Do Commercial Insect Repellents Provide Protection against the Tick Amblyomma sculptum (Acari: Ixodidae)?
Abstract
1. Introduction
2. Materials and Methods
2.1. Tick Colony
2.2. Repellents
2.3. Repellency of Bioassays
2.4. Filter Paper Bioassay
2.5. Fingertip Bioassay
2.6. Field Bioassay
2.7. Statistical Analysis
3. Results
3.1. Laboratory Bioassays
3.2. Field Bioassay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dantas-Torres, F.; Otranto, D. Ixodid and Argasid Ticks. J. Encycl. Infect. Immun. 2022, 2, 1049–1063. [Google Scholar]
- Dantas-Torres, F.; Martins, T.F.; Muñoz-Leal, S.; Onofrio, V.C.; Barros-Battesti, D.M. Ticks (Ixodida: Argasidae, Ixodidae) of Brazil: Updated species checklist and taxonomic keys. Ticks Tick Borne Dis. 2019, 10, 101–252. [Google Scholar] [CrossRef] [PubMed]
- Nava, S.; Beati, L.; Labruna, M.B.; Cáceres, A.G.; Mangold, A.J.; Guglielmone, A.A. Reassessment of the taxonomic status of Amblyomma cajennense (Fabricius, 1787) with the description of three new species, Amblyomma tonelliae n. sp., Amblyomma interandinum n. sp. and Amblyomma patinoi n. sp., and reinstatement of Amblyomma mixtum Koch, 1844, and Amblyomma sculptum Berlese, 1888 (Ixodida: Ixodidae). Ticks Tick Borne Dis. 2014, 5, 252–275. [Google Scholar] [PubMed]
- Bitencourth, K.; Voloch, C.M.; Serra-Freire, N.M.; Machado-Ferreira, E.; Amorim, M.; Gazêta, G.S. Analysis of Amblyomma sculptum haplotypes in an area endemic for Brazilian Spotted Fever. Med. Vet. Entomol. 2016, 30, 342–350. [Google Scholar] [CrossRef]
- Scoles, G.A.; Ueti, M.W. Amblyomma cajennense is na intrastadial biological vector of Theileria equi. Parasites Vectors 2013, 6, 306. [Google Scholar] [CrossRef]
- Labruna, M.B. Ecology of Rickettsia in South America. Ann. N. Y. Acad. Sci. 2009, 1166, 156–166. [Google Scholar] [CrossRef]
- Ministério da Saúde, Brasil. Guia de Vigilância em Saúde. Available online: http://bvsms.saude.gov.br/bvs/publicacoes/guia_vigilancia_saude_1ed_atual.pdf (accessed on 25 October 2019).
- Ministério da Saúde, Brasil. Febre Maculosa. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/f/febre-maculosa#:~:text=A%20febre%20maculosa%20%C3%A9%20uma,transmitida%20pela%20picada%20do%20carrapato (accessed on 1 April 2023).
- Rodrigues, C.M.; Geise, L.; Gazeta, G.S.; Oliveira, S.V. Estudo descritivo de casos notificados de febre maculosa em São Paulo, Rio de Janeiro e Minas Gerais entre 2007 e 2016. Cad. Saúde. Colet. 2023, 31, e31020104. [Google Scholar] [CrossRef]
- Ribeiro, V.L.S.; Weber, M.A.; Fetzer, L.O.; Vargas, C.R.B. Espécies e prevalência das infestações por carrapatos em cães de rua da cidade de Porto Alegre, RS, Brasil. Ciência Rural 1997, 27, 285–289. [Google Scholar] [CrossRef]
- Angerami, R.N.; Câmara, M.; Pacola, M.R.; Rezende, R.C.; Duarte, R.M.; Nascimento, E.M.; Colombo, S.; Santos, F.C.; Leite, R.M.; Katz, G.; et al. Features of Brazilian spotted fever in two different endemic areas in Brazil. Ticks Tick Borne Dis. 2012, 3, 346–348. [Google Scholar] [CrossRef]
- Donalisio, M.R.; Souza, C.E.; Angerami, R.N.; Samy, A.M. Mapping Brazilian spottedfever: Linking etiological agent, vectors, and hosts. Acta Trop. 2020, 207, 105496. [Google Scholar] [CrossRef]
- Barnard, D.R. Repellents and for Personal Protection. World Health Organization. Available online: https://iris.who.int/bitstream/handle/10665/66666/WHO_CDS_WHOPES_GCDPP_2000.5.pdf?sequence=1&isAllowed=y (accessed on 1 April 2023).
