The Digestive Vacuole of the Malaria Parasite: A Specialized Lysosome
Abstract
1. Introduction
2. Life Cycle
3. Endocytosis and the Digestive Vacuole
3.1. Molecular Components of Endocytosis
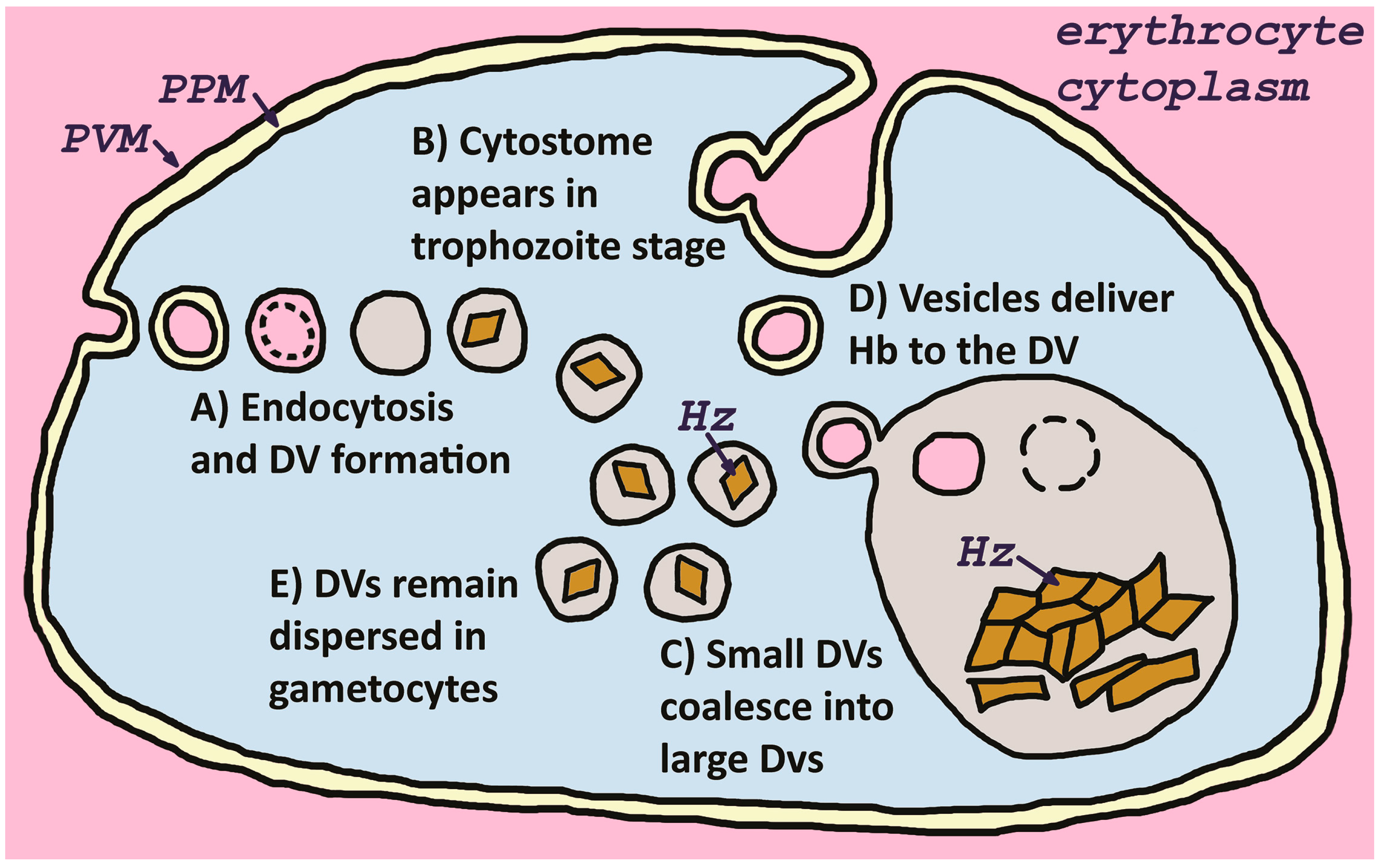
3.2. Digestive Vacuole Properties and Formation
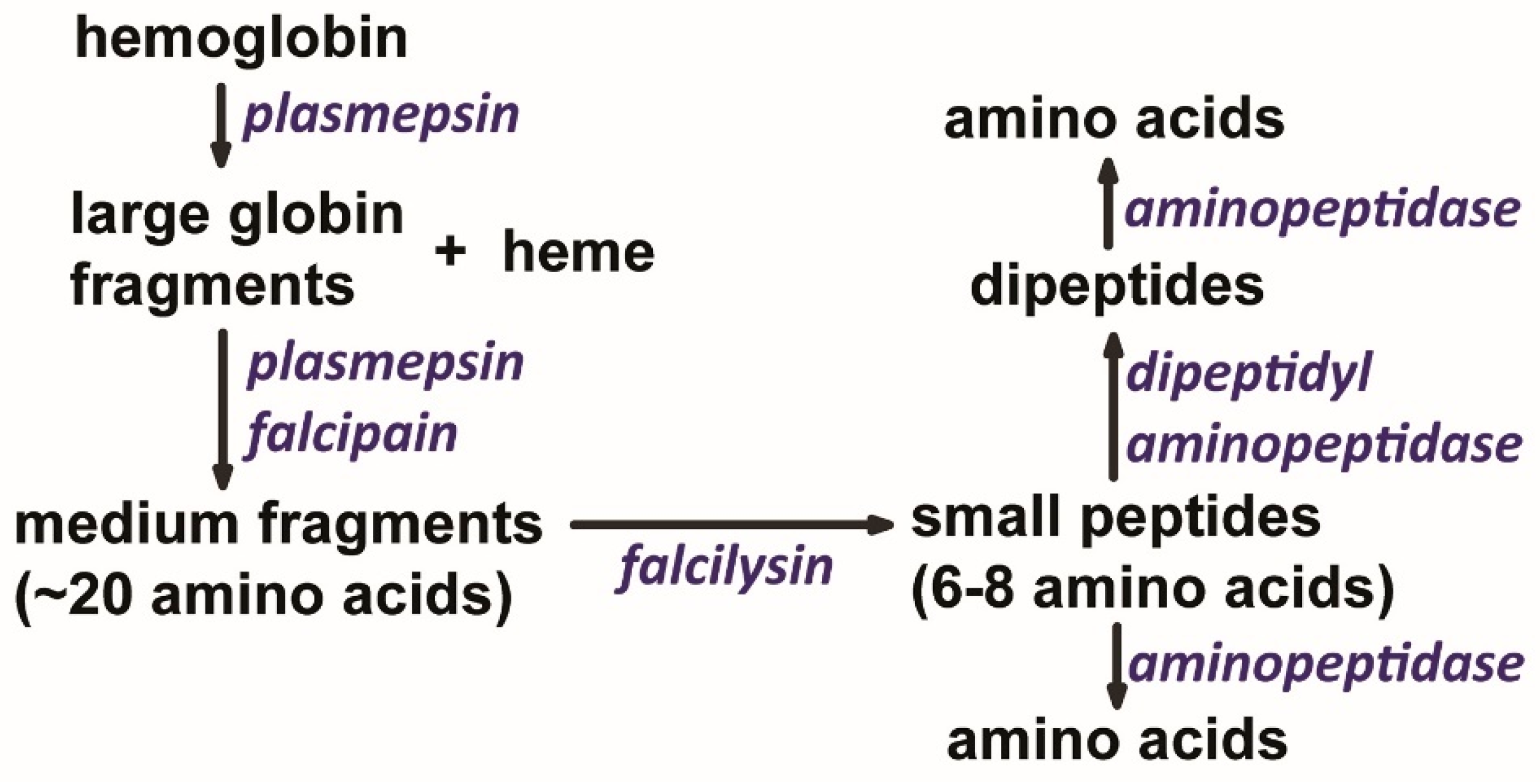
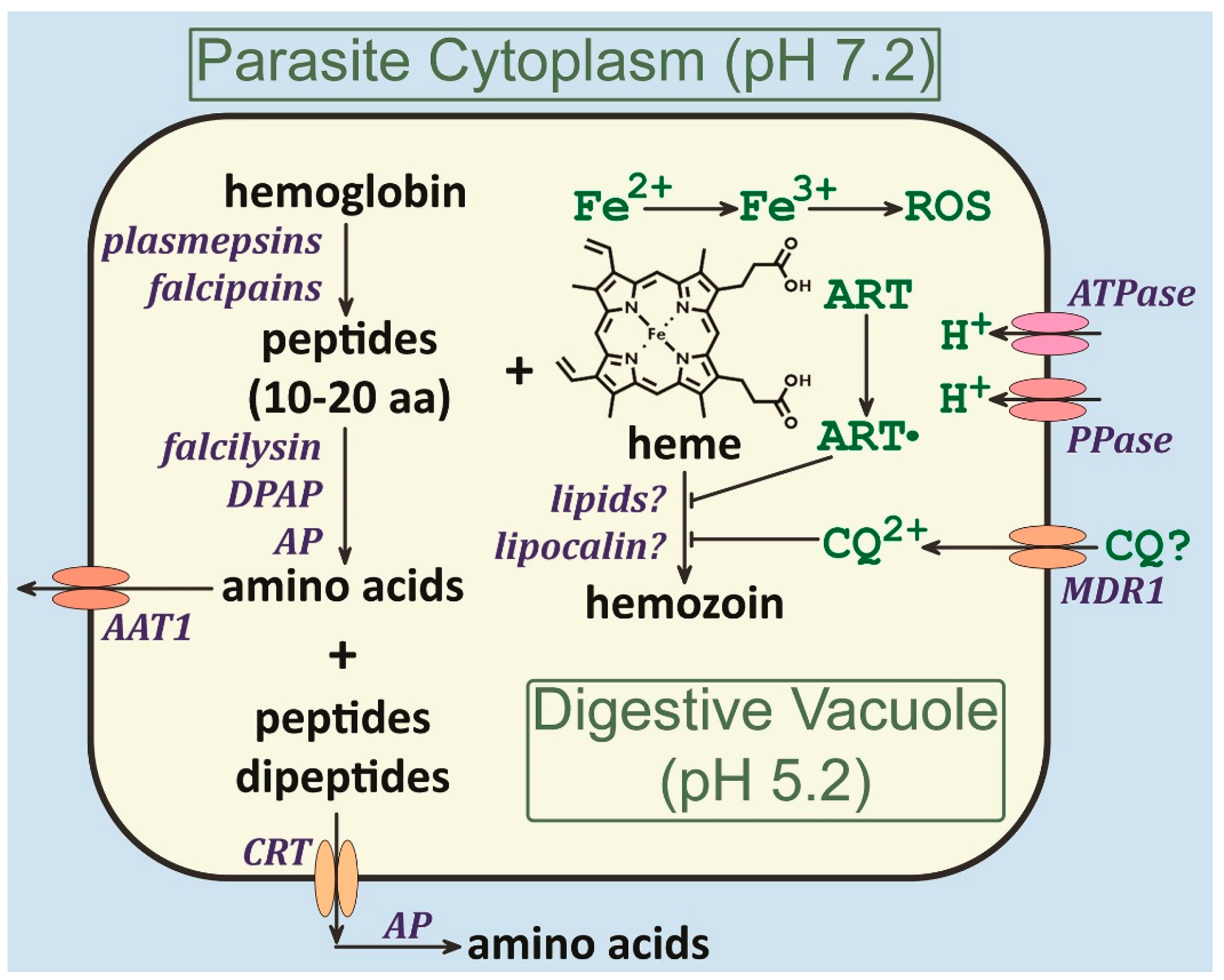
4. Hemoglobin Catabolism
4.1. Digestive Vacuole Membrane Transporters
4.2. Heme Detoxification
4.3. Hemozoin Formation
5. Anti-Malarials and the Digestive Vacuole
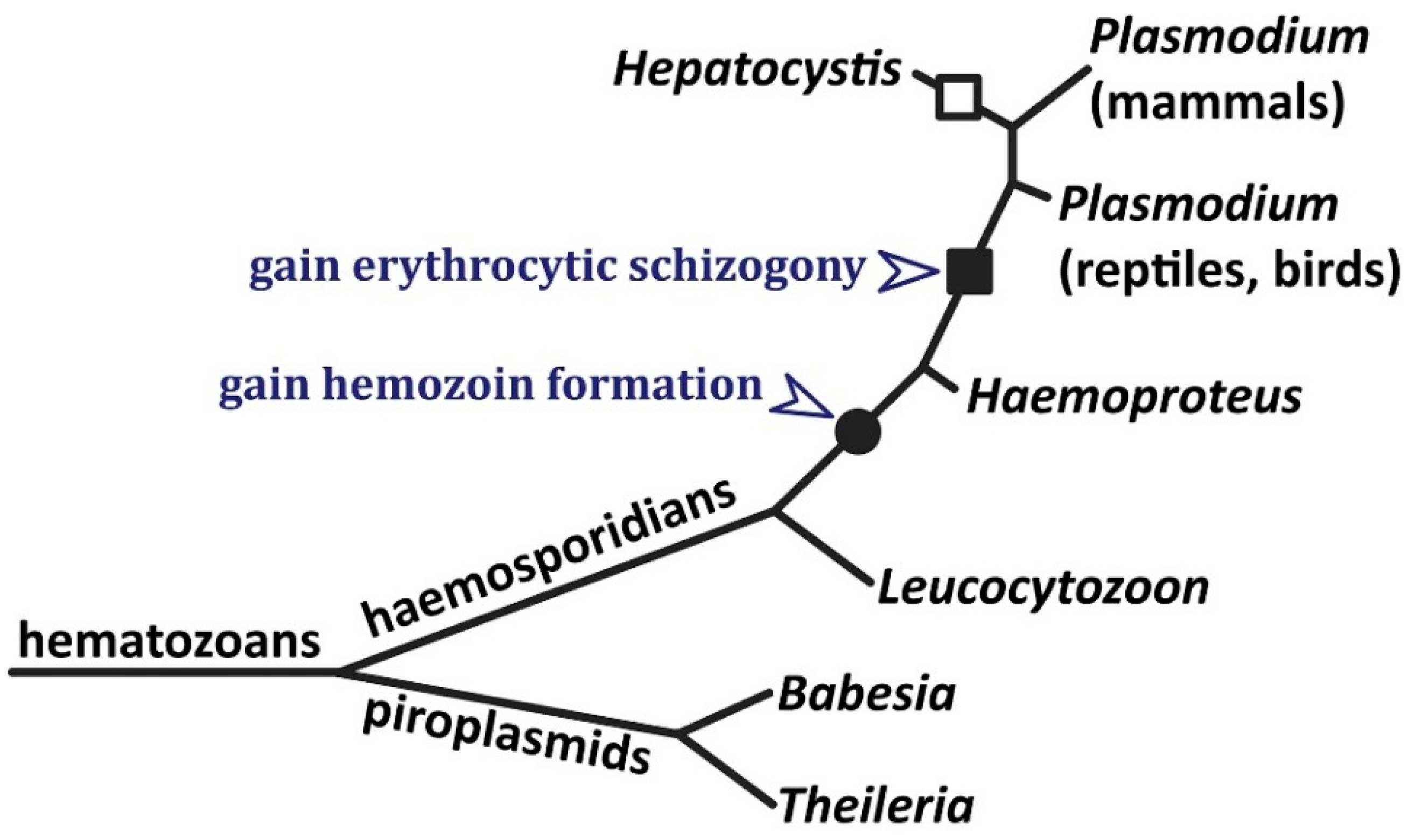
6. Other Intraerythrocytic Protozoan Parasites
7. Summary
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pacheco, M.A.; Escalante, A.A. Origin and diversity of malaria parasites and other Haemosporida. Trends Parasitol. 2023, 39, 501–516. [Google Scholar] [CrossRef]
- Wiser, M.F. Malaria. In Protozoa and Human Disease; Garland Science: New York, NY, USA, 2010; pp. 167–197. ISBN 9780429258282. [Google Scholar]
- Francis, S.E.; Sullivan, D.J.; Goldberg, D.E. Hemoglobin metabolism in the malaria parasite Plasmodium falciparum. Annu. Rev. Microbiol. 1997, 51, 97–123. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, J.; Rohrbach, P.; Dalton, J.P. The malaria digestive vacuole. Front. Biosci.-Sch. 2012, 4, 1424–1448. [Google Scholar] [CrossRef]
- Matz, J.M. Plasmodium’s bottomless pit: Properties and functions of the malaria parasite’s digestive vacuole. Trends Parasitol. 2022, 38, 525–543. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.A. Unique properties of nutrient channels on Plasmodium-infected erythrocytes. Pathogens 2023, 12, 1211. [Google Scholar] [CrossRef]
- Counihan, N.A.; Modak, J.K.; de Koning-Ward, T.F. How malaria parasites acquire nutrients from their host. Front. Cell Dev. Biol. 2021, 9, 649184. [Google Scholar] [CrossRef] [PubMed]
- Jeger, J.L. Endosomes, lysosomes, and the role of endosomal and lysosomal biogenesis in cancer development. Mol. Biol. Rep. 2020, 47, 9801–9810. [Google Scholar] [CrossRef] [PubMed]
- Elkin, S.R.; Lakoduk, A.M.; Schmid, S.L. Endocytic pathways and endosomal trafficking: A primer. Wiener Medizinische Wochenschrift 2016, 166, 196–204. [Google Scholar] [CrossRef]
- Kaksonen, M.; Roux, A. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2018, 19, 313–326. [Google Scholar] [CrossRef]
- Ferreira, A.P.A.; Boucrot, E. Mechanisms of carrier formation during clathrin-independent endocytosis. Trends Cell Biol. 2018, 28, 188–200. [Google Scholar] [CrossRef]
- Helenius, A.; Mellman, I.; Wall, D.; Hubbard, A. Endosomes. Trends Biochem. Sci. 1983, 8, 245–250. [Google Scholar] [CrossRef]
- Scott, C.C.; Vacca, F.; Gruenberg, J. Endosome maturation, transport and functions. Semin. Cell Dev. Biol. 2014, 31, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Rudzinska, M.A.; Trager, W.; Bray, R.S. Pinocytotic uptake and the digestion of hemoglobin in malaria parasites. J. Protozool. 1965, 12, 563–576. [Google Scholar] [CrossRef]
- Bannister, L.H.; Hopkins, J.M.; Margos, G.; Dluzewski, A.R.; Mitchell, G.H. Three-dimensional ultrastructure of the ring stage of Plasmodium falciparum: Evidence for export pathways. Microsc. Microanal. 2004, 10, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.E.; Zimmerberg, J. Hardly vacuous: The parasitophorous vacuolar membrane of malaria parasites. Trends Parasitol. 2020, 36, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Matz, J.M.; Beck, J.R.; Blackman, M.J. The parasitophorous vacuole of the blood-stage malaria parasite. Nat. Rev. Microbiol. 2020, 18, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Dluzewski, A.R.; Ling, I.T.; Hopkins, J.M.; Grainger, M.; Margos, G.; Mitchell, G.H.; Holder, A.A.; Bannister, L.H. Formation of the food vacuole in Plasmodium falciparum: A potential role for the 19 kDa fragment of merozoite surface protein 1 (MSP1(19)). PLoS ONE 2008, 3, e3085. [Google Scholar] [CrossRef]
