Molecular Mechanisms of Drug Resistance in Leishmania spp.
Abstract
1. Introduction
2. Available Treatments against Leishmaniasis
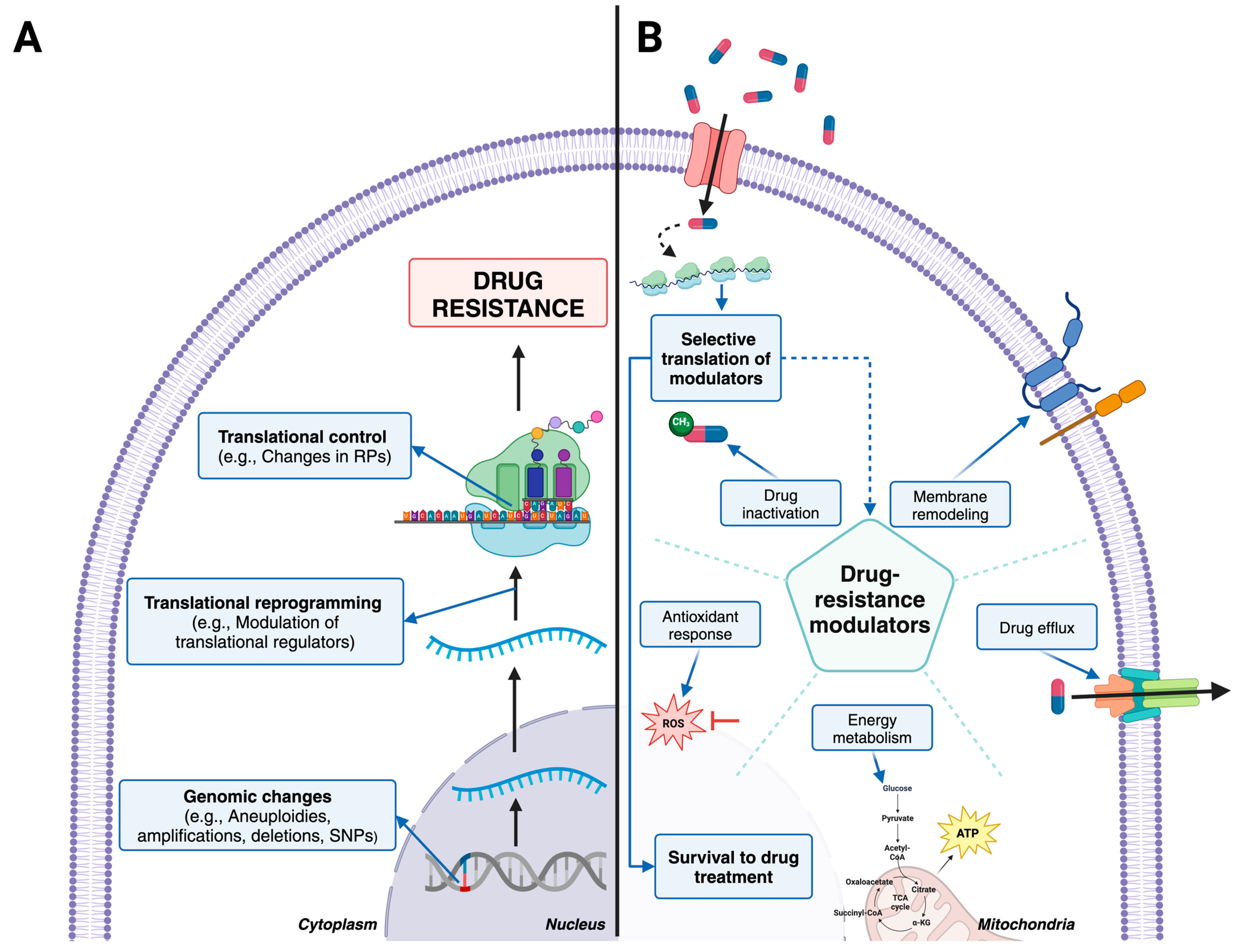
3. Genomic Changes and Drug Resistance
4. Changes in Transcriptomes Associated with Drug Resistance
5. Translational Control as a Major Driver of Drug Resistance
6. Changes in Metabolomes and Lipidomes Associated with Drug Resistance
7. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| aa-tRNA | Aminoacyl transfer ribonucleic acid |
| ABC | ATP binding cassette |
| AmB | Amphotericin B |
| APX | Ascorbate Peroxidase |
| AQP1 | Aquaglyceroporin 1 |
| ATP | Adenosine triphosphate |
| BCAT | Branched-chain amino acids |
| CDP | Cytidine-5’-diphosphate |
| CNV | Copy number variation |
| CTP | Cytidine-5’-triphosphate |
| CDPK1 | Calcium dependent protein kinase 1 |
| CL | Cutaneous Leishmaniasis |
| CRISPR-Cas9 | Clustered Regularly Interspaced Short Palindromic Repeats-Protein 9 |
| CYP51 | Sterol 14-demethylase |
| D-LDH | D-lactate dehydrogenase-like protein |
| DNA | Deoxyribonucleic Acid |
| DEGs | Differentially expressed genes |
| FDA | Food and Drug administration |
| G6PDH | Glucose-6-Phosphate Dehydrogenase |
| HAPT1 | High affinity pentamidine transporter |
| HIV | Human immunodeficiency virus |
| JAK-STAT | Janus kinase/signal transducers and activators of transcription |
| LMT | Miltefosine Transporter gene |
| MCL | Muco-Cutaneous Leishmaniasis |
| MDR1 | Multi-drug resistance 1 |
| MLT | Miltefosine |
| mRNA | Messenger ribonucleic acid |
| MRPA | Multidrug-resistance protein A |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NTD | Neglected tropical disease |
| PC | Phosphatidylcholines |
| PE | Phosphatidylethanolamines |
| PL | Phospholipids |
| PMM | Paromomycin |
| PRP1 | Pentamidine resistance protein 1 |
| PTM | Pentamidine |
| PTUs | Polycistronic transcription units |
| PDR-1 | Pectin degradation regulator-1 |
| RBP | RNA Binding protein |
| RBPs | RNA-binding proteins |
| RNAP II | RNA polymerase II |
| RPP | Ribosomal protection protein |
| rRNA | Ribosomal ribonucleic acid |
| SbV | Pentavalent antimony |
| SC5D | Sterol C5 -desaturase |
| SMT | 24-sterol methyltransferase |
| SNPs | Single nucleotide polymorphisms |
| VL | Visceral Leishmaniasis |
References
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 481–511. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial Resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef]
- Boyce, K.J. The Microevolution of Antifungal Drug Resistance in Pathogenic Fungi. Microorganisms 2023, 11, 2757. [Google Scholar] [CrossRef]
- Kanaujia, R.; Singh, S.; Rudramurthy, S.M. Aspergillosis: An Update on Clinical Spectrum, Diagnostic Schemes, and Management. Curr. Fungal Infect. Rep. 2023, 17, 144–155. [Google Scholar] [CrossRef]
- Cohen, N.R.; Lobritz, M.A.; Collins, J.J. Microbial persistence and the road to drug resistance. Cell Host Microbe 2013, 13, 632–642. [Google Scholar] [CrossRef]
- Tarannum, A.; Rodriguez-Almonacid, C.C.; Salazar-Bravo, J.; Karamysheva, Z.N. Molecular Mechanisms of Persistence in Protozoan Parasites. Microorganisms 2023, 11, 2248. [Google Scholar] [CrossRef]
- Peraman, R.; Sure, S.K.; Dusthackeer, V.N.A.; Chilamakuru, N.B.; Yiragamreddy, P.R.; Pokuri, C.; Kutagulla, V.K.; Chinni, S. Insights on recent approaches in drug discovery strategies and untapped drug targets against drug resistance. Future J. Pharm. Sci. 2021, 7, 56. [Google Scholar] [CrossRef]
- Vitiello, A.; Ferrara, F.; Boccellino, M.; Ponzo, A.; Cimmino, C.; Comberiati, E.; Zovi, A.; Clemente, S.; Sabbatucci, M. Antifungal Drug Resistance: An Emergent Health Threat. Biomedicines 2023, 11, 1063. [Google Scholar] [CrossRef]
- Gaurav, A.; Bakht, P.; Saini, M.; Pandey, S.; Pathania, R. Role of bacterial efflux pumps in antibiotic resistance, virulence, and strategies to discover novel efflux pump inhibitors. Microbiology 2023, 169, 001333. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, V.K.; Pathania, R. Efflux pump inhibitors for bacterial pathogens: From bench to bedside. Indian J. Med. Res. 2019, 149, 129–145. [Google Scholar] [CrossRef]