- Nentwig, G. Use of repellents as prophylactic agents. Parasitol. Res. 2003, 90, S40–S48. [Google Scholar] [CrossRef]
- Leal, W.S. The enigmatic reception of DEET—The gold standard of insect repellents. Curr. Opin. Insect Sci. 2014, 6, 93–98. [Google Scholar] [CrossRef]
- Miller, J.R.; Siegert, P.Y.; Amimo, F.A.; Walker, E.D. Designation of Chemicals in Terms of the Locomotor Responses They Elicit from Insects: An Update of Dethier et al. J. Econ. Entomol. 2009, 102, 2056–2060. [Google Scholar] [CrossRef]
- Ministério da Saúde, Brasil. Resolução—RDC no. 19, de 10 de Abril de 2013. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2013/rdc0019_10_04_2013.html (accessed on 25 October 2022).
- Szabó, M.P.J.; Olegário, M.M.M.; Santos, A.L.Q. Tick fauna from two locations in the Brazilian savannah. Exp. Appl. Acarol. 2007, 43, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.F.; Braga, R.S.; Ferreira, L.L.; Louly, C.C.B.; Sousa, L.A.D.; Silva, A.C.; Borges, L.M.F. Repellent activity of DEET against Amblyomma cajennense (Acari: Ixodidae) nymphs submitted to different laboratory bioassays. Rev. Bras. Parasitol. 2010, 19, 12–16. [Google Scholar] [CrossRef][Green Version]
- Carroll, J.F.; Solberg, V.B.; Klun, J.A.; Kramer, M.; Debboun, M. Comparative Activity of Deet and AI3-37220 Repellents Against the Ticks Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) in Laboratory Bioassays. J. Med. Entomol. 2004, 40, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Schreck, C.E. Techniques for the evaluation of insect repellents: A critical review. Annu. Rev. Entomol. 1977, 22, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Bissinger, B.W.; Apperson, C.S.; Watson, D.W.; Arellano, C.; Sonenshine, D.E.; Roe, R.M. Novel field assays and the comparative repellency of BioUD®, DEET and permethrin against Amblyomma americanum. Med. Vet. Entomol. 2011, 25, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Komagata, O.; Hayashi, T.; Itokawa, K.; Morikawa, S.; Sawabe, K.; Tomita, T. Field and Laboratory Evaluations of the Efficacy of DEET Repellent against Ixodes. Jpn, J. Infect. Dis. 2015, 69, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Pajuaba-Neto, A.A.; Ramos, V.D.N.; Martins, M.M.; Osava, C.F.; Pascoal, J.O.; Suzin, A.; Yokosawa, J.; Szabó, M.P.J. Influence of microhabitat use and behavior of Amblyomma sculptum and Amblyomma dubitatum nymphs (Acari: Ixodidae) on human risk for tick exposure, with notes on Rickettsia infection. Ticks Tick Borne Dis. 2018, 9, 67–71. [Google Scholar] [CrossRef]
- Martins, T.F.; Onofrio, V.C.; Barros-Battesti, D.M.; Labruna, M.B. Nymphs of the genus Amblyomma (Acari: Ixodidae) of Brazil: Descriptions, redescriptions, and identification key. Ticks Tick Borne Dis. 2010, 1, 75–99. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.F.; Barbieri, A.R.; Costa, F.B.; Terassini, F.A.; Camargo, L.M.; Peterka, C.R.C.; Pacheco, R.; Dias, R.A.; Nunes, P.H.; Marcili, A.; et al. Geographical distribution of Amblyomma cajennense (sensu lato) ticks (Parasitiformes: Ixodidae) in Brazil, with description of the nymph of A. cajennense (sensu stricto). Parasit. Vectors 2016, 9, 186. [Google Scholar] [CrossRef] [PubMed]
- R Project. The R Project for Statistical Computing. Available online: https://www.R-project.org (accessed on 1 April 2023).