- Bakar, N.A.; Klonis, N.; Hanssen, E.; Chan, C.; Tilley, L. Digestive-vacuole genesis and endocytic processes in the early intraerythrocytic stages of Plasmodium falciparum. J. Cell Sci. 2010, 123, 441–450. [Google Scholar] [CrossRef]
- Ngwa, C.J.; Rosa, T.F.; Pradel, G. The biology of malaria gametocytes. In Current Topics in Malaria; Rodriguez-Morales, A.J., Ed.; IntechOpen: Rijeka, Croatia, 2016; Chapter 7; ISBN 978-953-51-2790-1. [Google Scholar]
- Slomianny, C. Three-dimensional reconstruction of the feeding process of the malaria parasite. Blood Cells 1990, 16, 369–378. [Google Scholar]
- Milani, K.J.; Schneider, T.G.; Taraschi, T.F. Defining the morphology and mechanism of the hemoglobin transport pathway in Plasmodium falciparum-infected erythrocytes. Eukaryot. Cell 2015, 14, 415–426. [Google Scholar] [CrossRef]
- Elliott, D.A.; McIntosh, M.T.; Hosgood, H.D., 3rd; Chen, S.; Zhang, G.; Baevova, P.; Joiner, K.A. Four distinct pathways of hemoglobin uptake in the malaria parasite Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 2008, 105, 2463–2468. [Google Scholar] [CrossRef]
- Henrici, R.C.; Edwards, R.L.; Zoltner, M.; van Schalkwyk, D.A.; Hart, M.N.; Mohring, F.; Moon, R.W.; Nofal, S.D.; Patel, A.; Flueck, C.; et al. The Plasmodium falciparum artemisinin susceptibility-associated AP-2 adaptin μ subunit is clathrin independent and essential for schizont maturation. MBio 2020, 11, 10–1128. [Google Scholar] [CrossRef]
- Birnbaum, J.; Scharf, S.; Schmidt, S.; Jonscher, E.; Hoeijmakers, W.A.M.; Flemming, S.; Toenhake, C.G.; Schmitt, M.; Sabitzki, R.; Bergmann, B.; et al. A Kelch13-defined endocytosis pathway mediates artemisinin resistance in malaria parasites. Science 2020, 367, 51–59. [Google Scholar] [CrossRef]
- Robinson, M.S. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004, 14, 167–174. [Google Scholar] [CrossRef]
- Pieperhoff, M.S.; Schmitt, M.; Ferguson, D.J.P.; Meissner, M. The role of cathrin in post-Golgi trafficking in Toxoplasma gondii. PLoS ONE 2013, 8, e77620. [Google Scholar] [CrossRef]
- Jonscher, E.; Flemming, S.; Schmitt, M.; Sabitzki, R.; Reichard, N.; Birnbaum, J.; Bergmann, B.; Höhn, K.; Spielmann, T. PfVPS45 is required for host cell cytosol uptake by malaria blood stage parasites. Cell Host Microbe 2019, 25, 166–173.e5. [Google Scholar] [CrossRef]
- Zhou, H.; Gao, Y.; Zhong, X.; Wang, H. Dynamin like protein 1 participated in the hemoglobin uptake pathway of Plasmodium falciparum. Chin. Med. J. 2009, 122, 1686–1691. [Google Scholar] [PubMed]
- Smythe, W.A.; Joiner, K.A.; Hoppe, H.C. Actin is required for endocytic trafficking in the malaria parasite Plasmodium falciparum. Cell. Microbiol. 2008, 10, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Vaid, A.; Ranjan, R.; Smythe, W.A.; Hoppe, H.C.; Sharma, P. PfPI3K, a phosphatidylinositol-3 kinase from Plasmodium falciparum, is exported to the host erythrocyte and is involved in hemoglobin trafficking. Blood 2010, 115, 2500–2507. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Crochetière, M.-È.; Sergerie, A.; Amiar, S.; Thompson, L.A.; Ebrahimzadeh, Z.; Gagnon, D.; Lauruol, F.; Bourgeois, A.; Galaup, T.; et al. A phosphoinositide-binding protein acts in the trafficking pathway of hemoglobin in the malaria parasite Plasmodium falciparum. MBio 2022, 13, e0323921. [Google Scholar] [CrossRef]
