
- Wyres, K.L.; Lam, M.M.C.; Holt, K.E. Population genomics of Klebsiella pneumoniae. Nat. Rev. Microbiol. 2020, 18, 344–359. [Google Scholar] [CrossRef] [PubMed]
- Shadkam, S.; Goli, H.R.; Mirzaei, B.; Gholami, M.; Ahanjan, M. Correlation between antimicrobial resistance and biofilm formation capability among Klebsiella pneumoniae strains isolated from hospitalized patients in Iran. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- Nirwati, H.; Sinanjung, K.; Fahrunissa, F.; Wijaya, F.; Napitupulu, S.; Hati, V.P.; Hakim, M.S.; Meliala, A.; Aman, A.T.; Nuryastuti, T. Biofilm formation and antibiotic resistance of Klebsiella pneumoniae isolated from clinical samples in a tertiary care hospital, Klaten, Indonesia. BMC Proc. 2019, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Alcantar-Curiel, M.D.; Blackburn, D.; Saldana, Z.; Gayosso-Vazquez, C.; Iovine, N.M.; De la Cruz, M.A.; Girón, J.A. Multi-functional analysis of Klebsiella pneumoniae fimbrial types in adherence and biofilm formation. Virulence 2013, 4, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.N.; Mortensen, M.S.; Krogfelt, K.A.; Clegg, S. Role of Klebsiella pneumoniae type 1 and type 3 fimbriae in colonizing silicone tubes implanted into the bladders of mice as a model of catheter-associated urinary tract infections. Infect. Immun. 2013, 81, 3009–3017. [Google Scholar] [CrossRef] [PubMed]
- Wasfi, R.; Elkhatib, W.F.; Ashour, H.M. Molecular typing and virulence analysis of multidrug resistant Klebsiella pneumoniae clinical isolates recovered from Egyptianhospitals. Sci. Rep. 2016, 6, 38929. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.L.; da Silva, B.C.M.; Rezende, G.S.; Nakamura-Silva, R.; Pitondo-Silva, A.; Campanini, E.B.; Brito, M.C.A.; da Silva, E.M.L.; Freire, C.C.M.; da Cunha, A.F.; et al. High prevalence of multidrug-resistant Klebsiella pneumoniae harboring several virulence and β-lactamase encoding genes in a Brazilian intensive care unit. Front. Microbiol. 2019, 9, 3198. [Google Scholar] [CrossRef] [PubMed]
- Devanga Ragupathi, N.K.; Muthuirulandi Sethuvel, D.P.; Triplicane Dwarakanathan, H.; Murugan, D.; Umashankar, Y.; Monk, P.N.; Karunakaran, E.; Veeraraghavan, B. The influence of biofilms on carbapenem susceptibility and patient outcome in device associated K. pneumoniae infections: Insights into phenotype vs genome-wide analysis and correlation. Front. Microbiol. 2020, 11, 591679. [Google Scholar] [CrossRef] [PubMed]
- Ochońska, D.; Ścibik, Ł.; Brzychczy-Włoch, M. Biofilm formation of clinical Klebsiella pneumoniae strains isolated from tracheostomy tubes and their association with antimicrobial resistance, virulence and genetic diversity. Pathogens 2021, 10, 1345. [Google Scholar] [CrossRef]
- Makhrmash, J.H.; Al-Aidy, S.R.; Qaddoori, B.H. Investigation of biofilm virulence genes prevalence in Klebsiella pneumoniae isolated from the urinary tract infections. Arch. Razi Inst. 2022, 77, 1421–1427. [Google Scholar] [CrossRef]
- Li, Y.; Ni, M. Regulation of biofilm formation in Klebsiella pneumoniae. Front. Microbiol. 2023, 14, 1238482. [Google Scholar] [CrossRef] [PubMed]
- El Fertas-Aissani, R.; Messai, Y.; Alouache, S.; Bakour, R. Virulence profiles and antibiotic susceptibility patterns of Klebsiella pneumoniae strains isolated from different clinical specimens. Pathol. Biol. 2013, 61, 209–216. [Google Scholar] [CrossRef]
- Chen, K.M.; Chiang, M.K.; Wang, M.; Ho, H.C.; Lu, M.C.; Lai, Y.C. The role of pgaC in Klebsiella pneumoniae virulence and biofilm formation. Microb. Pathog. 2014, 77, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Mirzaie, A.; Ranjbar, R. Antibiotic resistance, virulence-associated genes analysis and molecular typing of Klebsiella pneumoniae strains recovered from clinical samples. AMB Express 2021, 11, 122. [Google Scholar] [CrossRef]
- Hafiz, T.A.; Alghamdi, G.S.; Alkudmani, Z.S.; Alyami, A.S.; AlMazyed, A.; Alhumaidan, O.S.; Mubaraki, M.A.; Alotaibi, F.E. Multidrug-resistant Proteus mirabilis infections and clinical outcome at tertiary hospital in Riyadh, Saudi Arabia. Infect. Drug. Resist. 2024, 17, 571–581. [Google Scholar] [CrossRef]
- Armbruster, C.E.; Smith, S.N.; Johnson, A.O.; DeOrnellas, V.; Eaton, K.A.; Yep, A.; Mody, L.; Wu, W.; Mobley, H.L.T. The Pathogenic potential of Proteus mirabilis is enhanced by other uropathogens during polymicrobial urinary tract infection. Infect. Immun. 2017, 85, e00808-16. [Google Scholar] [CrossRef]
- Al-Bassam, W.W.; Al-Kazaz, A.K. The isolation and characterization of Proteus mirabilis from different clinical samples. J. Biotechnol. Res. Cen. 2013, 7, 24–30. [Google Scholar] [CrossRef]
- Jabur, M.H.; AL-Saedi, E.A.; Trad, J.K. Isolation of Proteus mirabilis and Proteus vulgaris from different clinical sources and study of some virulence factors. J. Bab. Univ. Pure Applied. Sci. 2013, 21, 43–48. [Google Scholar]
- Schaffer, J.N.; Pearson, M.M. Proteus mirabilis and urinary tract infections. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef]
- Armbruster, C.E.; Forsyth-DeOrnellas, V.; Johnson, A.O.; Smith, S.N.; Zhao, L.; Wu, W.; Mobley, H.L.T. Genome-wide transposon mutagenesis of Proteus mirabilis: Essential genes, fitness factors for catheter-associated urinary tract infection, and the impact of polymicrobial infection on fitness requirements. PLoS Pathog. 2017, 13, e1006434. [Google Scholar] [CrossRef]
- Fusco, A.; Coretti, L.; Savio, V.; Buommino, E.; Lembo, F.; Donnarumma, G. Biofilm formation and immunomodulatory activity of Proteus mirabilis clinically isolated strains. Int. J. Mol. Sci. 2017, 18, 414. [Google Scholar] [CrossRef] [PubMed]
- Shelenkov, A.; Petrova, L.; Fomina, V.; Zamyatin, M.; Mikhaylova, Y.; Akimkin, V. Multidrug-resistant Proteus mirabilis strain with cointegrate plasmid. Microorganisms 2020, 8, 1775. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, D.W.; Lopes Barboza, M.G.; Faustino, G.; Yamanaka Inagaki, W.T.; Sanches, M.S.; Takayama Kobayashi, R.K.; Vespero, E.C.; Dejato Rocha, S.P. Virulence, resistance and clonality of Proteus mirabilis isolated from patients with community-acquired urinary tract infection (CA-UTI) in Brazil. Microb. Pathog. 2021, 152, 104642. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Cen, X.; He, M.; Wen, Y.; Zhang, H. Isolation, biological and whole genome characteristics of a Proteus mirabilis bacteriophage strain. BMC Microbiol. 2023, 23, 215. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wen, S.; Zhao, L.; Xia, Q.; Pan, Y.; Liu, H.; Wei, C.; Chen, H.; Ge, J.; Wang, H. Association among biofilm formation, virulence gene expression, and antibiotic resistance in Proteus mirabilis isolates from diarrhetic animals in Northeast China. BMC Vet. Res. 2020, 16, 176. [Google Scholar] [CrossRef] [PubMed]
- Scavone, P.; Iribarnegaray, V.; Caetano, A.L.; Schlapp, G.; Härtel, S.; Zunino, P. Fimbriae have distinguishable roles in Proteus mirabilis biofilm formation. Pathog. Dis. 2016, 74, ftw033. [Google Scholar] [CrossRef]
- Li, Y.; Yin, M.; Fang, C.; Fu, Y.; Dai, X.; Zeng, W.; Zhang, L. Genetic analysis of resistance and virulence characteristics of clinical multidrug-resistant Proteus mirabilis isolates. Front. Cell. Infect. Microbiol. 2023, 13, 1229194. [Google Scholar] [CrossRef] [PubMed]
- Talebi, A.; Momtaz, H.; Tajbakhsh, E. Frequency distribution of virulence factors and antibiotic resistance genes in uropathogenic Proteus species isolated from clinical samples. Lett. Appl. Microbiol. 2023, 76, ovac043. [Google Scholar] [CrossRef]
- Ciofu, O.; Tolker-Nielsen, T. Tolerance and resistance of Pseudomonas aeruginosa biofilms to antimicrobial agents- how P. aeruginosa can escape antibiotics. Front. Microbiol. 2019, 10, 913. [Google Scholar] [CrossRef]
- Tuon, F.F.; Dantas, L.R.; Suss, P.H.; Tasca Ribeiro, V.S. Pathogenesis of the Pseudomonas aeruginosa biofilm: A review. Pathogens 2022, 11, 300. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Litwin, A.; Rojek, S.; Gozdzik, W.; Duszynska, W. Pseudomonas aeruginosa device associated—Healthcare associated infections and its multidrug resistance at intensive care unit of University Hospital: Polish, 8.5-year, prospective, single-centre study. BMC Infect. Dis. 2021, 21, 180. [Google Scholar] [CrossRef] [PubMed]
- Edward, E.A.; El Shehawy, M.R.; Abouelfetouh, A.; Aboulmagd, E. Prevalence of different virulence factors and their association with antimicrobial resistance among Pseudomonas aeruginosa clinical isolates from Egypt. BMC Microbiol. 2023, 23, 161. [Google Scholar] [CrossRef] [PubMed]
- Asker, D.; Awad, T.S.; Raju, D.; Sanchez, H.; Lacdao, I.; Gilbert, S.; Sivarajah, P.; Andes, D.R.; Sheppard, D.C.; Howell, P.L.; et al. Preventing Pseudomonas aeruginosa biofilms on indwelling catheters by surface-bound enzymes. ACS Appl. Bio. Mater. 2021, 4, 8248–8258. [Google Scholar] [CrossRef] [PubMed]
- Asmare, Z.; Awoke, T.; Genet, C.; Admas, A.; Melese, A.; Mulu, W. Incidence of catheter-associated urinary tract infections by Gram-negative bacilli and their ESBL and carbapenemase production in specialized hospitals of Bahir Dar, northwest Ethiopia. Antimicrob. Resist. Infect. Control. 2024, 13, 10. [Google Scholar] [CrossRef]
- Cole, S.J.; Records, A.R.; Orr, M.W.; Linden, S.B.; Lee, V.T. Catheter-associated urinary tract infection by Pseudomonas aeruginosa is mediated by exopolysaccharide-independent biofilms. Infect. Immun. 2014, 82, 2048–2058. [Google Scholar] [CrossRef] [PubMed]
- Awoke, N.; Kassa, T.; Teshager, L. Magnitude of biofilm formation and antimicrobial resistance pattern of bacteria isolated from urinary catheterized inpatients of jimma university medical center, Southwest Ethiopia. Int. J. Microbiol. 2019, 2019, 5729568. [Google Scholar] [CrossRef] [PubMed]
- Karkee, P.; Dhital, D.; Madhup, S.K.; Sherchan, J.B. Catheter associated urinary tract infection: Prevalence, microbiological profile and antibiogram at a tertiary care hospital. Ann. Clin. Chem. Lab. Med. 2017, 3, 3–10. [Google Scholar] [CrossRef][Green Version]
- Kaur, M.; Gupta, V.; Gombar, S.; Chander, J.; Sahoo, T. Incidence, risk factors, microbiology of venous catheter associated bloodstream infections--a prospective study from a tertiary care hospital. Indian J. Med. Microbiol. 2015, 33, 248–254. [Google Scholar] [CrossRef]
