A Myeloid-Specific Lack of IL-4Rα Prevents the Development of Alternatively Activated Macrophages and Enhances Immunity to Experimental Cysticercosis
Abstract
1. Introduction
2. Materials and Methods
3. Results
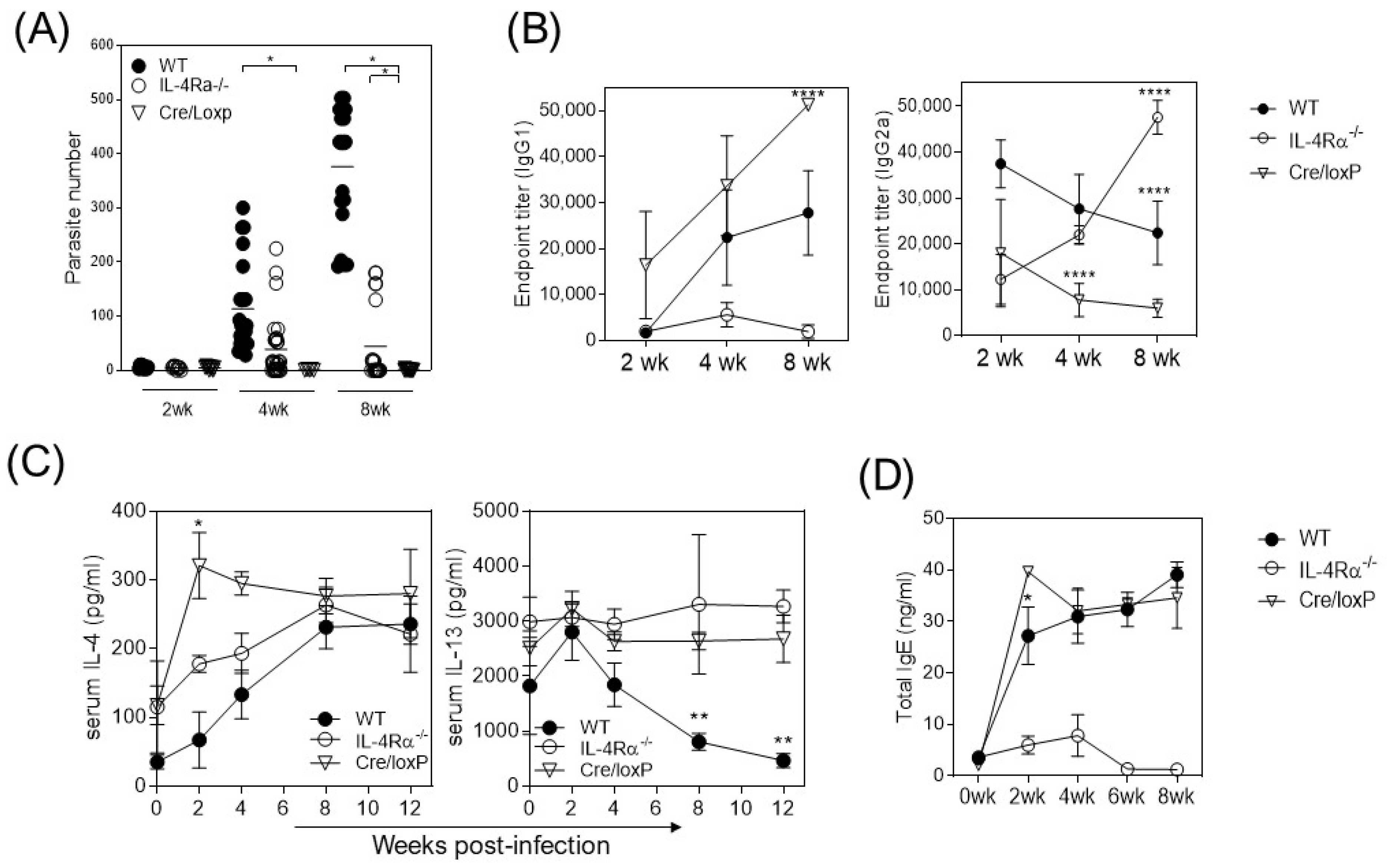
3.1. The Absence of IL-4Rα Favors Resistance to Cysticercosis
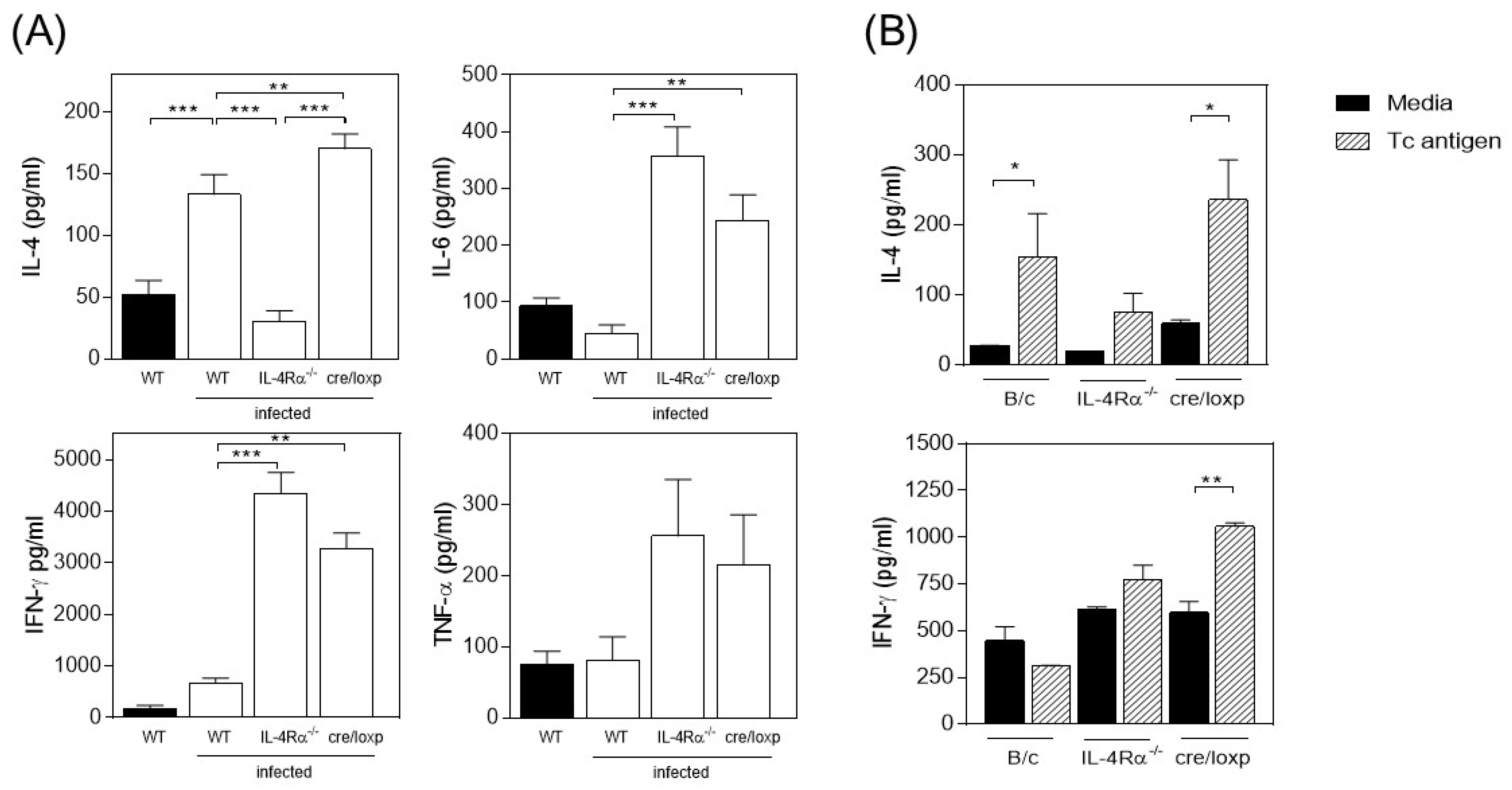
3.2. The Absence of Total IL-4Rα or the Myeloid Lineage-Specific Absence of IL-4Rα Favors IFN-γ and TNF-α Production in T. crassiceps-Infected Mice
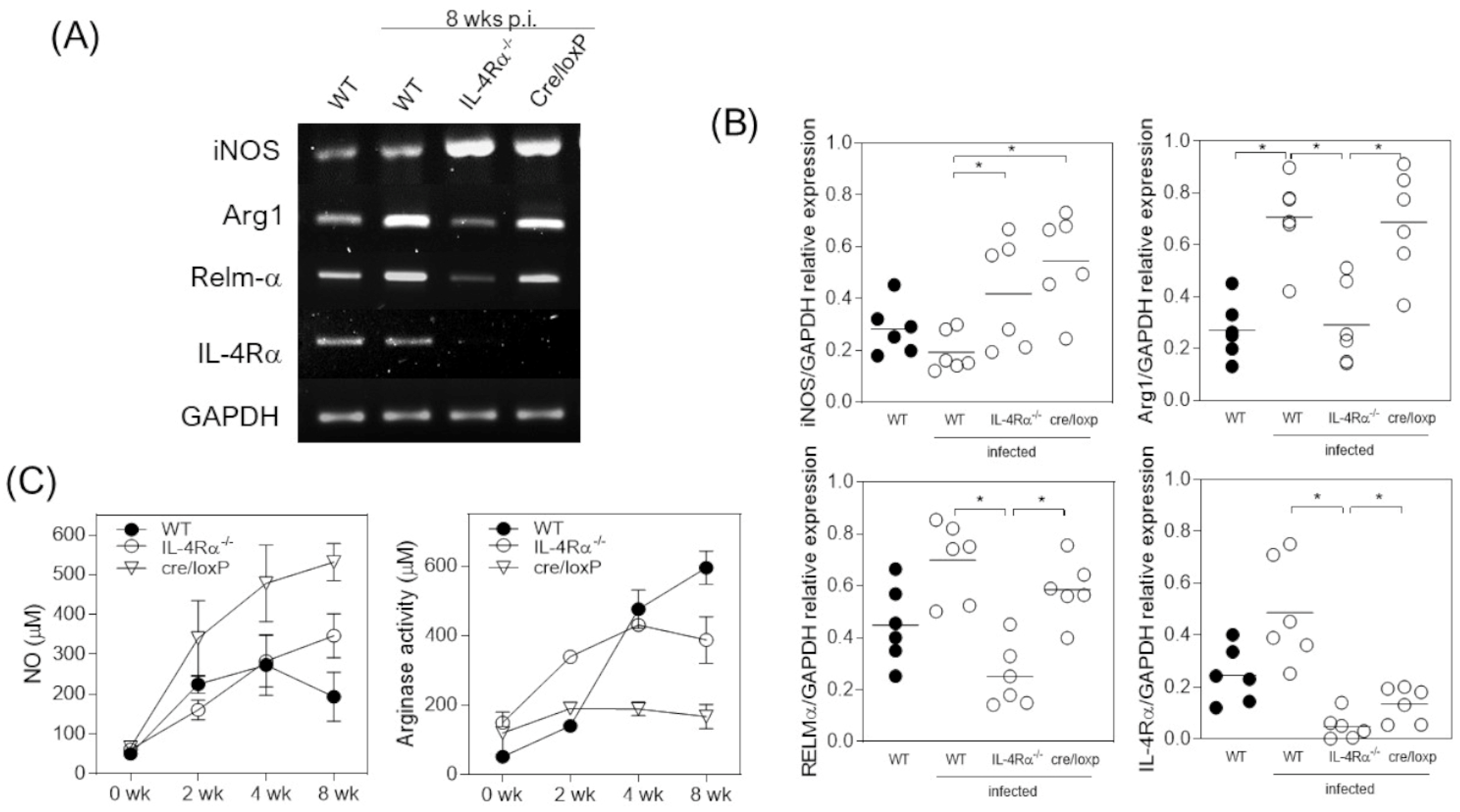
3.3. T. crassiceps-Infected cre/loxP Mice Maintain a Pro-Inflammatory Profile despite the Chronicity of the Infection
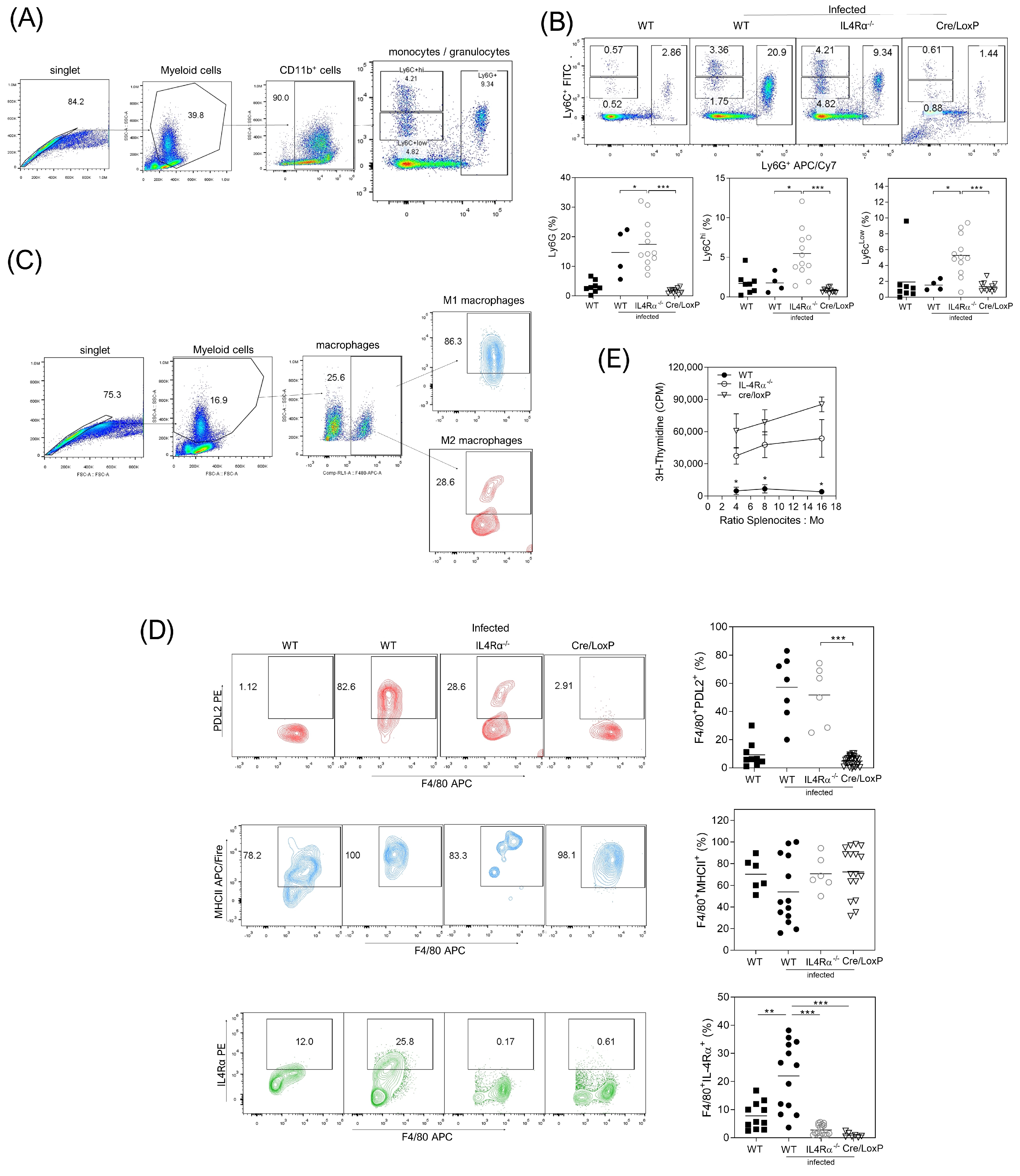
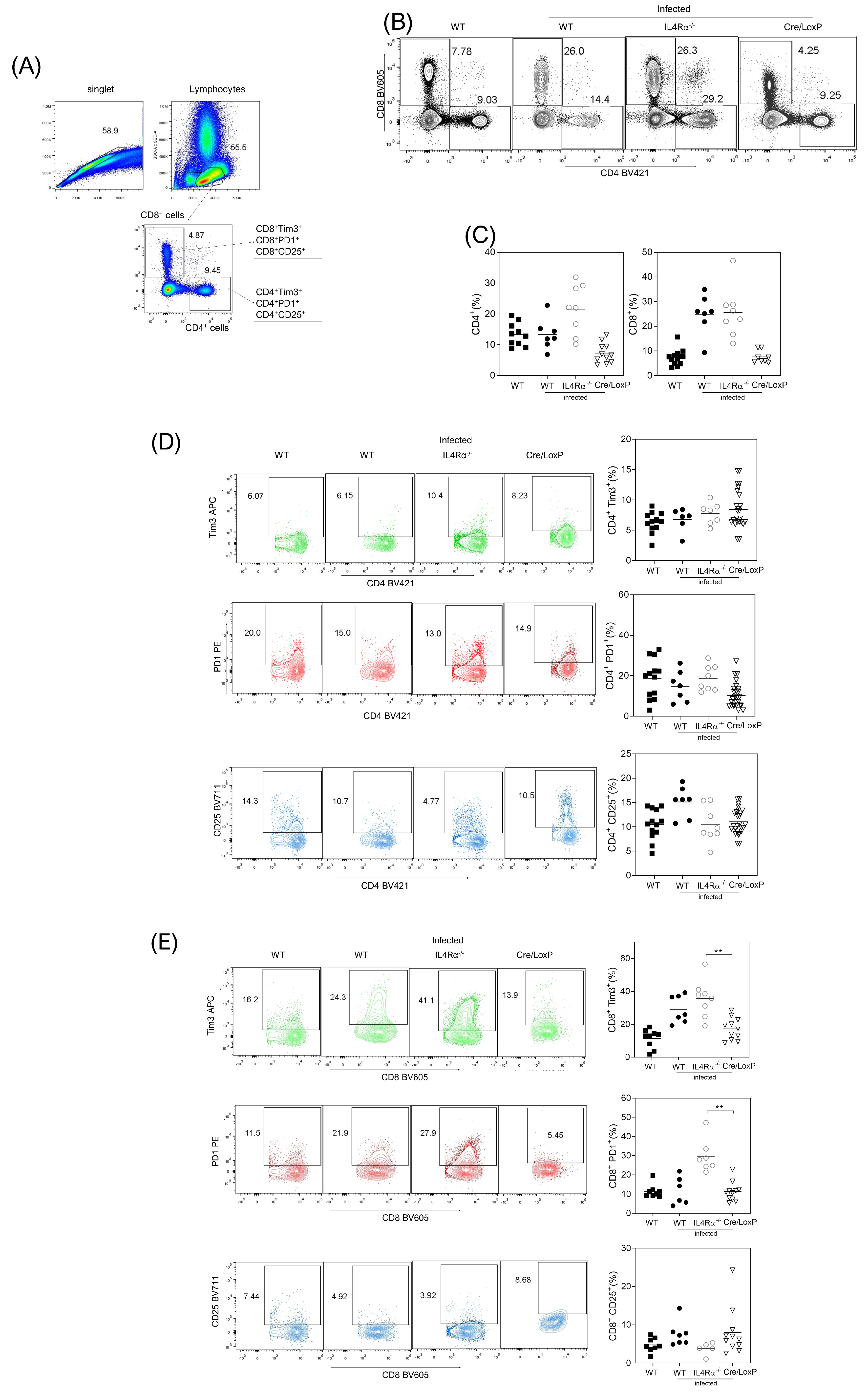
3.4. Cell Recruitment at the Site of Infection Is Altered in the Total or Cell Lineage-Specific Absence of IL-4Rα after T. crassiceps Infection
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Gazzinelli-Guimaraes, P.H.; Nutman, T.B. Helminth parasites and immune regulation. F1000Research 2018, 7, F1000 Faculty Rev-1685. [Google Scholar] [CrossRef]
- Maizels, R.M.; McSorley, H.J. Regulation of the host immune system by helminth parasites. J. Allergy Clin. Immunol. 2016, 138, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.H.; Tsai, K.W.; Wu, Y.J.; Liao, M.T.; Lu, K.C.; Hu, W.C. The Framework for Human Host Immune Responses to Four Types of Parasitic Infections and Relevant Key JAK/STAT Signaling. Int. J. Mol. Sci. 2021, 22, 13310. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, K.A.; Amici, S.A.; Webb, L.M.; Ruiz-Rosado Jde, D.; Popovich, P.G.; Partida-Sanchez, S.; Guerau-de-Arellano, M. Novel Markers to Delineate Murine M1 and M2 Macrophages. PLoS ONE 2015, 10, e0145342. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.G.; Du, Y.; Perrigoue, J.G.; Zaph, C.; Taylor, J.J.; Goldschmidt, M.; Swain, G.P.; Yancopoulos, G.D.; Valenzuela, D.M.; Murphy, A.; et al. Alternatively activated macrophage-derived RELM-alpha is a negative regulator of type 2 inflammation in the lung. J. Exp. Med. 2009, 206, 937–952. [Google Scholar] [CrossRef] [PubMed]
- Munder, M.; Eichmann, K.; Modolell, M. Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: Competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. J. Immunol. 1998, 160, 5347–5354. [Google Scholar] [CrossRef] [PubMed]
- Luzina, I.G.; Keegan, A.D.; Heller, N.M.; Rook, G.A.; Shea-Donohue, T.; Atamas, S.P. Regulation of inflammation by interleukin-4: A review of “alternatives”. J. Leukoc. Biol. 2012, 92, 753–764. [Google Scholar] [CrossRef]
- Nelms, K.; Keegan, A.D.; Zamorano, J.; Ryan, J.J.; Paul, W.E. The IL-4 receptor: Signaling mechanisms and biologic functions. Annu. Rev. Immunol. 1999, 17, 701–738. [Google Scholar] [CrossRef]
- Junttila, I.S. Tuning the Cytokine Responses: An Update on Interleukin (IL)-4 and IL-13 Receptor Complexes. Front. Immunol. 2018, 9, 888. [Google Scholar] [CrossRef]
- Finlay, C.M.; Walsh, K.P.; Mills, K.H. Induction of regulatory cells by helminth parasites: Exploitation for the treatment of inflammatory diseases. Immunol. Rev. 2014, 259, 206–230. [Google Scholar] [CrossRef] [PubMed]