- Howe, R.; Kelly, M.; Jimah, J.; Hodge, D.; Odom, A.R. Isoprenoid biosynthesis inhibition disrupts Rab5 localization and food vacuolar integrity in Plasmodium falciparum. Eukaryot. Cell 2013, 12, 215–223. [Google Scholar] [CrossRef]
- Siddiqui, G.; Srivastava, A.; Russell, A.S.; Creek, D.J. Multi-omics based identification of specific biochemical changes associated with PfKelch13-mutant artemisinin-resistant Plasmodium falciparum. J. Infect. Dis. 2017, 215, 1435–1444. [Google Scholar] [CrossRef]
- Yang, T.; Yeoh, L.M.; Tutor, M.V.; Dixon, M.W.; McMillan, P.J.; Xie, S.C.; Bridgford, J.L.; Gillett, D.L.; Duffy, M.F.; Ralph, S.A.; et al. Decreased K13 abundance reduces hemoglobin catabolism and proteotoxic stress, underpinning artemisinin resistance. Cell Rep. 2019, 29, 2917–2928.e5. [Google Scholar] [CrossRef]
- Thakur, V.; Asad, M.; Jain, S.; Hossain, M.E.; Gupta, A.; Kaur, I.; Rathore, S.; Ali, S.; Khan, N.J.; Mohmmed, A. Eps15 homology domain containing protein of Plasmodium falciparum (PfEHD) associates with endocytosis and vesicular trafficking towards neutral lipid storage site. Biochim. Biophys. Acta 2015, 1853, 2856–2869. [Google Scholar] [CrossRef]
- Goldberg, D.E.; Slater, A.F.; Cerami, A.; Henderson, G.B. Hemoglobin degradation in the malaria parasite Plasmodium falciparum: An ordered process in a unique organelle. Proc. Natl. Acad. Sci. USA 1990, 87, 2931–2935. [Google Scholar] [CrossRef]
- Saliba, K.J.; Allen, R.J.W.; Zissis, S.; Bray, P.G.; Ward, S.A.; Kirk, K. Acidification of the malaria parasite’s digestive vacuole by a H+-ATPase and a H+-pyrophosphatase. J. Biol. Chem. 2003, 278, 5605–5612. [Google Scholar] [CrossRef] [PubMed]
- Lamarque, M.; Tastet, C.; Poncet, J.; Demettre, E.; Jouin, P.; Vial, H.; Dubremetz, J.-F. Food vacuole proteome of the malarial parasite Plasmodium falciparum. Proteomics. Clin. Appl. 2008, 2, 1361–1374. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Liu, J.; Beatty, W.; Pelosof, L.; Klemba, M.; Goldberg, D.E. Four plasmepsins are active in the Plasmodium falciparum food vacuole, including a protease with an active-site histidine. Proc. Natl. Acad. Sci. USA 2002, 99, 990–995. [Google Scholar] [CrossRef]
- Dame, J.B.; Yowell, C.A.; Omara-Opyene, L.; Carlton, J.M.; Cooper, R.A.; Li, T. Plasmepsin 4, the food vacuole aspartic proteinase found in all Plasmodium spp. infecting man. Mol. Biochem. Parasitol. 2003, 130, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, P.J. Cysteine proteases of malaria parasites. Int. J. Parasitol. 2004, 34, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Shenai, B.R.; Sijwali, P.S.; Singh, A.; Rosenthal, P.J. Characterization of native and recombinant falcipain-2, a principal trophozoite cysteine protease and essential hemoglobinase of Plasmodium falciparum. J. Biol. Chem. 2000, 275, 29000–29010. [Google Scholar] [CrossRef]
- Sijwali, P.S.; Shenai, B.R.; Gut, J.; Singh, A.; Rosenthal, P.J. Expression and characterization of the Plasmodium falciparum haemoglobinase falcipain-3. Biochem. J. 2001, 360, 481–489. [Google Scholar] [CrossRef]
- Eggleson, K.K.; Duffin, K.L.; Goldberg, D.E. Identification and characterization of falcilysin, a metallopeptidase involved in hemoglobin catabolism within the malaria parasite Plasmodium falciparum. J. Biol. Chem. 1999, 274, 32411–32417. [Google Scholar] [CrossRef]
- Klemba, M.; Gluzman, I.; Goldberg, D.E. A Plasmodium falciparum dipeptidyl aminopeptidase I participates in vacuolar hemoglobin degradation. J. Biol. Chem. 2004, 279, 43000–43007. [Google Scholar] [CrossRef]
