Comparing the Fate and Transport of MS2 Bacteriophage and Sodium Fluorescein in a Karstic Chalk Aquifer
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Area Description
2.2. Materials
2.2.1. MS2 Bacteriophage Propagation
2.2.2. Bacteriophage Enumeration
2.2.3. Fluorescent Dye
2.3. Comparative Techniques
2.3.1. Transport Model Description
2.3.2. Transport Model Conditions
3. Results
3.1. Tracer Attenuation
3.2. Tracer Transport
3.2.1. Normalised Tracer Breakthrough Curves
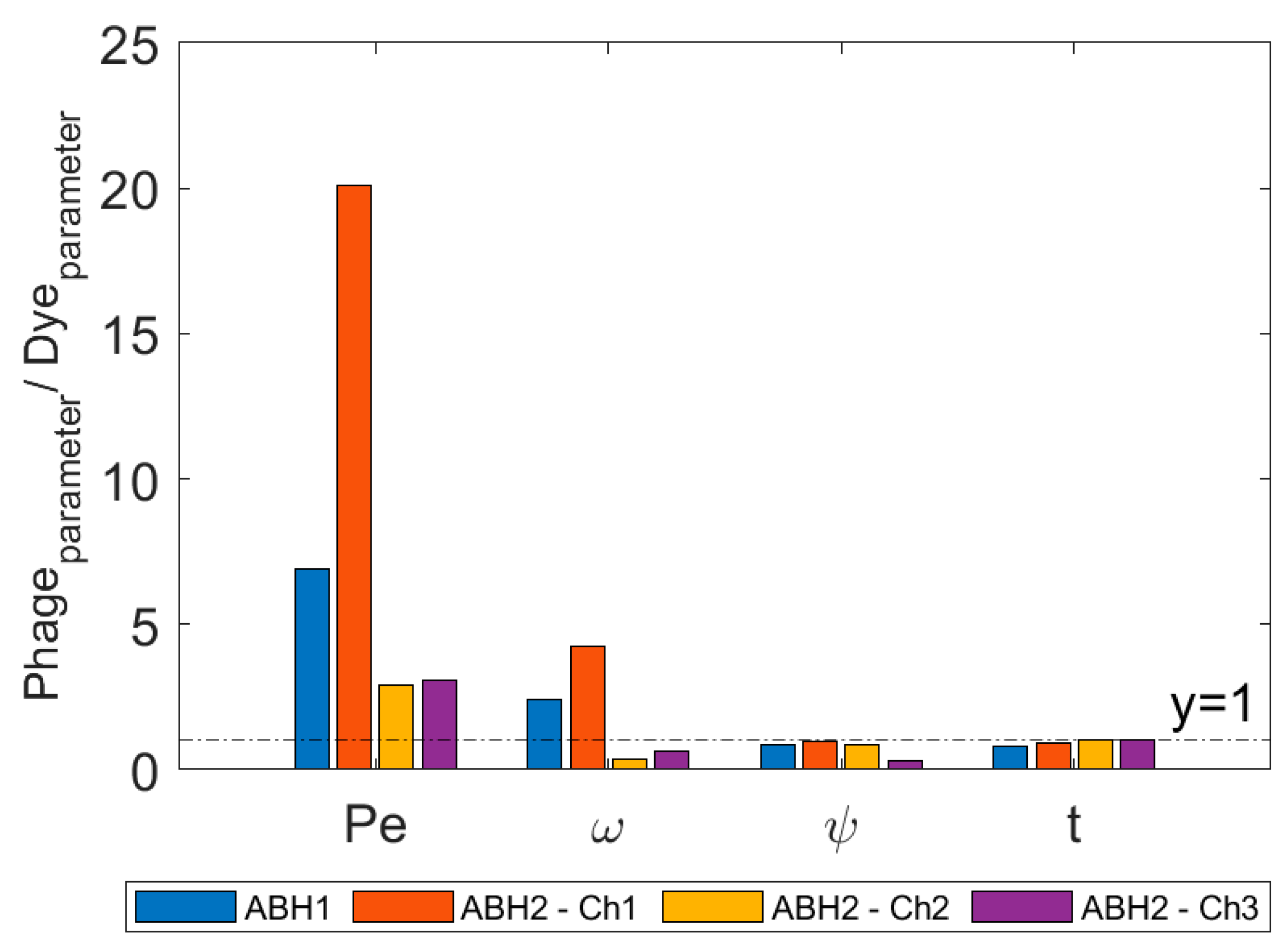
3.2.2. Transport Parameter Estimations
4. Discussion
4.1. Tracer Attenuation
4.2. Tracer Transport
4.3. MS2 Phage as a Tool for Karst Aquifer Vulnerability Assessments
5. Conclusions
- The attenuation of MS2 phage tracer is massive relative to the fluorescein dye tracer. The disparity is predominantly attributed to irreversible adsorption to immobile particles and decay. Phage attenuation is highly sensitive to the physicochemical conditions encountered along the flow path, and therefore, when applied as a tracer, the phage cannot provide quantitative information about aquifer vulnerability to solute contaminants.
- Transit times for MS2 phage tracer are consistently less than for fluorescein dye. This is attributed to the influence of exclusion processes in both the unsaturated zone and saturated zone. However, the transit time disparities are sufficiently small to render MS2 bacteriophage suitable for the characterisation of solute and groundwater transit times, and therefore for effective catchment delineation (e.g., source protection zone mapping).