- Christaki, E.; Marcou, M.; Tofarides, A. Antimicrobial Resistance in Bacteria: Mechanisms, Evolution, and Persistence. J. Mol. Evol. 2020, 88, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Alastruey-Izquierdo, A.; Berman, J.; Bicanic, T.; Bignell, E.M.; Bowyer, P.; Bromley, M.; Bruggemann, R.; Garber, G.; Cornely, O.A.; et al. Tackling the emerging threat of antifungal resistance to human health. Nat. Rev. Microbiol. 2022, 20, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Iwamoto, H.; Abe, M.; Yang, Y.; Cui, D.; Seki, T.; Nakamura, M.; Hosaka, K.; Lim, S.; Wu, J.; He, X.; et al. Cancer Lipid Metabolism Confers Antiangiogenic Drug Resistance. Cell Metab. 2018, 28, 104–117.e5. [Google Scholar] [CrossRef]
- Khan, S.U.; Fatima, K.; Aisha, S.; Malik, F. Unveiling the mechanisms and challenges of cancer drug resistance. Cell Commun. Signal 2024, 22, 109. [Google Scholar] [CrossRef]
- Schiliro, C.; Firestein, B.L. Mechanisms of Metabolic Reprogramming in Cancer Cells Supporting Enhanced Growth and Proliferation. Cells 2021, 10, 1056. [Google Scholar] [CrossRef]
- Kusnadi, E.P.; Trigos, A.S.; Cullinane, C.; Goode, D.L.; Larsson, O.; Devlin, J.R.; Chan, K.T.; De Souza, D.P.; McConville, M.J.; McArthur, G.A.; et al. Reprogrammed mRNA translation drives resistance to therapeutic targeting of ribosome biogenesis. EMBO J. 2020, 39, e105111. [Google Scholar] [CrossRef]
- Munday, J.C.; Settimo, L.; de Koning, H.P. Transport proteins determine drug sensitivity and resistance in a protozoan parasite, Trypanosoma brucei. Front. Pharmacol. 2015, 6, 32. [Google Scholar] [CrossRef]
- Karamysheva, Z.N.; Gutierrez Guarnizo, S.A.; Karamyshev, A.L. Regulation of Translation in the Protozoan Parasite Leishmania. Int. J. Mol. Sci. 2020, 21, 2981. [Google Scholar] [CrossRef] [PubMed]
- Maltezou, H.C. Drug resistance in visceral leishmaniasis. J. Biomed. Biotechnol. 2010, 2010, 617521. [Google Scholar] [CrossRef] [PubMed]
- Marquis, N.; Gourbal, B.; Rosen, B.P.; Mukhopadhyay, R.; Ouellette, M. Modulation in aquaglyceroporin AQP1 gene transcript levels in drug-resistant Leishmania. Mol. Microbiol. 2005, 57, 1690–1699. [Google Scholar] [CrossRef] [PubMed]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.C.; Barrett, M.P.; Lopez-Velez, R.; Garcia-Hernandez, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Negl. Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef]
- Légaré, D.; Ouellette, M. Drug Resistance in Leishmania. In Handbook of Antimicrobial Resistance; Springer: New York, NY, USA, 2014. [Google Scholar]
- Darby, E.M.; Trampari, E.; Siasat, P.; Gaya, M.S.; Alav, I.; Webber, M.A.; Blair, J.M.A. Molecular mechanisms of antibiotic resistance revisited. Nat. Rev. Microbiol. 2023, 21, 280–295. [Google Scholar] [CrossRef]
- Kamran, M.; Bhattacharjee, R.; Das, S.; Mukherjee, S.; Ali, N. The paradigm of intracellular parasite survival and drug resistance in leishmanial parasite through genome plasticity and epigenetics: Perception and future perspective. Front. Cell Infect. Microbiol. 2023, 13, 1001973. [Google Scholar] [CrossRef]
- Gutierrez Guarnizo, S.A.; Tikhonova, E.B.; Karamyshev, A.L.; Muskus, C.E.; Karamysheva, Z.N. Translational reprogramming as a driver of antimony-drug resistance in Leishmania. Nat. Commun. 2023, 14, 2605. [Google Scholar] [CrossRef]
- Shaw, C.D.; Lonchamp, J.; Downing, T.; Imamura, H.; Freeman, T.M.; Cotton, J.A.; Sanders, M.; Blackburn, G.; Dujardin, J.C.; Rijal, S.; et al. In vitro selection of miltefosine resistance in promastigotes of Leishmania donovani from Nepal: Genomic and metabolomic characterization. Mol. Microbiol. 2016, 99, 1134–1148. [Google Scholar] [CrossRef]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Shmueli, M.; Ben-Shimol, S. Review of Leishmaniasis Treatment: Can We See the Forest through the Trees? Pharmacy 2024, 12, 30. [Google Scholar] [CrossRef]
- Aronson, N.E.; Joya, C.A. Cutaneous Leishmaniasis: Updates in Diagnosis and Management. Infect. Dis. Clin. N. Am. 2019, 33, 101–117. [Google Scholar] [CrossRef] [PubMed]
- CDC. Clinical Care of Leishmaniasis. Available online: https://www.cdc.gov/leishmaniasis/hcp/clinical-care/index.html (accessed on 4 September 2024).