- Ragheb, D.; Bompiani, K.; Dalal, S.; Klemba, M. Evidence for catalytic roles for Plasmodium falciparum aminopeptidase P in the food vacuole and cytosol. J. Biol. Chem. 2009, 284, 24806–24815. [Google Scholar] [CrossRef] [PubMed]
- Harbut, M.B.; Velmourougane, G.; Dalal, S.; Reiss, G.; Whisstock, J.C.; Onder, O.; Brisson, D.; McGowan, S.; Klemba, M.; Greenbaum, D.C. Bestatin-based chemical biology strategy reveals distinct roles for malaria M1- and M17-family aminopeptidases. Proc. Natl. Acad. Sci. USA 2011, 108, E526–E534. [Google Scholar] [CrossRef]
- Klemba, M.; Beatty, W.; Gluzman, I.; Goldberg, D.E. Trafficking of plasmepsin II to the food vacuole of the malaria parasite Plasmodium falciparum. J. Cell Biol. 2004, 164, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Sijwali, P.S.; Rosenthal, P.J. Falcipain cysteine proteases require bipartite motifs for trafficking to the Plasmodium falciparum food vacuole. J. Biol. Chem. 2007, 282, 24961–24969. [Google Scholar] [CrossRef] [PubMed]
- Drew, M.E.; Banerjee, R.; Uffman, E.W.; Gilbertson, S.; Rosenthal, P.J.; Goldberg, D.E. Plasmodium food vacuole plasmepsins are activated by falcipains. J. Biol. Chem. 2008, 283, 12870–12876. [Google Scholar] [CrossRef]
- Goldberg, D.E. Hemoglobin degradation. Curr. Top. Microbiol. Immunol. 2005, 295, 275–291. [Google Scholar] [CrossRef]
- Omara-Opyene, A.L.; Moura, P.A.; Sulsona, C.R.; Bonilla, J.A.; Yowell, C.A.; Fujioka, H.; Fidock, D.A.; Dame, J.B. Genetic disruption of the Plasmodium falciparum digestive vacuole plasmepsins demonstrates their functional redundancy. J. Biol. Chem. 2004, 279, 54088–54096. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.E.; Slater, A.F.; Beavis, R.; Chait, B.; Cerami, A.; Henderson, G.B. Hemoglobin degradation in the human malaria pathogen Plasmodium falciparum: A catabolic pathway initiated by a specific aspartic protease. J. Exp. Med. 1991, 173, 961–969. [Google Scholar] [CrossRef]
- Dalal, S.; Klemba, M. Roles for two aminopeptidases in vacuolar hemoglobin catabolism in Plasmodium falciparum. J. Biol. Chem. 2007, 282, 35978–35987. [Google Scholar] [CrossRef] [PubMed]
- Krugliak, M.; Zhang, J.; Ginsburg, H. Intraerythrocytic Plasmodium falciparum utilizes only a fraction of the amino acids derived from the digestion of host cell cytosol for the biosynthesis of its proteins. Mol. Biochem. Parasitol. 2002, 119, 249–256. [Google Scholar] [CrossRef]
- Dalal, S.; Klemba, M. Amino acid efflux by asexual blood-stage Plasmodium falciparum and its utility in interrogating the kinetics of hemoglobin endocytosis and catabolism in vivo. Mol. Biochem. Parasitol. 2015, 201, 116–122. [Google Scholar] [CrossRef]
- Hanssen, E.; Knoechel, C.; Dearnley, M.; Dixon, M.W.A.; Le Gros, M.; Larabell, C.; Tilley, L. Soft X-ray microscopy analysis of cell volume and hemoglobin content in erythrocytes infected with asexual and sexual stages of Plasmodium falciparum. J. Struct. Biol. 2012, 177, 224–232. [Google Scholar] [CrossRef]
- Lew, V.L.; Tiffert, T.; Ginsburg, H. Excess hemoglobin digestion and the osmotic stability of Plasmodium falciparum-infected red blood cells. Blood 2003, 101, 4189–4194. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.E.; Marchetti, R.V.; Cowan, A.I.; Howitt, S.M.; Bröer, S.; Kirk, K. Chloroquine transport via the malaria parasite’s chloroquine resistance transporter. Science 2009, 325, 1680–1682. [Google Scholar] [CrossRef]
- Martin, R.E.; Kirk, K. The malaria parasite’s chloroquine resistance transporter is a member of the drug/metabolite transporter superfamily. Mol. Biol. Evol. 2004, 21, 1938–1949. [Google Scholar] [CrossRef]