- Hammer, O.; Harper, D.A.; Ryan, P.D. Palaeontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Jensenius, M.; Pretorius, A.M.; Clarke, F.; Myrvang, B. Repellent efficacy of four commercial DEET lotions against Amblyomma hebraeum (Acari: Ixodidae), the principal vector of Rickettsia africae in southern Africa. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.P.C.; Bankuti, R.M.; Moraes, C.A.P.; Seo, E.S.M. Critical Analysis of Mosquito Repellents Formulation In The Brazilian Market. Int. J. Dev. Res. 2022, 12, 55129–55131. [Google Scholar]
- Meng, H.; Li, A.Y.; Costa Junior, L.M.; Castro-Arellano, I.; Liu, J. Evaluation of DEET and eight essential oils for repellency against nymphs of the lone star tick, Amblyomma americanum (Acari: Ixodidae). Exp. Appl. Acarol. 2016, 68, 241–249. [Google Scholar] [CrossRef]
- Bissinger, B.W.; Apperson, C.S.; Sonenshine, D.E.; Watson, D.W.; Roe, R.M. Efficacy of the new repellent BioUD® against three species of ixodid ticks. Exp. Appl. Acarol. 2009, 48, 239–250. [Google Scholar] [CrossRef]
- Perez, C.A.; Omoto, C.; Carvalho, V.H.B.; Silva, M.S.T. Atividade de repelentes aplicados na pele e na roupa para a proteção contra o carrapato-estrela Amblyomma cajennense (Acari: Ixodidae). In Proceedings of the Anais do Congresso Brasileiro de Parasitologia Veterinária e Simpósio Latino-Americano de Rickettsioses, Ribeirão Preto, Brazil, 1 August 2006; Volume 198. [Google Scholar]
- Bissinger, B.W.; Roe, R.M. Tick repellents: Past, present, and future. Pestic. Biochem. Physiol. 2010, 96, 63–79. [Google Scholar] [CrossRef]