- A comparison of tracer attenuation and transit times suggests the following: i) MS2 bacteriophage attenuation is concentrated in the unsaturated zone and limited when in transit at karstic-scale migration rates in the conduit network, and ii) phage migrates faster through the unsaturated zone than the saturated conduit network, relative to fluorescein dye.
- The application of transport models to tracer BTCs in comparative tests can provide a quantitative basis for comparison. In this study, parameter estimates suggest that the phage tracer experiences significantly less dispersion along the flow paths than the non-reactive solute tracer.
- Future field investigations could focus on improving understanding of the potential for bacteriophage attenuation in the unsaturated zone and along karst flow pathways in the saturated zone. Comparative tracer migration experiments (i) from karst-impacted observation boreholes to karst-impacted abstraction boreholes or springs, and (ii) from stream sinks to monitoring sites at the base of the unsaturated zone (e.g., a shallow observation borehole) would help to isolate relative attenuation information to each zone. These tests should be accompanied by a detailed characterisation of physicochemical conditions to help identify the dominant factors controlling virus and solute attenuation in karst. Associated laboratory studies could isolate the impact of site-specific conditions on tracer attenuation (e.g., particle composition, type and size, pH, organic matter quantity and quality and advective velocity).
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stevanović, Z. Karst Waters in Potable Water Supply: A Global Scale Overview. Environ. Earth Sci. 2019, 78, 662. [Google Scholar] [CrossRef]
- Atkinson, T.C.; Smith, D.I. Rapid Groundwater Flow in Fissures in the Chalk: An Example from South Hampshire. Q. J. Eng. Geol. 1974, 7, 197–205. [Google Scholar] [CrossRef]
- Doerfliger, N.; Jeannin, P.-Y.; Zwahlen, F. Water Vulnerability Assessment in Karst Environments: A New Method of Defining Protection Areas Using a Multi-Attribute Approach and GIS Tools (EPIK Method). Environ. Geol. 1999, 39, 165–176. [Google Scholar] [CrossRef]
- Mahler, B.; Massei, N. Anthropogenic Contaminants as Tracers in an Urbanizing Karst Aquifer. J. Contam. Hydrol. 2007, 91, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Goldscheider, N.; Drew, D. Methods in Karst Hydrogeology: IAH: International Contributions to Hydrogeology, 26; CRC Press: Boca Raton, FL, USA, 2014; ISBN 978-1-4822-6602-3. [Google Scholar]
- Sinreich, M.; Zwahlen, F. Feasibility of Contaminant Specific Karst Groundwater Vulnerability Assessment. In Karst and Environment; Fundacion Cueva de Nerja: Málaga, Spain, 2002; pp. 53–59. [Google Scholar]
- Sinreich, M. Towards Developing Conceptual Models for Reactive Contaminant Transport in Karst. IAHS-AISH Publ. 2011, 342, 473–476. [Google Scholar]
- Kass, W. Tracing Technique in Geohydrology; Routledge: London, UK, 1998; ISBN 978-1-351-40733-5. [Google Scholar]
- Goldscheider, N.; Meiman, J.; Pronk, M.; Smart, C. Tracer Tests in Karst Hydrogeology and Speleology. Int. J. Speleol. 2008, 37, 27–40. [Google Scholar] [CrossRef]
- Aldous, P.; Fawell, J. The Use of Fluorescein Sodium (Uranine) for Groundwater Tracing Investigations in Potable Water Supplies; WRc Environment, Medmenham Lab, Medmenham: Bucks, UK, 1986. [Google Scholar]
- Worthington, S.R.H.; Smart, C.C. Determination of Tracer Mass for Effective Groundwater Tracer Tests. Carbonates Evaporites 2016, 31, 349–356. [Google Scholar] [CrossRef]
- Bergh, Ø.; BØrsheim, K.Y.; Bratbak, G.; Heldal, M. High Abundance of Viruses Found in Aquatic Environments. Nature 1989, 340, 467–468. [Google Scholar] [CrossRef]
- Ashelford, K.; Martin, D.; Fry, J. Elevated Abundance of Bacteriophage Infecting Bacteria in Soil. Appl. Environ. Microbiol. 2003, 69, 285–289. [Google Scholar] [CrossRef]
- Rossi, P.; Dörfliger, N.; Kennedy, K.; Müller, I.; Aragno, M. Bacteriophages as Surface and Ground Water Tracers. Hydrol. Earth Syst. Sci. 1998, 2, 101–110. [Google Scholar] [CrossRef]
- Harvey, R.W.; Harms, H. Tracers in Groundwater: Use of Microorganisms and Microspheres. Encycl. Environ. Microbiol. 2002, 6, 3194–3202. [Google Scholar]
- Sinreich, M. Contaminant Attenuation in Karst Aquifers: A Paradigm Shift. In H2Karst Research in Limestone Hydrogeology; Springer: Cham, Switzerland, 2014; pp. 175–184. [Google Scholar]
- Massei, N.; Wang, H.Q.; Field, M.S.; Dupont, J.P.; Bakalowicz, M.; Rodet, J. Interpreting Tracer Breakthrough Tailing in a Conduit-Dominated Karstic Aquifer. Hydrogeol. J. 2006, 14, 849–858. [Google Scholar] [CrossRef]
- Foley, A.; Cachandt, G.; Franklin, J.; Willmore, F.; Atkinson, T. Tracer Tests and the Structure of Permeability in the Corallian Limestone Aquifer of Northern England, UK. Hydrogeol. J. 2012, 20, 483–498. [Google Scholar] [CrossRef]