- Fidock, D.A.; Nomura, T.; Talley, A.K.; Cooper, R.A.; Dzekunov, S.M.; Ferdig, M.T.; Ursos, L.M.; Sidhu, A.B.; Naudé, B.; Deitsch, K.W.; et al. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol. Cell 2000, 6, 861–871. [Google Scholar] [CrossRef]
- Shafik, S.H.; Cobbold, S.A.; Barkat, K.; Richards, S.N.; Lancaster, N.S.; Llinás, M.; Hogg, S.J.; Summers, R.L.; McConville, M.J.; Martin, R.E. The natural function of the malaria parasite’s chloroquine resistance transporter. Nat. Commun. 2020, 11, 3922. [Google Scholar] [CrossRef] [PubMed]
- Lehane, A.M.; Hayward, R.; Saliba, K.J.; Kirk, K. A verapamil-sensitive chloroquine-associated H+ leak from the digestive vacuole in chloroquine-resistant malaria parasites. J. Cell Sci. 2008, 121, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Cowell, A.N.; Istvan, E.S.; Lukens, A.K.; Gomez-Lorenzo, M.G.; Vanaerschot, M.; Sakata-Kato, T.; Flannery, E.L.; Magistrado, P.; Owen, E.; Abraham, M.; et al. Mapping the malaria parasite druggable genome by using in vitro evolution and chemogenomics. Science 2018, 359, 191–199. [Google Scholar] [CrossRef]
- Amambua-Ngwa, A.; Button-Simons, K.A.; Li, X.; Kumar, S.; Brenneman, K.V.; Ferrari, M.; Checkley, L.A.; Haile, M.T.; Shoue, D.A.; McDew-White, M.; et al. Chloroquine resistance evolution in Plasmodium falciparum is mediated by the putative amino acid transporter AAT1. Nat. Microbiol. 2023, 8, 1213–1226. [Google Scholar] [CrossRef] [PubMed]
- Rohrbach, P.; Sanchez, C.P.; Hayton, K.; Friedrich, O.; Patel, J.; Sidhu, A.B.S.; Ferdig, M.T.; Fidock, D.A.; Lanzer, M. Genetic linkage of pfmdr1 with food vacuolar solute import in Plasmodium falciparum. EMBO J. 2006, 25, 3000–3011. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Tilley, L.; Vennerstrom, J.L.; Roberts, D.; Rogerson, S.; Ginsburg, H. Oxidative stress in malaria parasite-infected erythrocytes: Host-parasite interactions. Int. J. Parasitol. 2004, 34, 163–189. [Google Scholar] [CrossRef]
- Penketh, P.G. Hemoglobin is not a biological Fenton reagent. React. Oxyg. Species 2022, 12, c1–c5. [Google Scholar] [CrossRef]
- Kumar, S.; Bandyopadhyay, U. Free heme toxicity and its detoxification systems in human. Toxicol. Lett. 2005, 157, 175–188. [Google Scholar] [CrossRef]
- Egan, T.J. Haemozoin formation. Mol. Biochem. Parasitol. 2008, 157, 127–136. [Google Scholar] [CrossRef]
- Kapishnikov, S.; Hempelmann, E.; Elbaum, M.; Als-Nielsen, J.; Leiserowitz, L. Malaria pigment crystals: The Achilles’ heel of the malaria parasite. ChemMedChem 2021, 16, 1515–1532. [Google Scholar] [CrossRef]
- Sullivan, D.J. Theories on malarial pigment formation and quinoline action. Int. J. Parasitol. 2002, 32, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Dalapati, T.; Moore, J.M. Hemozoin: A complex molecule with complex activities. Curr. Clin. Microbiol. Rep. 2021, 8, 87–102. [Google Scholar] [CrossRef]
- Pham, T.-T.; Lamb, T.J.; Deroost, K.; Opdenakker, G.; Van den Steen, P.E. Hemozoin in malarial complications: More questions than answers. Trends Parasitol. 2021, 37, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Olivier, M.; Van Den Ham, K.; Shio, M.T.; Kassa, F.A.; Fougeray, S. Malarial pigment hemozoin and the innate inflammatory response. Front. Immunol. 2014, 5, 25. [Google Scholar] [CrossRef]
- Pagola, S.; Stephens, P.W.; Bohle, D.S.; Kosar, A.D.; Madsen, S.K. The structure of malaria pigment β-haematin. Nature 2000, 404, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Dorn, A.; Stoffel, R.; Matile, H.; Bubendorf, A.; Ridley, R.G. Malarial haemozoin/β-haematin supports haem polymerization in the absence of protein. Nature 1995, 374, 269–271. [Google Scholar] [CrossRef]
