Abstract
We analyzed the antimicrobial resistance (AMR) data of 6519 clinical isolates of Escherichia coli (n = 3985), Klebsiella pneumoniae (n = 775), Acinetobacter baumannii (n = 163), Pseudomonas aeruginosa (n = 781), Enterococcus faecium (n = 124), and Staphylococcus aureus (n = 691) from 43 centers in Mexico. AMR assays were performed using commercial microdilution systems (37/43) and the disk diffusion susceptibility method (6/43). The presence of carbapenemase-encoding genes was assessed using PCR. Data from centers regarding site of care, patient age, and clinical specimen were collected. According to the site of care, the highest AMR was observed in E. coli, K. pneumoniae, and P. aeruginosa isolates from ICU patients. In contrast, in A. baumannii, higher AMR was observed in isolates from hospitalized non-ICU patients. According to age group, the highest AMR was observed in the ≥60 years age group for E. coli, E. faecium, and S. aureus, and in the 19–59 years age group for A. baumannii and P. aeruginosa. According to clinical specimen type, a higher AMR was observed in E. coli, K. pneumoniae, and P. aeruginosa isolates from blood specimens. The most frequently detected carbapenemase-encoding gene in E. coli was blaNDM (84%).
1. Introduction
In an effort to promote drug research and development, the World Health Organization (WHO) has identified and classified priority antibiotic-resistant pathogens into three groups on the basis of the urgency of the need for novel antibiotics. The first and the second groups (critical- and high-priority pathogens, respectively) include, among others, carbapenem-resistant and extended-spectrum beta-lactamase-producing Klebsiella pneumoniae and Escherichia coli, carbapenem-resistant Acinetobacter baumannii and Pseudomonas aeruginosa, vancomycin-resistant Enterococcus faecium, and methicillin-resistant Staphylococcus aureus [1]. The surveillance of antimicrobial resistance (AMR) in these critical- and high-priority pathogens requires a multidisciplinary approach, including national and local strategies [2]. Previous studies have reported that several factors, such as increased age, gender, and various demographics and comorbidities, increase the risk of infections due to antibiotic-resistant organisms [3,4].
In 2018, the Network for the Research and Surveillance of Drug Resistance (Red Temática de Investigación y Vigilancia de la Farmacorresistencia in Spanish; INVIFAR) was created to comprehensively study AMR in Mexico. Although previous studies have reported several aspects of antibiotic resistance in Mexico [5,6,7,8,9,10,11], including reports of an increase in drug resistance during the COVID-19 pandemic [12], there is limited information regarding AMR according to age and site of care at the time of infection.
In the present study, we assessed AMR in clinical isolates of E. coli, K. pneumoniae, A. baumannii, P. aeruginosa, E. faecium, and S. aureus from Mexico on the basis of clinical specimens from which the isolates were recovered, patient age and site of care, and the presence of carbapenemase-encoding genes in the included Gram negatives.
2. Materials and Methods
2.1. Participating Centers and Data Collection
A total of 43 centers across 18 Mexican states participated in this study. We collected data from 34 hospital center-based and 9 ambulatory-care microbiology laboratories. Data regarding each center’s total number of beds and intensive care unit (ICU) capacity, clinical specimens from which the studied isolates were recovered, and patient age and site of care were collected. AMR data from E. coli, K. pneumoniae, A. baumannii, P. aeruginosa, E. faecium, and S. aureus recovered from urine, respiratory, and blood specimens between 1 January and 31 March 2023 were included. Pathogens with the result of AMR data of more than 10 isolates were included.
Data from each laboratory were deposited into WHONET 2022® software (WHO Collaborating Centre for the Surveillance of Antibiotic Resistance, Geneva, Switzerland). The extracted file was converted to the WHONET 2022 format through the data conversion utility BacLink 2022 (available online: http://www.whonet.org/, accessed on 1 June 2022). After conversion, all WHONET files for each hospital were combined and analyzed.
For the AMR analysis, only one isolate per patient was selected. The results were reported as antibiotic-susceptible, intermediate, or resistant according to the Clinical & Laboratory Standards Institute (CLSI) [13]. The data from patients were encrypted to protect personal information. Antimicrobial susceptibility testing (AST) was performed using amikacin (AMK), amoxicillin–clavulanic acid (AMC), ampicillin (AMP), aztreonam (ATM), ampicillin–sulbactam (SAM), cefepime (FEP), cefoxitin (FOX), ceftazidime (CAZ), ceftriaxone (CRO), cefuroxime (CXM), ciprofloxacin (CIP), clindamycin (CLI), cefotaxime (CTX), erythromycin (ERY), ertapenem (ETP), gentamicin (GEN), imipenem (IPM), linezolid (LNZ), levofloxacin (LVX), meropenem (MEM), oxacillin (OXA), tetracycline (TCY), tigecycline (TGC), piperacillin–tazobactam (TZP), tobramycin (TOB), trimethoprim–sulfamethoxazole (SXT), and vancomycin (VAN).
Using the WHONET software, Gram-negative isolates were classified as multi-drug-resistant (MDR) when non-susceptibility to at least one antibiotic in the three antimicrobial classes tested was documented, extensively drug-resistant (XDR) when non-susceptibility to at least one antibiotic in all but two or fewer antimicrobial classes was reported, and pan-drug-resistant (PDR) when non-susceptibility to all antibiotics in all antimicrobial classes tested was noted [14]. Because only one antimicrobial agent was evaluated for each antimicrobial class for some isolates, the categories of possible XDR and possible PDR were also described [14].
