Abstract
Ticks play a pivotal role in propagating a diverse spectrum of infectious agents that detrimentally affect the health of both humans and animals. In the present study, a molecular survey was executed of piroplasmids in ticks collected from small ruminants in four districts within Konya province, Turkey. Microscopic examination identified 1281 adult ticks, which were categorized into 357 pools based on their species, sexes, host animals, and collection site before DNA extraction. The infection rates were calculated by using a maximum likelihood estimate (MLE) with 95% confidence intervals (CI). Hyalomma detritum, H. excavatum, Rhipicephalus bursa, R. sanguineus, and R. turanicus were identified in this study. Among the five tick species identified here, R. turanicus exhibited the highest infestation rate in both goats and sheep. The presence of Babesia ovis and Theileria ovis based on 18S rRNA was confirmed using molecular assay. The overall MLE of infection rates for B. ovis and T. ovis was 2.49% (CI 1.72–3.46) and 1.46% (CI 0.87–2.23), respectively. The MLE of B. ovis and T. ovis infection rates in R. bursa was 10.80% (CI 7.43–14.90) and 0.33% (CI 0.02–1.42), respectively, while that in R. turanicus was 0.12% (CI 0.01–0.51) and 2.08% (CI 1.25–3.22). This study further confirms that R. turanicus and R. sanguineus can act as vectors for B. ovis, thus advancing our comprehension of tick-borne piroplasmids epidemiology and providing valuable insights for the development of effective control strategies for ticks and tick-borne diseases in Turkey.
1. Introduction
Piroplasms, Babesia spp., and Theileria spp., which are conveyed by ticks, serve as the etiologic agents of piroplasmosis that significantly impact the economy of the livestock industry, resulting in production losses, treatment and prevention costs, and increased morbidity and mortality [1,2,3]. Babesiosis and theileriosis are found in different hosts and are generally prevalent in tropical and subtropical zones [4]. T. parva and T. annulata cause bovine clinical theileriosis, while T. buffeli, T. orientalis, and T. sergenti are associated with benign theileriosis [5]. B. bovis, B. bigemina, B. divergens, B. major, B. occultans, and B. ovata are confirmed to infect cattle; however, only B. bovis, B. bigemina, and B. divergens are known to cause clinical babesiosis [6]. Moreover, a recent study provided evidence that B. naoakii (Babesia sp. Mymensingh) can cause acute clinical babesiosis in cattle [7]. In small ruminants, T. lestoquardi, T. luwenshuni, and T. uilenbergi are considered the pathogenic Theileria spp. causing malignant theileriosis [8,9]. On the other hand, B. ovis, B. motasi, B. crassa, and Babesia sp. Xinjiang are the causative agents that can induce severe babesiosis [10,11].
Ticks are essential hematophagous arachnid external parasites affiliated with the Ixodida suborder, deemed inferior only to mosquitoes in relevance as carriers of pathogenic entities significantly impacting animal and human health [12,13,14,15]. Ticks exhibit a broad spectrum of hosts, encompassing mammals, birds, and reptiles; during their blood meal, ticks can transmit a multitude of pathogens to their hosts [16,17]. According to the recent classification, the tick species are categorized into three families: Ixodidae, which included 702 species of hard ticks; Argasidae, including 193 species of soft ticks; and Nuttalliellidae, represented solely by Nuttalliella namaqua [18]. The prevalence of ticks fluctuates with the season [19,20]. While most tick species persist in low numbers throughout the year, their abundance spikes briefly under favorable climatic conditions. In 1984, the United Nations Food and Agricultural Organization (FAO) estimated that Ixodidae tick infestations resulted in a staggering global cost of $US 7.0 billion annually due to skin damage and tick-borne diseases [21].
Owing to the unique climatic conditions of Turkey, diverse vegetation, and fertile soil, the country possesses a substantial livestock resource, with small ruminants representing the primary asset and their total population exceeding 56 million in animal numbers [22,23]. These conditions within the country also provide a conducive environment for ticks and facilitate the maintenance of parasite-host-vector relationships [4,24]. To date, in Turkey, 51 tick species have been identified (43 from Ixodidae and 8 from Argasidae families) [25]. The most frequently encountered tick genera in this country include Hyalomma, Ixodes, Haemaphysalis, Dermacentor, and Rhipicephalus [26,27,28,29,30,31,32]. Several studies performed the epidemiological analysis of Babesia spp. and Theileria spp. in small ruminants in different regions of Turkey [8,33,34,35,36]. A comprehensive comprehension of tick-borne piroplasmid epidemiology is vital for disease control. However, data on tick vectors of piroplasmids infecting small ruminants in Turkey are still limited, and some provinces have not yet reported on occurrences of tick-borne piroplasmids. Therefore, this study aimed to conduct the molecular identification of Theileria and Babesia species in ixodid ticks collected from sheep and goats to enhance the understanding of their distribution in Turkey for their effective control.