- Terrazas, L.I.; Montero, D.; Terrazas, C.A.; Reyes, J.L.; Rodriguez-Sosa, M. Role of the programmed Death-1 pathway in the suppressive activity of alternatively activated macrophages in experimental cysticercosis. Int. J. Parasitol. 2005, 35, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Herbert, D.R.; Holscher, C.; Mohrs, M.; Arendse, B.; Schwegmann, A.; Radwanska, M.; Leeto, M.; Kirsch, R.; Hall, P.; Mossmann, H.; et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity 2004, 20, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Medina-Andrade, I.; Olguin, J.E.; Guerrero-Garcia, S.; Espinosa, J.A.; Garduno-Javier, E.; Hernandez-Gomez, V.; Vaca-Paniagua, F.; Rodriguez-Sosa, M.; Terrazas, L.I. Recruitment of M1 Macrophages May Not Be Critical for Protection against Colitis-Associated Tumorigenesis. Int. J. Mol. Sci. 2021, 22, 11204. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Diaz, M.; Ledesma-Soto, Y.; Olguin, J.E.; Sanchez-Barrera, A.; Mendoza-Rodriguez, M.G.; Reyes, S.; Satoskar, A.R.; Terrazas, L.I. STAT1-Dependent Recruitment of Ly6C(hi)CCR2(+) Inflammatory Monocytes and M2 Macrophages in a Helminth Infection. Pathogens 2021, 10, 1287. [Google Scholar] [CrossRef]
- Migliorini, P.; Corradin, G.; Corradin, S.B. Macrophage NO2− production as a sensitive and rapid assay for the quantitation of murine IFN-gamma. J. Immunol. Methods 1991, 139, 107–114. [Google Scholar] [CrossRef]
- Reyes, J.L.; Terrazas, C.A.; Vera-Arias, L.; Terrazas, L.I. Differential response of antigen presenting cells from susceptible and resistant strains of mice to Taenia crassiceps infection. Infect. Genet. Evol. 2009, 9, 1115–1127. [Google Scholar] [CrossRef]