2.2. Site of Care, Age, and Clinical Specimens
The frequency of resistance to distinct antimicrobials was adjusted to the patient’s site of care (ICU, hospitalized medical/surgical non-ICU, emergency room, and outpatient setting) and age group (0–18 years, 19–59 years, and ≥60 years), and clinical specimens from which the studied isolates were cultured, such as respiratory (endotracheal aspirate and bronchial lavage), blood, and urine specimens. A comparison of antibiotic resistance between sites of care, age groups, and clinical specimens was performed using chi-square or Fisher’s exact test as appropriate. A two-tailed p-value ≤ 0.05 was considered statistically significant. The statistical analyses were performed using the MedCalc software, V 22.009.
2.3. Carbapenemase-Encoding Genes
As part of the active surveillance performed by the INVIFAR network, centers sent relevant carbapenem-resistant Gram negatives to the coordinating laboratory. In the clinical isolates received from centers, polymerase chain reaction (PCR) was performed to detect the most frequent carbapenemase-encoding genes previously reported in this population [15], that is, blaNDM-1, blaKPC, blaVIM, blaIMP, and blaOXA-48-like [16,17] in E. coli and K. pneumoniae isolates; blaOXA-23 and blaOXA-24 in A. baumannii isolates [18,19]; and blaVIM, blaIMP, and blaGES in P. aeruginosa isolates [10].
3. Results
3.1. Participating Centers and Study Population
During the study period, 43 centers reported data, of which 32/43 (74.4%) belong to the national public health care system and 11/43 (25.6%) belong to private practice health care system. To perform AST, 37/43 (83.7%) laboratories used commercial microdilution systems, of which 30 used the VITEK 2 system (Biomérieux, Marcy l’ Etoile, France), 5 used MicroScan WalkAway (Siemens Healthcare Diagnostics, West Sacramento, CA, USA), and 2 used the Phoenix System (Becton-Dickinson, Sparks, MD, USA). Six laboratories used the disk diffusion susceptibility method. The characteristics of the participating centers are listed in Table 1. Most clinical specimens (57%) were obtained from females. In total, 14% of patients belonged to the 0–18 years age group, whereas 49% and 37% belonged to the 19–59 years age group and ≥60 years age group, respectively. Most isolates were recovered from patients hospitalized in non-ICU settings (44%), followed by those in outpatient (32%), emergency room (16%), and ICU (8%) settings. A total of 6519 strains from all participating laboratories were analyzed: E. coli (n = 3985), K. pneumoniae (n = 775), A. baumannii (n = 163), P. aeruginosa (n = 781), E. faecium (n = 124), and S. aureus (n = 691).

Table 1.
Distribution and characteristics of participating centers.
3.2. Percentages of Resistance Detected in Critical- and High-Priority Pathogens/Phenotypes
The results of the distribution of antibiotic resistance are shown in Supplementary Tables S1–S3 and Figure 1, Figure 2 and Figure 3. Third-generation cephalosporin resistance in K. pneumoniae was as high as 75% for CRO in isolates from the 0–18 years age group.
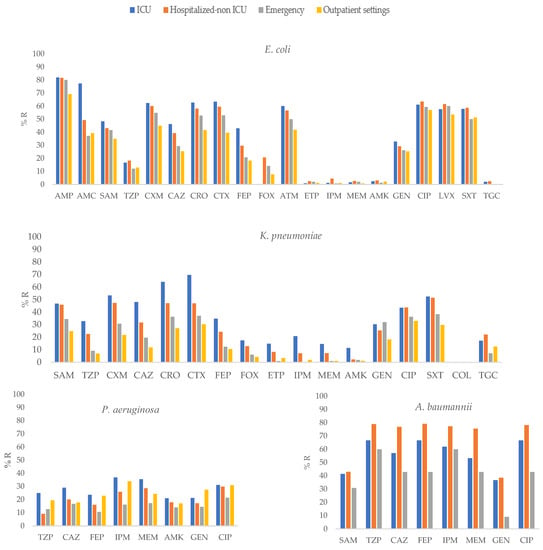
Figure 1.
Percentages of antimicrobial resistance for selected Gram-negative pathogens at 43 centers according to sites of care. AMK: amikacin, AMC: amoxicillin–clavulanic acid, AMP: ampicillin, SAM: ampicillin–sulbactam, FEP: cefepime, FOX: cefoxitin, CAZ: ceftazidime, CRO: ceftriaxone, CXM: cefuroxime, CIP: ciprofloxacin, CTX: cefotaxime, ETP: ertapenem, GEN: gentamicin, IPM: imipenem, LVX: levofloxacin, MEM: meropenem, TGC: tigecycline, TZP: piperacillin–tazobactam, SXT: trimethoprim-sulfamethoxazole.
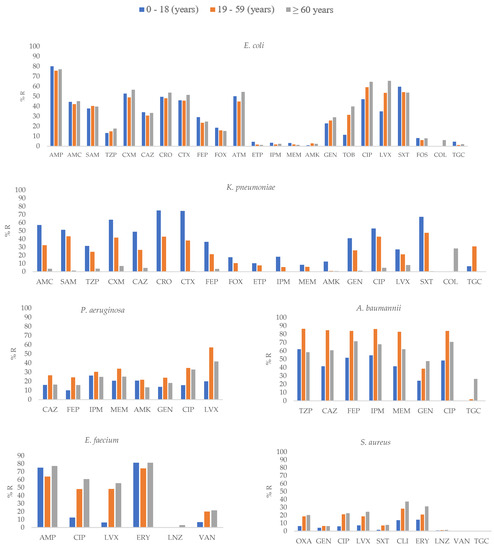
Figure 2.
Percentages of antimicrobial resistance for selected pathogens at 43 centers according to age groups. AMK: amikacin, AMC: amoxicillin–clavulanic acid, AMP: ampicillin, SAM: ampicillin–sulbactam, FEP: cefepime, FOX: cefoxitin, CAZ: ceftazidime, CRO: ceftriaxone, CXM: cefuroxime, CIP: ciprofloxacin, CLI: Clindamycin, CTX: cefotaxime, ERY: erythromycin, ETP: ertapenem, GEN: gentamicin, IPM: imipenem, LNZ: linezolid, LVX: levofloxacin, MEM: meropenem, OXA: oxacillin, TGC: tigecycline, TZP: piperacillin–tazobactam, TOB: tobramycin, SXT: trimethoprim-sulfamethoxazole, VAN: vancomycin.