2. Materials and Methods
2.1. Ethical Statement
The sheep and goat owners were duly informed about the research goals, and their consent was obtained before the tick samples collection. All procedures related to tick sampling and subsequent processing were as per the ethical guidelines established by Obihiro University of Agriculture and Veterinary Medicine. (Animal experiment approval number: 19–74; DNA experiment approval number: 1706-4, 1705-4, 1704-4).
2.2. Study Area
Konya, located between 36°41′ and 39°16′ north latitude and 31°14′ and 34°26′ east longitude, is the province with the largest land area in the southern part of the Central Anatolia region of Turkey. Its surface area is 38,873 km2 (excluding lakes). The average elevation is 1.016 m. Konya’s provincial territory has 60% cultivated and planted areas, 17% forests and heathlands, and 15% meadows and pastures. Konya resembles a big steppe. There is little forest. Konya province has harsh, cold, snowy winters and hot and dry summers. The average annual temperature is 11.5 °C, and the average relative humidity is 60. The average annual precipitation in Konya is 326 mm. Although only a few districts of Konya close to the Mediterranean region have a slightly Mediterranean climate, the districts of Kadınhanı, Karatay, Sarayönü, and Selçuklu (Figure 1), where ticks were collected in the study have the main climatic, vegetational, and geographical characteristics found in the province.
Figure 1.
Map showing the four districts (Kadınhanı, Karatay, Sarayönü, Selçuklu) of Konya Province, Turkey, where tick samples were collected.
2.3. Tick Collection and Identification
In 2013, tick samples were gathered between May and June. 614 and 667 ticks were randomly collected from apparently healthy 76 sheep and 29 goats, respectively. Each tick was carefully removed from the host’s skin with the livestock owner’s permission using a fine-tipped bent tweezer, making sure it was close to the dermis to minimize any harm and discomfort for the species. All tick specimens were then preserved in 70% ethanol. The identification of tick species was conducted employing a binocular microscope (Olympus SZX16, Tokyo, Japan), following the guidelines of standard taxonomic keys [37].
2.4. DNA Extraction
Upon microscopic identification, the tick specimens were grouped based on species, sexes, and site of collection, culminating in the formation of 357 pools, with each pool containing 1 to 10 ticks, guided by the sample size. The tick DNA was extracted following the methodology previously described by [38], using a NucleoSpin Tissue Kit (Macherey-Nagel, Düren, Germany), following the manufacturer’s instructions. The extracted DNA was eluted with 50 μL of double-distilled water (Invitrogen, UltrapureTM Distilled Water, DNAse, and RNAse, Free) and then preserved at −30 °C awaiting further utilization.
2.5. Molecular Identification of Tick-Borne Piroplasmids
All tick pool specimens underwent initial screening with a universal primer target at the 18S rRNA genes of both Babesia and Theileria [39], with a thermocycling protocol began with an initial denaturation at 95 °C for 3 min, followed by 35 cycles of 30-s denaturation at 95 °C, 30-s annealing at 55 °C, and 1 min extension at 72 °C. A final extension step was carried out at 72 °C for 7 min. The positive samples obtained from the initial screening were chosen for species-specific identification. The partial regions of several genes were amplified, including the apical membrane antigen-1 (AMA-1) of B. naoakii, the 18S rRNA of B. ovis, the merozoite surface antigen (Tlms) of T. lestoquardi, the 18S rRNA of T. luwenshuni, the 18S rRNA of T. ovis, and the 18S rRNA of T. uilenbergi. The PCR cycling conditions for species-specific piroplasmids screening mirrored those previously described, with the only variation being the annealing temperatures, which were adopted from the referenced publications and are elaborated upon in Table 1. The 10 µL final reaction mixture was used for all PCR screening that contained 1 µL of 10× AmpliTaq gold 360 buffer (Applied Biosystems, Waltham, MA, USA), 0.8 µL of 25 mM magnesium chloride (Applied Biosystems), 0.8 µL of dNTP mix (Applied Biosystems), 0.2 µM of each primer, 0.05 µL of 5 U/µL AmpliTaq gold 360 DNA polymerase (Applied Biosystems), 1 µL of DNA template, and 5.95 µL of double-distilled water. Positive controls included previously sequence-confirmed DNA templates and double-distilled water was used as the negative control.
2.6. Cloning and Sequencing Analysis
Selected partial positive PCR amplicons, based on the presence of piroplasmids detected from various tick species, were chosen for sequencing. To ensure adequate concentrations for sequencing, PCR was conducted in larger volumes. PCR amplicons were purified employing a gel extraction kit. (NucleoSpinTM Gel and PCR Clean Up, Macherey-Nagel, Germany). The concentration of eluted DNA was measured using a NanoDrop 2000 spectrophotometer. All PCR amplicons with an adequate DNA concentration were directly sequenced, and samples of low concentration were first cloned into a pGEM vector as per the commercial protocol of pGEM®-T Easy Vector System (Promega, Fitchburg, WI, USA). Recombinant clones resulting from this transformation were selected for sequencing. The plasmid was extracted from this culture using the Nucleospin® Plasmid QuickPure Kit (Macherey-Nagel-German), and the sequencing of samples was performed with the Big Dye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) on an ABI PRISM 3130xl Genetic Analyzer (Applied Biosystems).