- Pages, F.; Dautel, H.; Duvallet, G.; Kahl, O.; Gentile, L.; Boulanger, N. Ticks repellents for human use: Prevention of ticks bites and tick-borne diseases. Vector Borne Zoonotic Dis. 2014, 14, 85–93. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R. Repellence of essential oils and selected compounds against ticks—A systematic review. Acta Trop. 2018, 179, 47–54. [Google Scholar] [CrossRef]
- McMeniman, C.J.; Corfas, R.A.; Matthews, B.J.; Ritchie, S.A.; Vosshall, L.B. Multimodal Integration of Carbon Dioxide and Other Sensory Cues Drives Mosquito Attraction to Humans. Cell 2014, 156, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Leal, W.S.; Barbosa, R.M.; Zeng, F.; Faierstein, G.B.; Tan, K.; Paiva, M.H.; Guedes, D.R.; Crespo, M.M.; Ayres, C.F. Does Zika virus infection affect mosquito response to repellents? Sci. Rep. 2017, 16, 42826. [Google Scholar] [CrossRef] [PubMed]
- Deletre, E.; Schatz, B.; Bourguet, D.; Chandre, F.; Williams, L.; Ratnadass, A.; Thibaud, M. Prospects for repellent in pest control: Current developments and future challenges. Chemoecology 2016, 26, 127–142. [Google Scholar] [CrossRef]
- Stefani, G.P.; Pastorino, A.C.; Castro, A.P.B.M.; Fomin, A.B.F.; Jacob, C.M.A. Insect repellents: Recommendations for use in children. Rev. Paul. Pediatr. 2009, 27, 81–89. [Google Scholar] [CrossRef]

| Commercial Name | Way of Applying | Active Principle (Product Concentration) | Registration Number (ANVISA) | Manufacturing Company |
|---|---|---|---|---|
| 1 OFF!® Family | Spray | DEET (6.65%) | 201920481 | Reckitt Benckiser |