- Vincent, J.L.; Sakr, Y.; Singer, M.; Martin-Loeches, I.; Machado, F.R.; Marshall, J.C.; Finfer, S.; Pelosi, P.; Brazzi, L.; Aditianingsih, D.; et al. Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA 2020, 323, 1478–1487. [Google Scholar] [CrossRef]
- Jain, R.; Behrens, A.J.; Kaever, V.; Kazmierczak, B.I. Type IV pilus assembly in Pseudomonas aeruginosa over a broad range of cyclic di-GMP concentrations. J. Bacteriol. 2012, 194, 4285–4294. [Google Scholar] [CrossRef] [PubMed]
- Olejnickova, K.; Hola, V.; Ruzicka, F. Catheter-related infections caused by Pseudomonas aeruginosa: Virulence factors involved and their relationships. Pathog. Dis. 2014, 72, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Christopher, G.F. Role of flagella, type IV pili, biosurfactants, and extracellular polymeric substance polysaccharides on the formation of pellicles by Pseudomonas aeruginosa. Langmuir 2019, 35, 5294–5304. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, T.; Hébraud, M.; Alves, O.; Costa, E.; Maltez, L.; Pereira, J.E.; Martins, Â.; Igrejas, G.; Poeta, P. Study of antimicrobial resistance, biofilm formation, and motility of Pseudomonas aeruginosa derived from urine samples. Microorganisms 2023, 11, 1345. [Google Scholar] [CrossRef] [PubMed]
- Colvin, K.M.; Irie, Y.; Tart, C.S.; Urbano, R.; Whitney, J.C.; Ryder, C.; Howell, P.L.; Wozniak, D.J.; Parsek, M.R. The Pel and Psl polysaccharides provide Pseudomonas aeruginosa structural redundancy within the biofilm matrix. Environ. Microbiol. 2012, 14, 1913–1928. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Byrd, M.S.; Sergeant, S.; Azad, A.K.; Parsek, M.R.; McPhail, L.; Schlesinger, L.S.; Wozniak, D.J. Pseudomonas aeruginosa Psl polysaccharide reduces neutrophil phagocytosis and the oxidative response by limiting complement-mediated opsonization. Cell. Microbiol. 2012, 14, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, I.; Oliva, A.; Pages, R.; Sivori, F.; Truglio, M.; Fabrizio, G.; Pasqua, M.; Pimpinelli, F.; Di Domenico, E.G. Acinetobacter baumannii in the critically ill: Complex infections get complicated. Front. Microbiol. 2023, 14, 1196774. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Su, P.W.; Moi, S.H.; Chuang, L.Y. Biofilm formation in Acinetobacter Baumannii: Genotype-phenotype correlation. Molecules 2019, 24, 1849. [Google Scholar] [CrossRef] [PubMed]
- Zeighami, H.; Valadkhani, F.; Shapouri, R.; Samadi, E.; Haghi, F. Virulence characteristics of multidrug resistant biofilm forming Acinetobacter baumannii isolated from intensive care unit patients. BMC Infect Dis. 2019, 19, 629. [Google Scholar] [CrossRef] [PubMed]
- Ceparano, M.; Baccolini, V.; Migliara, G.; Isonne, C.; Renzi, E.; Tufi, D.; De Vito, C.; De Giusti, M.; Trancassini, M.; Alessandri, F.; et al. Acinetobacter baumannii Isolates from COVID-19 patients in a hospital intensive care unit: Molecular typing and risk factors. Microorganisms 2022, 10, 722. [Google Scholar] [CrossRef]
- Elbehiry, A.; Marzouk, E.; Moussa, I.; Mushayt, Y.; Algarni, A.A.; Alrashed, O.A.; Alghamdi, K.S.; Almutairi, N.A.; Anagreyyah, S.A.; Alzahrani, A.; et al. The Prevalence of Multidrug-Resistant Acinetobacter baumannii and Its Vaccination Status among Healthcare Providers. Vaccines 2023, 11, 1171. [Google Scholar] [CrossRef] [PubMed]
- Whiteway, C.; Breine, A.; Philippe, C.; Van der Henst, C. Acinetobacter baumannii. Trends Microbiol. 2022, 30, 199–200. [Google Scholar] [CrossRef] [PubMed]
- Pour, N.K.; Dusane, D.H.; Dhakephalkar, P.K.; Zamin, F.R.; Zinjarde, S.S.; Chopade, B.A. Biofilm formation by Acinetobacter baumannii strains isolated from urinary tract infection and urinary catheters. FEMS Immunol. Med. Microbiol. 2011, 62, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.F.; Lan, C.Y. Antimicrobial resistance in Acinetobacter baumannii: From bench to bedside. World J. Clin. Cases 2014, 2, 787. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.; Vadlamudi, G.; Newton, D.; Foxman, B.; Xi, C. The influence of biofilm formation and multidrug resistance on environmental survival of clinical and environmental isolates of Acinetobacter baumannii. Am. J. Infect. Control. 2016, 44, e65–e71. [Google Scholar] [CrossRef]
- Blot, K.; Hammami, N.; Blot, S.; Vogelaers, D.; Lambert, M.-L. Seasonal variation of hospital-acquired bloodstream infections: A national cohort study. Infect. Control Hosp. Epidemiol. 2021, 12, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Cho, S.I.; Bang, J.H. Risk factors associated with bloodstream infection among patients colonized by multidrug-resistant Acinetobacter baumannii: A 7-year observational study in a general hospital. Am. J. Infect. Control. 2020, 48, 581–583. [Google Scholar] [CrossRef]
- Di Domenico, E.G.; Marchesi, F.; Cavallo, I.; Toma, L.; Sivori, F.; Papa, E.; Spadea, A.; Cafarella, G.; Terrenato, I.; Prignano, G. The impact of bacterial biofilms on end-organ disease and mortality in patients with hematologic malignancies developing a bloodstream infection. Microbiol. Spectr. 2021, 9, e0055021. [Google Scholar] [CrossRef] [PubMed]
- Asaad, A.M.; Ansari, S.; Ajlan, S.E.; Awad, S.M. Epidemiology of biofilm producing Acinetobacter baumannii nosocomial isolates from a tertiary care hospital in Egypt: A cross-sectional study. Infect. Drug. Resist. 2021, 14, 709–717. [Google Scholar] [CrossRef]
- Opoku-Asare, B.; Boima, V.; Ganu, V.J.; Aboagye, E.; Asafu-Adjaye, O.; Asare, A.A.; Kyeremateng, I.; Kwakyi, E.; Agyei, A.; Sampane-Donkor, E.; et al. Catheter-related bloodstream infections among patients on maintenance haemodialysis: A cross-sectional study at a tertiary hospital in Ghana. BMC Infect. Dis. 2023, 23, 664. [Google Scholar] [CrossRef]
- Azizi, O.; Shahcheraghi, F.; Salimizand, H.; Modarresi, F.; Shakibaie, M.R.; Mansouri, S.H.; Ramazanzadeh, R.; Badmasti, F.; Nikbin, V. Molecular Analysis and Expression of bap Gene in Biofilm-Forming Multi-Drug-Resistant Acinetobacter baumannii. Rep. Biochem. Mol. Biol. 2016, 5, 62–72. [Google Scholar] [PubMed]
- Thummeepak, R.; Kongthai, P.; Leungtongkam, U.; Sitthisak, S. Distribution of virulence genes involved in biofilm formation in multi-drug resistant Acinetobacter baumannii clinical isolates. Int. Microbiol. 2016, 19, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, E.; Ghalavand, Z.; Goudarzi, H.; Yeganeh, F.; Hashemi, A.; Dabiri, H.; Mirsamadi, E.S.; Foroumand, M. Phenotypic and genotypic investigation of biofilm formation in clinical and environmental isolates of Acinetobacter baumannii. Arch. Clin. Infect. Dis. 2018, 13, 12914. [Google Scholar] [CrossRef]
- Khoshnood, S.; Savari, M.; Abbasi Montazeri, E.; Farajzadeh Sheikh, A. Survey on genetic diversity, biofilm formation, and detection of colistin resistance genes in clinical isolates of Acinetobacter baumannii. Infect. Drug. Resist. 2020, 13, 1547–1558. [Google Scholar] [CrossRef]
- Li, Z.; Ding, Z.; Liu, Y.; Jin, X.; Xie, J.; Li, T.; Zeng, Z.; Wang, Z.; Liu, J. Phenotypic and genotypic characteristics of biofilm formation in clinical isolates of Acinetobacter baumannii. Infect. Drug. Resist. 2021, 14, 2613–2624. [Google Scholar] [CrossRef]
- Kasperski, T.; Romaniszyn, D.; Jachowicz-Matczak, E.; Pomorska-Wesołowska, M.; Wójkowska-Mach, J.; Chmielarczyk, A. Extensive drug resistance of strong biofilm-producing Acinetobacter baumannii strains isolated from infections and colonization hospitalized patients in Southern Poland. Pathogens 2023, 12, 975. [Google Scholar] [CrossRef]
- Reddy, P.N.; Srirama, K.; Dirisala, V.R. An update on clinical burden, diagnostic tools, and therapeutic options of Staphylococcus aureus. Infect. Dis. 2017, 10, 1179916117703999. [Google Scholar]
- Kong, C.; Chee, C.F.; Richter, K.; Thomas, N.; Abd Rahman, N.; Nathan, S. Suppression of Staphylococcus aureus biofilm formation and virulence by a benzimidazole derivative, UM-C162. Sci. Rep. 2018, 8, 2758. [Google Scholar] [CrossRef]
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. [Google Scholar] [CrossRef] [PubMed]
- Graf, A.C.; Leonard, A.; Schäuble, M.; Rieckmann, L.M.; Hoyer, J.; Maass, S.; Lalk, M.; Becher, D.; Pané-Farré, J.; Riedel, K. Virulence factors produced by Staphylococcus aureus biofilms have a moonlighting function contributing to biofilm integrity. Mol. Cell. Proteomics 2019, 18, 1036–1053. [Google Scholar] [CrossRef]
- Kimmig, A.; Hagel, S.; Weis, S.; Bahrs, C.; Löffler, B.; Pletz, M.W. Management of Staphylococcus aureus bloodstream infections. Front. Med. 2021, 7, 616524. [Google Scholar] [CrossRef] [PubMed]
- Romero, L.C.; de Souza da Cunha, M.L.R. Insights into the epidemiology of community-associated methicillin-resistant Staphylococcus aureus in special populations and at the community-healthcare interface. Braz. J. Infect. Dis. 2021, 25, 101636. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J.; Geoghegan, J.A.; Ganesh, V.K.; Hook, M. Adhesion, invasion and evasion: The many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 2014, 12, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Pietrocola, G.; Nobile, G.; Rindi, S.; Speziale, P. Staphylococcus aureus manipulates innate immunity through own and host-expressed proteases. Front. Cell. Infect. Microbiol. 2017, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Furukawa, K. Staphylococcus aureus bacteremia due to central venous catheter infection: A clinical comparison of infections caused by methicillin-resistant and methicillin-susceptible strains. Cureus 2021, 13, e16607. [Google Scholar] [CrossRef] [PubMed]
- Mandolfo, S.; Anesi, A.; Maggio, M.; Rognoni, V.; Galli, F.; Forneris, G. High success rate in salvage of catheter-related bloodstream infections due to Staphylococcus aureus, on behalf of project group of Italian society of nephrology. J. Vasc. Access 2020, 21, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Bonnal, C.; Birgand, G.; Lolom, I.; Diamantis, S.; Dumortier, C.; L’Heriteau, F.; Armand-Lefevre, L.; Lucet, J.C. Staphylococcus aureus healthcare associated bacteraemia: An indicator of catheter related infections. Med. Mel. Infect. 2015, 45, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.; Borges, V.; Nascimento, M.; Martins, F.; Pessanha, M.A.; Faria, I.; Rodrigues, J.; Matias, R.; Gomes, J.P.; Jordao, L. Insights on catheter-related bloodstream infections: A prospective observational study on the catheter colonization and multidrug resistance. J. Hosp. Infect. 2022, 123, 43–51. [Google Scholar] [CrossRef]