2.7. Phylogenetic and Statistical Analysis
Nucleotide sequences garnered in this investigation were aligned with those previously submitted to GenBank via the BLASTn algorithm, facilitating the determination of identities and similarities. Phylogenetic trees for B. ovis (18S rRNA) and Babesia/Theileria (18S rRNA) were prepared using the maximum likelihood method facilitated using MEGA version X software. Bootstrap analysis with 1000 replications was employed to ascertain the confidence of the nodes and branches within the trees. Finally, sequences derived from the current study were submitted to the GenBank of the National Center for Biotechnology Information via BankIt. Statistical analysis was performed in R software, version 4.3.1 (The R Foundation for Statistical Computing). The maximum-likelihood estimate (MLE) of pooled prevalence was calculated using the “PoolTestR” package [40].
2.8. Accession Numbers
Sequences derived from this research have been submitted to GenBank and can be accessed using the following accession numbers: for B. ovis: OR394130, OR395165, OR395166, OR395167, OR395168, OR395169, and OR395170; for T. ovis: OR395235, OR395236, OR395237, OR395238, OR395239, OR395240, OR395241, OR395242, OR395243, OR395244, OR395245, OR395246, OR395247, OR395248, OR395249, OR395250, OR395251, and OR395252.

Table 1.
PCR primers were used for the piroplasmids identification in this study.
Table 1.
PCR primers were used for the piroplasmids identification in this study.
| Target Piroplasmid | Target Gene | Primer Sequence (5′ to 3′) | Size (bp) | Annealing Temperature (°C) | References |
|---|---|---|---|---|---|
| Babesia/Theileria spp. | 18S rRNA | GTCTTGTAATTGGAATGATGG TAGTTTATGGTTAGGACTACG | ~500 | 55 | [39] |
| B. naoakii | AMA-1 | TGGCGCCGACTTCCTGGAGCCCATCTCCAA AGCTGGGGCCCTCCTTCGATGAACCGTCGG | 371 | 64 | [41] |
| B. ovis | 18S rRNA | TGGGCAGGACCTTGGTTCTTCT CCGCGTAGCGCCGGCTAAATA | 549 | 62 | [10] |
| T. lestoquardi | Merozoite surface antigen (Tlms) | GTGCCGCAAGTGAGTCA GGACTGATGAGAAGACGATGAG | 730 | 54 | [42] |
| T. luwenshuni | 18S rRNA | GGTAGGGTATTGGCCTACTGA TCATCCGGATAATACAAG | 389 | 57 | [43] |
| T. ovis | 18S rRNA | TCGAGACCTTCGGGT TCCGGACATTGTAAAACAAA | 520 | 53 | [44] |
| T. uilenbergi | 18S rRNA | GGTAGGGTATTGGCCTACCGG ACACTCGGAAAATGCAAGCA | 388 | 55 | [43] |
3. Results
3.1. Tick Species Identification and Infestation Rate
In this study, morphological identification showed that the 1281 ixodid adult ticks gathered from 76 sheep and 29 goats belonged to two genera, Hyalomma and Rhipicephalus; among these, five species, H. detritum (n = 7), H. excavatum (n = 94), R. bursa (n = 308), R. sanguineus (n = 3), and R. turanicus (n = 869) were from the four districts of Konya province. The heaviest tick infestation in sheep and goats was observed in the Selçuklu district, followed by the Kadınhanı district.
The infestation rates of H. detritum, H. excavatum, R. bursa, R. sanguineus, and R. turanicus were 0.55, 0.23, 10.69, 0, and 40.59% in goats, and 0, 7.10, 13.35, 0.23, and 27.24% in sheep, respectively (Figure 2).
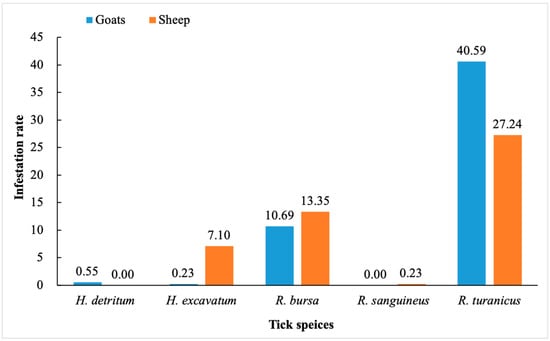
Figure 2.
Total infestation rate of H. detritum, H. excavatum, R. bursa, R. sanguineus, and R. turanicus in small ruminants in this study.