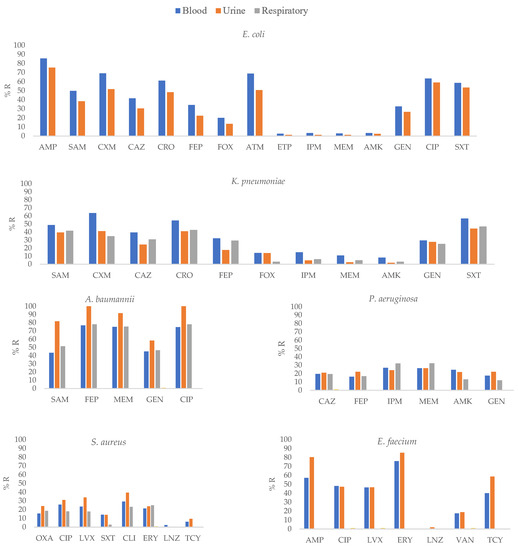
Figure 3.
Percentages of antimicrobial resistance for selected Gram-negative pathogens at 43 centers according to clinical specimens. AMK: amikacin, AMC: amoxicillin–clavulanic acid, AMP: ampicillin, SAM: ampicillin–sulbactam, FEP: cefepime, FOX: cefoxitin, CAZ: ceftazidime, CRO: ceftriaxone, CXM: cefuroxime, CIP: ciprofloxacin, CLI: clindamycin, CTX: cefotaxime, ERY: erythromycin, ETP: ertapenem, GEN: gentamicin, IPM: imipenem, LNZ: linezolid, LVX: levofloxacin, MEM: meropenem, OXA: oxacillin, TGC: tigecycline, TZP: piperacillin–tazobactam, TOB: tobramycin, SXT: trimethoprim–sulfamethoxazole, VAN: vancomycin.
For K. pneumoniae, the highest frequency of carbapenem resistance was detected in 20.8% of the isolates recovered from ICU-admitted patients. For A. baumannii, carbapenem resistance was reported to be 86.2% in the 19–50 group and 36.8% for P. aeruginosa recovered from patients in the ICU. The methicillin resistance was found in 20.3% S. aureus isolates recovered from patients aged ≥60 years.
3.3. Antimicrobial Resistance of Selected Pathogens According to Site of Care
E. coli isolates obtained from ICU patients showed a higher frequency of resistance to AMP, AMC, SAM, CXM, CAZ, CRO, CTX, FEP, IPM, and GEN than other E. coli isolates (p ≤ 0.01; Supplementary Table S1, Figure 1).
In K. pneumoniae, isolates recovered from ICU patients showed higher resistance to SAM, TZP, CAZ, CRO, CTX, FEP, ETP, IPM, MEM, AMK, and SXT (p ≤ 0.01), than isolates from other groups.
In P. aeruginosa, isolates recovered from ICU patients showed higher resistance to TZP, FEP, IPM, and MEM (p ≤ 0.05) than isolates obtained from other groups. In contrast, in A. baumannii, isolates obtained from hospitalized non-ICU patients showed higher resistance to CAZ, CRO, MEM, FEP and CIP (p ≤ 0.01) than other isolates.
3.4. Antimicrobial Resistance of Selected Pathogens According to Patient Age Group
E. coli isolates recovered from the ≥60 years age group showed higher resistance to most antibiotics, including CXM, CIP, LVX (p ≤ 0.01) and TOB (p = 0.01; Supplementary Table S2, Figure 2). In contrast, in K. pneumoniae, isolates obtained from the 0–18 years age group showed higher resistance to SAM, CXM, CAZ, CRO, CTX, FEP, AMK, GEN, and SXT (p ≤ 0.01), ETP, and IPM (p ≤ 0.05). Regarding A. baumannii, higher resistance to CAZ, FEP, IPM, MEM, and CIP (p ≤ 0.01) in the 19-59 years group was found; similar results were observed for P. aeruginosa. For E. faecium and S. aureus, isolates recovered from the ≥60 years age group showed higher resistance to CIP and LVX (p ≤ 0.01) and OXA, CIP, LVX, CLI, and ERY (p ≤ 0.01), respectively.
3.5. Antimicrobial Resistance of Selected Pathogens According to Clinical Specimen Type
E. coli isolates from blood specimens showed higher resistance to SAM, CAZ, CRO, and FEP (p ≤ 0.01) and CXM, IPM, MEM, and GEN (p ≤ 0.05) than isolates from other clinical specimens. K. pneumoniae isolates from blood specimens showed higher resistance to CAZ, FEP, ETP, MEM, and AMK (p ≤ 0.01) and CXM, CRO, and IPM (p ≤ 0.05) than K. pneumoniae isolates from other clinical specimens (Supplementary Table S3, Figure 3).
In P. aeruginosa, isolates obtained from blood and urine specimens showed higher resistance to AMK and GEN (p ≤ 0.05) than other isolates. S. aureus isolates from blood specimens showed higher resistance to SXT (p ≤ 0.01) than isolates from other clinical specimens.
3.6. MDR, XDR, and PDR Isolates
MDR, true XDR, possible XDR, and possible PDR isolates were detected among samples obtained from patients in all studied settings. Isolates recovered from ICU patients showed the highest frequency of MDR in E. coli (62.9%), K. pneumoniae (50.5%), and P. aeruginosa (29.7%). For A. baumannii, the highest frequency of MDR was observed among isolates cultured from non-ICU hospitalized patients (79.1%; Table 2).

Table 2.