- Cuervo, G.; Camoez, M.; Shaw, E.; Dominguez, M.Á.; Gasch, O.; Padilla, B.; Pintado, V.; Almirante, B.; Molina, J.; López-Medrano, F. Methicillin-resistant Staphylococcus aureus (MRSA) catheter-related bacteraemia in haemodialysis patients. BMC Infect. Dis. 2015, 15, 484. [Google Scholar] [CrossRef]
- Hogan, S.; Zapotoczna, M.; Stevens, N.T.; Humphreys, H.; O’Gara, J.P.; O’Neill, E. Eradication of Staphylococcus aureus catheter-related biofilm infections using ML:8 and citrox. Antimicrob. Agents Chemother. 2016, 60, 5968–5975. [Google Scholar] [CrossRef][Green Version]
- Bolormaa, E.; Kang, C.; Choe, Y.J.; Heo, J.S.; Cho, H. Epidemiology of catheter-related bloodstream infections in neonatal intensive care units: A rapid systematic literature review. Korean J. Health. Assoc. Infect. Control Prev. 2023, 28, 113–125. [Google Scholar] [CrossRef]
- Weldetensae, M.K.; Weledegebriel, M.G.; Nigusse, A.T.; Berhe, E.; Gebrearegay, H. Catheter-related blood stream infections and associated factors among hemodialysis patients in a tertiary care hospital. Infect. Drug. Resist. 2023, 16, 3145–3156. [Google Scholar] [CrossRef] [PubMed]
- Mazuel, M.; Moulier, V.; Bourrel, A.S.; Guillier, C.; Tazi, A.; Jarreau, P.H.; Chollat, C. Systematic culture of central catheters and infections related to catheters in a neonatal intensive care unit: An observational study. Sci. Rep. 2024, 14, 8647. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.N.; Flores-Mireles, A.L.; Pinkner, C.L.; Schreiber, H.L.; Joens, M.S.; Park, A.M.; Potretzke, A.M.; Bauman, T.M.; Pinkner, J.S.; Fitzpatrick, J.A.J.; et al. Catheterization alters bladder ecology to potentiate Staphylococcus aureus infection of the urinary tract. Proc. Natl. Acad. Sci. USA 2017, 114, E8721–E8730. [Google Scholar] [CrossRef] [PubMed]
- Alshomrani, M.K.; Alharbi, A.A.; Alshehri, A.A.; Arshad, M.; Dolgum, S. Isolation of Staphylococcus aureus urinary tract infections at a community-based healthcare center in Riyadh. Cureus 2023, 15, e35140. [Google Scholar] [CrossRef]
- Mason, C.Y.; Sobti, A.; Goodman, A.L. Staphylococcus aureus bacteriuria: Implications and management. JAC Antimicrob. Resist. 2023, 5, dlac123. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, G.; Khadka, P.; Siddhi Shilpakar, G.; Chapagain, G.; Dhungana, G.R. Catheter-Associated Urinary Tract Infection and Obstinate Biofilm Producers. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 7624857. [Google Scholar] [CrossRef]
- Bagali, S.; Mantur, P.G. Bacteriological profile of catheter associated urinary tract infection and its antimicrobial susceptibility pattern in a Tertiary Care Hospital. Int. J. Health Clin. Res. 2021, 4, 268–271. [Google Scholar]
- Seng, R.; Kitti, T.; Thummeepak, R.; Kongtha, P.; Leungtongkam, U.; Wannalerdsakun, S. Biofilm formation of methicillin-resistant coagulase negative staphylococci (MR-CoNS) isolated from community and hospital environments. PLoS ONE 2017, 12, e184172. [Google Scholar] [CrossRef]
- Foster, C.E.; Kok, M.; Flores, A.R.; Minard, C.G.; Luna, R.A.; Lamberth, L.B.; Kaplan, S.L.; Hulten, K.G. Adhesin genes and biofilm formation among pediatric Staphylococcus aureus isolates from implant-associated infections. PLoS ONE 2020, 15, e0235115. [Google Scholar] [CrossRef]
- François, P.; Schrenzel, J.; Götz, F. Biology and Regulation of Staphylococcal Biofilm. Int. J. Mol. Sci. 2023, 24, 5218. [Google Scholar] [CrossRef]
- Mirzaee, M.; Najar-Peerayeh, S.H.; Behmanesh, M.; Forouzandeh, M.M.; Ghasemian, A.M. Detection of intercellular adhesion (ica) gene and biofilm formation Staphylococcus aureus isolates from clinical blood cultures. J. Med. Bacteriol. 2014, 3, 1–7. [Google Scholar]
- Torlak, E.; Korkut, E.; Uncu, A.T.; Şener, Y. Biofilm formation by Staphylococcus aureus isolates from a dental clinic in Konya, Turkey. J. Infect. Public Health 2017, 10, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, S.; Singh, A.; Varma, A.; Pandey, S.; Shrivastava, N. Evaluation of methods to detect in vitro biofilm formation by staphylococcal clinical isolates. BMC Res. Notes 2018, 11, 19. [Google Scholar] [CrossRef]
- Omidi, M.; Firoozeh, F.; Saffari, M.; Sedaghat, H.; Zibaei, M.; Khaledi, A. Ability of biofilm production and molecular analysis of spa and ica genes among clinical isolates of methicillin-resistant Staphylococcus aureus. BMC Res. Notes 2020, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Kot, B.; Sytykiewicz, H.; Sprawka, I. Expression of the Biofilm-Associated Genes in Methicillin-Resistant Staphylococcus aureus in Biofilm and Planktonic Conditions. Int. J. Mol. Sci. 2018, 19, 3487. [Google Scholar] [CrossRef] [PubMed]
- Uribe-García, A.; Paniagua-Contreras, G.L.; Monroy-Pérez, E.; Bustos-Martínez, J.; Hamdan-Partida, A.; Garzón, J.; Alanís, J.; Quezada, R.; Vaca-Paniagua, F.; Vaca, S. Frequency and expression of genes involved in adhesion and biofilm formation in Staphylococcus aureus strains isolated from periodontal lesions. J. Microbiol. Immunol. Infect. 2021, 54, 267–275. [Google Scholar] [CrossRef]
- Giulieri, S.G.; Holmes, N.E.; Stinear, T.P.; Howden, B.P. Use of bacterial whole-genome sequencing to understand and improve the management of invasive Staphylococcus aureus infections. Expert Rev. Anti Infect. Ther. 2016, 14, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
- San-Juan, R.; Pérez-Montarelo, D.; Viedma, E.; Lalueza, A.; Fortún, J.; Loza, E.; Pujol, M.; Ardanuy, C.; Morales, I.; de Cueto, M.; et al. Pathogen-related factors affecting outcome of catheter-related bacteremia due to methicillin-susceptible Staphylococcus aureus in a Spanish multicenter study. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1757–1765. [Google Scholar] [CrossRef]
- Pérez-Montarelo, D.; Viedma, E.; Larrosa, N.; Gómez-González, C.; Ruiz de Gopegui, E.; Muñoz-Gallego, I.; San Juan, R.; Fernández-Hidalgo, N.; Almirante, B.; Chaves, F. Molecular epidemiology of Staphylococcus aureus bacteremia: Association of molecular factors with the source of infection. Front. Microbiol. 2018, 9, 2210. [Google Scholar] [CrossRef]
- Spoto, M.; Riera Puma, J.P.; Fleming, E.; Guan, C.; OndouahNzutchi, Y.; Kim, D.; Oh, J. Large-scale CRISPRi and transcriptomics of Staphylococcus epidermidis identify genetic factors implicated in lifestyle versatility. mBio 2022, 13, e0263222. [Google Scholar] [CrossRef]
- Yogo, A.; Yamamoto, S.; Sumiyoshi, S.; Iwamoto, N.; Aoki, K.; Motobayashi, H.; Tochitani, K.; Shimizu, T.; Murashima, T.; Nishikawa, N.; et al. Two cases of pyelonephritis with bacteremia by Staphylococcus epidermidis in male patients with nephrolithiasis: Case reports and a literature review. J. Infect. Chemother. 2022, 28, 1189–1192. [Google Scholar] [CrossRef] [PubMed]
- Pouget, C.; Chatre, C.; Lavigne, J.-P.; Pantel, A.; Reynes, J.; Dunyach-Remy, C. Effect of antibiotic exposure on Staphylococcus epidermidis responsible for catheter-related bacteremia. Int. J. Mol. Sci. 2023, 24, 1547. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.M.; Kim, J.S.; Park, M.G.; Hwang, J.; Kim, W.Y.; Seo, D.W. Incidence and short-term outcomes of central line-related bloodstream infection in patients admitted to the emergency department: A single-center retrospective study. Sci. Rep. 2023, 13, 3867. [Google Scholar] [CrossRef] [PubMed]
- Lunnemar, P.; Taxbro, K.; Hammarskjöld, F. An analysis of central venous catheter-related bloodstream infections in patients treated in a Swedish Covid-19 intensive care unit. SAGE Open Med. 2024, 12, 20503121241233213. [Google Scholar] [CrossRef]
- Strasheim, W.; Kock, M.M.; Ueckermann, V.; Hoosien, E.; Dreyer, A.W.; Ehlers, M.M. Surveillance of catheter-related infections: The supplementary role of the microbiology laboratory. BMC Infect. Dis. 2015, 15, 5. [Google Scholar] [CrossRef][Green Version]
- Ehlers, M.M.; Strasheim, W.; Lowe, M.; Ueckermann, V.; Kock, M.M. Molecular Epidemiology of Staphylococcus epidermidis Implicated in Catheter-Related Bloodstream Infections at an Academic Hospital in Pretoria, South Africa. Front. Microbiol. 2018, 9, 417. [Google Scholar] [CrossRef]
- de la Cruz-Hernández, I.; Cornejo-Juárez, P.; Tellez-Miranda, O.; Barrera-Pérez, L.; Sandoval-Hernández, S.; Vilar-Compte, D.; Velázquez-Acosta, C.; Volkow, P. Microbiology and prevalence of E2SKAPE-resistant strains in catheter-related bloodstream infections in patients with cancer. Am. J. Infect. Control 2020, 48, 40–45. [Google Scholar] [CrossRef]
- Kochanowicz, J.F.; Nowicka, A.; Al-Saad, S.R.; Karbowski, L.M.; Gadzinowski, J.; Szpecht, D. Catheter-related bloodstream infections in infants hospitalized in neonatal intensive care units: A single center study. Sci. Rep. 2022, 12, 13679. [Google Scholar] [CrossRef]
- Zaragoza Rodríguez, R.M.; Santos Flores, J.A.; Moreno Zaragoza, A.N. #3139 Prevalence of infections associated with the he-modialysis catheter in Mexico city. Nephrol. Dial. Transplant. 2023, 38, gfad063c_3139. [Google Scholar] [CrossRef]
- Cabrera-Contreras, R.; Santamaría, R.I.; Bustos, P.; Martínez-Flores, I.; Meléndez-Herrada, E.; Morelos-Ramírez, R.; Barbosa-Amezcua, M.; González-Covarrubias, V.; Silva-Herzog, E.; Soberón, X.; et al. Genomic diversity of prevalent Staphylococcus epidermidis multidrug-resistant strains isolated from a Children’s Hospital in México City in an eight-years survey. PeerJ 2019, 7, e8068. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, J.; Zhang, T.; He, S.; Li, Y.; Zhang, W.; Ye, X.; Yao, Z. Environmental contamination prevalence, antimicrobial resistance and molecular characteristics of methicillin-resistant Staphylococcus Aureus and Staphylococcus Epidermidis isolated from secondary schools in Guangzhou, China. Int. J. Environ. Res. Public Health 2020, 17, 623. [Google Scholar] [CrossRef]