3.2. Detection of Piroplasms in Ticks
Among the 357 tick pools analyzed in this study, 49 pools had piroplasms. B. ovis and T. ovis were identified in 31/357 and 18/357 pools, respectively, and no other piroplasma species were detected. The MLE for B. ovis was 2.49% (CI 1.72–3.46), while for T. ovis, it was 1.46% (CI 0.87–2.23).
Three tick species, R. bursa, R. sanguineus, and R. turanicus were detected that carried Babesia or Theileria in which B. ovis was present in all three species; in contrast, T. ovis was identified only in R. bursa and R. turanicus. The MLE of B. ovis in R. bursa (29/118 pools) and R. turanicus (1/169 pools) were 10.80% (CI 7.43–14.90) and 0.12% (CI 0.01–0.51), respectively. Due to significant variation in the number of pools for R. sanguineus compared to that of the other two species, the MLE for B. ovis in R. sanguineus pools was not estimated. Furthermore, the estimated MLE for T. ovis in R. bursa (1/118 pools) and R. turanicus (17/169 pools) was 0.33% (CI 0.02–1.42) and 2.08% (CI 1.25–3.22), respectively.
The presence of piroplasma was noted across all four examined districts. B. ovis was absent in the Kadınhanı district but was identified in the other three districts. T. ovis was found in the Kadınhanı and Selçuklu districts. A detailed distribution of piroplasma detection rates, based on district and tick species, is presented in Table 2.

Table 2.
The collected tick distribution and infection rates of B. ovis and T. ovis within the four districts in Konya province.
3.3. Phylogenetic Analyses of B. ovis and T. ovis
In this study, phylogenetic trees using the B. ovis 18S rRNA (BoSSUrRNA) gene sequence were constructed. The BoSSUrRNA sequences of B. ovis from R. bursa, R. sanguineus, and R. turanicus, identified in this study, were phylogenetically clustered within the same clade as sheep and tick isolates from Turkey, and sheep and goat isolates from various other countries, including Bosnia and Herzegovina, Iran, Iraq, Spain, Tunisia, and Uganda. However, three sequences (KT587793, MG920541, MK713823) exhibited divergent clustering from R. sanguineus and R. bursa in Palestine and Turkey, which formed a separate clade (Figure 3).

Figure 3.
Phylogenetic analysis of B. ovis based on the 18S rRNA. Sequences identified in the current investigation are highlighted in bold. Node numbers indicate the percentage frequency of clades across 1000 bootstrap replicates of the taxa.
Eighteen positive samples for the Babesia/Theileria 18S rRNA gene using a universal primer; however, these were negative when species-specific primers were used and included in a phylogenetic tree prepared using the universal primer for the 18S rRNA gene. These eighteen samples formed a distinct clade and clustered with T. ovis sequences from Ghana (OQ7669770), Egypt (OP389065), India (OM666861), and Turkey (OM066224, OM066211). This clade was distinct from T. luwenshuni, T. annulata, B. naoakii, B. ovis, and B. bovis sequences from samples of other countries (Figure 4).

Figure 4.
Phylogenetic analysis of Babesia and Theileira species based on the 18S rRNA. Sequences identified in the current investigation are highlighted in bold. Node numbers indicate the percentage frequency of clades across 1000 bootstrap replicates of the taxa.
4. Discussion
Ticks are pivotal in veterinary and human health, serving as carriers for a variety of infectious agents, which include bacteria, helminths, protozoa, and viruses. In recent years, Turkey has documented an uptick in the incidence of tick-borne diseases, a phenomenon also observed in numerous regions globally [45]. This increase is significantly influenced by climate change, which alters tick-friendly ecological niches and might lengthen tick’s active seasons. A further level of intricacy is added by avian migration patterns. Birds can spread ticks across large geographic distances, possibly introducing or increasing the incidence of ticks-associated pathogens. [46]. Notably, Babesia spp. and Theileria spp., tick-borne piroplasmids, rank the most hemoparasites impacting the economy of small ruminant industries, with a worldwide distribution [1]. To mitigate tick-borne piroplasmids transmission, it is imperative to understand the intricate associations between ticks and piroplasms, given their significance to public health. In the present study, we conducted a molecular examination of piroplasmids in ticks collected from small ruminants in four districts (Kadınhanı, Karatay, Sarayönü, Selçuklu) in the Konya province of Turkey. Microscopic examination revealed two genera, Hyalomma and Rhipicephalus, including five species: H. detritum, H. excavatum, R. bursa, R. sanguineus, and R. turanicus. Molecular assays further identified two piroplasms, B. ovis, and T. ovis.