Distribution of MDR, possible XDR, and possible PDR bacteria among species studied.
The highest frequency of possible XDR isolates of E. coli was detected in isolates from ambulatory-care patients (14%); for K. pneumoniae and P. aeruginosa, possible XDR was detected in 37.6% and 27.1% of the isolates cultured from ICU-admitted patients, respectively. The highest frequency of possible XDR isolates of A. baumannii was detected in isolates from hospitalized non-ICU patients (78.2%). True XDR P. aeruginosa was detected across samples obtained from patients in all settings (Table 2).
3.7. Carbapenemase-Encoding Genes
In K. pneumoniae and E. coli, the most frequently detected carbapenemase-encoding gene was blaNDM (50%, 22/44 and 84%, 63/75, respectively) in the strains evaluated. The second most frequent carbapenemase-encoding genes detected in K. pneumoniae and E. coli were blaKPC (27.3%, 12/44) and blaOXA-48-like (12%, 9/75; Table 3), respectively. In A. baumannii, the most frequent carbapenemase-encoding gene was blaOXA24.

Table 3.
Distribution of genes encoding carbapenemase among bacterial species investigated in this study. ND—Not determined.
4. Discussion
The Infectious Diseases Society of America recognizes antimicrobial resistance as threat to human health worldwide [20]. In the present study, we studied AMR in five pathogenic species considered critical and high-priority by the WHO [1] via the analysis of consolidated data on antibiotic resistance by specimen, according to patient age and site of care, increasing the value and usefulness of the data generated.
Among the organisms/phenotypes to be surveyed according to the WHO recommendations, in E. coli and K. pneumoniae, the highest value of third-generation cephalosporin resistance was observed in isolates obtained from ICU patients (63.4%) and from the 0–18 years group (75%), respectively. A significant increase in the prevalence of extended-spectrum beta-lactamase-producing Enterobacterales in children has been reported in the USA, and this increase has been correlated with the spread of ST131 CTX-M-producing E. coli strains [21].
Recently, 24 E. coli strains from the same population were sequenced and the majority of them, 11 (45.8%), were detected to be ST2 (Pasteur)-ST167 (Warwick), followed by ST650 (Pasteur)-ST 361 (Warwick) (16.7%), with only one strain detected to be ST131, suggesting that ST131 has no impact in the alarmingly high levels of cephalosporin resistance.
In the present study, K. pneumoniae isolates obtained from ICU patients showed the highest carbapenem resistance (20.8%). This result is highly relevant because the hospital mortality of patients infected with carbapenem-resistant K. pneumoniae isolates has been reported to be 48%, in contrast with the 20% mortality reported for patients infected with carbapenem-susceptible K. pneumoniae [22].
AMR is considered one of the key determinants of patient outcome, and patients in the ICU are at a higher risk of acquiring antimicrobial-resistant infections, owing to the use of invasive devices, clinical condition, and increased exposure to antibiotics [23]. In our study, we detected higher antibiotic resistance in isolates obtained from patients in ICU settings than those in other settings for E. coli and K. pneumoniae (resistance to SAM, CAZ, CRO, CTX, FEP, and IPM; p ≤ 0.01) and for P. aeruginosa (resistance to TZP, FEP, IPM, and MEM, p ≤ 0.05).
The relevance of E. coli in the ICU has been reported previously. Although antibiotic resistance may affect any patient in the hospital, a nationwide study on bloodstream infections in ICUs in Swiss hospitals during 2008–2017 reported that the most common antibiotic-resistant species was E. coli (23.2%, 910), with resistance to first- and second-line antibiotics increasing linearly during hospitalization [24].
Previous studies have identified a predominance of A. baumannii in ICUs, especially in patients who have undergone invasive procedures and those with a prolonged ICU stay and prior use of broad-spectrum antimicrobial agents [25,26,27] constituting 7.9% of ventilator-associated pneumonia and up to 15.7% of bloodstream infections [28,29]. This bacterial species has been designated a human “red alarm” pathogen mainly because of its broad antibiotic resistance [30]. A high frequency of drug resistance in A. baumannii has been previously reported in Mexico [31,32,33,34,35,36,37], but there is little information on drug resistance in hospital settings. In our study, A. baumannii isolates from hospitalized non-ICU patients showed higher resistance to carbapenems and quinolones than isolates from ICU patients. It has been reported that the efficacy of antimicrobial agents is affected by hygiene quality [38]. The high drug resistance of this bacterial species in non-ICU hospitalized patients is relevant because it suggests that factors other than ICU stay such as antimicrobial stewardship, hand sanitization, and isolation of patients may play an important role in reducing infections by this bacterial species.
Some studies have investigated carbapenem resistance in Enterobacterales in Mexico, including carbapenemase production and genes encoding these enzymes, especially in K. pneumoniae and E. coli [7,39]. In the present study, we confirmed that, among Enterobacterales, K. pneumoniae show higher carbapenem resistance, and a high distribution of blaNDM was observed in both K. pneumoniae and E. coli. The detection of blaNDM in both E. coli and K. pneumoniae complicates the treatment of patients infected with these isolates because few therapeutic options are available for metallo-β-lactamase-producing bacteria; for example, the ceftazidime/avibactam + aztreonam combination [40] is not available in most centers in Mexico. A previous study that included a global collection of 81, 781 isolates of Enterobacterales collected from 39 countries in five geographic regions from 2012 to 2017 also reported that K. pneumoniae had a higher number of meropenem-nonsusceptible isolates (76.7%). In contrast, the majority of meropenem-nonsusceptible Enterobacterales were found to carry KPC-type carbapenemases (47.4%), followed by metallo-β-lactamases (20.6%) or OXA-48-like β-lactamases (19.0%) [41].
In a percentage of our isolates resistant to carbapenems, mainly Enterobacterales, we did not find any gene that codes for carbapenemase. Thus, carbapenem resistance may be porin- or efflux-pump-associated.
