A Study of Cross-Protection between Eimeria maxima Immunovariants
Abstract
1. Introduction
2. Materials and Methods
2.1. Parasites
Eimeria maxima
2.2. Evaluation of Cross-Protection among E. maxima APU1, APU2, and APU3
2.3. Ethics Statement
2.4. Statistical Comparisons
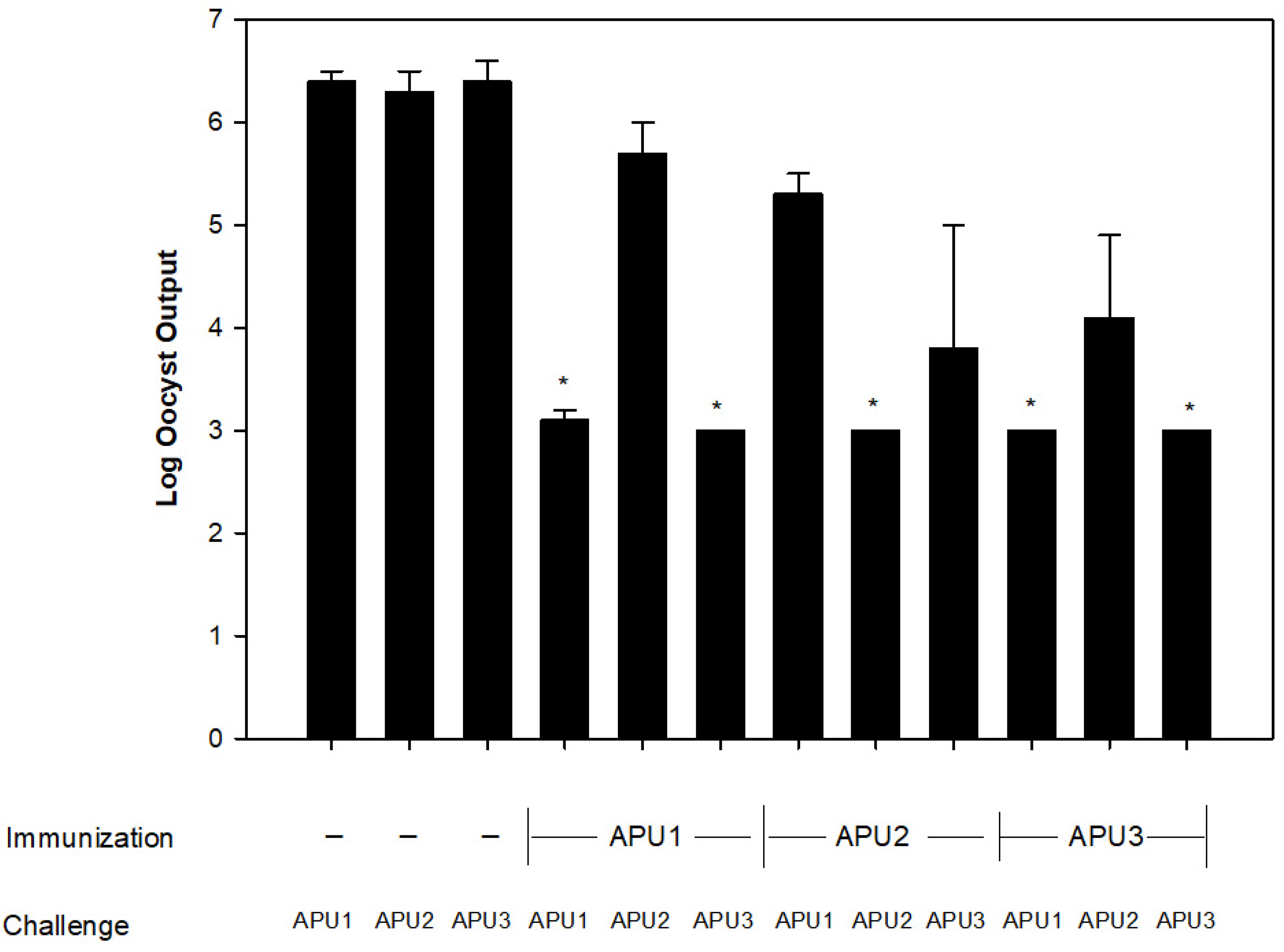
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McDonald, V.; Shirley, M.W.; Bellatti, M.A. Eimeria maxima: Characteristics of attenuated lines obtained by selection for precocious development in the chicken. Exp. Parasitol. 1986, 61, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Jenkins, M.C. Re-evaluation of the life cycle of Eimeria maxima Tyzzer, 1929 in chickens (Gallus domesticus). Parasitology 2018, 145, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Barta, J.R.; Coles, B.A.; Schito, M.L.; Fernando, M.A.; Martin, A.; Danforth, H.D. Analysis of infraspecific variation among five strains of Eimeria maxima from North America. Int. J. Parasitol. 1998, 28, 485–492. [Google Scholar] [CrossRef]
- Allen, P.C.; Jenkins, M.C.; Miska, K.B. Cross protection studies with Eimeria maxima strains. Parasitol. Res. 2005, 97, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, R.S.; Jenkins, M.C.; Klopp, S.; Miska, K.B. Genomic analysis of Eimeria spp. populations in relation to performance levels of broiler chicken farms in Arkansas and North Carolina. J. Parasitol. 2009, 95, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, M.C.; Dubey, J.P.; Miska, K.; Fetterer, R. Differences in fecundity of Eimeria maxima strains exhibiting different levels of pathogenicity in its avian host. Vet. Parasitol. 2017, 236, 1–6. [Google Scholar] [CrossRef]
- Schnitzler, B.E.; Shirley, M.W. Immunological aspects of infections with Eimeria maxima: A short review. Avian Pathol. 1999, 28, 537–543. [Google Scholar] [CrossRef]
- Chapman, H.D.; Matsler, P.L.; Muthavarapu, V.K.; Chapman, M.E. Acquisition of immunity to Eimeria maxima in newly hatched chickens given 100 oocysts. Avian Dis. 2005, 49, 426–429. [Google Scholar] [CrossRef]
- Chapman, H.D.; Rayavarapu, S. Acquisition of immunity to Eimeria maxima in newly hatched chickens reared on new or reused litter. Avian Pathol. 2007, 36, 319–323. [Google Scholar] [CrossRef]
- Norton, C.C.; Hein, H.E. Eimeria maxima: A comparison of two laboratory strains with a fresh isolate. Parasitology 1976, 72, 345–354. [Google Scholar] [CrossRef]