Climatic factors like rainfall, vegetation, altitude, and temperature, along with host availability, influence the distribution trends of ixodid ticks and the spread of tick-borne diseases. [47]. Tick infestation in sheep and goats in Turkey is common due to geographic conditions and management systems [4]. In the present study, both goats and sheep exhibited a pronounced infestation rate of R. turanicus, with 40.59% in goats and 27.24% in sheep, followed by R. bursa (goats: 10.69%; sheep: 13.35%). A previous study identified R. turanicus, Ha. parva, and R. bursa as the predominant species of sheep and goats in the Black Sea Region of Turkey [32]. Ceylan et al. 2021 [48] reported R. turanicus as the most prevalent tick species among sheep in Turkey, consistent with our finding. Interestingly, H. excavatum demonstrated a relatively higher infestation rate (7.10%) in our study compared to those conducted earlier [32,48] and Ha. parva was not detected, possibly attributable to geographical and seasonal variations. Of note, despite the comparable climatic conditions across the four selected districts, the presence of R. turanicus was solely identified in the Kadınhanı district. To comprehensively understand the influence of geographical disparities across the remaining districts, in-depth investigations are warranted. Such studies would contribute valuable insights into vector distribution patterns and potential ramifications for disease transmission dynamics.
The most important Babesia species associated with babesiosis in small ruminants are considered B. ovis, B. crassa, B. motasi, and Babesia sp. Xinjiang [11,49,50]. Among these, B. ovis is particularly pathogenic in sheep and goats [51], and ovine babesiosis caused by this piroplasma exhibits a state of nationwide endemic instability in Turkey [52]. Although molecular detection of B. ovis in sheep and goats has been reported in various provinces of Turkey [8,35,53,54], no earlier study had identified tick vectors of this parasite in the Konya province. In this study, we discovered that R. bursa, R. sanguineus, and R. turanicus can serve as vectors for B. ovis in this region. This parasite is documented to be carried by R. bursa, R. sanguineus, R. turanicus, Ha. parva, H. marginatum, and H. excavatum [31,55,56,57]. Our findings indicate that B. ovis was detected in 29 out of 118 R. bursa pools (10.80%, CI 7.43–14.90), 1 out of 169 R. turanicus pools (0.12%, CI 0.01–0.51), and 1 out of 3 R. sanguineus pools. This observation particularly of B. ovis in R. bursa, is consistent with that of prior studies [31,55]. There is one report each of the detection of B. ovis in R. turanicus and R. sanguineus in Turkey [55,57]. The present study substantiates these findings, suggesting that R. turanicus and R. sanguineus may act as vectors for B. ovis in this country. Moreover, phylogenetic analyses using 18S rRNA gene sequences formed two diverged clades of B. ovis. Further investigation into the genetic diversity of B. ovis is warranted. Molecular diagnostic techniques employed in the study of Babesia species have facilitated the identification of previously unrecognized species, including B. naoakii, implicated in cases of acute babesiosis [7]. Although its incidence in a few nations has been verified, no reports have indicated that it exists in Turkey. One of our goals in doing this experiment was to determine whether this piroplasmid might exist in Turkey. All samples, though, came out negative for this parasite. A recent study provided molecular evidence that B. naoakii was detected in Ha. bispinosa [58]. At the same time, there is no current evidence to suggest the presence of Ha. bispinosa in Turkey, the transmission of pathogens might be mediated by another tick vector, especially given the absence of the primary vector in the region [59]. Consequently, future studies should be maintaining a monitor of this parasite in both mammalian and potential vector hosts in the country.
Due to their varied pathogenic effects on small ruminants, Theileria species have long been a focus of research. T. lestoquardi, T. luwenshuni, and T. uilenbergi are known to cause severe theileriosis, which can harm the health and production of these animals. In contrast, T. ovis is generally considered a non-pathogenic species of Theileria [8]. However, it is noteworthy that T. ovis can switch from a benign to a pathogenic state under certain predisposing factors, primarily stressful situations or physiological states, leading to severe theileriosis. [60]. Therefore, it should not be ignored in the epidemiology. Past research in Turkey has reported the presence of T. ovis, T. luwenshuni, T. uilenbergi, and certain Theileria isolates, such as Theileria sp. MK, Theileria sp. OT1, and Theileria sp. OT3 in small ruminants [8,33,61]. Consequently, it is expected that the vectors of these piroplasmids are expected to exist within the country. However, in our study, only T. ovis was identified in the Rhipicephalus ticks, possibly because the primary transmission vector was not collected in this study [62]. The prevalence of T. ovis in R. bursa and R. turanicus pools were 0.33% (1/118, CI 0.02–1.42) and 2.08% (17/169, CI 1.25–3.22), respectively. In a prior study from the same province as our tick sampling site, T. ovis was detected in sheep with an infection rate of 21.05% [36], which is relatively higher than our current findings. As documented in a previous study, Rhipicephalus ticks are the vectors for T. ovis transmission in Turkey [53], consistent with the results of this study. The vector for carrying piroplasmids may vary due to geographical differences. Therefore, future studies should explore the relationship between tick species and Theileria spp., which were not detected in this study, should be explored in future studies to provide more comprehensive information for controlling theileriosis.