- Xu, Z.; Cave, R.; Chen, L.; Yangkyi, T.; Liu, Y.; Li, K.; Meng, G.; Niu, K.; Zhang, W.; Tang, N.; et al. Antibiotic resistance and molecular characteristics of methicillin-resistant Staphylococcus epidermidis recovered from hospital personnel in China. J. Glob. Antimicrob. Resist. 2020, 22, 195–201. [Google Scholar] [CrossRef]
- Siciliano, V.; Passerotto, R.A.; Chiuchiarelli, M.; Leanza, G.M.; Ojetti, V. Difficult-to-treat pathogens: A review on the management of multidrug-resistant Staphylococcus epidermidis. Life 2023, 13, 1126. [Google Scholar] [CrossRef] [PubMed]
- Skovdal, S.M.; Jørgensen, N.P.; Meyer, R.L. JMM Profile: Staphylococcus epidermidis. J. Med. Microbiol. 2022, 71, 10–1099. [Google Scholar] [CrossRef]
- Ardon, C.B.; Prens, E.P.; Fuursted, K.; Ejaz, R.N.; Shailes, J.; Jenssen, H.; Jemec, G.B.E. Biofilm production and antibiotic susceptibility of Staphylococcus epidermidis strains from Hidradenitis Suppurativa lesions. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 170–177. [Google Scholar] [CrossRef]
- Ahmad, S.; Rahman, H.; Qasim, M.; Nawab, J.; Alzahrani, K.J.; Alsharif, K.F.; Alzahrani, F.M. Staphylococcus epidermidis Pathogenesis: Interplay of icaADBC Operon and MSCRAMMs in Biofilm Formation of Isolates from Pediatric Bacteremia in Peshawar, Pakistan. Medicina 2022, 58, 1510. [Google Scholar] [CrossRef] [PubMed]
- Cherifi, S.; Byl, B.; Deplano, A.; Nonhoff, C.; Denis, O.; Hallin, M. Comparative epidemiology of Staphylococcus epidermidis isolates from patients with catheter-related bacteremia and from healthy volunteers. J. Clin. Microbiol. 2013, 51, 1541–1547. [Google Scholar] [CrossRef]
- Dai, L.; Yang, L.; Parsons, C.; Findlay, V.J.; Molin, S.; Qin, Z. Staphylococcus epidermidis recovered from indwelling catheters exhibit enhanced biofilm dispersal and “self-renewal” through downregulation of agr. BMC Microbiol 2012, 12, 102. [Google Scholar] [CrossRef]
- García-Solache, M.; Rice, L.B. The Enterococcus: A model of adaptability to its environment. Clin. Microbiol. Rev. 2019, 32, e058-18. [Google Scholar] [CrossRef]
- Cui, P.; Feng, L.; Zhang, L.; He, J.; An, T.; Fu, X.; Li, C.; Zhao, X.; Zhai, Y.; Li, H.; et al. Antimicrobial resistance, virulence genes, and biofilm formation capacity among Enterococcus species from yaks in Aba Tibetan autonomous prefecture, China. Front. Microbiol. 2020, 11, 1250. [Google Scholar] [CrossRef] [PubMed]
- Top, J.; Sinnige, J.C.; Brouwer, E.C.; Werner, G.; Corander, J.; Severin, J.A.; Jansen, R.; Bathoorn, E.; Bonten, M.J.M.; Rossen, J.W.A.; et al. Identification of a novel genomic island associated with vanD-type vancomycin resistance in six dutch vancomycin-resistant Enterococcus faecium isolates. Antimicrob. Agents Chemother. 2018, 62, e01793-17. [Google Scholar] [CrossRef] [PubMed]
- Arshadi, M.; Mahmoudi, M.; Motahar, M.S.; Soltani, S.; Pourmand, M.R. Virulence determinants and antimicrobial resistance patterns of vancomycin-resistant Enterococcus faecium isolated from different sources in Southwest Iran. Iran J. Public Health 2018, 47, 264–272. [Google Scholar] [PubMed]
- Georges, M.; Odoyo, E.; Matano, D.; Tiria, F.; Kyany’a, C.; Mbwika, D.; Mutai, W.C.; Musila, L. Determination of Enterococcus faecalis and Enterococcus faecium antimicrobial resistance and virulence factors and their association with clinical and demographic factors in Kenya. J. Pathog. 2022, 2022, 3129439. [Google Scholar] [CrossRef] [PubMed]
- Aung, M.S.; Urushibara, N.; Kawaguchiya, M.; Ohashi, N.; Hirose, M.; Kudo, K.; Tsukamoto, N.; Ito, M.; Kobayashi, N. Antimicrobial resistance, virulence factors, and genotypes of Enterococcus faecalis and Enterococcus faecium clinical isolates in Northern Japan: Identification of optrA in ST480 E. faecalis. Antibiotics 2023, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Farman, M.; Yasir, M.; Al-Hindi, R.R.; Farraj, S.A.; Jiman-Fatani, A.A.; Alawi, M.; Azhar, E.I. Genomic analysis of multidrug-resistant clinical Enterococcus faecalis isolates for antimicrobial resistance genes and virulence factors from the western region of Saudi Arabia. Antimicrob. Resist. Infect. Control 2019, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Boccella, M.; Santella, B.; Pagliano, P.; De Filippis, A.; Casolaro, V.; Galdiero, M.; Borrelli, A.; Capunzo, M.; Boccia, G.; Franci, G. Prevalence and antimicrobial resistance of Enterococcus Species: A retrospective cohort study in Italy. Antibiotics 2021, 10, 1552. [Google Scholar] [CrossRef] [PubMed]
- Putta, S.; Gandhi, A.; Kulkarni, V.; Jangale, N.; Walawalkar, A. Prevalence of Vancomycin Resistant Enterococci (VRE) in catheter associated urinary tract infections (CAUTI) with special reference to biofilm formation. Int. J. Med. Microbiol. Trop. Dis. 2018, 4, 191–195. [Google Scholar] [CrossRef]
- Patel, C.; Shah, M.B.; Singh, S.; Modi, S.; Shah, P. Biofilm production and antimicrobial resistance in catheter associated urinary tract infection (CAUTI) pathogens isolated from ICU patients. Eur. J. Mol. Clin. Med. 2021, 8, 3. [Google Scholar]
- Bahuleyan, A.; Harshan, K.H.; Bhai, G. Identification and antibiogram of gram positive cocci from catheter associated urinary tract infection (CAUTI) in intensive care units of a tertiary care hospital. Indian J. Microbiol. Res. 2019, 6, 78–81. [Google Scholar] [CrossRef]
- Perdana, M.A.; Wahyuni, D.D.; Yunita, R. Characteristics and susceptibility pattern of catheter-associated urinary tract infections (CAUTI) bacteria in Indonesia: A study in a national reference hospital of Sumatra region 2020-2021. Narra J. 2023, 3, e436. [Google Scholar] [CrossRef] [PubMed]
- Gul, A.; Khan, M.; Ali, M.; Shuaib, S.L.; Sahar, S. Catheter associated urinary tract infection: Characterization of bacterial pathogens and their antimicrobial susceptibility pattern at two Major tertiary care hospitals. Int. J. Pathol. 2021, 19, 194–199. [Google Scholar]
- Singh, D.; Umrao, P.D.; Kaistha, S.D. Multiple antibiotic resistance and biofilm formation of catheter associated urinary tract infection (CAUTI) causing microorganisms. J. Bacteriol. Mycol. 2018, 6, 217–221. [Google Scholar] [CrossRef]
- Tomar, S.; Lodha, R.; Das, B.; Sood, S.; Kapil, A. Central line-associated bloodstream infections (CLABSI): Microbiology and antimicrobial resistance pattern of isolates from the Pediatric ICU of a tertiary care Indian hospital. Clin. Epidemiol. Glob. Health 2015, 3, 16–19. [Google Scholar] [CrossRef]
- Awadh, H.; Chaftari, A.M.; Khalil, M.; Fares, J.; Jiang, Y.; Deeba, R.; Ali, S.; Hachem, R.; Raad, I.I. Management of enterococcal central line-associated bloodstream infections in patients with cancer. BMC Infect. Dis. 2021, 21, 643. [Google Scholar] [CrossRef] [PubMed]
- Markwart, R.; Willrich, N.; Haller, S.; Noll, I.; Koppe, U.; Werner, G.; Eckmanns, T.; Reuss, A. The rise in vancomycin-resistant Enterococcus faecium in Germany: Data from the German Antimicrobial Resistance Surveillance (ARS). Antimicrob. Resist. Infect. Control 2019, 8, 147. [Google Scholar] [CrossRef]
- Ramos, S.; Silva, V.; Dapkevicius, M.d.L.E.; Igrejas, G.; Poeta, P. Enterococci, from harmless bacteria to a pathogen. Microorganisms 2020, 8, 1118. [Google Scholar] [CrossRef] [PubMed]
- Monteiro Marques, J.; Coelho, M.; Santana, A.R.; Pinto, D.; Semedo-Lemsaddek, T. Dissemination of Enterococcal genetic lineages: A one health perspective. Antibiotics 2023, 12, 1140. [Google Scholar] [CrossRef]
- Kresken, M.; Klare, I.; Wichelhaus, T.A.; Wohlfarth, E.; Layer-Nicolaou, F.; Neumann, B.; Werner, G.; Study Group ‘Antimicrobial Resistance’ of the Paul-Ehrlich-Society for Chemotherapy. Glycopeptide resistance in Enterococcus spp. and coagulase-negative staphylococci from hospitalised patients in Germany: Occurrence, characteristics and dalbavancin susceptibility. J. Glob. Antimicrob. Resist. 2022, 28, 102–107. [Google Scholar] [CrossRef]
- Smout, E.; Palanisamy, N.; Valappil, S.P. Prevalence of vancomycin-resistant Enterococci in India between 2000 and 2022: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2023, 12, 79. [Google Scholar] [CrossRef]
- Kim, M.A.; Rosa, V.; Min, K.S. Characterization of Enterococcus faecalis in different culture conditions. Sci Rep. 2020, 10, 21867. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.A.; Alorabi, J.A.; Al-Otaibi, L.M.; Ali, S.S.; Elsilk, S.E. Antibiotic resistance and biofilm formation in Enterococcus spp. isolated from urinary tract infections. Pathogens 2022, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.O.; Fedi, A.C.; Reiter, K.C.; Caierão, J.; d’Azevedo, P.A. Correlation between biofilm formation and gelE, esp, and agg genes in Enterococcus spp. clinical isolates. Virulence 2014, 5, 634–637. [Google Scholar] [CrossRef]
- Kafil, H.S.; Mobarez, A.M. Assessment of biofilm formation by Enterococci isolates from urinary tract infections with different virulence profiles. J. King Saud Univ. Sci. 2015, 27, 312–317. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Pinkner, J.S.; Caparon, M.G.; Hultgren, S.J. EbpA vaccine antibodies block binding of Enterococcus faecalis to fibrinogen to prevent catheter-associated bladder infection in mice. Sci. Transl. Med. 2014, 6, 254ra127. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.L.; Walker, J.N.; Potretzke, A.; Schreiber, H.L.; Pinkner, J.S.; Bauman, T.M.; Park, A.M.; Desai, A.; Hultgren, S.J.; Caparon, M.G. Antibody-Based Therapy for Enterococcal Catheter-Associated Urinary Tract Infections. mBio 2016, 7, e01653-16. [Google Scholar] [CrossRef]
- Xu, W.; Flores-Mireles, A.L.; Cusumano, Z.T.; Takagi, E.; Hultgren, S.J.; Caparon, M.G. Host and bacterial proteases influence biofilm formation and virulence in a murine model of enterococcal catheter-associated urinary tract infection. npj Biofilms Microbiomes 2017, 3, 28. [Google Scholar] [CrossRef] [PubMed]
- Shahriar, A.; Rob Siddiquee, M.F.; Ahmed, H.; Mahmud, A.R.; Ahmed, T.; Mahmud, M.R.; Acharjee, M. Catheter-associated urinary tract infections: Etiological analysis, biofilm formation, antibiotic resistance, and a novel therapeutic era of phage. Int. J. One Health 2022, 8, 86–100. [Google Scholar] [CrossRef]