- Pisciotta, J.M.; Coppens, I.; Tripathi, A.K.; Scholl, P.F.; Shuman, J.; Bajad, S.; Shulaev, V.; Sullivan, D.J., Jr. The role of neutral lipid nanospheres in Plasmodium falciparum haem crystallization. Biochem. J. 2007, 402, 197–204. [Google Scholar] [CrossRef]
- Kapishnikov, S.; Weiner, A.; Shimoni, E.; Guttmann, P.; Schneider, G.; Dahan-Pasternak, N.; Dzikowski, R.; Leiserowitz, L.; Elbaum, M. Oriented nucleation of hemozoin at the digestive vacuole membrane in Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 2012, 109, 11188–11193. [Google Scholar] [CrossRef]
- Hoang, A.N.; Ncokazi, K.K.; de Villiers, K.A.; Wright, D.W.; Egan, T.J. Crystallization of synthetic haemozoin (β-haematin) nucleated at the surface of lipid particles. Dalt. Trans. 2010, 39, 1235–1244. [Google Scholar] [CrossRef]
- Ambele, M.A.; Egan, T.J. Neutral lipids associated with haemozoin mediate efficient and rapid β-haematin formation at physiological pH, temperature and ionic composition. Malar. J. 2012, 11, 337. [Google Scholar] [CrossRef]
- Fitch, C.D.; Cai, G.Z.; Chen, Y.F.; Shoemaker, J.D. Involvement of lipids in ferriprotoporphyrin IX polymerization in malaria. Biochim. Biophys. Acta 1999, 1454, 31–37. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Burda, P.-C.; Crosskey, T.; Lauk, K.; Zurborg, A.; Söhnchen, C.; Liffner, B.; Wilcke, L.; Pietsch, E.; Strauss, J.; Jeffries, C.M.; et al. Structure-based identification and functional characterization of a lipocalin in the malaria parasite Plasmodium falciparum. Cell Rep. 2020, 31, 107817. [Google Scholar] [CrossRef]
- Matz, J.M.; Drepper, B.; Blum, T.B.; van Genderen, E.; Burrell, A.; Martin, P.; Stach, T.; Collinson, L.M.; Abrahams, J.P.; Matuschewski, K.; et al. A lipocalin mediates unidirectional heme biomineralization in malaria parasites. Proc. Natl. Acad. Sci. USA 2020, 117, 16546–16556. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.J.; Gluzman, I.Y.; Goldberg, D.E. Plasmodium hemozoin formation mediated by histidine-rich proteins. Science 1996, 271, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Jani, D.; Nagarkatti, R.; Beatty, W.; Angel, R.; Slebodnick, C.; Andersen, J.; Kumar, S.; Rathore, D. HDP-a novel heme detoxification protein from the malaria parasite. PLoS Pathog. 2008, 4, e1000053. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.S.; Counihan, N.A.; McGowan, S.; de Koning-Ward, T.F. Methods used to investigate the Plasmodium falciparum digestive vacuole. Front. Cell. Infect. Microbiol. 2021, 11, 829823. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.J.J.; Gluzman, I.Y.; Russell, D.G.; Goldberg, D.E. On the molecular mechanism of chloroquine’s antimalarial action. Proc. Natl. Acad. Sci. USA 1996, 93, 11865–11870. [Google Scholar] [CrossRef]
- Bray, P.G.; Janneh, O.; Raynes, K.J.; Mungthin, M.; Ginsburg, H.; Ward, S.A. Cellular uptake of chloroquine is dependent on binding to ferriprotoporphyrin IX and is independent of NHE activity in Plasmodium falciparum. J. Cell Biol. 1999, 145, 363–376. [Google Scholar] [CrossRef]
- Geary, T.G.; Jensen, J.B.; Ginsburg, H. Uptake of [3H]chloroquine by drug-sensitive and -resistant strains of the human malaria parasite Plasmodium falciparum. Biochem. Pharmacol. 1986, 35, 3805–3812. [Google Scholar] [CrossRef]
- Chong, C.R.; Sullivan, D.J. Inhibition of heme crystal growth by antimalarials and other compounds: Implications for drug discovery. Biochem. Pharmacol. 2003, 66, 2201–2212. [Google Scholar] [CrossRef]
- Vippagunta, S.R.; Dorn, A.; Matile, H.; Bhattacharjee, A.K.; Karle, J.M.; Ellis, W.Y.; Ridley, R.G.; Vennerstrom, J.L. Structural specificity of chloroquine-hematin binding related to inhibition of hematin polymerization and parasite growth. J. Med. Chem. 1999, 42, 4630–4639. [Google Scholar] [CrossRef]