- Rossi, P. Advances in Biological Trace Techniques for Hydrology and Hydrogeology Using Bacteriophages; University of Neuchatel: Neuchatel, Switzerland, 1994. [Google Scholar]
- Auckenthaler, A.; Raso, G.; Huggenberger, P. Particle Transport in a Karst Aquifer: Natural and Artificial Tracer Experiments with Bacteria, Bacteriophages and Microspheres. Water Sci. Technol. 2002, 46, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Flynn, R.M.; Sinreich, M. Characterisation of Virus Transport and Attenuation in Epikarst Using Short Pulse and Prolonged Injection Multi-Tracer Testing. Water Res. 2010, 44, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Maurice, L.; Atkinson, T.C.; Williams, A.T.; Barker, J.A.; Farrant, A.R. Catchment Scale Tracer Testing from Karstic Features in a Porous Limestone. J. Hydrol. 2010, 389, 31–41. [Google Scholar] [CrossRef]
- Goeppert, N.; Goldscheider, N. Solute and Colloid Transport in Karst Conduits under Low- and High-Flow Conditions. Groundwater 2008, 46, 61–68. [Google Scholar] [CrossRef]
- Schiperski, F.; Zirlewagen, J.; Scheytt, T. Transport and Attenuation of Particles of Different Density and Surface Charge: A Karst Aquifer Field Study. Environ. Sci. Technol. 2016, 50, 8028–8035. [Google Scholar] [CrossRef]
- Bales, R.C.; Gerba, C.P.; Grondin, G.H.; Jensen, S.L. Bacteriophage Transport in Sandy Soil and Fractured Tuff. Appl. Environ. Microbiol. 1989, 55, 2061–2067. [Google Scholar] [CrossRef]
- McKay, L.D.; Cherry, J.A.; Bales, R.C.; Yahya, M.T.; Gerba, C.P. A Field Example of Bacteriophage as Tracers of Fracture Flow. Environ. Sci. Technol. 1993, 27, 1075–1079. [Google Scholar] [CrossRef]
- Bales, R.C.; Li, S.; Yeh, T.-C.J.; Lenczewski, M.E.; Gerba, C.P. Bacteriophage and Microsphere Transport in Saturated Porous Media: Forced-Gradient Experiment at Borden, Ontario. Water Resour. Res. 1997, 33, 639–648. [Google Scholar] [CrossRef]
- Flynn, R.M.; Mallèn, G.; Engel, M.; Ahmed, A.; Rossi, P. Characterizing Aquifer Heterogeneity Using Bacterial and Bacteriophage Tracers. J. Environ. Qual. 2015, 44, 1448–1458. [Google Scholar] [CrossRef]
- Yao, K.-M.; Habibian, M.T.; O’Melia, C.R. Water and Waste Water Filtration. Concepts and Applications. Environ. Sci. Technol. 1971, 5, 1105–1112. [Google Scholar] [CrossRef]
- Yates, M.V.; Gerba, C.P.; Kelley, L.M. Virus Persistence in Groundwater. Appl. Environ. Microbiol. 1985, 49, 778–781. [Google Scholar] [CrossRef]
- Gordon, C.; Toze, S. Influence of Groundwater Characteristics on the Survival of Enteric Viruses. J. Appl. Microbiol. 2003, 95, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Formentin, K.; Rossi, P.; Aragno, M.; Müller, I. Determination of Bacteriophage Migration and Survival Potential in Karstic Groundwaters Using Batch Agitated Experiments and Mineral Colloidal Particles. In Tracer Hydrology 97; CRC Press: Boca Raton, FL, USA, 1997; ISBN 978-1-00-307814-2. [Google Scholar]
- Grant, S.B.; List, E.J.; Lidstrom, M.E. Kinetic Analysis of Virus Adsorption and Inactivation in Batch Experiments. Water Resour. Res. 1993, 29, 2067–2085. [Google Scholar] [CrossRef]
- Payment, P.; Morin, E.; Trudel, M. Coliphages and Enteric Viruses in the Particulate Phase of River Water. Can. J. Microbiol. 1988, 34, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Auset, M.; Keller, A.A. Pore-Scale Processes That Control Dispersion of Colloids in Saturated Porous Media: COLLOID DISPERSION AT PORE SCALE. Water Resour. Res. 2004, 40. [Google Scholar] [CrossRef]
- Keller, A.A.; Sirivithayapakorn, S.; Chrysikopoulos, C.V. Early Breakthrough of Colloids and Bacteriophage MS2 in a Water-Saturated Sand Column. Water Resour. Res. 2004, 40. [Google Scholar] [CrossRef]
- Goeppert, N.; Goldscheider, N. Improved Understanding of Particle Transport in Karst Groundwater Using Natural Sediments as Tracers. Water Res. 2019, 166, 115045. [Google Scholar] [CrossRef]
- Harold, H. Thirty-Second Annual Report on the Results of the Chemical and Bacteriological Examination of the London Waters for the Twelve Months Ended 31st December, 1937; P.S. King & Son, Ltd.: London, UK, 1937. [Google Scholar]
- Cook, S.J.; Fitzpatrick, C.M.; Burgess, W.G.; Lytton, L.; Bishop, P.; Monkhouse, R.A. Modelling the Influence of Solution-Enhanced Conduits on Catchment-Scale Contaminant Transport in the Hertfordshire Chalk Aquifer; Geological Society, London, Special Publications: London, UK, 2012; Volume 364, pp. 205–225. [Google Scholar] [CrossRef]
- Maurice, L.; Farrant, A.R.; Mathewson, E.; Atkinson, T. Karst Hydrogeology of the Chalk and Implications for Groundwater Protection; Geological Society, London, Special Publications: London, UK, 2022; Volume 517, pp. 39–62. [Google Scholar] [CrossRef]
- Allen, D.J.; Brewerton, L.J.; Coleby, L.M.; Gibbs, B.R.; Lewis, M.A.; MacDonald, A.M.; Wagstaff, S.J.; Williams, A.T. The Physical Properties of Major Aquifers in England and Wales; British Geological Survey: Nottingham, UK, 1997.