- Lloyd, C.M.; Snelgrove, R.J. Type 2 immunity: Expanding our view. Sci. Immunol. 2018, 3, 1–11. [Google Scholar] [CrossRef]
- Vacca, F.; Le Gros, G. Tissue-specific immunity in helminth infections. Mucosal Immunol. 2022, 15, 1212–1223. [Google Scholar] [CrossRef]
- Peng, J.; Federman, H.G.; Hernandez, C.M.; Siracusa, M.C. Communication is key: Innate immune cells regulate host protection to helminths. Front. Immunol. 2022, 13, 995432. [Google Scholar] [CrossRef]
- Voehringer, D. Basophils in immune responses against helminths. Microbes Infect. 2011, 13, 881–887. [Google Scholar] [CrossRef]
- Walker, J.A.; McKenzie, A.N.J. T(H)2 cell development and function. Nat. Rev. Immunol. 2018, 18, 121–133. [Google Scholar] [CrossRef]
- Annunziato, F.; Romagnani, C.; Romagnani, S. The 3 major types of innate and adaptive cell-mediated effector immunity. J. Allergy Clin. Immunol. 2015, 135, 626–635. [Google Scholar] [CrossRef]
- Tanaka, S.; Furuta, K. Roles of IgE and Histamine in Mast Cell Maturation. Cells 2021, 10, 2170. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C.; Oeser, K.; Prazeres da Costa, C.; Layland, L.E.; Voehringer, D. T cell-derived IL-4/IL-13 protects mice against fatal Schistosoma mansoni infection independently of basophils. J. Immunol. 2014, 193, 3590–3599. [Google Scholar] [CrossRef] [PubMed]
- Obata-Ninomiya, K.; Ishiwata, K.; Tsutsui, H.; Nei, Y.; Yoshikawa, S.; Kawano, Y.; Minegishi, Y.; Ohta, N.; Watanabe, N.; Kanuka, H.; et al. The skin is an important bulwark of acquired immunity against intestinal helminths. J. Exp. Med. 2013, 210, 2583–2595. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Nagatake, T.; Saika, A.; Kawai, S.; Node, E.; Hosomi, K.; Kunisawa, J. Induction of unique macrophage subset by simultaneous stimulation with LPS and IL-4. Front. Immunol. 2023, 14, 1111729. [Google Scholar] [CrossRef] [PubMed]