B. ovis has been detected in all developmental stages of R. bursa, demonstrating both transovarial and transstadial transmission capabilities, as previously documented [63]. In contrast, T. ovis is acquired by the immature stages of R. bursa and transmitted by the subsequent adults [64]. Hence, the vital role of tick larvae and nymphs in piroplasm transmission is well-established. However, in our study, only adult ticks were collected. To provide a comprehensive understanding of piroplasmid prevalence, future investigations should prioritize the assessment of piroplasmids across various life stages of ticks. It is worth noting that no existing reports detail the development and transmission of B. ovis and T. ovis in R. turanicus and R. sanguineus. As a result, further research is warranted to delve into the intricate dynamics of B. ovis and T. ovis development and transmission in these tick species.
5. Conclusions
In conclusion, we investigated the distribution and prevalence of tick-borne piroplasmids in ticks infesting sheep and goats within the Konya province of Turkey, a region where no previous reports identified tick vectors for such piroplasmids. The presence of B. ovis and T. ovis was confirmed in tick vectors in this province. Furthermore, this study corroborates that R. turanicus and R. sanguineus can serve as potential vectors for B. ovis transmission in this region. This research augments our comprehension of the epidemiology of piroplasmids in tick vectors. This research augments our comprehension of the epidemiology of piroplasmids in tick vectors and is expected to contribute valuable insights toward the development of effective strategies for the control of ticks and tick-borne diseases.
Author Contributions
Conceptualization, Z.M., O.C., M.L., F.S. and X.X.; methodology, Z.M., E.M.G., O.C., M.L. and X.X.; software, Z.M., S.J., T.T.D. and M.L.; validation, Z.M., M.L. and S.A.E.-S.E.-S.; formal analysis, Z.M., U.K.M. and I.Z.; investigation, Z.M.; resources, O.C., F.S. and X.X.; data curation, Z.M., M.L. and X.X.; writing—original draft preparation, Z.M.; writing—review and editing, Z.M., O.C., E.M.G., M.L., I.Z., R.U.-S., S.A.E.-S.E.-S., F.S. and X.X.; visualization, Z.M., H.L., T.T.D. and S.J.; supervision, project administration, and funding acquisition, X.X. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a Grant-in-Aid for Scientific Research (26304036, 18KK0188) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, and a grant from JSPS Core-to-Core Program, Japan.
Institutional Review Board Statement
This study was approved by the Ethics Committee of Obihiro University of Agriculture and Veterinary Medicine (animal experiment approval number: 19–74; DNA experiment approval number: 1706-4, 1705-4, 1704-4).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank Professor Naoaki Yokoyama for the positive sample of Babesia naoakii provision.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Uilenberg, G. International collaborative research: Significance of tick-borne hemoparasitic diseases to world animal health. Vet. Parasitol. 1995, 57, 19–41. [Google Scholar] [CrossRef]
- Minjauw, B.; McLeod, A. Tick-borne diseases and poverty: The impact of ticks and tick-borne diseases on the livelihoods of small-scale and marginal livestock owners in India and Eastern and Southern Africa. In Tick-Borne Diseases and Poverty: The Impact of Ticks and Tick-Borne Diseases on the Livelihoods of Small-Scale and Marginal Livestock Owners in India and Eastern and Southern Africa; DFID Animal Health Programme, Centre for Tropical Veterinary Medicine: Edinburgh, UK, 2003. [Google Scholar]
- McLeod, R.; Kristjanson, P. Economic Impact of Ticks and Tick-Borne Diseases to Livestock in Africa, Asia and Australia; International Livestock Research Institute: Nairobi, Kenya, 1999. [Google Scholar]
- Ceylan, O.; Xuan, X.; Sevinc, F. Primary tick-borne protozoan and rickettsial infections of animals in Turkey. Pathogens 2021, 10, 231. [Google Scholar] [CrossRef] [PubMed]
- Minami, T.; Fujinaga, T.; Furuya, K.; Ishihara, T. Clinico-hematologic and serological comparison of Japanese and Russian strains of Theileria sergenti. Natl. Inst. Anim. Health Q. 1980, 20, 44–52. [Google Scholar]