- Met Office UK Climate Averages. Available online: https://www.metoffice.gov.uk/research/climate/maps-and-data/uk-climate-averages/gcpwxz1ey (accessed on 6 February 2024).
- Maurice, L.D.; Atkinson, T.C.; Barker, J.A.; Bloomfield, J.P.; Farrant, A.R.; Williams, A.T. Karstic Behaviour of Groundwater in the English Chalk. J. Hydrol. 2006, 330, 63–70. [Google Scholar] [CrossRef]
- Farrant, A. Mapping of Karst Features and Identification of Preferential Pollutant Pathways; British Geological Survey: Nottingham, UK, 2017; p. 166.
- Farrant, A.R.; Maurice, L.; Ballesteros, D.; Nehme, C. The Genesis and Evolution of Karstic Conduit Systems in the Chalk; Geological Society, London, Special Publications: London, UK, 2023; Volume 517, pp. 15–37. [Google Scholar] [CrossRef]
- Adams, M. Bacteriophages; Interscience Publishers: New York, NY, USA, 1959. [Google Scholar]
- Purnell, S.E.; Ebdon, J.E.; Taylor, H.D. Bacteriophage Lysis of Enterococcus Host Strains: A Tool for Microbial Source Tracking? Environ. Sci. Technol. 2011, 45, 10699–10705. [Google Scholar] [CrossRef] [PubMed]
- Toride, N.; Leij, F.J.; van Genuchten, M.T. The CXTFIT Code for Estimating Transport Parameters from Laboratory or Field Tracer Experiments; US Salinity Laboratory: Riverside, CA, USA, 1993. [Google Scholar]
- Maloszewski, P.; Harum, T.; Benischke, R. Mathematical Modelling of Tracer Experiments in the Karst of Lurbach System. Beitr. Z. Hydrogeol. 1992, 43, 116–136. [Google Scholar]
- Watson, S.J. Solute Transport and Hydrodynamic Characteristics in the Chalk Aquifer at Tilmanstone, Kent; University of London, University College London (United Kingdom): London, UK, 2006. [Google Scholar]
- Cook, S.J. The Hydrogeology of Bromate Contamination in the Hertfordshire Chalk: Incorporating Karst in Predictive Models. Ph.D. Dissertation, University College London, London, UK, 2010. [Google Scholar]
- Bodin, J. MFIT 1.0.0: Multi-Flow Inversion of Tracer Breakthrough Curves in Fractured and Karst Aquifers. Geosci. Model Dev. 2020, 13, 2905–2924. [Google Scholar] [CrossRef]
- Maurice, L. Investigations of Rapid Groundwater Flow and Karst in the Chalk. Ph.D. Thesis, University College London, London, UK, 2009. [Google Scholar]
- CS Countryside Survey (CS) Topsoil Maps for Great Britain 2007. Available online: https://www.ukso.org/static-maps/countryside-survey-topsoil.html (accessed on 2 October 2023).
- Cranfield University. The Soils Guide 2023; Cranfield University: Cranfield, UK, 2023. [Google Scholar]
- Edmunds, W.M.; Darling, W.G.; Kinniburgh, D.G.; Dever, L.; Vachier, P. Chalk Groundwater in England and France: Hydrochemistry and Water Quality; British Geological Survey: Nottingham, UK, 1992.