- Woitschach, F.; Kloss, M.; Schlodder, K.; Borck, A.; Grabow, N.; Reisinger, E.C.; Sombetzki, M. Bacterial adhesion and biofilm formation of Enterococcus faecalis on zwitterionic methylmethacrylat and polysulfones. Front. Cell. Infect. Microbiol. 2022, 12, 868338. [Google Scholar] [CrossRef]
- Neoh, K.G.; Li, M.; Kang, E.-T.; Chiong, E.; Tambyah, P.A. Surface modification strategies for combating catheter-related complications: Recent advances and challenges. J. Mater. Chem. B. 2017, 5, 2045–2067. [Google Scholar] [CrossRef]
- Rupp, M.E.; Karnatak, R. Intravascular catheter–related bloodstream infections. Infect. Dis. Clin. N. Am. 2018, 32, 765–787. [Google Scholar] [CrossRef] [PubMed]
- Soi, V.; Moore, C.L.; Kumbar, L.; Yee, J. Prevention of catheter-related bloodstream infections in patients on hemodialysis: Challenges and management strategies. Int. J. Nephrol. Renov. Dis. 2016, 9, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Lebeaux, D.; Ghigo, J.M.; Beloin, C. Biofilm-related infections: Bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Jones, A.; McLean, A.; Sterner, M.; Robbins, C.; Cunningham, M.; Walters, M.; Doddapaneni, K.; Keitel, I.; Gallagher, C. Slippery liquid infused fluoropolymer coating for central lines to reduce catheter associated clotting and infections. Sci. Rep. 2020, 10, 14973. [Google Scholar] [CrossRef] [PubMed]
- Köves, B.; Magyar, A.; Tenke, P. Spectrum and antibiotic resistance of catheter-associated urinary tract infections. GMS Infect. Dis. 2017, 5, Doc06. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.L.; Walker, J.N.; Bauman, T.M.; Potretzke, A.M.; Schreiber, H.L.; Park, A.M.; Pinkner, J.S.; Caparon, M.G.; Hultgren, S.J.; Desai, A. Fibrinogen release and deposition on urinary catheters placed during urological procedures. J. Urol. 2016, 196, 416–421. [Google Scholar] [CrossRef]
- Pickering, A.C.; Vitry, P.; Prystopiuk, V.; Garcia, B.; Höök, M.; Schoenebeck, J.; Geoghegan, J.A.; Dufrêne, Y.F.; Fitzgerald, J.R. Host-specialized fibrinogen-binding by a bacterial surface protein promotes biofilm formation and innate immune evasion. PLoS Pathog. 2019, 15, e1007816. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.; Walker, J.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- O’Grady, N.P.; Alexander, M.; Burns, L.A.; Dellinger, E.P.; Garland, J.; Heard, S.O.; Lipsett, P.A.; Masur, H.; Mermel, L.A.; Pearson, M.L.; et al. Guidelines for the prevention of intravascular catheter-related infections. Clin. Infect. Dis. 2011, 52, e162–e193. [Google Scholar] [CrossRef]
- Chen, S.; Yao, J.; Chen, J.; Liu, L.; Miu, A.; Jiang, Y.; Chen, Y. Knowledge of “Guidelines for the prevention of intravascular catheter-related infections (2011)”: A survey of intensive care unit nursing staffs in China. Int. J. Nurs. Sci. 2015, 2, 383–388. [Google Scholar] [CrossRef]
- Böll, B.; Schalk, E.; Buchheidt, D.; Hasenkamp, J.; Kiehl, M.; Kiderlen, T.R.; Kochanek, M.; Koldehoff, M.; Kostrewa, P.; Claßen, A.Y.; et al. Central venous catheter-related infections in hematology and oncology: 2020 updated guidelines on diagnosis, management, and prevention by the infectious diseases working party (AGIHO) of the German Society of Hematology and Medical Oncology (DGHO). Ann. Hematol. 2021, 100, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, R.; Pasciuto, G.; Magnini, D.; Cavalletti, M.; Scoppettuolo, G.; Montemurro, G.; Smargiassi, A.; Torelli, R.; Sanguinetti, M.; Spanu, T. Educational interventions alone and combined with port protector reduce the rate of central venous catheter infection and colonization in respiratory semi-intensive care unit. BMC Infect Dis. 2019, 19, 215. [Google Scholar] [CrossRef] [PubMed]
- Mimoz, O.; Lucet, J.-C.; Kerforne, T.; Pascal, J.; Souweine, B.; Goudet, V.; Mercat, A.; Bouadma, L.; Lasocki, S.; Alfandari, S. Skin antisepsis with chlorhexidine–alcohol versus povidone iodine–alcohol, with and without skin scrubbing, for prevention of intravascular-catheter-related infection (CLEAN): An open-label, multicentre, randomised, controlled, two-by-two factorial trial. Lancet 2015, 386, 2069–2077. [Google Scholar] [PubMed]
- Buetti, N.; Timsit, J.-F. Management and prevention of central venous catheter-related infections in the ICU. Semin. Respir. Crit. Care. Med. 2019, 40, 508–523. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, D.; Belmar Campos, C.E.; Ehrhardt, S.; Kock, T.; Weber, C.; Rohde, H.; Kluge, S. Efficacy of introducing a checklist to reduce central venous line associated bloodstream infections in the ICU caring for adult patients. BMC Infect Dis. 2018, 18, 267. [Google Scholar] [CrossRef] [PubMed]
- Timsit, J.F.; Bouadma, L.; Mimoz, O.; Parienti, J.J.; Garrouste-Orgeas, M.; Alfandari, S.; Plantefeve, G.; Bronchard, R.; Troche, G.; Gauzit, R.; et al. Jugular versus femoral short-term catheterization and risk of infection in intensive care unit patients. Causal analysis of two randomized trials. Am. J. Respir. Crit. Care. Med. 2013, 188, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Parienti, J.J.; Mongardon, N.; Mégarbane, B.; Mira, J.P.; Kalfon, P.; Gros, A.; Marqué, S.; Thuong, M.; Pottier, V.; Ramakers, M.; et al. Intravascular complications of central venous catheterization by insertion site. N. Engl. J. Med. 2015, 373, 1220–1229. [Google Scholar] [CrossRef]
- Snarski, E.; Stringer, J.; Mikulska, M.; Gil, L.; Tridello, G.; Bosman, P.; Lippinkhof, A.; Hoek, J.; Karas, M.; Zver, S.; et al. Risk of infectious complications in adult patients after allogeneic hematopoietic stem cell transplantation depending on the site of central venous catheter insertion—Multicenter prospective observational study, from the IDWP EBMT and Nurses Group of EBMT. Bone Marrow Transplant. 2021, 56, 2929–2933. [Google Scholar] [CrossRef]
- Vassallo, M.; Dunais, B.; Roger, P.-M. Antimicrobial lock therapy in central-line associated bloodstream infections: A systematic review. Infection 2015, 43, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Norris, L.B.; Kablaoui, F.; Brilhart, M.K.; Bookstaver, P.B. Systematic review of antimicrobial lock therapy for prevention of central-line-associated bloodstream infections in adult and pediatric cancer patients. Int. J. Antimicrob. Agents 2017, 50, 308–317. [Google Scholar]
- Dang, F.; Li, H.; Tian, J.; Wang, R.; Ren, J. What is the best catheter lock solution in preventing catheter-related blood infections? A protocol for a Bayesian network meta-analysis of randomised controlled trials. BMJ Open 2019, 9, e030019. [Google Scholar] [CrossRef]
- López-Briz, E.; Ruiz Garcia, V.; Cabello, J.B.; Bort-Martí, S.; Carbonell Sanchis, R.; Burls, A. Heparin versus 0.9% sodium chloride locking for prevention of occlusion in central venous catheters in adults. Cochrane Database Syst. Rev. 2018, 7, CD008462. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Jiang, X.; Meng, L.B.; Di, C.Y.; Guo, P.; Qiu, Y.; Dai, Y.L.; Lv, X.Q.; Shi, C.J. Reducing catheter-associated complications using 4% sodium citrate versus sodium heparin as a catheter lock solution. J. Int. Med. Res. 2019, 47, 4204–4214. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Yang, J.W.; Boddu, S.H.; Jung, R.; Churchwell, M.D. Compatibility, stability, and efficacy of vancomycin combined with gentamicin or ethanol in sodium citrate as a catheter lock solution. Hosp. Pharm. 2017, 52, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Zhang, L.; Guo, X.; Sun, L. Vancomycin-lock therapy for prevention of catheter-related bloodstream infection in very low body weight infants. BMC Pediatr. 2021, 21, 3. [Google Scholar] [CrossRef]
- Quenot, J.P.; Helms, J.; Bourredjem, A.; Dargent, A.; Meziani, F.; Badie, J.; Blasco, G.; Piton, G.; Capellier, G.; Mezher, C.; et al. Trisodium citrate 4% versus heparin as a catheter lock for non-tunneled hemodialysis catheters in critically ill patients: A multicenter, randomized clinical trial. Ann. Intensive Care 2019, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, B.; Wang, J.; Yang, Q. Ethanol locks for the prevention of catheter-related infection in patients with central venous catheter: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2019, 14, e0222408. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.K.; Roy-Chaudhury, P.; Mounts, P.; Hurlburt, E.; Pfaffle, A.; Poggio, E.C. Taurolidine/heparin lock solution and catheter-related bloodstream infection in hemodialysis: A randomized, double-blind, active-control, phase 3 study. Clin. J. Am. Soc. Nephrol. 2023, 18, 1446–1455. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Massoumi, H.; Chug, M.K.; Brisbois, E.J. S-Nitroso-N-Acetyl-l-Cysteine Ethyl Ester (SNACET) Catheter lock solution to reduce catheter-associated infections. ACS Appl. Mater. Interfaces 2021, 13, 25813–25824. [Google Scholar] [CrossRef]
- de Campos Pereira Silveira, R.C.; Dos Reis, P.E.D.; Ferreira, E.B.; Braga, F.T.M.M.; Galvão, C.M.; Clark, A.M. Dressings for the central venous catheter to prevent infection in patients undergoing hematopoietic stem cell transplantation: A systematic review and meta-analysis. Support. Care Cancer 2020, 28, 425–438. [Google Scholar] [CrossRef]
- Jones, A. Dressings for the management of catheter sites: A review. J. Assoc. Vasc. Acc. 2004, 9, 26–33. [Google Scholar] [CrossRef]
- Timsit, J.F.; Bouadma, L.; Ruckly, S.; Schwebel, C.; Garrouste-Orgeas, M.; Bronchard, R.; Calvino-Gunther, S.; Laupland, K.; Adrie, C.; Thuong, M.; et al. Dressing disruption is a major risk factor for catheter-related infections. Crit. Care. Med. 2012, 40, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Ryder, M.; Duley, C. Evaluation of compatibility of a gum mastic liquid zdhesive and liquid adhesive remover with an alcoholic chlorhexidine gluconate skin preparation. J. Infus. Nurs. 2017, 40, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Safdar, N.; O’Horo, J.C.; Ghufran, A.; Bearden, A.; Didier, M.E.; Chateau, D.; Maki, D.G. Chlorhexidine-impregnated dressing for prevention of catheter-related bloodstream infection: A meta-analysis. Crit. Care Med. 2014, 42, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Scheithauer, S.; Lewalter, K.; Schröder, J.; Koch, A.; Häfner, H.; Krizanovic, V.; Nowicki, K.; Hilgers, R.D.; Lemmen, S.W. Reduction of central venous line-associated bloodstream infection rates by using a chlorhexidine-containing dressing. Infection 2014, 42, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Puig-Asensio, M.; Marra, A.R.; Childs, C.A.; Kukla, M.E.; Perencevich, E.N.; Schweizer, M.L. Effectiveness of chlorhexidine dressings to prevent catheter-related bloodstream infections. Does one size fit all? a systematic literature review and meta-analysis. Infect. Control Hosp. Epidemiol. 2020, 41, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Ullman, A.J.; Cooke, M.L.; Mitchell, M.; Lin, F.; New, K.; Long, D.A.; Mihala, G.; Rickard, C.M. Dressing and securement for central venous access devices (CVADs): A Cochrane systematic review. Int. J. Nurs. Stud. 2016, 59, 177–196. [Google Scholar] [PubMed]