- Bock, R.; Jackson, L.; de Vos, A.; Jorgensen, W. Babesiosis of cattle. Parasitology 2004, 129, S247–S269. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, T.; Tuvshintulga, B.; Otgonsuren, D.; Batmagnai, E.; Ahedor, B.; Kothalawala, H.; Vimalakumar, S.C.; Silva, S.S.P.; Yamagishi, J.; Yokoyama, N. Phylogenetic analyses of the mitochondrial, plastid, and nuclear genes of Babesia sp. Mymensingh and its naming as Babesia naoakii n. sp. Parasit. Vectors 2022, 15, 299. [Google Scholar] [CrossRef] [PubMed]
- Bilgic, H.B.; Bakırcı, S.; Kose, O.; Unlu, A.H.; Hacılarlıoglu, S.; Eren, H.; Weir, W.; Karagenc, T. Prevalence of tick-borne haemoparasites in small ruminants in Turkey and diagnostic sensitivity of single-PCR and RLB. Parasit. Vectors 2017, 10, 211. [Google Scholar] [CrossRef] [PubMed]
- Morel, P.C.; Uilenberg, G. The nomenclature of some Theileria species (Sporozoa, Babesioidea) of domestic ruminants (Author’s Transl). Rev. Elev. Med. Vet. Pays. Trop. 1981, 34, 139–143. [Google Scholar]
- Aktaş, M.; Altay, K.; Dumanli, N. Development of a polymerase chain reaction method for diagnosis of Babesia ovis infection in sheep and goats. Vet. Parasitol. 2005, 133, 277–281. [Google Scholar] [CrossRef]
- Liu, A.H.; Yin, H.; Guan, G.Q.; Schnittger, L.; Liu, Z.J.; Ma, M.L.; Dang, Z.S.; Liu, J.L.; Ren, Q.Y.; Bai, Q.; et al. At least two genetically distinct large Babesia species infective to sheep and goats in China. Vet. Parasitol. 2007, 147, 246–251. [Google Scholar] [CrossRef]
- Köseoğlu, A.E.; Can, H.; Güvendi, M.; Erkunt Alak, S.; Kandemir, Ç.; Taşkın, T.; Demir, S.; Akgül, G.; Değirmenci Döşkaya, A.; Karakavuk, M.; et al. Molecular investigation of bacterial and protozoal pathogens in ticks collected from different hosts in Turkey. Parasit. Vectors 2021, 14, 270. [Google Scholar] [CrossRef]
- De la Fuente, J.; Estrada-Pena, A.; Venzal, J.M.; Kocan, K.M.; Sonenshine, D.E. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 2008, 13, 6938–6946. [Google Scholar] [CrossRef] [PubMed]
- Nava, S.; Guglielmone, A.A.; Mangold, A.J. An overview of systematics and evolution of ticks. Front. Biosci. 2009, 14, 2857–2877. [Google Scholar] [CrossRef] [PubMed]
- Reuben Kaufman, W. Ticks: Physiological aspects with implications for pathogen transmission. Ticks Tick Borne Dis. 2010, 1, 11–22. [Google Scholar] [CrossRef]
- Guo, H.; Adjou Moumouni, P.F.; Thekisoe, O.; Gao, Y.; Liu, M.; Li, J.; Galon, E.M.; Efstratiou, A.; Wang, G.; Jirapattharasate, C.; et al. Genetic characterization of tick-borne pathogens in ticks infesting cattle and sheep from three South African provinces. Ticks Tick Borne Dis. 2019, 10, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Shi, Y.; Zhao, N.; Zhang, X.; Jin, X.; He, J.; Xu, B.; Qin, T. Molecular detection of tick-borne bacterial and protozoan pathogens in Haemaphysalis longicornis (Acari: Ixodidae) ticks from free-ranging domestic sheep in Hebei Province, China. Pathogens 2023, 12, 763. [Google Scholar] [CrossRef] [PubMed]
- Guglielmone, A.A.; Robbins, R.G.; Apanaskevich, D.A.; Petney, T.N.; Estrada-peña, A.; Horak, I.G.; Shao, R.; Barker, S.C. The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida) of the world: A list of valid species names. Zootaxa 2010, 2528, 1–28. [Google Scholar] [CrossRef]
- Randolph, S.E.; Green, R.M.; Hoodless, A.N.; Peacey, M.F. An empirical quantitative framework for the seasonal population dynamics of the tick Ixodes ricinus. Int. J. Parasitol. 2002, 32, 979–989. [Google Scholar] [CrossRef]
- Guglielmone, A.A. The seasonal occurrence of Amblyomma triguttatum triguttatum Koch (Acari, Ixodidae). Acarologia 1994, 35, 107–113. [Google Scholar]
- Abunna, F.; Kasasa, D.; Shelima, B.; Megersa, B.; Regassa, A.; Amenu, K. Survey of tick infestation in small ruminants of Miesso district, West Harergie, Oromia Region, Ethiopia. Trop Anim. Health Prod. 2009, 41, 969–972. [Google Scholar] [CrossRef]
- Sevinc, F.; Xuan, X. Major tick-borne parasitic diseases of animals: A frame of references in Turkey. Eurasian J. Vet. Sci. 2015, 31, 132. [Google Scholar] [CrossRef]
- Turkish Statistical Institute. Available online: http://www.turkstat.gov.tr/ (accessed on 9 February 2023).