- Maarouf, M.; de Kouchkovsky, Y.; Brown, S.; Petit, P.X.; Robert-Gero, M. In vivo interference of paromomycin with mitochondrial activity of Leishmania. Exp. Cell Res. 1997, 232, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Bharadava, K.; Upadhyay, T.K.; Kaushal, R.S.; Ahmad, I.; Alraey, Y.; Siddiqui, S.; Saeed, M. Genomic Insight of Leishmania Parasite: In-Depth Review of Drug Resistance Mechanisms and Genetic Mutations. ACS Omega 2024, 9, 12500–12514. [Google Scholar] [CrossRef] [PubMed]
- Faris, R.M.; Jarallah, J.S.; Khoja, T.A.; al-Yamani, M.J. Intralesional treatment of cutaneous leishmaniasis with sodium stibogluconate antimony. Int. J. Dermatol. 1993, 32, 610–612. [Google Scholar] [CrossRef]
- Solomon, M.; Baum, S.; Barzilai, A.; Pavlotsky, F.; Trau, H.; Schwartz, E. Treatment of cutaneous leishmaniasis with intralesional sodium stibogluconate. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 1189–1192. [Google Scholar] [CrossRef]
- Herwaldt, B.L.; Berman, J.D. Recommendations for treating leishmaniasis with sodium stibogluconate (Pentostam) and review of pertinent clinical studies. Am. J. Trop. Med. Hyg. 1992, 46, 296–306. [Google Scholar] [CrossRef]
- Heleine, M.; Elenga, N.; Njuieyon, F.; Martin, E.; Piat, C.; Pansart, C.; Couppie, P.; Hernandez, M.; Demar, M.; Blaizot, R. Using pentamidine to treat cutaneous leishmaniasis in children: A 10-year study in French Guiana. Clin. Exp. Dermatol. 2023, 48, 913–915. [Google Scholar] [CrossRef]
- Lai, A.F.E.J.; Vrede, M.A.; Soetosenojo, R.M.; Lai, A.F.R.F. Pentamidine, the drug of choice for the treatment of cutaneous leishmaniasis in Surinam. Int. J. Dermatol. 2002, 41, 796–800. [Google Scholar] [CrossRef]
- Piccica, M.; Lagi, F.; Bartoloni, A.; Zammarchi, L. Efficacy and safety of pentamidine isethionate for tegumentary and visceral human leishmaniasis: A systematic review. J. Travel. Med. 2021, 28, taab065. [Google Scholar] [CrossRef]
- Pokharel, P.; Ghimire, R.; Lamichhane, P. Efficacy and Safety of Paromomycin for Visceral Leishmaniasis: A Systematic Review. J. Trop. Med. 2021, 2021, 8629039. [Google Scholar] [CrossRef]
- Soto, J.; Soto, P.; Ajata, A.; Luque, C.; Tintaya, C.; Paz, D.; Rivero, D.; Berman, J. Topical 15% Paromomycin-Aquaphilic for Bolivian Leishmania braziliensis Cutaneous Leishmaniasis: A Randomized, Placebo-controlled Trial. Clin. Infect. Dis. 2019, 68, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Sosa, N.; Pascale, J.M.; Jimenez, A.I.; Norwood, J.A.; Kreishman-Detrick, M.; Weina, P.J.; Lawrence, K.; McCarthy, W.F.; Adams, R.C.; Scott, C.; et al. Topical paromomycin for New World cutaneous leishmaniasis. PLoS Negl. Trop. Dis. 2019, 13, e0007253. [Google Scholar] [CrossRef] [PubMed]
- Soto, J.; Toledo, J.; Gutierrez, P.; Nicholls, R.S.; Padilla, J.; Engel, J.; Fischer, C.; Voss, A.; Berman, J. Treatment of American cutaneous leishmaniasis with miltefosine, an oral agent. Clin. Infect. Dis. 2001, 33, E57–E61. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Report of a WHO Informal Consultation on Liposomal Amphotericin B in the Treatment of Visceral Leishmaniasis; WHO/CDS/NTD/IDM/2007.4; WHO: Geneva, Switzerland, 16 April 2005. [Google Scholar]
- Frezard, F.; Aguiar, M.M.G.; Ferreira, L.A.M.; Ramos, G.S.; Santos, T.T.; Borges, G.S.M.; Vallejos, V.M.R.; De Morais, H.L.O. Liposomal Amphotericin B for Treatment of Leishmaniasis: From the Identification of Critical Physicochemical Attributes to the Design of Effective Topical and Oral Formulations. Pharmaceutics 2022, 15, 99. [Google Scholar] [CrossRef]
- Battista, T.; Colotti, G.; Ilari, A.; Fiorillo, A. Targeting Trypanothione Reductase, a Key Enzyme in the Redox Trypanosomatid Metabolism, to Develop New Drugs against Leishmaniasis and Trypanosomiases. Molecules 2020, 25, 1924. [Google Scholar] [CrossRef]
- Kumari, D.; Perveen, S.; Sharma, R.; Singh, K. Advancement in leishmaniasis diagnosis and therapeutics: An update. Eur. J. Pharmacol. 2021, 910, 174436. [Google Scholar] [CrossRef]
- Pinto-Martinez, A.K.; Rodriguez-Duran, J.; Serrano-Martin, X.; Hernandez-Rodriguez, V.; Benaim, G. Mechanism of Action of Miltefosine on Leishmania donovani Involves the Impairment of Acidocalcisome Function and the Activation of the Sphingosine-Dependent Plasma Membrane Ca2+ Channel. Antimicrob. Agents Chemother. 2018, 62, 1. [Google Scholar] [CrossRef]
- Rakotomanga, M.; Blanc, S.; Gaudin, K.; Chaminade, P.; Loiseau, P.M. Miltefosine affects lipid metabolism in Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 2007, 51, 1425–1430. [Google Scholar] [CrossRef]
- Shirzadi, M.R. Lipsosomal amphotericin B: A review of its properties, function, and use for treatment of cutaneous leishmaniasis. Res. Rep. Trop. Med. 2019, 10, 11–18. [Google Scholar] [CrossRef]
- Stone, N.R.; Bicanic, T.; Salim, R.; Hope, W. Liposomal Amphotericin B (AmBisome((R))): A Review of the Pharmacokinetics, Pharmacodynamics, Clinical Experience and Future Directions. Drugs 2016, 76, 485–500. [Google Scholar] [CrossRef]
- Shalev-Benami, M.; Zhang, Y.; Rozenberg, H.; Nobe, Y.; Taoka, M.; Matzov, D.; Zimmerman, E.; Bashan, A.; Isobe, T.; Jaffe, C.L.; et al. Atomic resolution snapshot of Leishmania ribosome inhibition by the aminoglycoside paromomycin. Nat. Commun. 2017, 8, 1589. [Google Scholar] [CrossRef] [PubMed]