MDR bacteria are well-recognized to be one of the most important health problems. In a previous study on clinical samples in Iran, 16.50% of P. aeruginosa isolates and 74.75% of A. baumannii isolates were MDR [42]. Furthermore, a systematic review that included eight articles showed that the prevalence of MDR Gram-negative bacilli was from 11.2 to 59.1% in nursing home residents [43] and colonization by MDR P. aeruginosa and A. baumannii was reported in 5.4% and 15.0% of residents in long-term care facilities in North America [44]. In the present study, similar results were obtained, including clinical samples with 29.7% MDR isolates of P. aeruginosa and 79.1% MDR isolates of A. baumannii. MDR in bacterial species makes the control of infectious diseases difficult, worsening the effectiveness of treatment and increasing the likelihood of proliferation of resistant pathogens, leading to an extended time of infection in patients [45]. Thus, exhaustive infection control measures should be implemented when these organisms are detected.
Independent predictors of mortality have been associated with ICU stay, antimicrobial therapy, and clinical factors such as sepsis, the use of medical devices, and the presence of immunosuppression [46,47,48,49] that have also been associated with high multidrug resistance [50]. Our study did not analyze clinical data, but including these variables may provide a better idea of their impact on drug resistance in Mexican hospitals.
In our study, we performed AMR surveillance by collecting data from a limited number of sentinel healthcare facilities (43 centers), and the use of WHONET facilitated the analysis by obtaining the data directly from automated instruments. However, there are invisible areas in regions of the country that need to be included to have a better idea of the actual situation of drug resistance [51].
In our study, different AST methods were used in each laboratory. This variability introduced a bias in the results. However, to minimize the effect of this variation, all values were interpreted according to CLSI document M100-S33.
5. Conclusions
This study has certain limitations, including that colonizing and infection-causing isolates were not distinguished, the selection of the participating centers introduces possible biases, and the study includes data for a relatively short period (January to March 2023).
The highest carbapenem resistance in Enterobacterales and P. aeruginosa was detected in isolates from ICU patients, and the highest carbapenem resistance in A. baumannii was detected in isolates recovered from hospitalized non-ICU patients. Furthermore, the high cephalosporin resistance detected in the 0–18 years age group deserves special attention. The blaNDM gene was the most frequently detected carbapenemase-encoding gene among carbapenem-resistant K. pneumoniae and E. coli, followed by blaKPC.
Our results may facilitate the implementation of direct and specific antibiotic resistance control measures according to the site of care and patient age group. They may have potential clinical implications by allowing the guidance of empirical therapies that may be more effective and more useful for the establishment of public health policies at a national level.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens12091144/s1, Table S1: Percentages of antimicrobial resistance for selected pathogens at 43 centers according to sites of care; Table S2: Percentages of antimicrobial resistance for selected pathogens at 43 centers according to age groups; Table S3: Percentages of antimicrobial resistance for selected Gram-negative pathogens at 43 centers according to clinical specimens.
Author Contributions
Data curation, E.G.-G.; formal analysis, E.G.-G.; investigation, F.R.-L., B.A.M.-G., L.E.L.-J., E.B.-M., M.d.R.V.-L., M.d.C.V.-A., D.R.-R., C.D.M.-D., S.Q.-P., J.M.F.-G., J.M.P.-H., Y.P.C.-L., E.L.-G., E.R.-N., E.G.-D., E.V.C.-C., J.P.M.-R., V.A.M.-C., A.P.-d.-L.-G., M.A.-E., L.K.A.-B., L.J.Q.-C., E.R.-A., J.M.B.-M., C.P.-I., M.B.B.-S., N.A.A.-S., C.T.M.-d.-l.-P., M.G.-M., T.P.-V., G.J.-B., M.I.M.-M., M.d.l.L.M.-P., M.G.-B., X.Y.S.-G., N.V.N.-V., L.E.M.-B., A.D.-B., M.A.S.-C., I.L.-O., A.M.-C., J.R.-Z., R.F.-C., S.M.-M., I.C.M.-A., M.L.-G., L.S.D.-M., C.M.C.-U., I.E.B.-H.-y.-C. and L.I.L.-M.; methodology, L.E.L.-J. and E.G.-G.; resources, E.G.-G.; writing—original draft, E.G.-G.; writing—review and editing, F.R.-L. and B.A.M.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially supported by Pfizer (Grant 72469575). No person from Pfizer has reviewed this manuscript, and there has been no interference with the information presented. The researchers received no salary or payment.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional ethics committee) of the Hospital Civil de Guadalajara “Fray Antonio Alcalde”, Jalisco, Mexico (protocol code 129/22 and date of approval 2 May 2022).
Informed Consent Statement
Approved this study (129/22). The need for informed consent was waived by the ethics committee.
Data Availability Statement
All research data is included in Supplementary Tables S1–S3.