| 2 Repelex® Super | Spray | DEET (6.79%) | 203450001 | Reckitt Benckiser |
| 3 Exposis® Extreme | Spray | Icaridina (20.00%) | 203451022 | Reckitt Benckiser |
| 4 SBP® Advanced Family | Spray | Icaridina (9.98%) | 03451022 | Reckitt Benckiser |
| 5 Johnsons® Baby Loção | Lotion | IR3535 (12.50%) | 200920551 | Johnson & Johnson |
| 6 Henlau® Repelente | Spray | IR3535 (20.00%) | 227430204 | Henlau |
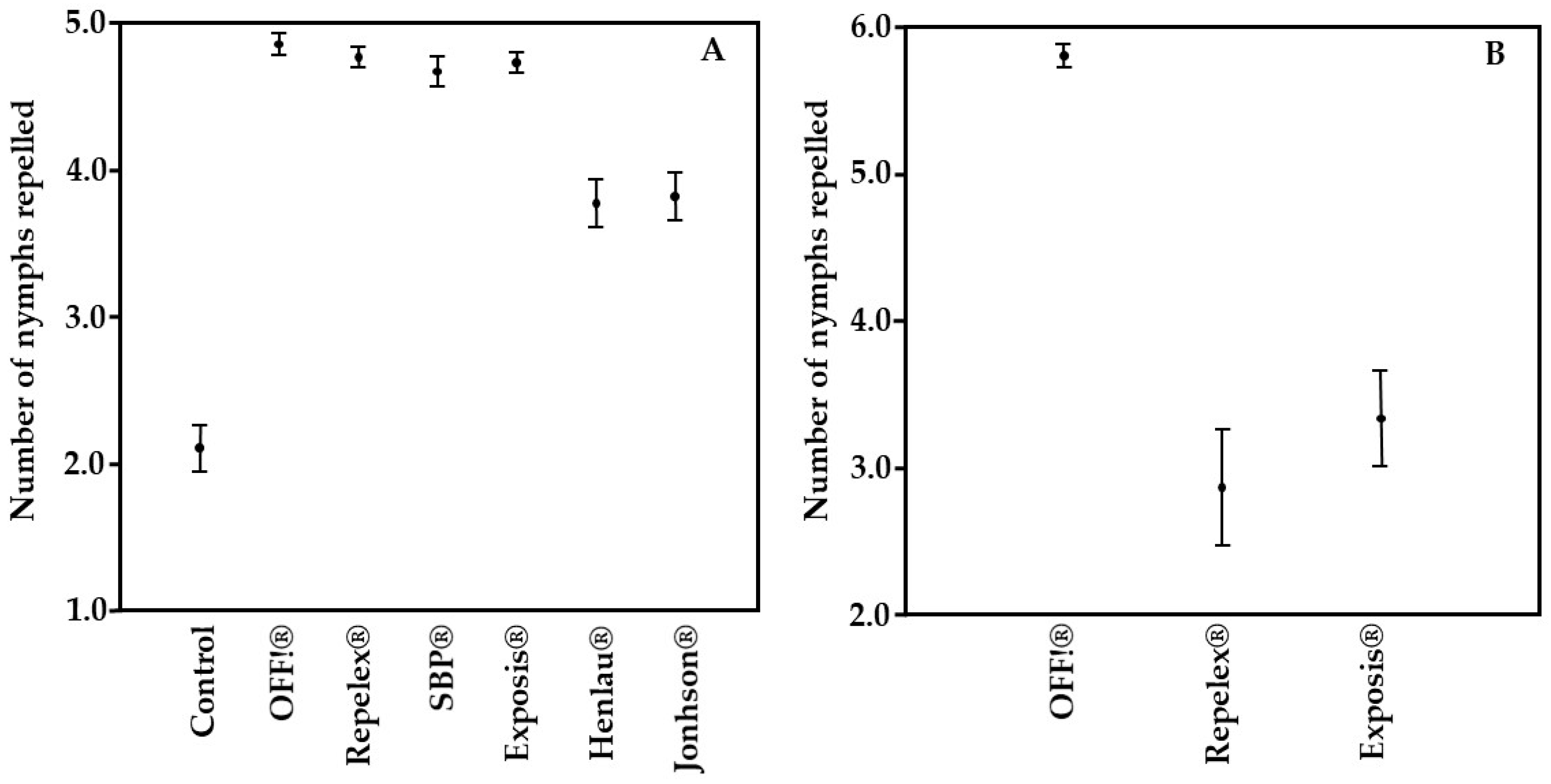
| Repellent | Total of Nymphs Repelled | Repellency (%) | Mean * | Standard Deviation * | Coefficient of Variance * |
|---|---|---|---|---|---|
| Control | 101 | 43.33 | 2.10 a | 1.08 | 51.17 |
| OFF!® | 233 | 97.08 | 4.50 b | 0.50 | 10.40 |
| Repelex® | 229 | 95.42 | 4.77 b | 0.47 | 9.90 |
| SBP® | 225 | 93.33 | 4.67 b | 0.72 | 15.52 |
| Exposis® | 227 | 94.58 | 4.73 b | 0.45 | 9.50 |
| Henlau® | 179 | 75.42 | 3.77 c | 1.13 | 30.08 |
| Jonhson® | 183 | 76.25 | 3.81 c | 1.12 | 29.47 |
| Repellent | Total of Nymphs Repelled | Repellency (%) | Mean * | Standard Deviation * | Coefficient of Variance * |
|---|---|---|---|---|---|
| OFF!® | 174 | 96.6 | 5.80 a | 0.41 | 7.01 |
| Repelex® | 86 | 47.7 | 2.87 b | 2.15 | 74.84 |
| Exposis | 100 | 55.5 | 3.33 b | 1.79 | 53.63 |
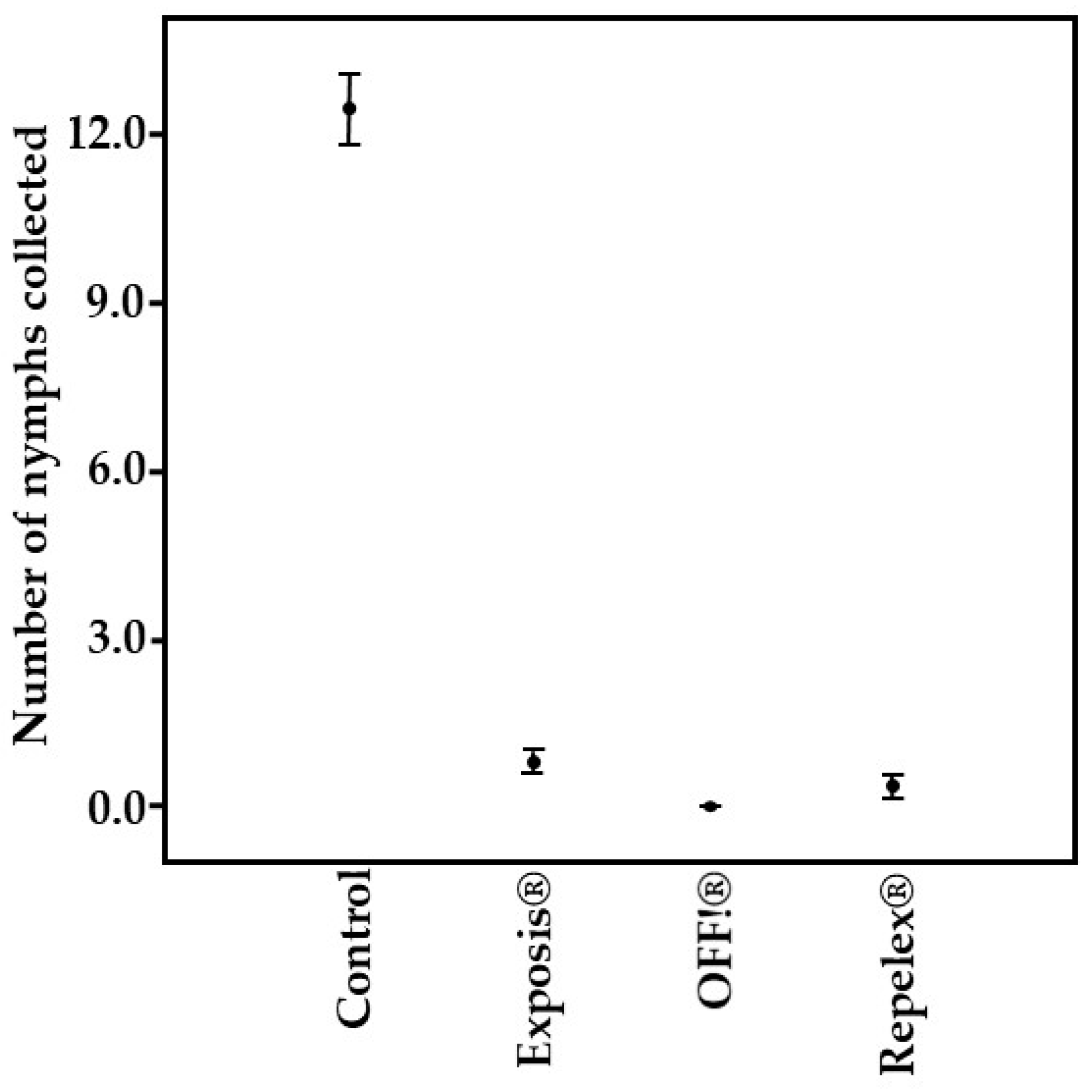
| Repellent | Total of Nymphs Collected | Repellency (%) | Mean | Standard Deviation | Coefficient of Variance |
|---|---|---|---|---|---|
| Control | 434 | 0 | 12.44 a | 4.18 | 33.60 |
| OFF!® | 0 | 100 | 0.00 b | 0.00 | - |
| Repelex® | 14 | 96.8 | 0.38 b | 1.37 | 362.63 |