- Bales, R.C.; Hinkle, S.R.; Kroeger, T.W.; Stocking, K.; Gerba, C.P. Bacteriophage Adsorption during Transport through Porous Media: Chemical Perturbations and Reversibility. Environ. Sci. Technol. 1991, 25, 2088–2095. [Google Scholar] [CrossRef]
- Walshe, G.E.; Pang, L.; Flury, M.; Close, M.E.; Flintoft, M. Effects of pH, Ionic Strength, Dissolved Organic Matter, and Flow Rate on the Co-Transport of MS2 Bacteriophages with Kaolinite in Gravel Aquifer Media. Water Res. 2010, 44, 1255–1269. [Google Scholar] [CrossRef] [PubMed]
- Schijven, J.F.; Hassanizadeh, S.M. Removal of Viruses by Soil Passage: Overview of Modeling, Processes, and Parameters. Crit. Rev. Environ. Sci. Technol. 2000, 30, 49–127. [Google Scholar] [CrossRef]
- Camesano, T.A.; Logan, B.E. Influence of Fluid Velocity and Cell Concentration on the Transport of Motile and Nonmotile Bacteria in Porous Media. Environ. Sci. Technol. 1998, 32, 1699–1708. [Google Scholar] [CrossRef]
- McKay, L.D.; Harton, A.D.; Wilson, G.V. Influence of Flow Rate on Transport of Bacteriophage in Shale Saprolite. J. Environ. Qual. 2002, 31, 1095–1105. [Google Scholar] [CrossRef]
- Collins, K.E.; Cronin, A.A.; Rueedi, J.; Pedley, S.; Joyce, E.; Humble, P.J.; Tellam, J.H. Fate and Transport of Bacteriophage in UK Aquifers as Surrogates for Pathogenic Viruses. Eng. Geol. 2006, 85, 33–38. [Google Scholar] [CrossRef]
- Shand, P.; Tyler-Whittle, R.; Besien, T.; Peach, D.W.; Lawrence, A.R.; Lewis, H.O.; Hargreaves, R.; Barron, M. Baseline Report Series: 6. The Chalk of the Colne and Lee River Catchments; British Geological Survey; Nottingham, UK; Environment Agency: Bristol, UK, 2003.