- Olafson, K.N.; Ketchum, M.A.; Rimer, J.D.; Vekilov, P.G. Mechanisms of hematin crystallization and inhibition by the antimalarial drug chloroquine. Proc. Natl. Acad. Sci. USA 2015, 112, 4946–4951. [Google Scholar] [CrossRef]
- Wang, J.; Xu, C.; Wong, Y.K.; Li, Y.; Liao, F.; Jiang, T.; Tu, Y. Artemisinin, the magic drug discovered from traditional chinese medicine. Engineering 2019, 5, 32–39. [Google Scholar] [CrossRef]
- Klonis, N.; Crespo-Ortiz, M.P.; Bottova, I.; Abu-Bakar, N.; Kenny, S.; Rosenthal, P.J.; Tilley, L. Artemisinin activity against Plasmodium falciparum requires hemoglobin uptake and digestion. Proc. Natl. Acad. Sci. USA 2011, 108, 11405–11410. [Google Scholar] [CrossRef]
- Meunier, B.; Robert, A. Heme as trigger and target for trioxane-containing antimalarial drugs. Acc. Chem. Res. 2010, 43, 1444–1451. [Google Scholar] [CrossRef]
- Robert, A.; Benoit-Vical, F.; Claparols, C.; Meunier, B. The antimalarial drug artemisinin alkylates heme in infected mice. Proc. Natl. Acad. Sci. USA 2005, 102, 13676–13680. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, C.-J.; Chia, W.N.; Loh, C.C.Y.; Li, Z.; Lee, Y.M.; He, Y.; Yuan, L.-X.; Lim, T.K.; Liu, M.; et al. Haem-activated promiscuous targeting of artemisinin in Plasmodium falciparum. Nat. Commun. 2015, 6, 10111. [Google Scholar] [CrossRef]
- O’Donoghue, P. Haemoprotozoa: Making biological sense of molecular phylogenies. Int. J. Parasitol. Parasites Wildl. 2017, 6, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Martinsen, E.S.; Perkins, S.L.; Schall, J.J. A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): Evolution of life-history traits and host switches. Mol. Phylogenet. Evol. 2008, 47, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M.A.; Matta, N.E.; Valkiūnas, G.; Parker, P.G.; Mello, B.; Stanley Jr, C.E.; Lentino, M.; Garcia-Amado, M.A.; Cranfield, M.; Kosakovsky Pond, S.L.; et al. Mode and rate of evolution of haemosporidian mitochondrial genomes: Timing the radiation of avian parasites. Mol. Biol. Evol. 2018, 35, 383–403. [Google Scholar] [CrossRef]
- Votýpka, J.; Modrý, D.; Oborník, M.; Šlapeta, J.; Lukeš, J. Apicomplexa. In Handbook of the Protists; Archibald, J.M., Simpson, A.G.B., Slamovits, C.H., Margulis, L., Melkonian, M., Chapman, D.J., Corliss, J.O., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–58. ISBN 978-3-319-32669-6. [Google Scholar]
- Sojka, D.; Jalovecká, M.; Perner, J. Babesia, Theileria, Plasmodium and hemoglobin. Microorganisms 2022, 10, 1651. [Google Scholar] [CrossRef] [PubMed]
- Langreth, S.G. Feeding mechanisms in extracellular Babesia microti and Plasmodium lophurae. J. Protozool. 1976, 23, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Potgieter, F.T.; Els, H.J. The fine structure of intra-erythrocytic stages of Babesia bigemina. Onderstepoort J. Vet. Res. 1977, 44, 157–168. [Google Scholar] [PubMed]
- Uchida, T.A.; Yanagawa, H.; Mori, T.; Shiraishi, S. Ultrastructural observations of the intra-erythrocytic merozoite in Theileria sergenti. Trop. Med. Parasitol. 1985, 36, 35–38. [Google Scholar] [PubMed]
- Fawcett, D.W.; Conrad, P.A.; Grootenhuis, J.G.; Morzaria, S.P. Ultrastructure of the intra-erythrocytic stage of Theileria species from cattle and waterbuck. Tissue Cell 1987, 19, 643–655. [Google Scholar] [CrossRef]
- Rudzinska, M.A. Ultrastructure of intraerythrocytic Babesia microti with emphasis on the feeding mechanism. J. Protozool. 1976, 23, 224–233. [Google Scholar] [CrossRef]