- Ergul, A.B.; Gokcek, I.; Ozcan, A.; Cetin, S.; Gultekin, N.; Torun, Y.A. Use of a chlorhexidine-impregnated dressing reduced catheter-related bloodstream infections caused by Gram-positive microorganisms. Pak. J. Med. Sci. 2018, 34, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Pedrolo, E.; Danski, M.T.R.; Wiens, A.; Boostel, R. Cost effectiveness of dressing in the prevention of catheter-related infection in critically ill patients. J. Infect. Dev. Ctries. 2018, 12, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Li, Y.; Li, X.; Bian, L.; Wen, Z.; Li, M. Chlorhexidine-impregnated dressing for the prophylaxis of central venous catheter-related complications: A systematic review and meta-analysis. BMC Infect Dis. 2019, 19, 429. [Google Scholar] [CrossRef]
- Hou, Y.; Griffin, L.; Bernatchez, S.F.; Hommes, J.; Kärpänen, T.; Palka-Santini, M. Comparative effectiveness of 2 chlorhexidine gluconate-containing dressings in reducing central line-associated bloodstream infections, hospital stay, and costs. Inquiry 2023, 60, 469580231214751. [Google Scholar] [CrossRef] [PubMed]
- Sylvia, C.J.J.R.; Wagel, M.A.; Giare-Patel, K.; Spangler, T.A.; Breznock, E.M.; Gupta, N. Chlorhexidine-coated peripherally inserted central catheters reduce fibroblastic sleeve formation in an in vivo ovine model. J. Vasc. Access 2018, 19, 644–650. [Google Scholar] [CrossRef]
- Faustino, C.M.C.; Lemos, S.M.C.; Monge, N.; Ribeiro, I.A.C. A scope at antifouling strategies to prevent catheter-associated infections. Adv. Colloid Interface Sci. 2020, 284, 102230. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.J.; Flores-Mireles, A.L. Urinary catheter coating modifications: The race against catheter-associated infections. Coatings 2020, 10, 23. [Google Scholar] [CrossRef]
- Kanti, S.P.Y.; Csóka, I.; Jójárt-Laczkovich, O.; Adalbert, L. Recent advances in antimicrobial coatings and material modification strategies for preventing urinary catheter-associated complications. Biomedicines 2022, 10, 2580. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, S.I.C.; Anjos, I.I.L.; Monge, N.; Faustino, C.M.C.; Ribeiro, I.A.C. A glance at antimicrobial strategies to prevent catheter-associated medical infections. ACS Infect. Dis. 2020, 6, 3109–3130. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Tay, F.R.; Niu, L.N.; Chen, J.H. Advancing antimicrobial strategies for managing oral biofilm infections. Int. J. Oral Sci. 2019, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhang, L.; Bai, R.; Zhuang, Z.; Zhang, Y.; Yu, T.; Peng, L.; Xin, T.; Chen, S.; Han, B. Recent Progress in Antimicrobial Strategies for Resin-Based Restoratives. Polymers 2021, 13, 1590. [Google Scholar] [CrossRef]
- Lai, N.M.; Chaiyakunapruk, N.; Lai, N.A.; O’Riordan, E.; Pau, W.S.; Saint, S. Catheter impregnation, coating or bonding for reducing central venous catheter-related infections in adults. Cochrane Database Syst. Rev. 2016, 3, CD007878. [Google Scholar] [CrossRef]
- Bell, T.; O’Grady, N.P. Prevention of central line-associated bloodstream infections. Infect. Dis. Clin. N. Am. 2017, 31, 551–559. [Google Scholar] [CrossRef]
- Chong, H.Y.; Lai, N.M.; Apisarnthanarak, A.; Chaiyakunapruk, N. Comparative efficacy of antimicrobial central venous catheters in reducing catheter-related bloodstream infections in adults: Abridged cochrane systematic review and network meta-analysis. Clin. Infect. Dis. 2017, 64, S131–S140. [Google Scholar] [CrossRef] [PubMed]
- Abouleish, Y.Z.; Oldfield, E.C.; Marik, P.E. Comparison of central-line–associated bloodstream infections between central venous catheters lined by combined chlorhexidine and silver sulfadiazine versus silver ionotrophes alone: A before–after–before retrospective study. Infect. Control Hosp. Epidemiol. 2021, 42, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shukla, S.; Vera-González, N.; Tharmalingam, N.; Mylonakis, E.; Fuchs, B.B.; Shukla, A. Auranofin releasing antibacterial and antibiofilm polyurethane intravascular catheter coatings. Front. Cell. Infect. Microbiol. 2019, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Felix, L.; Whitely, C.; Tharmalingam, N.; Mishra, B.; Vera-Gonzalez, N.; Mylonakis, E.; Shukla, A.; Fuchs, B.B. Auranofin coated catheters inhibit bacterial and fungal biofilms in a murine subcutaneous model. Front. Cell. Infect. Microbiol. 2023, 13, 1135942. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Liu, L.; Yang, H.; Zhou, R.; Che, C.; Li, X.; Li, C.; Luan, S.; Yin, J.; Shi, H. Water-insoluble polymeric guanidine derivative and application in the preparation of antibacterial coating of catheter. ACS Appl. Mater. Interfaces 2018, 10, 39257–39267. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Liu, L.; Li, X.; Zhou, R.; Yan, S.; Li, C.; Luan, S.; Yin, J.; Shi, H. Fabrication of polylysine based antibacterial coating for catheters by facile electrostatic interaction. Chem. Eng. J. 2019, 360, 1030–1041. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhao, Y.Q.; Zhang, Y.; Wang, A.; Ding, X.; Li, Y.; Duan, S.; Ding, X.; Xu, F.J. Antimicrobial peptide-conjugated hierarchical antifouling polymer brushes for functionalized catheter surfaces. Biomacromolecules 2019, 20, 4171–4179. [Google Scholar] [CrossRef]
- Naga, V.; Nehate, S.D.; Saikumar, A.K.; Sundaram, K.B. Boron carbon nitride (BCN) nano-coatings of central venous catheters inhibits bacterial colonization. ECS J. Solid State Sci. Technol. 2020, 9, 115018. [Google Scholar] [CrossRef]
- Pant, J.; Goudie, M.J.; Chaji, S.M.; Johnson, B.W.; Handa, H. Nitric oxide releasing vascular catheters for eradicating bacterial infection. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2849–2857. [Google Scholar] [CrossRef]
- Maharubin, S.; Nayak, C.; Phatak, O.; Kurhade, A.; Singh, M.; Zhou, Y.; Tan, G. Polyvinylchloride coated with silver nanoparticles and zinc oxide nanowires for antimicrobial applications. Mater. Lett. 2019, 249, 108–111. [Google Scholar] [CrossRef]
- Lotlikar, S.R.; Gallaway, E.; Grant, T.; Popis, S.; Whited, M.; Guragain, M.; Rogers, R.; Hamilton, S.; Gerasimchuk, N.G.; Patrauchan, M.A. Polymeric composites with silver (I) cyanoximates inhibit biofilm formation of gram-positive and gram-negative bacteria. Polymers 2019, 11, 1018. [Google Scholar] [CrossRef] [PubMed]
- Zander, Z.K.; Chen, P.; Hsu, Y.H.; Dreger, N.Z.; Savariau, L.; McRoy, W.C.; Cerchiari, A.E.; Chambers, S.D.; Barton, H.A.; Becker, M.L. Post-fabrication QAC-functionalized thermoplastic polyurethane for contact-killing catheter applications. Biomaterials 2018, 178, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.N.; Borges, I.; Pereira, A.T.; Maia, A.F.; Pestana, M.; Magalhães, F.D.; Pinto, A.M.; Gonçalves, I.C. Antimicrobial graphene nanoplatelets coatings for silicone catheters. Carbon N. Y. 2018, 139, 635–647. [Google Scholar] [CrossRef]
- Dong, J.J.; Muszanska, A.; Xiang, F.; Falkenberg, R.; van de Belt-Gritter, B.; Loontjens, T. Contact killing of Gram-positive and Gram-negative bacteria on PDMS provided with immobilized hyperbranched antibacterial coatings. Langmuir 2019, 35, 14108–14116. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Ding, X.; Zhu, Y.; Wu, S.; Ding, X.; Li, Y.; Yu, B.; Xu, F.-J. Facile surface multi-functionalization of biomedical catheters with dual-microcrystalline broad-spectrum antibacterial drugs and antifouling poly(ethylene glycol) for effective inhibition of bacterial infections. ACS Appl. Bio Mater. 2019, 2, 1348–1356. [Google Scholar] [PubMed]
- Zhang, W.; Du, J.; Zhu, T.; Wang, R. SiO2 nanosphere coated tough catheter with superhydrophobic surface for improving the antibacterial and hemocompatibility. Front. Bioeng. Biotechnol. 2023, 10, 1067139. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.L.; Deval, P.; Tsai, W.B. Fabrication of transparent PEGylated antifouling coatings via one-step pyrogallol deposition. Polymers 2023, 15, 2731. [Google Scholar] [CrossRef] [PubMed]
- Kovach, K.M.; Capadona, J.R.; Sen Gupta, A.; Potkay, J.A. The effects of PEG-based surface modification of PDMS microchannels on long-term hemocompatibility. J. Biomed. Mater. Res. A 2014, 102, 4195–4205. [Google Scholar] [CrossRef] [PubMed]
- Atkins, L.; Sallis, A.; Chadborn, T.; Shaw, K.; Schneider, A.; Hopkins, S.; Bunten, A.; Michie, S.; Lorencatto, F. Reducing catheter-associated urinary tract infections: A systematic review of barriers and facilitators and strategic behavioural analysis of interventions. Implementation Sci. 2020, 15, 44. [Google Scholar] [CrossRef]
- Chenoweth, C.E.; Gould, C.V.; Saint, S. Diagnosis, management, and prevention of catheter-associated urinary tract infections. Infect. Dis. Clin. N. Am. 2014, 28, 105–119. [Google Scholar] [CrossRef]
- Nicolle, L.E. Catheter associated urinary tract infections. Antimicrob. Resist. Infect. Control 2014, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Meddings, J.; Rogers, M.A.; Krein, S.L.; Fakih, M.G.; Olmsted, R.N.; Saint, S. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: An integrative review. BMJ Qual. Saf. 2014, 23, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Gould, C.V.; Umscheid, C.A.; Agarwal, R.K.; Kuntz, G.; Pegues, D.A.; The Healthcare Infection Control Practices Advisory Committee (HICPAC). Guideline for Prevention of Catheter-Associated Urinary Tract Infections 2009. 2019. Available online: https://www.cdc.gov/infectioncontrol/guidelines/cauti/ (accessed on 18 April 2024).