Acknowledgments
We thank all members of the Network for the Research and Surveillance of Drug Resistance (INVIFAR).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Gould, I. The epidemiology of antibiotic resistance. Int. J. Antimicrob. Agents 2008, 32 (Suppl. 1), S2–S9. [Google Scholar] [CrossRef]
- Arslan, H.; Azap, K.; Ergönül, O.; Timurkaynak, F.; Group UTIS. Risk factors for ciprofloxacin resistance among Escherichia coli strains isolated from community-acquired urinary tract infections in Turkey. J. Antimicrob. Chemother. 2005, 56, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Colgan, R.; Johnson, J.R.; Kuskowski, M.; Gupta, K. Risk factors for trimethoprim-sulfamethoxazole resistance in patients with acute uncomplicated cystitis. Antimicrob. Agents Chemother. 2008, 52, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Barrios, H.; Garza-Ramos, U.; Reyna-Flores, F.; Sanchez-Perez, A.; Rojas-Moreno, T.; Garza-Gonzalez, E.; Llaca-Diaz, J.M.; Camacho-Ortiz, A.; Guzmán-López, S.; Silva-Sanchez, J. Isolation of carbapenem-resistant NDM-1-positive Providencia rettgeri in Mexico. J. Antimicrob. Chemother. 2013, 68, 1934–1936. [Google Scholar] [CrossRef]
- Barrios, H.; Silva-Sanchez, J.; Reyna-Flores, F.B.; Sanchez-Perez, A.Q.; Sanchez-Francia, D.Q.; Aguirre-Torres, J.A.T.; Sánchez-Rogel, J.T.; Garza-Ramos, U. Detection of a NDM-1-producing Klebsiella pneumoniae (ST22) clinical isolate at a pediatric hospital in Mexico. Pediatr. Infect. Dis. J. 2014, 33, 335. [Google Scholar] [CrossRef]
- Aquino-Andrade, A.; Merida-Vieyra, J.; de la Garza, E.A.; Arzate-Barbosa, P.; Ranero, A.D.C. Carbapenemase-producing Enterobacteriaceae in Mexico: Report of seven non-clonal cases in a pediatric hospital. BMC Microbiol. 2018, 18, 38. [Google Scholar] [CrossRef]
- Garza-Ramos, U.; Morfin-Otero, R.; Sader, H.S.; Jones, R.N.; Hernandez, E.; Rodriguez-Noriega, E.; Sanchez, A.; Carrillo, B.; Esparza-Ahumada, S.; Silva-Sanchez, J. Metallo-beta-lactamase gene bla(IMP-15) in a class 1 integron, In95, from Pseudomonas aeruginosa clinical isolates from a hospital in Mexico. Antimicrob. Agents Chemother. 2008, 52, 2943–2946. [Google Scholar] [CrossRef]
- Bocanegra-Ibarias, P.; Pena-López, C.; Camacho-Ortiz, A.; Llaca-Díaz, J.; Silva-Sánchez, J.; Barrios, H.; Garza-Ramos, U.; Rodríguez-Flores, A.M.; Garza-González, E. Genetic characterisation of drug resistance and clonal dynamics of Acinetobacter baumannii in a hospital setting in Mexico. Int. J. Antimicrob. Agents 2015, 45, 309–313. [Google Scholar] [CrossRef]
- Garza-González, E.; Bocanegra-Ibarias, P.; Bobadilla-del-Valle, M.; Ponce-de-León-Garduño, L.A.; Esteban-Kenel, V.; Silva-Sánchez, J.; Garza-Ramos, U.; Barrios-Camacho, H.; López-Jácome, L.E.; Colin-Castro, C.A.; et al. Drug resistance phenotypes and genotypes in Mexico in representative Gram-negative species: Results from the infivar network. PLoS ONE 2021, 16, e0248614. [Google Scholar] [CrossRef]
- Garza-González, E.; Morfín-Otero, R.; Mendoza-Olazarán, S.; Bocanegra-Ibarias, P.; Flores-Treviño, S.; Rodríguez-Noriega, E.; Ponce-De-León, A.; Sanchez-Francia, D.; Franco-Cendejas, R.; Arroyo-Escalante, S.; et al. A snapshot of antimicrobial resistance in Mexico. Results from 47 centers from 20 states during a six-month period. PLoS ONE 2019, 14, e0209865. [Google Scholar] [CrossRef] [PubMed]
- López-Jácome, L.E.; Fernández-Rodríguez, D.; Franco-Cendejas, R.; Camacho-Ortiz, A.; Morfin-Otero, M.D.R.; Rodríguez-Noriega, E.; Ponce-De-León, A.; Ortiz-Brizuela, E.; Rojas-Larios, F.; Velázquez-Acosta, M.D.C.; et al. Increment Antimicrobial Resistance During the COVID-19 Pandemic: Results from the Invifar Network. Microb. Drug Resist. 2021, 28, 338–345. [Google Scholar] [PubMed]
- CLSI. M100-S33; Performance Standards for Antimicrobial Susceptibility Testing. Twenty-Second Informational Supplement; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022.