- Long, P.L.; Millard, B.J. Immunological differences in Eimeria maxima: Effect of a mixed immunizing inoculum on heterologous challenge. Parasitology 1979, 79, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H.D.; Cherry, T.E.; Danforth, H.D.; Richards, G.; Shirley, M.W.; Williams, R.B. Sustainable coccidiosis control in poultry production: The role of live vaccines. Int. J. Parasitol. 2002, 32, 617–629. [Google Scholar] [CrossRef]
- Williams, R.B. Fifty years of anticoccidial vaccines for poultry (1952–2002). Avian Dis. 2002, 46, 775–802. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.B. Anticoccidial vaccines for broiler chickens: Pathways to success. Avian Pathol. 2002, 31, 317–353. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, X.; Zhao, X.; Zhu, X.Q.; Suo, X. Live attenuated anticoccidial vaccines for chickens. Trends Parasitol. 2023, 39, 1087–1099. [Google Scholar] [CrossRef] [PubMed]
- Fitz-Coy, S.H. Antigenic variation among strains of Eimeria maxima and E. tenella of the chicken. Avian Dis. 1992, 36, 40–43. [Google Scholar] [CrossRef]
- Martin, A.G.; Danforth, H.D.; Barta, J.R.; Fernando, M.A. Analysis of immunological cross-protection and sensitivities to anticoccidial drugs among five geographical and temporal strains of Eimeria maxima. Int. J. Parasitol. 1997, 27, 527–533. [Google Scholar] [CrossRef]
- Smith, A.L.; Hesketh, P.; Archer, A.; Shirley, M.W. Antigenic diversity in Eimeria maxima and the influence of host genetics and immunization schedule on cross-protective immunity. Infect. Immun. 2002, 70, 2472–2479. [Google Scholar] [CrossRef]
- Boulton, K.; Nolan, M.J.; Wu, Z.; Riggio, V.; Matika, O.; Harman, K.; Hocking, P.M.; Bumstead, N.; Hesketh, P.; Archer, A.; et al. Dissecting the genomic architecture of resistance to Eimeria maxima parasitism in the chicken. Front. Genet. 2018, 9, 528. [Google Scholar] [CrossRef]
- Shirley, M.W.; Bellatti, M.A. Live attenuated coccidiosis vaccine: Selection of a second precocious line of Eimeria maxima. Res. Vet. Sci. 1988, 44, 25–28. [Google Scholar] [CrossRef]