- Capela, R.; Moreira, R.; Lopes, F. An Overview of Drug Resistance in Protozoal Diseases. Int. J. Mol. Sci. 2019, 20, 5748. [Google Scholar] [CrossRef] [PubMed]
- Chawla, B.; Jhingran, A.; Panigrahi, A.; Stuart, K.D.; Madhubala, R. Paromomycin affects translation and vesicle-mediated trafficking as revealed by proteomics of paromomycin -susceptible -resistant Leishmania donovani. PLoS ONE 2011, 6, e26660. [Google Scholar] [CrossRef]
- Nguewa, P.A.; Fuertes, M.A.; Cepeda, V.; Iborra, S.; Carrion, J.; Valladares, B.; Alonso, C.; Perez, J.M. Pentamidine is an antiparasitic and apoptotic drug that selectively modifies ubiquitin. Chem. Biodivers. 2005, 2, 1387–1400. [Google Scholar] [CrossRef]
- Wong, I.L.; Chan, K.F.; Zhao, Y.; Chan, T.H.; Chow, L.M. Quinacrine and a novel apigenin dimer can synergistically increase the pentamidine susceptibility of the protozoan parasite Leishmania. J. Antimicrob. Chemother. 2009, 63, 1179–1190. [Google Scholar] [CrossRef]
- Maheshwari, A.; Seth, A.; Kaur, S.; Aneja, S.; Rath, B.; Basu, S.; Patel, R.; Dutta, A.K. Cumulative cardiac toxicity of Sodium Stibogluconate and Amphotericin B in treatment of Kala-Azar. Pediatr. Infect. Dis. J. 2011, 30, 180–181. [Google Scholar] [CrossRef]
- Garza-Tovar, T.F.; Sacriste-Hernandez, M.I.; Juarez-Duran, E.R.; Arenas, R. An overview of the treatment of cutaneous leishmaniasis. Fac. Rev. 2020, 9, 28. [Google Scholar] [CrossRef]
- Ashutosh; Sundar, S.; Goyal, N. Molecular mechanisms of antimony resistance in Leishmania. J. Med. Microbiol. 2007, 56, 143–153. [Google Scholar] [CrossRef]
- Magalhaes, L.S.; Bomfim, L.G.; Mota, S.G.; Cruz, G.S.; Correa, C.B.; Tanajura, D.M.; Lipscomb, M.W.; Borges, V.M.; Jesus, A.R.; Almeida, R.P.; et al. Increased thiol levels in antimony-resistant Leishmania infantum isolated from treatment-refractory visceral leishmaniasis in Brazil. Mem. Inst. Oswaldo Cruz 2018, 113, 119–125. [Google Scholar] [CrossRef]
- Wyllie, S.; Cunningham, M.L.; Fairlamb, A.H. Dual action of antimonial drugs on thiol redox metabolism in the human pathogen Leishmania donovani. J. Biol. Chem. 2004, 279, 39925–39932. [Google Scholar] [CrossRef]
- Pan American Health Organization. Leishmaniasis in the Americas: Treatment recommendations.; PAHO: Washington, DC, USA, 2018. [Google Scholar]
- Velez, I.; Lopez, L.; Sanchez, X.; Mestra, L.; Rojas, C.; Rodriguez, E. Efficacy of miltefosine for the treatment of American cutaneous leishmaniasis. Am. J. Trop. Med. Hyg. 2010, 83, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Dorlo, T.P.; Balasegaram, M.; Beijnen, J.H.; de Vries, P.J. Miltefosine: A review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J. Antimicrob. Chemother. 2012, 67, 2576–2597. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Prada, C.; Vincent, I.M.; Brotherton, M.C.; Roberts, M.; Roy, G.; Rivas, L.; Leprohon, P.; Smith, T.K.; Ouellette, M. Different Mutations in a P-type ATPase Transporter in Leishmania Parasites are Associated with Cross-resistance to Two Leading Drugs by Distinct Mechanisms. PLoS Negl. Trop. Dis. 2016, 10, e0005171. [Google Scholar] [CrossRef]
- Younis, B.M.; Mudawi Musa, A.; Monnerat, S.; Abdelrahim Saeed, M.; Awad Gasim Khalil, E.; Elbashir Ahmed, A.; Ahmed Ali, M.; Noureldin, A.; Muthoni Ouattara, G.; Nyakaya, G.M.; et al. Safety and efficacy of paromomycin/miltefosine/liposomal amphotericin B combinations for the treatment of post-kala-azar dermal leishmaniasis in Sudan: A phase II, open label, randomized, parallel arm study. PLoS Negl. Trop. Dis. 2023, 17, e0011780. [Google Scholar] [CrossRef]
- Hendrickx, S.; Van den Kerkhof, M.; Mabille, D.; Cos, P.; Delputte, P.; Maes, L.; Caljon, G. Combined treatment of miltefosine and paromomycin delays the onset of experimental drug resistance in Leishmania infantum. PLoS Negl. Trop. Dis. 2017, 11, e0005620. [Google Scholar] [CrossRef]
- Bussotti, G.; Piel, L.; Pescher, P.; Domagalska, M.A.; Rajan, K.S.; Cohen-Chalamish, S.; Doniger, T.; Hiregange, D.G.; Myler, P.J.; Unger, R.; et al. Genome instability drives epistatic adaptation in the human pathogen Leishmania. Proc. Natl. Acad. Sci. USA 2021, 118, e2113744118. [Google Scholar] [CrossRef]
- Leprohon, P.; Fernandez-Prada, C.; Gazanion, E.; Monte-Neto, R.; Ouellette, M. Drug resistance analysis by next generation sequencing in Leishmania. Int. J. Parasitol. Drugs Drug Resist. 2015, 5, 26–35. [Google Scholar] [CrossRef]
- Santi, A.M.M.; Murta, S.M.F. Impact of Genetic Diversity and Genome Plasticity of Leishmania spp. in Treatment and the Search for Novel Chemotherapeutic Targets. Front. Cell Infect. Microbiol. 2022, 12, 826287. [Google Scholar] [CrossRef]
- Grunebast, J.; Clos, J. Leishmania: Responding to environmental signals and challenges without regulated transcription. Comput. Struct. Biotechnol. J. 2020, 18, 4016–4023. [Google Scholar] [CrossRef]
- Assis, L.H.C.; de Paiva, S.C.; Cano, M.I.N. Behind Base J: The Roles of JBP1 and JBP2 on Trypanosomatids. Pathogens 2023, 12, 467. [Google Scholar] [CrossRef]
- Ubeda, J.M.; Legare, D.; Raymond, F.; Ouameur, A.A.; Boisvert, S.; Rigault, P.; Corbeil, J.; Tremblay, M.J.; Olivier, M.; Papadopoulou, B.; et al. Modulation of gene expression in drug resistant Leishmania is associated with gene amplification, gene deletion and chromosome aneuploidy. Genome Biol. 2008, 9, R115. [Google Scholar] [CrossRef] [PubMed]