- Florin-Christensen, M.; Wieser, S.N.; Suarez, C.E.; Schnittger, L. In silico survey and characterization of Babesia microti functional and non-functional proteases. Pathogens 2021, 10, 1457. [Google Scholar] [CrossRef]
- Slomianny, C.; Charet, P.; Prensier, G. Ultrastructural localization of enzymes involved in the feeding process in Plasmodium chabaudi and Babesia hylomysci. J. Protozool. 1983, 30, 376–382. [Google Scholar] [CrossRef]
- Weiss, L.M. Babesiosis in humans: A treatment review. Expert Opin. Pharmacother. 2002, 3, 1109–1115. [Google Scholar] [CrossRef]
- Frerichs, W.M.; Holbrook, A.A. Feeding mechanisms of Babesia equi. J. Protozool. 1974, 21, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, S.; Kawamura, S.; Hanamatsu, K.; Yasuda, Y. Electron microscopy of Theileria sergenti in bovine erythrocytes. Nihon Juigaku Zasshi. 1984, 46, 745–748. [Google Scholar] [CrossRef] [PubMed]
| Protein | Comments |
|---|---|
| vacuolar sorting protein 45 | Inactivation leads to the accumulation of vesicles and prevents the delivery of hemoglobin to the digestive vacuole [28] |
| Rab5a | Implicated in hemoglobin uptake and transport to digestive vacuole [23] |
| dynamin like protein-1 | Inhibitors reduce hemoglobin uptake [29] |
| actin | Inhibition of actin polymerization increases hemoglobin containing vesicles [22,30] |
| phosphatidylinositol-3 kinase | Inhibitors block trafficking of hemoglobin to the digestive vacuole [31] |
| phosphoinositide-binding protein | Localized to the digestive vacuole and plays a role in trafficking of hemoglobin [32] |
| protein prenylation | Inhibitors cause deformation of digestive vacuole and mislocalization of Rab5 [33] |
| μ subunit of AP-2 adaptin complex | Implicated in clathrin-independent endocytosis [24] |
| Kelch13 | Localized to cytostomes [25] and mutations [34] or reduced abundance [35] impairs hemoglobin catabolism |
| Eps15 | Localized to a large multi-vesicular structure near the digestive vacuole [36] |
| Class | Protease | Comments |
|---|---|---|
| Aspartic Proteases [40] | Plasmepsin-1 (PM1) | PM4 is found in all Plasmodium species and PM1, PM2, and HAP are paralogs of PM4 and are only found in P. falciparum [41] |
| Plasmepsin-2 (PM2) | ||
| Plasmepsin-4 (PM4) | ||
| Histoaspartic protease (HAP), aka plasmepsin-3 | ||
| Cysteine proteases [42] | Falcipain-2 [43] | Falcipain-2 and -3 exhibit 70% sequence identity and falcipain-2′ is a nearly identical copy of falcipain-2 |
| Falcipain-2′ | ||
| Falcipain-3 [44] | ||
| Metalloprotease | Falcilysin [45] | Prefers peptides < 20 amino acids long |
| Exopeptidases | Dipeptidyl aminopeptidase-1 [46] | Generates dipeptides from hemoglobin-derived peptides |
| Aminopeptidase P [47] | Possibly functions in both the digestive vacuole and the parasite cytoplasm | |
| M1 alanyl aminopeptidase [48] | Inhibition caused swelling of the parasite digestive vacuole and prevented proteolysis of hemoglobin-derived peptides |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiser, M.F. The Digestive Vacuole of the Malaria Parasite: A Specialized Lysosome. Pathogens 2024, 13, 182. https://doi.org/10.3390/pathogens13030182
Wiser MF. The Digestive Vacuole of the Malaria Parasite: A Specialized Lysosome. Pathogens. 2024; 13(3):182. https://doi.org/10.3390/pathogens13030182
Chicago/Turabian StyleWiser, Mark F. 2024. "The Digestive Vacuole of the Malaria Parasite: A Specialized Lysosome" Pathogens 13, no. 3: 182. https://doi.org/10.3390/pathogens13030182
APA StyleWiser, M. F. (2024). The Digestive Vacuole of the Malaria Parasite: A Specialized Lysosome. Pathogens, 13(3), 182. https://doi.org/10.3390/pathogens13030182