- Assadi, F. Strategies for preventing catheter-associated urinary tract infections. Int. J. Prev. Med. 2018, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.; Lange, D.; Chew, B.H. Ureteral stents and foley catheters-associated urinary tract infections: The role of coatings and materials in infection prevention. Antibiotics 2014, 3, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, S.; Keatch, R.; Corner, G.; Nabi, G.; Murdoch, S.; Davidson, F.; Zhao, Q. In vitro antibacterial and anti-encrustation performance of silver-polytetrafluoroethylene nanocomposite coated urinary catheters. J. Hosp. Infect. 2019, 103, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Rajaramon, S.; Shanmugam, K.; Dandela, R.; Solomon, A.P. Emerging evidence-based innovative approaches to control catheter-associated urinary tract infection: A review. Front. Cell. Infect. Microbiol. 2023, 13, 1134433. [Google Scholar] [CrossRef] [PubMed]
- Rigo, S.; Cai, C.; Gunkel-Grabole, G.; Maurizi, L.; Zhang, X.; Xu, J.; Palivan, C.G. Nanoscience-based strategies to engineer antimicrobial surfaces. Adv Sci. 2018, 8, 1700892. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhang, D.; Gu, Q.; Miao, J.; Cen, X.; Golodok, R.P.; Savich, V.V.; Ilyushchenko, A.P.; Zhou, Z.; Wang, R. One-step coordination of metal-phenolic networks as antibacterial coatings with sustainable and controllable copper release for urinary catheter applications. RSC Adv. 2022, 12, 15685–15693. [Google Scholar] [CrossRef] [PubMed]
- Mandakhalikar, K.D.; Wang, R.; Rahmat, J.N.; Chiong, E.; Neoh, K.G.; Tambyah, P.A. Restriction of in vivo infection by antifouling coating on urinary catheter with controllable and sustained silver release: A proof of concept study. BMC Infect. Dis. 2018, 18, 370. [Google Scholar] [CrossRef]
- Shalom, Y.; Perelshtein, I.; Perkas, N.; Gedanken, A.; Banin, E. Catheters coated with Zn-doped CuO nanoparticles delay the onset of catheter-associated urinary tract infections. Nano Res. 2017, 10, 520–533. [Google Scholar] [CrossRef]
- Ivanova, A.; Ivanova, K.; Perelshtein, I.; Gedanken, A.; Todorova, K.; Milcheva, R.; Dimitrov, P.; Popova, T.; Tzanov, T. Sonochemically engineered nano-enabled zinc oxide/amylase coatings prevent the occurrence of catheter-associated urinary tract infections. Mater. Sci. Eng. C 2021, 131, 112518. [Google Scholar] [CrossRef] [PubMed]
- Srisang, S.; Nasongkla, N. Spray coating of foley urinary catheter by chlorhexidine-loadedpoly(epsilon-caprolactone) nanospheres: Effect of lyoprotectants, characteristics, and antibacterial activity evaluation. Pharm. Dev. Technol. 2019, 24, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.; Surabhi, S.; Lepoittevin, B.; Philippe, R.; Manoj, P.; Bhuvanesh, G. Biomodification strategies for the development of antimicrobial urinary catheters: Overview and advances. Glob. Chall. 2018, 2, 1700068. [Google Scholar] [CrossRef] [PubMed]
- Kowalczuk, D.; Ginalska, G.; Piersiak, T.; Miazga-Karska, M. Prevention of biofilm formation on urinary catheters: Comparison of the sparfloxacin-treated long-term antimicrobial catheters with silver-coated ones. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1874–1882. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Lo, J.C.; Yan, M.; Yang, X.; Brooks, D.E.; Hancock, R.E.; Lange, D.; Kizhakkedathu, J.N. Anti-adhesive antimicrobial peptide coating prevents catheter associated infection in a mouse urinary infection model. Biomaterials 2017, 116, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.; Costa, F.; Pirttila, A.M.; Tejesvi, M.V.; Martins, M.C.L. Prevention of urinary catheter-associated infections by coating antimicrobial peptides from crowberry endophytes. Sci. Rep. 2019, 9, 10753. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Basu, A.; Chua, R.R.Y.; Saravanan, R.; Tambyah, P.A.; Ho, B.; Chang, M.W.; Leong, S.S.J. Site specific immobilization of a potent antimicrobial peptide onto silicone catheters: Evaluation against urinary tract infection pathogens. J. Mater. Chem. B 2014, 2, 1706. [Google Scholar] [CrossRef]
- Regev-Shoshani, G.; Ko, M.; Miller, C.; Av-Gay, Y. Slow release of nitric oxide from charged catheters and its effect on biofilm formation by Escherichia coli. Antimicrob. Agents Chemother. 2010, 54, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Lehman, S.M.; Donlan, R.M. Bacteriophage-mediated control of a two-species biofilm formed by microorganisms causing catheter-associated urinary tract infections in an in vitro urinary catheter model. Antimicrob. Agents Chemother. 2015, 59, 1127–1137. [Google Scholar] [CrossRef]
- Szell, T.; Dressler, F.F.; Goelz, H.; Bluemel, B.; Miernik, A.; Brandstetter, T.; Scherag, F.; Schoeb, D.S. In vitro effects of a novel coating agent on bacterial biofilm development on ureteral stents. J. Endourol. 2019, 33, 225–231. [Google Scholar] [CrossRef]
- Yong, Y.; Qiao, M.; Chiu, A.; Fuchs, S.; Liu, Q.; Pardo, Y.; Worobo, R.; Liu, Z.; Ma, M. Conformal hydrogel coatings on catheters to reduce biofouling. Langmuir 2019, 35, 1927–1934. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Alzahrani, A.; Khoddami, S.; Ferreira, D.; Scotland, K.B.; Cheng, J.T.J.; Yazdani-Ahmadabadi, H.; Mei, Y.; Gill, A.; Takeuchi, L.E.; et al. Self-limiting mussel inspired thin antifouling coating with broad-spectrum resistance to biofilm formation to prevent catheter-associated infection in mouse and porcine models. Adv. Healthc. Mater. 2021, 10, e2001573. [Google Scholar] [CrossRef] [PubMed]
- Tailly, T.; MacPhee, R.A.; Cadieux, P.; Burton, J.P.; Dalsin, J.; Wattengel, C.; Koepsel, J.; Razvi, H. Evaluation of polyethylene glycol-based antimicrobial coatings on urinary catheters in the prevention of Escherichia coli infections in a rabbit model. J. Endourol. 2021, 35, 116–121. [Google Scholar] [CrossRef]
- Diaz Blanco, C.; Ortner, A.; Dimitrov, R.; Navarro, A.; Mendoza, E.; Tzanov, T. Building an antifouling zwitterionic coating on urinary catheters using an enzymatically triggered bottom-up approach. ACS Appl. Mater. Interfaces 2014, 6, 11385–11393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, L.; Liang, X.; Vorstius, J.; Keatch, R.; Corner, G.; Nabi, G.; Davidson, F.; Gadd, G.M.; Zhao, Q. Enhanced antibacterial and antiadhesive activities of silver-PTFE nanocomposite coating for urinary catheters. ACS Biomater. Sci. Eng. 2019, 5, 2804–2814. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, K.; Fernandes, M.M.; Mendoza, E.; Tzanov, T. Enzyme multilayer coatings inhibit Pseudomonas aeruginosa biofilm formation on urinary catheters. Appl. Microbiol. Biotechnol. 2015, 99, 4373–4385. [Google Scholar] [CrossRef] [PubMed]
- Catto, C.; Secundo, F.; James, G.; Villa, F.; Cappitelli, F. Alpha-chymotrypsin immobilized on a low-Density polyethylene surface successfully weakens Escherichia coli biofilm formation. Int. J. Mol. Sci. 2018, 19, 4003. [Google Scholar] [CrossRef]
- Asker, D.; Awad, T.S.; Baker, P.; Howell, P.L.; Hatton, B.D. Non-eluting, surface-bound enzymes disrupt surface attachment of bacteria by continuous biofilm polysaccharide degradation. Biomaterials 2018, 167, 168–176. [Google Scholar] [CrossRef]
- Thallinger, B.; Brandauer, M.; Burger, P.; Sygmund, C.; Ludwig, R.; Ivanova, K.; Kun, J.; Scaini, D.; Burnet, M.; Tzanov, T.; et al. Cellobiose dehydrogenase functionalized urinary catheter as novel antibiofilm system. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1448–1456. [Google Scholar] [CrossRef]
- Domingues, B.; Silva, J.M.; Aroso, I.M.; Lima, E.; Barros, A.A.; Reis, R.L. Coatings for Urinary Stents: Current State and Future Directions. In Urinary Stents; Soria, F., Rako, D., de Graaf, P., Eds.; Springer: Cham, Switzerland, 2022; pp. 209–223. [Google Scholar] [CrossRef]
- Leuck, A.M.; Johnson, J.R.; Hunt, M.A.; Dhody, K.; Kazempour, K.; Ferrieri, P.; Kline, S. Safety and efficacy of a novel silver-impregnated urinary catheter system for preventing catheter-associated bacteriuria: A pilot randomized clinical trial. Am. J. Infect. Control. 2015, 43, 260–265. [Google Scholar] [CrossRef]
- Chung, P.H.; Wong, C.W.; Lai, C.K.; Siu, H.K.; Tsang, D.N.; Yeung, K.Y.; Ip, D.K.; Tam, P.K. A prospective interventional study to examine the effect of a silver alloy and hydrogel-coated catheter on the incidence of catheter-associated urinary tract infection. Hong Kong Med. J. 2017, 23, 239–245. [Google Scholar] [CrossRef][Green Version]
- Francolini, I.; Vuotto, C.; Piozzi, A.; Donelli, G. Antifouling and antimicrobial biomaterials: An overview. APMIS 2017, 125, 392–417. [Google Scholar] [CrossRef]
- Ogilvie, A.T.; Brisson, B.A.; Gow, W.R.; Wainberg, S.; Singh, A.; Weese, J.S. Effects of the use of silver-coated urinary catheters on the incidence of catheter-associated bacteriuria and urinary tract infection in dogs. J. Am. Vet. Med. Assoc. 2018, 253, 1289–1293. [Google Scholar] [CrossRef]
- Sun, Y.; Ren, P.; Long, X. Role of noble metal-coated catheters for short-term urinary catheterization of adults: A meta-analysis. PLoS ONE 2020, 15, e0233215. [Google Scholar] [CrossRef]
- Wang, R.; Neoh, K.G.; Kang, E.T.; Tambyah, P.A.; Chiong, E. Antifouling coating with controllable and sustained silver release for long-term inhibition of infection and encrustation in urinary catheters. J. Biomed. Mater. Res. B. Appl. Biomater. 2015, 103, 519–528. [Google Scholar] [CrossRef]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical applications of silver nanoparticles: An up-to-date overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef]
- Kędziora, A.; Speruda, M.; Krzy, E.; Rybka, J.; Łukowiak, A.; Bugla-Płoskónska, G. Molecular sciences similarities and differences between silver ions and silver in nanoforms as antibacterial agents. Int. J. Mol. Sci. 2018, 19, 444. [Google Scholar] [CrossRef]
- Yassin, M.A.; Elkhooly, T.A.; Elsherbiny, S.M.; Reicha, F.M.; Shokeir, A.A. Facile coating of urinary catheter with bio–inspired antibacterial coating. Heliyon 2019, 5, e02986. [Google Scholar] [CrossRef]
- Rahuman, H.B.H.; Dhandapani, R.; Palanivel, V.; Thangavelu, S.; Paramasivam, R.; Muthupandian, S. Bioengineered phytomolecules-capped silver nanoparticles using carissa carandas leaf extract to embed on to urinary catheter to combat UTI pathogens. PLoS ONE 2021, 16, e0256748. [Google Scholar] [CrossRef]
- Goda, R.M.; El-Baz, A.M.; Khalaf, E.M.; Alharbi, N.K.; Elkhooly, T.A.; Shohayeb, M.M. Combating bacterial biofilm formation in urinary catheter by green silver nanoparticle. Antibiotics 2022, 11, 495. [Google Scholar] [CrossRef]