- Leprohon, P.; Legare, D.; Raymond, F.; Madore, E.; Hardiman, G.; Corbeil, J.; Ouellette, M. Gene expression modulation is associated with gene amplification, supernumerary chromosomes and chromosome loss in antimony-resistant Leishmania infantum. Nucleic Acids Res. 2009, 37, 1387–1399. [Google Scholar] [CrossRef] [PubMed]
- Patino, L.H.; Imamura, H.; Cruz-Saavedra, L.; Pavia, P.; Muskus, C.; Mendez, C.; Dujardin, J.C.; Ramirez, J.D. Major changes in chromosomal somy, gene expression and gene dosage driven by Sb(III) in Leishmania braziliensis and Leishmania panamensis. Sci. Rep. 2019, 9, 9485. [Google Scholar] [CrossRef] [PubMed]
- Patino, L.H.; Muskus, C.; Munoz, M.; Ramirez, J.D. Genomic analyses reveal moderate levels of ploidy, high heterozygosity and structural variations in a Colombian isolate of Leishmania (Leishmania) amazonensis. Acta Trop. 2020, 203, 105296. [Google Scholar] [CrossRef]
- Laffitte, M.N.; Leprohon, P.; Papadopoulou, B.; Ouellette, M. Plasticity of the Leishmania genome leading to gene copy number variations and drug resistance. F1000Research 2016, 5, 2350. [Google Scholar] [CrossRef]
- Dumetz, F.; Cuypers, B.; Imamura, H.; Zander, D.; D’Haenens, E.; Maes, I.; Domagalska, M.A.; Clos, J.; Dujardin, J.C.; De Muylder, G. Molecular Preadaptation to Antimony Resistance in Leishmania donovani on the Indian Subcontinent. mSphere 2018, 3. [Google Scholar] [CrossRef]
- Shaw, C.D.; Imamura, H.; Downing, T.; Blackburn, G.; Westrop, G.D.; Cotton, J.A.; Berriman, M.; Sanders, M.; Rijal, S.; Coombs, G.H.; et al. Genomic and Metabolomic Polymorphism among Experimentally Selected Paromomycin-Resistant Leishmania donovani Strains. Antimicrob. Agents Chemother. 2019, 64. [Google Scholar] [CrossRef]
- Gazanion, E.; Fernandez-Prada, C.; Papadopoulou, B.; Leprohon, P.; Ouellette, M. Cos-Seq for high-throughput identification of drug target and resistance mechanisms in the protozoan parasite Leishmania. Proc. Natl. Acad. Sci. USA 2016, 113, E3012–E3021. [Google Scholar] [CrossRef]
- Monte-Neto, R.; Laffitte, M.C.; Leprohon, P.; Reis, P.; Frezard, F.; Ouellette, M. Intrachromosomal amplification, locus deletion and point mutation in the aquaglyceroporin AQP1 gene in antimony resistant Leishmania (Viannia) guyanensis. PLoS Negl. Trop. Dis. 2015, 9, e0003476. [Google Scholar] [CrossRef]
- Mukherjee, A.; Boisvert, S.; Monte-Neto, R.L.; Coelho, A.C.; Raymond, F.; Mukhopadhyay, R.; Corbeil, J.; Ouellette, M. Telomeric gene deletion and intrachromosomal amplification in antimony-resistant Leishmania. Mol. Microbiol. 2013, 88, 189–202. [Google Scholar] [CrossRef]
- Xiang, L.; Laranjeira-Silva, M.F.; Maeda, F.Y.; Hauzel, J.; Andrews, N.W.; Mittra, B. Ascorbate-Dependent Peroxidase (APX) from Leishmania amazonensis Is a Reactive Oxygen Species-Induced Essential Enzyme That Regulates Virulence. Infect. Immun. 2019, 87. [Google Scholar] [CrossRef] [PubMed]
- Abadi, M.F.S.; Moradabadi, A.; Vahidi, R.; Shojaeepour, S.; Rostami, S.; Rad, I.; Dabiri, S. High resolution melting analysis and detection of Leishmania resistance: The role of multi drug resistance 1 gene. Genes Environ. 2021, 43, 36. [Google Scholar] [CrossRef] [PubMed]
- Rastrojo, A.; Garcia-Hernandez, R.; Vargas, P.; Camacho, E.; Corvo, L.; Imamura, H.; Dujardin, J.C.; Castanys, S.; Aguado, B.; Gamarro, F.; et al. Genomic and transcriptomic alterations in Leishmania donovani lines experimentally resistant to antileishmanial drugs. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 246–264. [Google Scholar] [CrossRef] [PubMed]
- Mwenechanya, R.; Kovarova, J.; Dickens, N.J.; Mudaliar, M.; Herzyk, P.; Vincent, I.M.; Weidt, S.K.; Burgess, K.E.; Burchmore, R.J.S.; Pountain, A.W.; et al. Sterol 14alpha-demethylase mutation leads to amphotericin B resistance in Leishmania mexicana. PLoS Negl. Trop. Dis. 2017, 11, e0005649. [Google Scholar] [CrossRef]
- Coelho, A.C.; Boisvert, S.; Mukherjee, A.; Leprohon, P.; Corbeil, J.; Ouellette, M. Multiple mutations in heterogeneous miltefosine-resistant Leishmania major population as determined by whole genome sequencing. PLoS Negl. Trop. Dis. 2012, 6, e1512. [Google Scholar] [CrossRef]
- Morais-Teixeira, E.; Damasceno, Q.S.; Galuppo, M.K.; Romanha, A.J.; Rabello, A. The in vitro leishmanicidal activity of hexadecylphosphocholine (miltefosine) against four medically relevant Leishmania species of Brazil. Mem. Inst. Oswaldo Cruz 2011, 106, 475–478. [Google Scholar] [CrossRef]
- Hendrickx, S.; Reis-Cunha, J.L.; Forrester, S.; Jeffares, D.C.; Caljon, G. Experimental Selection of Paromomycin Resistance in Leishmania donovani Amastigotes Induces Variable Genomic Polymorphisms. Microorganisms 2021, 9, 1546. [Google Scholar] [CrossRef]
- Rugani, J.N.; Gontijo, C.M.F.; Frezard, F.; Soares, R.P.; Monte-Neto, R.L.D. Antimony resistance in Leishmania (Viannia) braziliensis clinical isolates from atypical lesions associates with increased ARM56/ARM58 transcripts and reduced drug uptake. Mem. Inst. Oswaldo Cruz 2019, 114, e190111. [Google Scholar] [CrossRef]
- Cordeiro, A.T.; Thiemann, O.H.; Michels, P.A. Inhibition of Trypanosoma brucei glucose-6-phosphate dehydrogenase by human steroids and their effects on the viability of cultured parasites. Bioorg. Med. Chem. 2009, 17, 2483–2489. [Google Scholar] [CrossRef]