- Mahler, B.J.; Bennett, P.C.; Zimmerman, M. Lanthanide-Labeled Clay: A New Method for Tracing Sediment Transport in Karst. Groundwater 1998, 36, 835–843. [Google Scholar] [CrossRef]
- Massei, N.; Wang, H.Q.; Dupont, J.P.; Rodet, J.; Laignel, B. Assessment of Direct Transfer and Resuspension of Particles during Turbid Floods at a Karstic Spring. J. Hydrol. 2003, 275, 109–121. [Google Scholar] [CrossRef]
- Field, M.S.; Pinsky, P.F. A Two-Region Nonequilibrium Model for Solute Transport in Solution Conduits in Karstic Aquifers. J. Contam. Hydrol. 2000, 44, 329–351. [Google Scholar] [CrossRef]
- Duran, A.E.; Muniesa, M.; Mocé-Llivina, L.; Campos, C.; Jofre, J.; Lucena, F. Usefulness of Different Groups of Bacteriophages as Model Micro-organisms for Evaluating Chlorination. J. Appl. Microbiol. 2003, 95, 29–37. [Google Scholar] [CrossRef]
- Jofre, J.; Lucena, F.; Blanch, A.R.; Muniesa, M. Coliphages as Model Organisms in the Characterization and Management of Water Resources. Water 2016, 8, 199. [Google Scholar] [CrossRef]
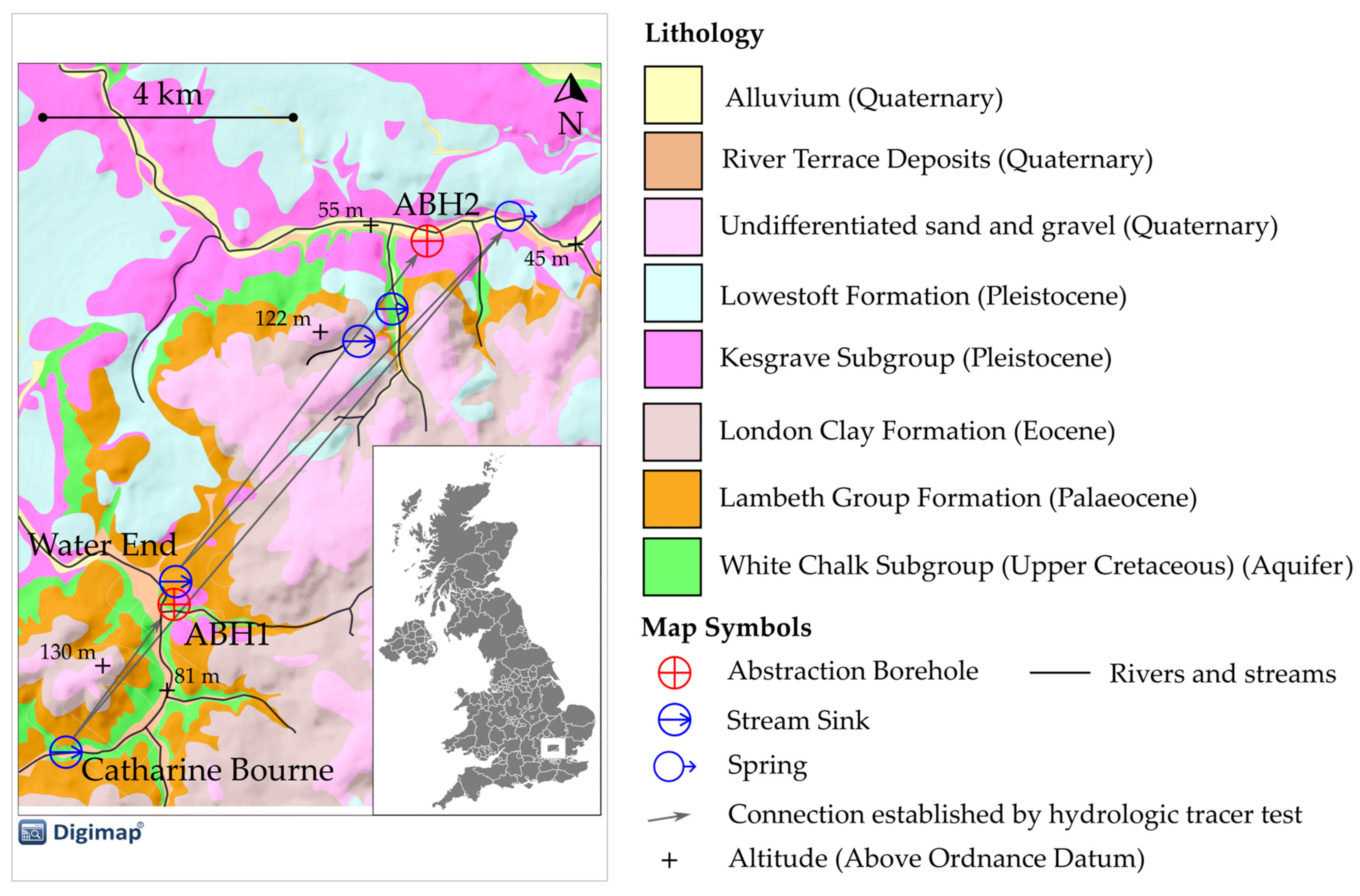
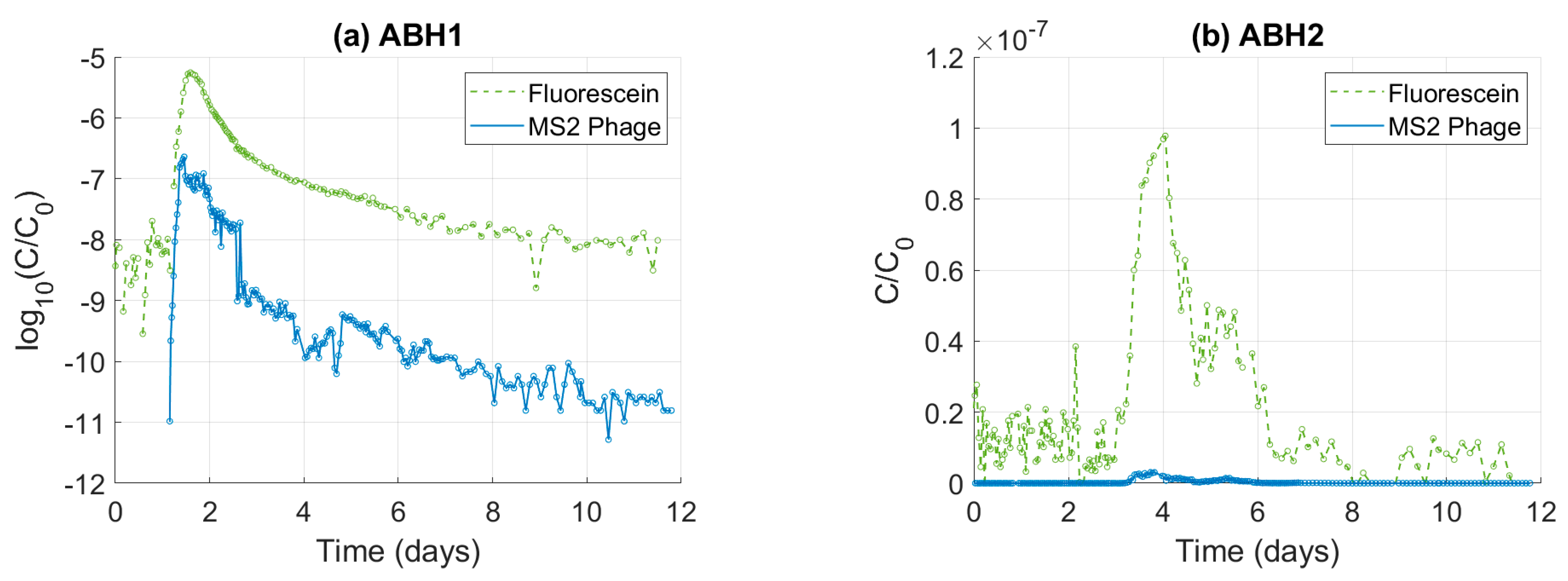
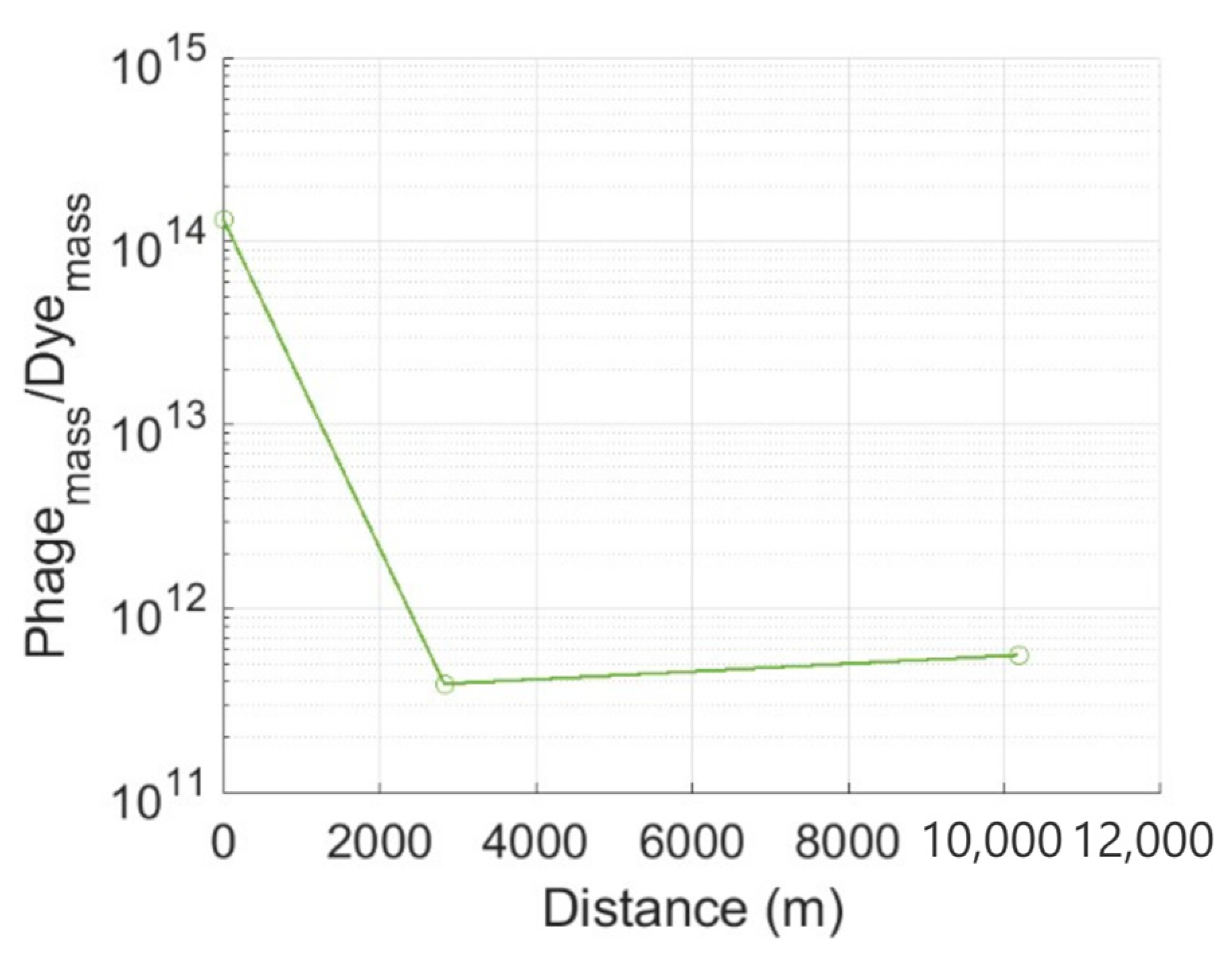
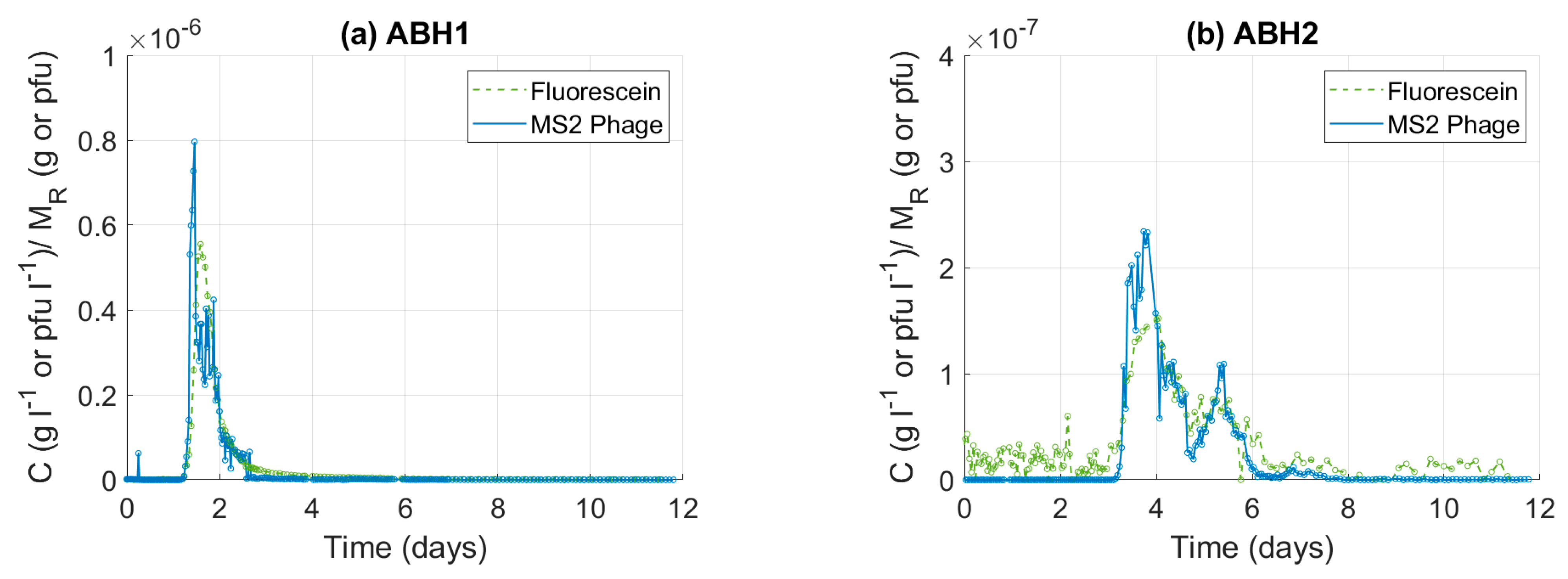

| Bedrock Geology | Test Type | l 4 (m) | Q 5 (l s−1) | Phage | RP 6 (%) | Solute | RS 7 (%) | Publication |
|---|---|---|---|---|---|---|---|---|
| Limestone | S-S 1 | 6250 | 1180 | H40/1 | 21 | SR-G 8 | 80 | Rossi [19] |
| Limestone | S-S | 6250 | 1180 | H6/1 | 1 | SR-G | 80 | Rossi [19] |