- Arunachalam, K.D.; Annamalai, S.K.; Arunachalam, A.M.; Raghavendra, R.S.; Kennedy, S. One step green synthesis of phytochemicals mediated gold nanoparticles from Aegle marmales for the prevention of urinary catheter infection. Int. J. Pharm. Pharm. Sci. 2014, 6, 700–706. [Google Scholar]
- Rtimi, S.; Sanjines, R.; Pulgarin, C.; Kiwi, J. Quasi-instantaneous bacterial inactivation on Cu-Ag nanoparticulate 3D catheters in the dark and under light: Mechanism and dynamics. ACS Appl. Mater. Interfaces 2016, 8, 47–55. [Google Scholar] [CrossRef]
- Agarwala, M.; Choudhury, B.; Yadav, R.N. Comparative study of antibiofilm activity of copper oxide and iron oxide nanoparticles against multidrug resistant biofilm forming uropathogens. Indian J. Microbiol. 2014, 54, 365–368. [Google Scholar] [CrossRef]
- Singha, P.; Locklin, J.; Handa, H. A review of the recent advances in antimicrobial coatings for urinary catheters. Acta Biomater. 2017, 50, 20–40. [Google Scholar] [CrossRef]
- Lam, T.B.; Omar, M.I.; Fisher, E.; Gillies, K.; MacLennan, S. Types of indwelling urethral catheters for short-term catheterisation in hospitalised adults. Cochrane Database Syst. Rev. 2014, CD004013. [Google Scholar] [CrossRef]
- Kart, D.; Kustimur, A.S.; Sağıroğlu, M.; Kalkancı, A. Evaluation of antimicrobial durability and anti-Biofilm effects in urinary catheters against Enterococcus faecalis clinical isolates and reference strains. Balk. Med. J. 2017, 34, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Menezes, F.G.; Correa, L.; Medina-Pestana, J.O.; Aguiar, W.F.; Camargo, L.F.A. A randomized clinical trial comparing Nitrofurazone-coated and uncoated urinary catheters in kidney transplant recipients: Results from a pilot study. Transpl. Infect. Dis. 2019, 21, e13031. [Google Scholar] [CrossRef]
- Rafienia, M.; Zarinmehr, B.; Poursamar, S.A.; Bonakdar, S.; Ghavami, M.; Janmaleki, M. Coated urinary catheter by PEG/PVA/gentamicin with drug delivery capability against hospital infection. Iranian Polymer J. 2013, 22, 75–83. [Google Scholar] [CrossRef]
- Mitchell, B.G.; Fasugba, O.; Cheng, A.C.; Gregory, V.; Koerner, J.; Collignon, P.; Gardner, A.; Graves, N. Chlorhexidine versus saline in reducing the risk of catheter associated urinary tract infection: A cost-effectiveness analysis. Int. J. Nurs. Stud. 2019, 97, 1–6. [Google Scholar] [CrossRef]
- Saini, H.; Vadekeetil, A.; Chhibber, S.; Harjai, K. Azithromycin-ciprofloxacin-impregnated urinary catheters avert bacterial colonization, biofilm formation, and inflammation in a murine model of foreign-body-associated urinary tract infections caused by Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2017, 61, 10–1128. [Google Scholar] [CrossRef]
- Saini, H.; Chhibber, S.; Harjai, K. Antimicrobial and antifouling efficacy of urinary catheters impregnated with a combination of macrolide and fluoroquinolone antibiotics against Pseudomonas aeruginosa. Biofouling 2016, 32, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Yueh, M.-F.; Tukey, R.H. Triclosan: A widespread environmental toxicant with many biological effects. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Westfall, C.; Flores-Mireles, A.L.; Robinson, J.I.; Lynch, A.J.L.; Hultgren, S.; Henderson, J.P.; Levin, P.A. The Widely used antimicrobial triclosan induces high levels of antibiotic tolerance in vitro and reduces antibiotic efficacy up to 100-fold in vivo. Antimicrob. Agents Chemother. 2019, 63, e02312-18. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Panda, A.K.; De Mandal, S.; Shakeel, M.; Bisht, S.S.; Khan, J. Natural anti-biofilm agents: Strategies to control biofilm-forming pathogens. Front. Microbiol. 2020, 11, 566325. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.; Yang, P.; Lei, J.; Zhao, J. Biological function of antimicrobial peptides on suppressing pathogens and improving host immunity. Antibiotics 2023, 12, 1037. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, P.; Saravanan, R.; Basu, A.; Mishra, B.; Lim, S.H.; Su, X.; Tambyah, P.A.; Leong, S.S. Antimicrobial functionalization of silicone surfaces with engineered short peptides having broad spectrum antimicrobial and salt-resistant properties. Acta Biomater. 2014, 10, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; Chua, R.R.; Bow, H.; Tambyah, P.A.; Hadinoto, K.; Leong, S.S. Development of a catheter functionalized by a polydopamine peptide coating with antimicrobial and antibiofilm properties. Acta Biomater. 2015, 15, 127–138. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q.; Tian, Y.; Jian, Z.; Li, H.; Wang, K. Biodegradable hydrophilic polyurethane PEGU25 loading antimicrobial peptide Bmap-28: A sustained-release membrane able to inhibit bacterial biofilm formation in vitro. Sci Rep. 2015, 5, 8634. [Google Scholar] [CrossRef] [PubMed]
- Margel, D.; Mizrahi, M.; Regev-Shoshani, G.; Ko, M.; Moshe, M.; Ozalvo, R.; Shavit-Grievink, L.; Baniel, J.; Kedar, D.; Yossepowitch, O.; et al. Nitric oxide charged catheters as a potential strategy for prevention of hospital acquired infections. PLoS ONE 2017, 12, e0174443. [Google Scholar] [CrossRef]
- Homeyer, K.H.; Goudie, M.J.; Singha, P.; Handa, H. Liquid-infused nitric-oxide-releasing silicone foley urinary catheters for prevention of catheter-associated urinary tract infections. ACS Biomater. Sci. Eng. 2019, 5, 2021–2029. [Google Scholar] [CrossRef]
- Chug, M.K.; Brisbois, E.J. Smartphone compatible nitric oxide releasing insert to prevent catheter-associated infections. J. Control. Release 2022, 349, 227–240. [Google Scholar] [CrossRef]
- Yao, Q.; Wu, C.; Yu, X.; Chen, X.; Pan, G.; Chen, B. Current material engineering strategies to prevent catheter encrustation in urinary tracts. Mater. Today Bio. 2022, 16, 100413. [Google Scholar] [CrossRef] [PubMed]
- Townsend, E.M.; Moat, J.; Jameson, E. CAUTI’s next top model—Model dependent Klebsiella biofilm inhibition by bacteriophages and antimicrobials. Biofilm 2020, 2, 100038. [Google Scholar] [CrossRef] [PubMed]
- Bseikri, H.; Michniewski, S.; Serrano, E.G.; Jameson, E. Phages prevent biofilm formation on catheters under flow. bioRxiv 2023, 7, 550655. [Google Scholar] [CrossRef]
- Liao, K.S.; Lehman, S.M.; Tweardy, D.J.; Donlan, R.M.; Trautner, B.W. Bacteriophages are synergistic with bacterial interference for the prevention of Pseudomonas aeruginosa biofilm formation on urinary catheters. J. Appl. Microbiol. 2012, 113, 1530–1539. [Google Scholar] [CrossRef]
- Maszewska, A.; Zygmunt, M.; Grzejdziak, I.; Różalski, A. Use of polyvalent bacteriophages to combat biofilm of Proteus mirabilis causing catheter-associated urinary tract infections. J. Appl. Microbiol. 2018, 125, 1253–1265. [Google Scholar] [CrossRef] [PubMed]
- Ujmajuridze, A.; Chanishvili, N.; Goderdzishvili, M.; Leitner, L.; Mehnert, U.; Chkhotua, A.; Kessler, T.M.; Sybesma, W. Adapted bacteriophages for treating urinary tract infections. Front. Microbiol. 2018, 9, 1832. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Wagemans, J.; Esfahani, B.N.; Lavigne, R.; Moghim, S. A phage cocktail to control surface colonization by proteus mirabilis in catheter-associated urinary tract infections. Microbiol. Spectr. 2022, 10, e02092-22. [Google Scholar] [CrossRef] [PubMed]
- Teixeira-Santos, R.; Gomes, L.C.; Mergulhão, F.J.M. Recent advances in antimicrobial surfaces for urinary catheters. Curr. Opin. Biomed. Eng. 2022, 22, 100394. [Google Scholar] [CrossRef]
- Mosayyebi, A.; Manes, C.; Carugo, D. Somani. B.K. Advances in ureteral stent design and materials. Curr. Urol. Rep. 2018, 19, 35. [Google Scholar] [CrossRef]
- Dai, S.; Gao, Y.; Duan, L. Recent advances in hydrogel coatings for urinary catheters. J. Appl. Polym. Sci. 2023, 140, e53701. [Google Scholar] [CrossRef]
- Grigore, N.; Pirvut, V.; Mihai, I.; Hasegan, A.; Mitariu, S.I.C. Side-effects of polyurethane ureteral stents with or without hydrogel coating in urologic pathology. Mater. Plast. 2017, 54, 517–519. [Google Scholar] [CrossRef]
- Yang, K.; Kim, K.; Lee, E.A.; Liu, S.S.; Kabli, S.; Alsudir, S.A.; Albrahim, S.; Zhou, A.; Park, T.G.; Lee, H. Robust low friction antibiotic coating of urethral catheters using a catechol-functionalized polymeric hydrogel film. Front. Materials 2019, 6, 2019. [Google Scholar] [CrossRef]
- Tarawneh, O.; Abu Mahfouz, H.; Hamadneh, L.; Deeb, A.A.; Al-Sheikh, I.; Alwahsh, W.; Abed, A.F. Assessment of persistent antimicrobial and anti-biofilm activity of p-HEMA hydrogel loaded with rifampicin and cefixime. Sci. Rep. 2022, 12, 3900. [Google Scholar] [CrossRef]
- Kazmierska, K.A.; Thompson, R.; Morris, N.; Long, A.; Ciach, T. In vitro multicompartmental bladder model for assessing blockage of urinary catheters: Effect of hydrogel coating on dynamics of Proteus mirabilis growth. Urology 2010, 76, 515.e15–515.e20. [Google Scholar] [CrossRef] [PubMed]
- Pickard, R.; Lam, T.; MacLennan, G.; Starr, K.; Kilonzo, M.; McPherson, G.; Gillies, K.; McDonald, A.; Walton, K.; Buckley, B. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterisation in hospital: A multicentre randomised controlled trial. Lancet 2012, 380, 1927–1935. [Google Scholar] [CrossRef] [PubMed]
- Kilonzo, M.; Vale, L.; Pickard, R.; Lam, T.; N’Dow, J.; Catheter Trial Group. Cost effectiveness of antimicrobial catheters for adults requiring short-term catheterisation in hospital. Eur. Urol. 2014, 66, 615–618. [Google Scholar] [CrossRef]
- Wang, X.-w.; Wang, J.; Yu, Y.; Yu, L.; Wang, Y.-x.; Ren, K.-f.; Ji, J. A polyzwitterion-based antifouling and flexible bilayer hydrogel coating. Compos. B Eng. 2022, 244, 110164. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Q.; Han, Y.; Sun, Y.; Zhang, Y.; Zhang, H. Bioinspired surface functionalization of titanium alloy for enhanced lubrication and bacterial resistance. Langmuir 2019, 35, 13189–13195. [Google Scholar] [CrossRef]
- Li, S.; Huang, P.; Ye, Z.; Wang, Y.; Wang, W.; Kong, D.; Zhang, J.; Deng, L.; Dong, A. Layer-by-layer zwitterionic modification of diverse substrates with durable anti-corrosion and anti-fouling properties. J. Mater. Chem. B 2019, 7, 6024–6034. [Google Scholar] [CrossRef]
- Leigh, B.L.; Cheng, E.; Xu, L.; Derk, A.; Hansen, M.R.; Guymon, C.A. Antifouling photograftable zwitterionic coatings on PDMS substrates. Langmuir 2019, 35, 1100–1110. [Google Scholar] [CrossRef]
- Buchholz, N.; de Graaf, P.; de la Cruz, J.E.; Kram, W.; Skovorodkin, I.; Soaria, F.; Vainio, S. Preventing biofilm formation and encrustation on urinary implants: (bio)coatings and tissue engineering. In Urinary Stents; Soria, F., Rako, D., de Graaf, P., Eds.; Springer: Cham, Switzerland, 2022; pp. 427–435. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).