| Exposis® | 10 | 93.1 | 0.82 b | 1.09 | 132.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Barcelos, B.R.; Coelho, N.G.S.S.; Santos, M.M.B.; Vale, F.L.; Teixeira, A.L.C.; Pereira e Souza, L.M.; Zeringóta, V.; de Oliveira Monteiro, C.M.; Eugenio, C.U.O.; Obara, M.T. Do Commercial Insect Repellents Provide Protection against the Tick Amblyomma sculptum (Acari: Ixodidae)? Pathogens 2024, 13, 9. https://doi.org/10.3390/pathogens13010009
de Barcelos BR, Coelho NGSS, Santos MMB, Vale FL, Teixeira ALC, Pereira e Souza LM, Zeringóta V, de Oliveira Monteiro CM, Eugenio CUO, Obara MT. Do Commercial Insect Repellents Provide Protection against the Tick Amblyomma sculptum (Acari: Ixodidae)? Pathogens. 2024; 13(1):9. https://doi.org/10.3390/pathogens13010009
Chicago/Turabian Stylede Barcelos, Beatriz Rodrigues, Nathália Gabriela Silva Santos Coelho, Mayara Macedo Barrozo Santos, Francisca Letícia Vale, Ana Lúcia Coutinho Teixeira, Lainny Martins Pereira e Souza, Viviane Zeringóta, Caio Márcio de Oliveira Monteiro, Chesterton Ulysses Orlando Eugenio, and Marcos Takashi Obara. 2024. "Do Commercial Insect Repellents Provide Protection against the Tick Amblyomma sculptum (Acari: Ixodidae)?" Pathogens 13, no. 1: 9. https://doi.org/10.3390/pathogens13010009
APA Stylede Barcelos, B. R., Coelho, N. G. S. S., Santos, M. M. B., Vale, F. L., Teixeira, A. L. C., Pereira e Souza, L. M., Zeringóta, V., de Oliveira Monteiro, C. M., Eugenio, C. U. O., & Obara, M. T. (2024). Do Commercial Insect Repellents Provide Protection against the Tick Amblyomma sculptum (Acari: Ixodidae)? Pathogens, 13(1), 9. https://doi.org/10.3390/pathogens13010009