| Limestone | S-S | 6250 | 1180 | f1 | 0.15 | SR-G | 80 | Rossi [19] |
| Limestone | S-S | 6500 | 11,000 | H40/1 | 51 | Uranine | 56 | Rossi et al. [14] |
| Limestone | S-S | 6500 | 11,000 | H6/1 | 25 | Uranine | 56 | Rossi et al. [14] |
| Chalk | S-S | 5100 | 200 | ϕX174 | 0.87 | Uranine | 25 | Maurice et al. [22] |
| Limestone | T-S 2 | 1250 | 32 | H4 | 2.5 | Uranine | 23 | Auckenthaler et al. [20] |
| Limestone | T-S | 1250 | 32 | H40/1 | 2.5 | Uranine | 23 | Auckenthaler et al. [20] |
| Limestone (Epikarst) | Su-Tu 3 | 12 | Seepage | H40/1 | 84 | Iodide | 59 | Flynn and Sinreich [21] |
| Limestone (Epikarst) | Su-Tu | 12 | Seepage | T7 | 17 | Iodide | 59 | Flynn and Sinreich [21] |
| Parameter | DyeABH1 | PhageABH1 | DyeABH2 | PhageABH2 |
|---|---|---|---|---|
| ta 1 (h) | 29.8 | 27.7 | 79.3 | 75.7 |
| tm 2 (h) | 38.2 | 35 | 97.2 | 89.7 |
| r 3 (g/pfu 4) | 66.2 | 2.77 × 1013 | 2.25 | 1.26 × 1012 |
| r (%) | 189 * | 0.29 | 6.43 | 0.013 |
| Parameter | Channel | DyeABH1 | PhageABH1 | DyeABH2 | PhageABH2 |
|---|---|---|---|---|---|
| t (h) | 1 | 38.8 | 33.7 | 90 | 80.9 |
| Pe (-) | 1 | 250.1 | 1681.4 | 247.7 | 4974.3 |
| ψ (-) | 1 | 0.89 | 0.79 | 0.89 | 0.85 |
| ω (m−1) | 1 | 2.34 × 10−4 | 5.37 × 10−4 | 8.05 × 10−5 | 3.29 × 10−4 |
| t (h) | 2 | - | - | 130 | 129.6 |
| Pe (-) | 2 | - | - | 260 | 743.4 |
| ψ (-) | 2 | - | - | 0.9 | 0.76 |
| ω (m−1) | 2 | - | - | 3.99 × 10−5 | 1.6 × 10−5 |
| t (h) | 3 | - | - | 165 | 167.7 |
| Pe (-) | 3 | - | - | 25,803 | 799.6 |
| ψ (-) | 3 | - | - | 0.9 | 0.3 |
| ω (m−1) | 3 | - | - | 5.14 × 10−5 | 3.41 × 10−5 |