- Strizova, Z.; Benesova, I.; Bartolini, R.; Novysedlak, R.; Cecrdlova, E.; Foley, L.K.; Striz, I. M1/M2 macrophages and their overlaps—Myth or reality? Clin. Sci. 2023, 137, 1067–1093. [Google Scholar] [CrossRef] [PubMed]
- Dzik, J.M. Evolutionary roots of arginase expression and regulation. Front. Immunol. 2014, 5, 544. [Google Scholar] [CrossRef] [PubMed]
- Dowling, J.K.; Afzal, R.; Gearing, L.J.; Cervantes-Silva, M.P.; Annett, S.; Davis, G.M.; De Santi, C.; Assmann, N.; Dettmer, K.; Gough, D.J.; et al. Mitochondrial arginase-2 is essential for IL-10 metabolic reprogramming of inflammatory macrophages. Nat. Commun. 2021, 12, 1460. [Google Scholar] [CrossRef]
- Wills-Karp, M.; Finkelman, F.D. Untangling the complex web of IL-4- and IL-13-mediated signaling pathways. Sci. Signal. 2008, 1, pe55. [Google Scholar] [CrossRef]
- Lee, C.M.; He, C.H.; Nour, A.M.; Zhou, Y.; Ma, B.; Park, J.W.; Kim, K.H.; Dela Cruz, C.; Sharma, L.; Nasr, M.L.; et al. IL-13Ralpha2 uses TMEM219 in chitinase 3-like-1-induced signalling and effector responses. Nat. Commun. 2016, 7, 12752. [Google Scholar] [CrossRef]
- Fichtner-Feigl, S.; Strober, W.; Kawakami, K.; Puri, R.K.; Kitani, A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat. Med. 2006, 12, 99–106. [Google Scholar] [CrossRef]