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Garza-Ramos, U.; Silva-Sánchez, J.; López-Jácome, L.E.; Hernández-Durán, M.; Colín-Castro, C.A.; Sánchez-Pérez, A.; Rodríguez-Santiago, J.; Morfín-Otero, R.; Rodriguez-Noriega, E.; Velázquez-Acosta, M.D.; et al. Carbapenemase-Encoding Genes and Colistin Resistance in Gram-Negative Bacteria During the COVID-19 Pandemic in Mexico: Results from the Invifar Network. Microb. Drug Resist. 2023, 29, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Bogaerts, P.; Rezende de Castro, R.; de Mendonça, R.; Huang, T.D.; Denis, O.; Glupczynski, Y. Validation of carbapenemase and extended-spectrum β-lactamase multiplex endpoint PCR assays according to ISO 15189. J. Antimicrob. Chemother. 2013, 68, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.S.; Kim, K.; Huh, J.Y.; Jung, B.; Kang, M.S.; Hong, S.G. Multiplex PCR for rapid detection of genes encoding class A carbapenemases. Ann. Lab. Med. 2012, 32, 359–361. [Google Scholar] [CrossRef]
- Perez, F.; Hujer, A.M.; Hujer, K.M.; Decker, B.K.; Rather, P.N.; Bonomo, R.A. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2007, 51, 3471–3484. [Google Scholar] [CrossRef]
- Mulvey, M.R.; Grant, J.M.; Plewes, K.; Roscoe, D.; Boyd, D.A. New Delhi metallo-β-lactamase in Klebsiella pneumoniae and Escherichia coli, Canada. Emerg. Infect. Dis. 2011, 17, 103–106. [Google Scholar] [CrossRef]
- Spellberg, B.; Blaser, M.; Guidos, R.J.; Boucher, H.W.; Bradley, J.S.; Eisenstein, B.I.; Gerding, D.; Lynfield, R.; Reller, L.B.; Rex, J.; et al. Combating antimicrobial resistance: Policy recommendations to save lives. Clin. Infect. Dis. 2011, 52 (Suppl. 5), S397–S428. [Google Scholar]
- Dortet, L.; Poirel, L.; Nordmann, P. Worldwide dissemination of the NDM-type carbapenemases in gram-negative bacteria. BioMed Res. Int. 2014, 2014, 249856. [Google Scholar] [CrossRef]
- Patel, G.; Huprikar, S.; Factor, S.H.; Jenkins, S.G.; Calfee, D.P. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect. Control. Hosp. Epidemiol. 2008, 29, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- De Waele, J.J.; Akova, M.; Antonelli, M.; Canton, R.; Carlet, J.; De Backer, D.; Dimopoulos, G.; Garnacho-Montero, J.; Kesecioglu, J.; Lipman, J.; et al. Antimicrobial resistance and antibiotic stewardship programs in the ICU: Insistence and persistence in the fight against resistance. A position statement from ESICM/ESCMID/WAAAR round table on multi-drug resistance. Intensive Care Med. 2018, 44, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Sommerstein, R.; Damonti, L.; Marschall, J.; Harbarth, S.; Gasser, M.; Kronenberg, A.; Buetti, N. Distribution of pathogens and antimicrobial resistance in ICU-bloodstream infections during hospitalization: A nationwide surveillance study. Sci. Rep. 2021, 11, 16876. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.F.; Lan, C.Y. Antimicrobial resistance in Acinetobacter baumannii: From bench to bedside. World J. Clin. Cases 2014, 2, 787–814. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, R.B.; Dhakephalkar, P.K.; Niphadkar, K.B.; Chopade, B.A. A study on nosocomial pathogens in ICU with special reference to multiresistant Acinetobacter baumannii harbouring multiple plasmids. Indian J. Med. Res. 2008, 128, 178–187. [Google Scholar]
- Uwingabiye, J.; Frikh, M.; Lemnouer, A.; Bssaibis, F.; Belefquih, B.; Maleb, A.; Dahraoui, S.; Belyamani, L.; Bait, A.; Haimeur, C.; et al. Acinetobacter infections prevalence and frequency of the antibiotics resistance: Comparative study of intensive care units versus other hospital units. Pan Afr. Med. J. 2016, 23, 191. [Google Scholar] [CrossRef]
- Kalanuria, A.; Zai, W.; Mirski, M. Ventilator-associated pneumonia in the ICU. Crit. Care 2014, 18, 208. [Google Scholar] [CrossRef]
- Timsit, J.-F.; Soubirou, J.-F.; Voiriot, G.; Chemam, S.; Neuville, M.; Mourvillier, B.; Sonneville, R.; Mariotte, E.; Bouadma, L.; Wolff, M. Treatment of bloodstream infections in ICUs. BMC Infect. Dis. 2014, 14, 489. [Google Scholar] [CrossRef]
- Liu, C.; Chang, Y.; Xu, Y.; Luo, Y.; Wu, L.; Mei, Z.; Li, S.; Wang, R.; Jia, X. Distribution of virulence-associated genes and antimicrobial susceptibility in clinical. Oncotarget 2018, 9, 21663–21673. [Google Scholar] [CrossRef]
- Morfin-Otero, R.; Tinoco-Favila, J.C.; Sader, H.S.; Salcido-Gutierrez, L.; Perez-Gomez, H.R.; Gonzalez-Diaz, E.; Petersen, L.; Rodriguez-Noriega, E. Resistance trends in gram-negative bacteria: Surveillance results from two Mexican hospitals, 2005–2010. BMC Res. Notes 2012, 5, 277. [Google Scholar] [CrossRef]
- Morfín-Otero, R.; Curiel, A.; Rocha, M.; Alpuche-Aranda, C.; Santos-Preciado, J.; Gayosso-Vázquez, C.; Araiza-Navarro, J.; Flores-Vaca, M.; Esparza-Ahumada, S.; González-Díaz, E.; et al. Acinetobacter baumannii infections in a tertiary care hospital in Mexico over the past 13 years. Chemotherapy 2013, 59, 57–65. [Google Scholar] [CrossRef]
- Alcántar-Curiel, M.D.; García-Torres, L.F.; González-Chávez, M.I.; Morfín-Otero, R.; Gayosso-Vázquez, C.; Jarillo-Quijada, M.D.; Fernández-Vázquez, J.L.; Giono-Cerezo, S.; Rodríguez-Noriega, E.; Santos-Preciado, J.I. Molecular mechanisms associated with nosocomial carbapenem-resistant Acinetobacter baumannii in Mexico. Arch. Med. Res. 2014, 45, 553–560. [Google Scholar] [CrossRef]