- Pountain, A.W.; Weidt, S.K.; Regnault, C.; Bates, P.A.; Donachie, A.M.; Dickens, N.J.; Barrett, M.P. Genomic instability at the locus of sterol C24-methyltransferase promotes amphotericin B resistance in Leishmania parasites. PLoS Neglected Trop. Dis. 2019, 13, e0007052. [Google Scholar] [CrossRef]
- Singh, A.K.; Papadopoulou, B.; Ouellette, M. Gene amplification in amphotericin B-resistant Leishmania tarentolae. Exp. Parasitol. 2001, 99, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Douanne, N.; Dong, G.; Amin, A.; Bernardo, L.; Blanchette, M.; Langlais, D.; Olivier, M.; Fernandez-Prada, C. Leishmania parasites exchange drug-resistance genes through extracellular vesicles. Cell Rep. 2022, 40, 111121. [Google Scholar] [CrossRef] [PubMed]
- Gommers-Ampt, J.; Lutgerink, J.; Borst, P. A novel DNA nucleotide in<i>Trypanosoma brucei</i>only present in the mammalian phase of the life-cycle. Nucleic Acids Res. 1991, 19, 1745–1751. [Google Scholar] [CrossRef]
- Gommers-Ampt, J.H.; Van Leeuwen, F.; De Beer, A.L.J.; Vliegenthart, J.F.G.; Dizdaroglu, M.; Kowalak, J.A.; Crain, P.F.; Borst, P. β-d-glucosyl-hydroxymethyluracil: A novel modified base present in the DNA of the parasitic protozoan T. brucei. Cell 1993, 75, 1129–1136. [Google Scholar] [CrossRef]
- van Luenen, H.G.; Farris, C.; Jan, S.; Genest, P.-A.; Tripathi, P.; Velds, A.; Ron, M.K.; Nieuwland, M.; Haydock, A.; Ramasamy, G.; et al. Glucosylated Hydroxymethyluracil, DNA Base J, Prevents Transcriptional Readthrough in Leishmania. Cell 2012, 150, 909–921. [Google Scholar] [CrossRef]
- Haile, S.; Papadopoulou, B. Developmental regulation of gene expression in trypanosomatid parasitic protozoa. Curr. Opin. Microbiol. 2007, 10, 569–577. [Google Scholar] [CrossRef]
- Cortazzo da Silva, L.; Aoki, J.I.; Floeter-Winter, L.M. Finding Correlations Between mRNA and Protein Levels in Leishmania Development: Is There a Discrepancy? Front. Cell Infect. Microbiol. 2022, 12, 852902. [Google Scholar] [CrossRef]
- Andrade, J.M.; Goncalves, L.O.; Liarte, D.B.; Lima, D.A.; Guimaraes, F.G.; de Melo Resende, D.; Santi, A.M.M.; de Oliveira, L.M.; Velloso, J.P.L.; Delfino, R.G.; et al. Comparative transcriptomic analysis of antimony resistant and susceptible Leishmania infantum lines. Parasit. Vectors 2020, 13, 600. [Google Scholar] [CrossRef]
- Patino, L.H.; Muskus, C.; Ramirez, J.D. Transcriptional responses of Leishmania (Leishmania) amazonensis in the presence of trivalent sodium stibogluconate. Parasit. Vectors 2019, 12, 348. [Google Scholar] [CrossRef]
- García-Hernández, R.; Perea-Martínez, A.; Manzano, J.I.; Terrón-Camero, L.C.; Andrés-León, E.; Gamarro, F. Transcriptome Analysis of Intracellular Amastigotes of Clinical Leishmania infantum Lines from Therapeutic Failure Patients after Infection of Human Macrophages. Microorganisms 2022, 10, 1304. [Google Scholar] [CrossRef]
- Medina, J.; Cruz-Saavedra, L.; Patiño, L.H.; Muñoz, M.; Ramírez, J.D. Comparative analysis of the transcriptional responses of five Leishmania species to trivalent antimony. Parasit. Vectors 2021, 14, 419. [Google Scholar] [CrossRef] [PubMed]
- Laffitte, M.-C.N.; Leprohon, P.; Légaré, D.; Ouellette, M. Deep-sequencing revealing mutation dynamics in the miltefosine transporter gene in Leishmania infantum selected for miltefosine resistance. Parasitol. Res. 2016, 115, 3699–3703. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Victoria, F.J.; Gamarro, F.; Ouellette, M.; Castanys, S. Functional Cloning of the Miltefosine Transporter. J. Biol. Chem. 2003, 278, 49965–49971. [Google Scholar] [CrossRef]
- Pérez-Victoria, F.J.; Sánchez-Cañete, M.P.; Castanys, S.; Gamarro, F. Phospholipid Translocation and Miltefosine Potency Require Both L. donovani Miltefosine Transporter and the New Protein LdRos3 in Leishmania Parasites. J. Biol. Chem. 2006, 281, 23766–23775. [Google Scholar] [CrossRef]
- Seifert, K.; Pérez-Victoria, F.J.; Stettler, M.; Sánchez-Cañete, M.P.; Castanys, S.; Gamarro, F.; Croft, S.L. Inactivation of the miltefosine transporter, LdMT, causes miltefosine resistance that is conferred to the amastigote stage of Leishmania donovani and persists in vivo. Int. J. Antimicrob. Agents 2007, 30, 229–235. [Google Scholar] [CrossRef]
- Espada, C.R.; Magalhaes, R.M.; Cruz, M.C.; Machado, P.R.; Schriefer, A.; Carvalho, E.M.; Hornillos, V.; Alves, J.M.; Cruz, A.K.; Coelho, A.C.; et al. Investigation of the pathways related to intrinsic miltefosine tolerance in Leishmania (Viannia) braziliensis clinical isolates reveals differences in drug uptake. Int. J. Parasitol. Drugs Drug Resist. 2019, 11, 139–147. [Google Scholar] [CrossRef]
- Kulshrestha, A.; Sharma, V.; Singh, R.; Salotra, P. Comparative transcript expression analysis of miltefosine-sensitive and miltefosine-resistant Leishmania donovani. Parasitol. Res. 2014, 113, 1171–1184. [Google Scholar] [CrossRef]
- Suman, S.S.; Equbal, A.; Zaidi, A.; Ansari, M.Y.; Singh, K.P.; Singh, K.; Purkait, B.; Sahoo, G.C.; Bimal, S.; Das, P.; et al. Up-regulation of cytosolic tryparedoxin in Amp B resistant isolates of Leishmania donovani and its interaction with cytosolic tryparedoxin peroxidase. Biochimie 2016, 121, 312–325. [Google Scholar] [CrossRef]
- Purkait, B.; Singh, R.; Wasnik, K.; Das, S.; Kumar, A.; Paine, M.; Dikhit, M.; Singh, D.; Sardar, A.H.; Ghosh, A.K.; et al. Up-regulation of silent information regulator 2 (Sir2) is associated with amphotericin B resistance in clinical isolates of Leishmania donovani. J. Antimicrob. Chemother. 2015, 70, 1343–1356. [Google Scholar] [CrossRef]