- Jaen, M.; Martin-Regalado, A.; Bartolome, R.A.; Robles, J.; Casal, J.I. Interleukin 13 receptor alpha 2 (IL13Ralpha2): Expression, signaling pathways and therapeutic applications in cancer. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188802. [Google Scholar] [CrossRef]
- Kitazawa, S.; Ross, F.P.; McHugh, K.; Teitelbaum, S.L. Interleukin-4 induces expression of the integrin alpha v beta 3 via transactivation of the beta 3 gene. J. Biol. Chem. 1995, 270, 4115–4120. [Google Scholar] [CrossRef] [PubMed]
- Szeto, A.C.H.; Ferreira, A.C.F.; Mannion, J.; Clark, P.A.; Sivasubramaniam, M.; Heycock, M.W.D.; Crisp, A.; Jolin, H.E.; Kozik, P.; Knolle, M.D.; et al. An alphavbeta3 integrin checkpoint is critical for efficient T(H)2 cell cytokine polarization and potentiation of antigen-specific immunity. Nat. Immunol. 2023, 24, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Aghbali, A.; Rafieyan, S.; Mohamed-Khosroshahi, L.; Baradaran, B.; Shanehbandi, D.; Kouhsoltani, M. IL-4 induces the formation of multinucleated giant cells and expression of beta5 integrin in central giant cell lesion. Med. Oral Patol. Oral Cir. Bucal 2017, 22, e1–e6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stafford, J.L.; Neumann, N.F.; Belosevic, M. Macrophage-mediated innate host defense against protozoan parasites. Crit. Rev. Microbiol. 2002, 28, 187–248. [Google Scholar] [CrossRef] [PubMed]
- Zhai, W.; Wu, F.; Zhang, Y.; Fu, Y.; Liu, Z. The Immune Escape Mechanisms of Mycobacterium Tuberculosis. Int. J. Mol. Sci. 2019, 20, 340. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, K.; Anagnostou, T.; Boussiotis, V.A. The PD1:PD-L1/2 Pathway from Discovery to Clinical Implementation. Front. Immunol. 2016, 7, 550. [Google Scholar] [CrossRef] [PubMed]