- Jenkins, M.C.; Parker, C.C.; O’Brien, C.N.; Ritter, D. Viable Eimeria oocysts in poultry house litter at the time of chick placement. Poult. Sci. 2019, 98, 3176–3180. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, M.C.; Miska, K.; Klopp, S. Improved polymerase chain reaction technique for determining the species composition of Eimeria in poultry litter. Avian Dis. 2006, 50, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Fetterer, R.H.; Barfield, R.C. Characterization of a developmentally regulated oocyst protein from Eimeria tenella. J. Parasitol. 2003, 89, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Guizetti, J.; Scherf, A. Silence, activate, poise and switch! Mechanisms of antigenic variation in Plasmodium falciparum. Cell. Microbiol. 2013, 15, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Higgins, M.K.; Carrington, M. Sequence variation and structural conservation allows development of novel function and immune evasion in parasite surface protein families. Protein. Sci. 2014, 23, 354–365. [Google Scholar] [CrossRef]
- Reid, A.J. Large, rapidly evolving gene families are at the forefront of host-parasite interactions in Apicomplexa. Parasitology 2015, 142 (Suppl. S1), S57–S70. [Google Scholar] [CrossRef]
- Su, X.Z.; Lane, K.D.; Xia, L.; Sá, J.M.; Wellems, T.E. Plasmodium genomics and genetics: New insights into malaria pathogenesis, drug resistance, epidemiology, and evolution. Clin. Microbiol. Rev. 2019, 32, e00019-19. [Google Scholar] [CrossRef]
- Shirley, M.W.; Hoyle, S.R. The antigenicity of Eimeria maxima populations obtained from commercial farms. J. Parasitol. 1981, 67, 587–588. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jenkins, M.C.; O'Brien, C.N.; Parker, C.C.; Tucker, M.S. A Study of Cross-Protection between Eimeria maxima Immunovariants. Pathogens 2024, 13, 66. https://doi.org/10.3390/pathogens13010066
Jenkins MC, O'Brien CN, Parker CC, Tucker MS. A Study of Cross-Protection between Eimeria maxima Immunovariants. Pathogens. 2024; 13(1):66. https://doi.org/10.3390/pathogens13010066
Chicago/Turabian StyleJenkins, Mark C., Celia N. O'Brien, Carolyn C. Parker, and Matthew S. Tucker. 2024. "A Study of Cross-Protection between Eimeria maxima Immunovariants" Pathogens 13, no. 1: 66. https://doi.org/10.3390/pathogens13010066
APA StyleJenkins, M. C., O'Brien, C. N., Parker, C. C., & Tucker, M. S. (2024). A Study of Cross-Protection between Eimeria maxima Immunovariants. Pathogens, 13(1), 66. https://doi.org/10.3390/pathogens13010066