- Verma, A.; Bhandari, V.; Deep, D.K.; Sundar, S.; Dujardin, J.C.; Singh, R.; Salotra, P. Transcriptome profiling identifies genes/pathways associated with experimental resistance to paromomycin in Leishmania donovani. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 370–377. [Google Scholar] [CrossRef]
- Chu, J.; Pelletier, J. Therapeutic Opportunities in Eukaryotic Translation. Cold Spring Harb. Perspect. Biol. 2018, 10, a032995. [Google Scholar] [CrossRef] [PubMed]
- Arribere, J.A.; Kuroyanagi, H.; Hundley, H.A. mRNA Editing, Processing and Quality Control in Caenorhabditis elegans. Genetics 2020, 215, 531–568. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Almonacid, C.C.; Kellogg, M.K.; Karamyshev, A.L.; Karamysheva, Z.N. Ribosome Specialization in Protozoa Parasites. Int. J. Mol. Sci. 2023, 24, 7484. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Leprohon, P.; Bigot, S.; Padmanabhan, P.K.; Mukherjee, A.; Roy, G.; Gingras, H.; Mestdagh, A.; Papadopoulou, B.; Ouellette, M. Coupling chemical mutagenesis to next generation sequencing for the identification of drug resistance mutations in Leishmania. Nat. Commun. 2019, 10, 5627. [Google Scholar] [CrossRef]
- Singh, A.K.; Roberts, S.; Ullman, B.; Madhubala, R. A quantitative proteomic screen to identify potential drug resistance mechanism in alpha-difluoromethylornithine (DFMO) resistant Leishmania donovani. J. Proteom. 2014, 102, 44–59. [Google Scholar] [CrossRef]
- Gutierrez Guarnizo, S.A.; Tikhonova, E.B.; Zabet-Moghaddam, M.; Zhang, K.; Muskus, C.; Karamyshev, A.L.; Karamysheva, Z.N. Drug-Induced Lipid Remodeling in Leishmania Parasites. Microorganisms 2021, 9, 790. [Google Scholar] [CrossRef]
- Gutierrez Guarnizo, S.A.; Karamysheva, Z.N.; Galeano, E.; Muskus, C.E. Metabolite Biomarkers of Leishmania Antimony Resistance. Cells 2021, 10, 1063. [Google Scholar] [CrossRef]
- Lee, L.J.; Papadopoli, D.; Jewer, M.; Del Rincon, S.; Topisirovic, I.; Lawrence, M.G.; Postovit, L.M. Cancer Plasticity: The Role of mRNA Translation. Trends Cancer 2021, 7, 134–145. [Google Scholar] [CrossRef]
- T’Kindt, R.; Scheltema, R.A.; Jankevics, A.; Brunker, K.; Rijal, S.; Dujardin, J.C.; Breitling, R.; Watson, D.G.; Coombs, G.H.; Decuypere, S. Metabolomics to unveil and understand phenotypic diversity between pathogen populations. PLoS Negl. Trop. Dis. 2010, 4, e904. [Google Scholar] [CrossRef]
- Scheltema, R.A.; Decuypere, S.; T’Kindt, R.; Dujardin, J.C.; Coombs, G.H.; Breitling, R. The potential of metabolomics for Leishmania research in the post-genomics era. Parasitology 2010, 137, 1291–1302. [Google Scholar] [CrossRef]
- Canuto, G.A.; Castilho-Martins, E.A.; Tavares, M.; Lopez-Gonzalvez, A.; Rivas, L.; Barbas, C. CE-ESI-MS metabolic fingerprinting of Leishmania resistance to antimony treatment. Electrophoresis 2012, 33, 1901–1910. [Google Scholar] [CrossRef] [PubMed]
- Berg, M.; Mannaert, A.; Vanaerschot, M.; Van Der Auwera, G.; Dujardin, J.C. (Post-) Genomic approaches to tackle drug resistance in Leishmania. Parasitology 2013, 140, 1492–1505. [Google Scholar] [CrossRef] [PubMed]
- Berg, M.; Vanaerschot, M.; Jankevics, A.; Cuypers, B.; Maes, I.; Mukherjee, S.; Khanal, B.; Rijal, S.; Roy, S.; Opperdoes, F.; et al. Metabolic adaptations of Leishmania donovani in relation to differentiation, drug resistance, and drug pressure. Mol. Microbiol. 2013, 90, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Vincent, I.M.; Weidt, S.; Rivas, L.; Burgess, K.; Smith, T.K.; Ouellette, M. Untargeted metabolomic analysis of miltefosine action in Leishmania infantum reveals changes to the internal lipid metabolism. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 20–27. [Google Scholar] [CrossRef]
- Glatz, J.F. Challenges in Fatty Acid and lipid physiology. Front. Physiol. 2011, 2, 45. [Google Scholar] [CrossRef]
- Rakotomanga, M.; Saint-Pierre-Chazalet, M.; Loiseau, P.M. Alteration of fatty acid and sterol metabolism in miltefosine-resistant Leishmania donovani promastigotes and consequences for drug-membrane interactions. Antimicrob. Agents Chemother. 2005, 49, 2677–2686. [Google Scholar] [CrossRef]
- Mbongo, N.; Loiseau, P.M.; Billion, M.A.; Robert-Gero, M. Mechanism of amphotericin B resistance in Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 1998, 42, 352–357. [Google Scholar] [CrossRef]
- de Azevedo, A.F.; Dutra, J.L.; Santos, M.L.; Santos Dde, A.; Alves, P.B.; de Moura, T.R.; de Almeida, R.P.; Fernandes, M.F.; Scher, R.; Fernandes, R.P. Fatty acid profiles in Leishmania spp. isolates with natural resistance to nitric oxide and trivalent antimony. Parasitol. Res. 2014, 113, 19–27. [Google Scholar] [CrossRef]
- Basselin, M.; Robert-Gero, M. Alterations in membrane fluidity, lipid metabolism, mitochondrial activity, and lipophosphoglycan expression in pentamidine-resistant Leishmania. Parasitol. Res. 1998, 84, 78–83. [Google Scholar] [CrossRef]
- Wassef, M.K.; Fioretti, T.B.; Dwyer, D.M. Lipid analyses of isolated surface membranes of Leishmania donovani promastigotes. Lipids 1985, 20, 108–115. [Google Scholar] [CrossRef]