- Guasconi, L.; Chiapello, L.S.; Masih, D.T. Fasciola hepatica excretory-secretory products induce CD4+T cell anergy via selective up-regulation of PD-L2 expression on macrophages in a Dectin-1 dependent way. Immunobiology 2015, 220, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Sosa, M.; Satoskar, A.R.; Calderon, R.; Gomez-Garcia, L.; Saavedra, R.; Bojalil, R.; Terrazas, L.I. Chronic helminth infection induces alternatively activated macrophages expressing high levels of CCR5 with low interleukin-12 production and Th2-biasing ability. Infect. Immun. 2002, 70, 3656–3664. [Google Scholar] [CrossRef] [PubMed]
- Papasavvas, E.; Surrey, L.F.; Glencross, D.K.; Azzoni, L.; Joseph, J.; Omar, T.; Feldman, M.D.; Williamson, A.L.; Siminya, M.; Swarts, A.; et al. High-risk oncogenic HPV genotype infection associates with increased immune activation and T cell exhaustion in ART-suppressed HIV-1-infected women. Oncoimmunology 2016, 5, e1128612. [Google Scholar] [CrossRef]
- Lopez-Medina, M.; Carrillo-Martin, I.; Leyva-Rangel, J.; Alpuche-Aranda, C.; Ortiz-Navarrete, V. Salmonella impairs CD8 T cell response through PD-1: PD-L axis. Immunobiology 2015, 220, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J. T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine 2015, 75, 14–24. [Google Scholar] [CrossRef]
- Rodriguez-Sosa, M.; David, J.R.; Bojalil, R.; Satoskar, A.R.; Terrazas, L.I. Cutting edge: Susceptibility to the larval stage of the helminth parasite Taenia crassiceps is mediated by Th2 response induced via STAT6 signaling. J. Immunol. 2002, 168, 3135–3139. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olguín, J.E.; Corano-Arredondo, E.; Hernández-Gómez, V.; Rivera-Montoya, I.; Rodríguez, M.A.; Medina-Andrade, I.; Arendse, B.; Brombacher, F.; Terrazas, L.I. A Myeloid-Specific Lack of IL-4Rα Prevents the Development of Alternatively Activated Macrophages and Enhances Immunity to Experimental Cysticercosis. Pathogens 2024, 13, 169. https://doi.org/10.3390/pathogens13020169
Olguín JE, Corano-Arredondo E, Hernández-Gómez V, Rivera-Montoya I, Rodríguez MA, Medina-Andrade I, Arendse B, Brombacher F, Terrazas LI. A Myeloid-Specific Lack of IL-4Rα Prevents the Development of Alternatively Activated Macrophages and Enhances Immunity to Experimental Cysticercosis. Pathogens. 2024; 13(2):169. https://doi.org/10.3390/pathogens13020169
Chicago/Turabian StyleOlguín, Jonadab E., Edmundo Corano-Arredondo, Victoria Hernández-Gómez, Irma Rivera-Montoya, Mario A. Rodríguez, Itzel Medina-Andrade, Berenice Arendse, Frank Brombacher, and Luis I. Terrazas. 2024. "A Myeloid-Specific Lack of IL-4Rα Prevents the Development of Alternatively Activated Macrophages and Enhances Immunity to Experimental Cysticercosis" Pathogens 13, no. 2: 169. https://doi.org/10.3390/pathogens13020169
APA StyleOlguín, J. E., Corano-Arredondo, E., Hernández-Gómez, V., Rivera-Montoya, I., Rodríguez, M. A., Medina-Andrade, I., Arendse, B., Brombacher, F., & Terrazas, L. I. (2024). A Myeloid-Specific Lack of IL-4Rα Prevents the Development of Alternatively Activated Macrophages and Enhances Immunity to Experimental Cysticercosis. Pathogens, 13(2), 169. https://doi.org/10.3390/pathogens13020169