| r2 | - | 0.998 | 0.97 | 0.98 | 0.98 |
| Parameter | Phage vs. Dye | Possible Related Processes |
|---|---|---|
| Recovery | ↓↓↓ * | Irreversible adsorption Temperature- or adsorption-driven decay |
| Transit time | ↓ | Exclusion processes |
| Péclet number | ↑↑ | Fast transport limiting dispersion |
| First-order mass transfer | ↑↑ | Reversible adsorption Temporary settling in slow-flow zones |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matthews, D.; Bottrell, S.; West, L.J.; Maurice, L.; Farrant, A.; Purnell, S.; Coffey, D. Comparing the Fate and Transport of MS2 Bacteriophage and Sodium Fluorescein in a Karstic Chalk Aquifer. Pathogens 2024, 13, 168. https://doi.org/10.3390/pathogens13020168
Matthews D, Bottrell S, West LJ, Maurice L, Farrant A, Purnell S, Coffey D. Comparing the Fate and Transport of MS2 Bacteriophage and Sodium Fluorescein in a Karstic Chalk Aquifer. Pathogens. 2024; 13(2):168. https://doi.org/10.3390/pathogens13020168
Chicago/Turabian StyleMatthews, Daniel, Simon Bottrell, Landis Jared West, Louise Maurice, Andrew Farrant, Sarah Purnell, and Danny Coffey. 2024. "Comparing the Fate and Transport of MS2 Bacteriophage and Sodium Fluorescein in a Karstic Chalk Aquifer" Pathogens 13, no. 2: 168. https://doi.org/10.3390/pathogens13020168
APA StyleMatthews, D., Bottrell, S., West, L. J., Maurice, L., Farrant, A., Purnell, S., & Coffey, D. (2024). Comparing the Fate and Transport of MS2 Bacteriophage and Sodium Fluorescein in a Karstic Chalk Aquifer. Pathogens, 13(2), 168. https://doi.org/10.3390/pathogens13020168