- Zhang, K.; Beverley, S.M. Phospholipid and sphingolipid metabolism in Leishmania. Mol. Biochem. Parasitol. 2010, 170, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Goad, L.J.; Holz, G.G., Jr.; Beach, D.H. Sterols of Leishmania species. Implications for biosynthesis. Mol. Biochem. Parasitol. 1984, 10, 161–170. [Google Scholar] [CrossRef] [PubMed]
- de Souza, W.; Rodrigues, J.C. Sterol Biosynthesis Pathway as Target for Anti-trypanosomatid Drugs. Interdiscip. Perspect. Infect. Dis. 2009, 2009, 642502. [Google Scholar] [CrossRef]
- Mathur, R.; Das, R.P.; Ranjan, A.; Shaha, C. Elevated ergosterol protects Leishmania parasites against antimony-generated stress. FASEB J. 2015, 29, 4201–4213. [Google Scholar] [CrossRef]
- Cauchetier, E.; Loiseau, P.M.; Lehman, J.; Rivollet, D.; Fleury, J.; Astier, A.; Deniau, M.; Paul, M. Characterisation of atovaquone resistance in Leishmania infantum promastigotes. Int. J. Parasitol. 2002, 32, 1043–1051. [Google Scholar] [CrossRef]
- Imbert, L.; Cojean, S.; Libong, D.; Chaminade, P.; Loiseau, P.M. Sitamaquine-resistance in Leishmania donovani affects drug accumulation and lipid metabolism. Biomed. Pharmacother. 2014, 68, 893–897. [Google Scholar] [CrossRef]
- Bringaud, F.; Barrett, M.P.; Zilberstein, D. Multiple roles of proline transport and metabolism in trypanosomatids. Front. Biosci. 2012, 17, 349–374. [Google Scholar] [CrossRef] [PubMed]
- Rojo, D.; Canuto, G.A.; Castilho-Martins, E.A.; Tavares, M.F.; Barbas, C.; Lopez-Gonzalvez, A.; Rivas, L. A Multiplatform Metabolomic Approach to the Basis of Antimonial Action and Resistance in Leishmania infantum. PLoS ONE 2015, 10, e0130675. [Google Scholar] [CrossRef]
- Colotti, G.; Ilari, A. Polyamine metabolism in Leishmania: From arginine to trypanothione. Amino Acids 2011, 40, 269–285. [Google Scholar] [CrossRef]
- Karamysheva, Z.N.; Tikhonova, E.B.; Grozdanov, P.N.; Huffman, J.C.; Baca, K.R.; Karamyshev, A.; Denison, R.B.; MacDonald, C.C.; Zhang, K.; Karamyshev, A.L. Polysome Profiling in Leishmania, Human Cells and Mouse Testis. J. Vis. Exp. 2018, 134, e57600. [Google Scholar] [CrossRef]

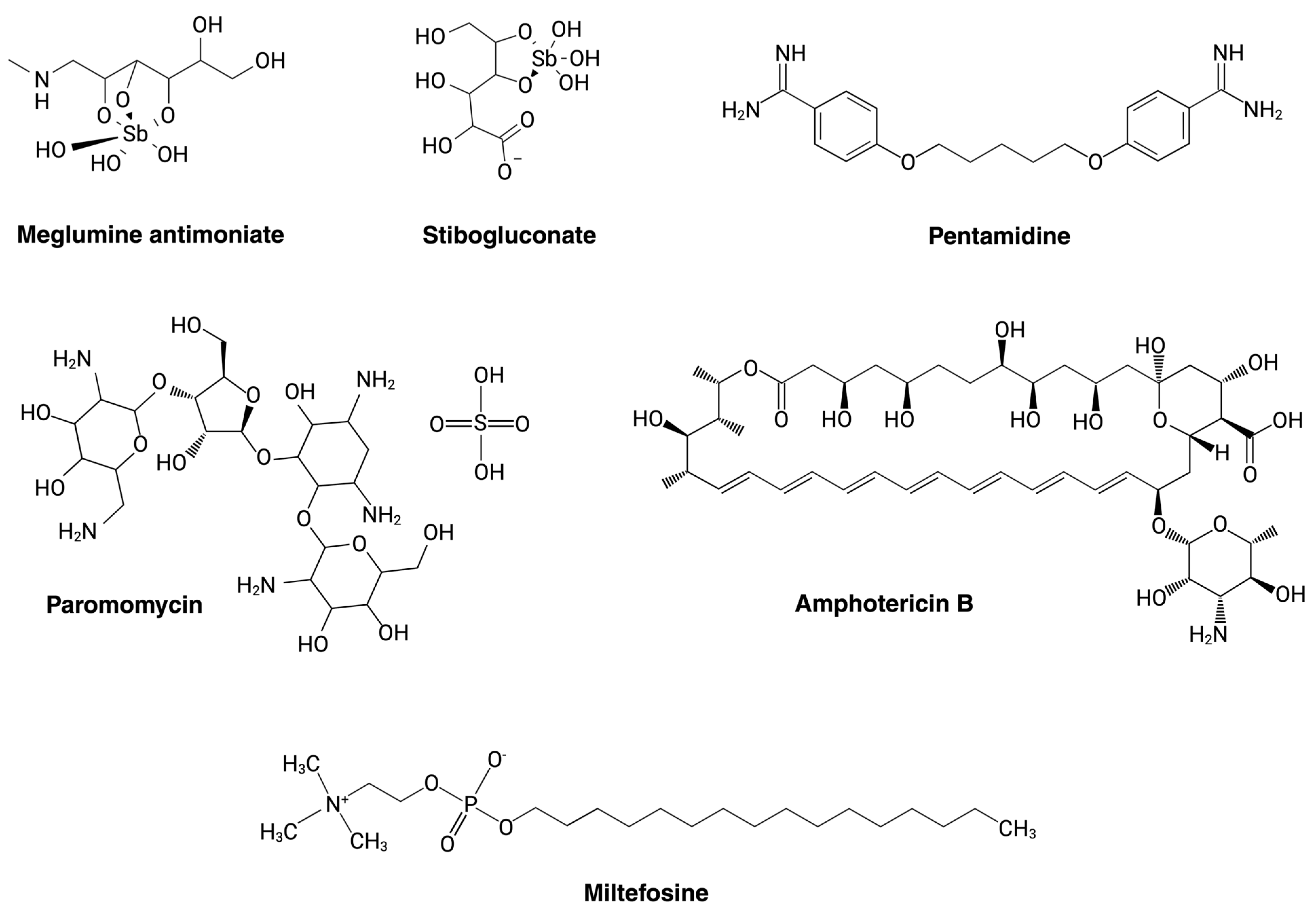
| Current Drugs for Leishmaniasis Treatment | Mode of Action and Parasite Targeting | References |
|---|---|---|
| Pentavalent Antimony (SbV) | Inhibits the mitochondrial enzyme trypanothione reductase, increasing the parasite’s susceptibility to oxidative stress generated by the macrophage during infection. It can obstruct major energy-driven pathways such as fatty acid oxidation and glycolysis. | [35,48,49] |
| Miltefosine (MLT) | Inhibits the enzyme cytochrome c oxidase located in the mitochondria, directly affecting energy production in the parasite. Also inhibits phosphatidylcholine synthesis, which affects lipid metabolism through the CDP-choline pathway by acting on CTP-phosphocholine cytidylyltransferase activity. | [50,51] |
| Liposomal amphotericin B (AmB) | Forms transmembrane channels through the cell wall and is known to have a high affinity for ergosterol, causing micropores in the membrane, increasing permeability and ion loss, and resulting in cell death. | [52,53] |
| Paromomycin (PMM) | Inhibits the cytosolic ribosome, affecting protein synthesis through binding to the 16S ribosomal unit and creating an alteration in its structure. | [54,55,56] |
| Pentamidine (PTM) | Inhibits DNA and protein synthesis and causes cell-cycle arrest in the G2/M phase. Inhibits RNA polymerase, leading to apoptosis. Inhibits arginine transport. | [57,58] |
| Drug | Gene Name | Genomic Changes | Effect Associated with Drug Resistance | Reference |
|---|---|---|---|---|
| Antimony | MRPA | Amplification | Increases drug efflux | [80,92] |
| APX | Amplification | Protection from ROS accumulation | [84] | |
| G6PDH | Amplification | Protection from ROS accumulation | [84,93] | |
| AQP1 | Amplification, Deletion | Reduces drug uptake | [83] | |
| MDR1 | Point mutation | Increases drug efflux | [86] | |
| AmB | SMT | Deletion | Reduces drug uptake | [87] |
| SC5D | Point mutation | Alters sterol biosynthesis | [94] | |
| CYP51 | Point mutation | Alters sterol biosynthesis | [88] | |
| LMT | Deletion, Point mutation | Alters sterol biosynthesis | [67] | |
| Miltefosine | LMT | Deletion | Reduces drug uptake | [89] |
| Paromomycin | Gene 18S RNA | Point mutation | Decreases binding of PMM | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moncada-Diaz, M.J.; Rodríguez-Almonacid, C.C.; Quiceno-Giraldo, E.; Khuong, F.T.H.; Muskus, C.; Karamysheva, Z.N. Molecular Mechanisms of Drug Resistance in Leishmania spp. Pathogens 2024, 13, 835. https://doi.org/10.3390/pathogens13100835
Moncada-Diaz MJ, Rodríguez-Almonacid CC, Quiceno-Giraldo E, Khuong FTH, Muskus C, Karamysheva ZN. Molecular Mechanisms of Drug Resistance in Leishmania spp. Pathogens. 2024; 13(10):835. https://doi.org/10.3390/pathogens13100835
Chicago/Turabian StyleMoncada-Diaz, Maria Juliana, Cristian Camilo Rodríguez-Almonacid, Eyson Quiceno-Giraldo, Francis T. H. Khuong, Carlos Muskus, and Zemfira N. Karamysheva. 2024. "Molecular Mechanisms of Drug Resistance in Leishmania spp." Pathogens 13, no. 10: 835. https://doi.org/10.3390/pathogens13100835
APA StyleMoncada-Diaz, M. J., Rodríguez-Almonacid, C. C., Quiceno-Giraldo, E., Khuong, F. T. H., Muskus, C., & Karamysheva, Z. N. (2024). Molecular Mechanisms of Drug Resistance in Leishmania spp. Pathogens, 13(10), 835. https://doi.org/10.3390/pathogens13100835