- Ponce-de-Leon, A.; Rodríguez-Noriega, E.; Morfín-Otero, R.; Cornejo-Juárez, D.P.; Tinoco, J.C.; Martínez-Gamboa, A.; Gaona-Tapia, C.J.; Guerrero-Almeida, M.L.; Martin-Onraët, A.; Vallejo Cervantes, J.L.; et al. Antimicrobial susceptibility of Gram-negative bacilli isolated from intra-abdominal and urinary-tract infections in Mexico from 2009 to 2015: Results from the Study for Monitoring Antimicrobial Resistance Trends (SMART). PLoS ONE 2018, 13, e0198621. [Google Scholar] [CrossRef] [PubMed]
- Diekema, D.; Pfaller, M.; Jones, R.; Doern, G.; Kugler, K.; Beach, M.; Sader, H. Trends in antimicrobial susceptibility of bacterial pathogens isolated from patients with bloodstream infections in the USA, Canada and Latin America. SENTRY Participants Group. Int. J. Antimicrob. Agents 2000, 13, 257–271. [Google Scholar] [CrossRef]
- Reinert, R.R.; Low, D.E.; Rossi, F.; Zhang, X.; Wattal, C.; Dowzicky, M.J. Antimicrobial susceptibility among organisms from the Asia/Pacific Rim, Europe and Latin and North America collected as part of TEST and the in vitro activity of tigecycline. J. Antimicrob. Chemother. 2007, 60, 1018–1029. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nelson, R.E.; Slayton, R.B.; Stevens, V.W.; Jones, M.M.; Khader, K.; Rubin, M.A.; Jernigan, J.A.; Samore, M.H. Attributable Mortality of Healthcare-Associated Infections Due to Multidrug-Resistant Gram-Negative Bacteria and Methicillin-Resistant Staphylococcus aureus. Infect. Control. Hosp. Epidemiol. 2017, 38, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ye, W.; Qi, Y.; Ying, Y.; Xia, Z. Overcoming Multidrug Resistance in Bacteria through Antibiotics Delivery in Surface-Engineered Nano-Cargos: Recent Developments for Future Nano-Antibiotics. Front. Bioeng. Biotechnol. 2021, 9, 696514. [Google Scholar] [CrossRef] [PubMed]
- Bocanegra-Ibarias, P.; Garza-González, E.; Morfín-Otero, R.; Barrios, H.; Villarreal-Treviño, L.; Rodríguez-Noriega, E.; Garza-Ramos, U.; Petersen-Morfin, S.; Silva-Sanchez, J. Molecular and microbiological report of a hospital outbreak of NDM-1-carrying Enterobacteriaceae in Mexico. PLoS ONE 2017, 12, e0179651. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Daikos, G.L.; Tiseo, G.; Bassoulis, D.; Giordano, C.; Galfo, V.; Leonildi, A.; Tagliaferri, E.; Barnini, S.; Sani, S.; et al. Efficacy of Ceftazidime-avibactam Plus Aztreonam in Patients With Bloodstream Infections Caused by Metallo-β-lactamase–Producing Enterobacterales. Clin. Infect. Dis. 2021, 72, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Kazmierczak, K.M.; Karlowsky, J.A.; de Jonge, B.L.M.; Stone, G.G.; Sahm, D.F. Epidemiology of Carbapenem Resistance Deter-minants Identified in Meropenem-Nonsusceptible. Antimicrob. Agents Chemother. 2021, 65, e0200020. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, B.; Bazgir, Z.N.; Goli, H.R.; Iranpour, F.; Mohammadi, F.; Babaei, R. Prevalence of multi-drug resistant (MDR) and extensively drug-resistant (XDR) phenotypes of Pseudomonas aeruginosa and Acinetobacter baumannii isolated in clinical samples from Northeast of Iran. BMC Res. Notes 2020, 13, 380. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, S.; Smaldone, A.; Larson, E. Prevalence of multidrug-resistant gram-negative bacteria among nursing home residents: A systematic review and meta-analysis. Am. J. Infect. Control 2017, 45, 512–518. [Google Scholar] [CrossRef]
- Rodríguez-Villodres, Á.; Martín-Gandul, C.; Peñalva, G.; Guisado-Gil, A.B.; Crespo-Rivas, J.C.; Pachón-Ibáñez, M.E.; Lepe, J.A.; Cisneros, J.M. Prevalence and Risk Factors for Multidrug-Resistant Organisms Colonization in Long-Term Care Facilities around the World: A Review. Antibiotics 2021, 10, 680. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; McNeil, E.B.; Huang, Z.; Chen, L.; Lu, X.; Wang, C.; Chen, H.; Chongsuvivatwong, V. Household financial burden among multidrug-resistant tuberculosis patients in Guizhou province, China: A cross-sectional study. Medicine 2020, 99, e21023. [Google Scholar] [CrossRef] [PubMed]
- Punpanich, W.; Nithitamsakun, N.; Treeratweeraphong, V.; Suntarattiwong, P. Risk factors for carbapenem non-susceptibility and mortality in Acinetobacter baumannii bacteremia in children. Int. J. Infect. Dis. 2012, 16, e811–e815. [Google Scholar] [CrossRef] [PubMed]
- Özgür, E.S.; Horasan, E.S.; Karaca, K.; Ersöz, G.; Atış, S.N.; Kaya, A. Ventilator-associated pneumonia due to extensive drug-resistant Acinetobacter baumannii: Risk factors, clinical features, and outcomes. Am. J. Infect. Control. 2014, 42, 206–208. [Google Scholar] [CrossRef]
- Townsend, J.; Na Park, A.; Gander, R.; Orr, K.; Arocha, D.; Zhang, S.; Greenberg, D.E. Acinetobacter infections and outcomes at an academic medical center: A disease of long-term care. Open Forum Infect. Dis. 2015, 2, ofv023. [Google Scholar] [CrossRef]
- Liu, Q.; Li, W.; Du, X.; Li, W.; Zhong, T.; Tang, Y.; Feng, Y.; Tao, C.; Xie, Y. Risk and Prognostic Factors for Multidrug-Resistant Acinetobacter baumannii Complex Bacteremia: A Retrospective Study in a Tertiary Hospital of West China. PLoS ONE 2015, 10, e0130701. [Google Scholar] [CrossRef]
- Rice, L.B. Progress and challenges in implementing the research on ESKAPE pathogens. Infect. Control Hosp. Epidemiol. 2010, 31 (Suppl. 1), S7–S10. [Google Scholar] [CrossRef]
- O’Brien, T.F.; Clark, A.; Peters, R.; Stelling, J. Why surveillance of antimicrobial resistance needs to be automated and comprehensive. J. Glob. Antimicrob. Resist. 2019, 17, 8–15. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).