- Bursali, A.; Keskin, A.; Tekin, S. A review of the ticks (Acari: Ixodida) of Turkey: Species diversity, hosts and geographical distribution. Exp. Appl. Acarol. 2012, 57, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Ceylan, O.; Ma, Z.; Galon, E.M.; Zafar, I.; Li, H.; Hasegawa, Y.; Sevinc, M.; Masatani, T.; Iguchi, A.; et al. Protozoan and rickettsial pathogens in ticks collected from infested cattle from Turkey. Pathogens 2022, 11, 500. [Google Scholar] [CrossRef] [PubMed]
- Aktas, M. A survey of ixodid tick species and molecular identification of tick-borne pathogens. Vet. Parasitol. 2014, 200, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Aktas, M.; Altay, K.; Dumanli, N.; Kalkan, A. Molecular detection and identification of Ehrlichia and Anaplasma species in ixodid ticks. Parasitol. Res. 2009, 104, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Aktas, M.; Altay, K.; Ozubek, S.; Dumanli, N. A survey of ixodid ticks feeding on cattle and prevalence of tick-borne pathogens in the Black Sea Region of Turkey. Vet. Parasitol. 2012, 187, 567–571. [Google Scholar] [CrossRef]
- Aktas, M.; Altay, K.; Dumanli, N. PCR-based detection of Theileria ovis in Rhipicephalus bursa adult ticks. Vet. Parasitol. 2006, 140, 259–263. [Google Scholar] [CrossRef]
- Aktas, M.; Ozübek, S.; Ipek, D.N.S. Molecular investigations of Hepatozoon species in dogs and developmental stages of Rhipicephalus sanguineus. Parasitol. Res. 2013, 112, 2381–2385. [Google Scholar] [CrossRef]
- Altay, K.; Aktas, M.; Dumanli, N. Detection of Babesia ovis by PCR in Rhipicephalus bursa collected from naturally infested sheep and goats. Res. Vet. Sci. 2008, 85, 116–119. [Google Scholar] [CrossRef]
- Aydin, M.; Aktas, M.; Dumanli, N. Tick infestations on sheep and goats in the Black Sea Region of Türkiye. Kafkas Univ. Vet. Fak. Derg. 2012, 18, A17–A22. [Google Scholar] [CrossRef]
- Ozubek, S.; Aktas, M. Molecular and parasitological survey of ovine piroplasmosis, including the first report of Theileria annulata (Apicomplexa: Theileridae) in sheep and goats from Turkey. J. Med. Entomol. 2017, 54, 212–220. [Google Scholar] [CrossRef]
- Benedicto, B.; Ceylan, O.; Moumouni, P.F.A.; Lee, S.-H.; Tumwebaze, M.A.; Li, J.; Galon, E.M.; Liu, M.; Li, Y.; Ji, S.; et al. Molecular detection and assessment of risk factors for tick-borne diseases in sheep and goats from Turkey. Acta Parasitol. 2020, 65, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, O.; Byamukama, B.; Ceylan, C.; Galon, E.M.; Liu, M.; Masatani, T.; Xuan, X.; Sevinc, F. Tick-borne hemoparasites of sheep: A molecular research in Turkey. Pathogens 2021, 10, 162. [Google Scholar] [CrossRef] [PubMed]
- Sevinc, F.; Zhou, M.; Cao, S.; Ceylan, O.; Aydin, M.F.; Sevinc, M.; Xuan, X. Haemoparasitic agents associated with ovine babesiosis: A possible negative interaction between Babesia ovis and Theileria ovis. Vet. Parasitol. 2018, 252, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Horak, I.G.; Heyne, H.; Williams, R.; Gallivan, G.J.; Spickett, A.M.; Bezuidenhout, J.D.; Estrada-Peña, A. The Ixodid Ticks (Acari: Ixodidae) of Southern Africa; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-70640-5. [Google Scholar]
- Adjou Moumouni, P.F.; Terkawi, M.A.; Jirapattharasate, C.; Cao, S.; Liu, M.; Nakao, R.; Umemiya-Shirafuji, R.; Yokoyama, N.; Sugimoto, C.; Fujisaki, K.; et al. Molecular detection of spotted fever group rickettsiae in Amblyomma variegatum ticks from Benin. Ticks Tick Borne Dis. 2016, 7, 828–833. [Google Scholar] [CrossRef]
- Casati, S.; Sager, H.; Gern, L.; Piffaretti, J.-C. Presence of potentially pathogenic Babesia sp. for Human in Ixodes ricinus in Switzerland. Ann. Agric. Environ. Med. 2006, 13, 65–70. [Google Scholar]
- McLure, A.; O’Neill, B.; Mayfield, H.; Lau, C.; McPherson, B. PoolTestR: An R package for estimating prevalence and regression modelling for molecular xenomonitoring and other applications with pooled samples. Environ. Model. Softw. 2021, 145, 105158. [Google Scholar] [CrossRef]
- Sivakumar, T.; Tuvshintulga, B.; Zhyldyz, A.; Kothalawala, H.; Yapa, P.R.; Kanagaratnam, R.; Vimalakumar, S.C.; Abeysekera, T.S.; Weerasingha, A.S.; Yamagishi, J.; et al. Genetic analysis of Babesia isolates from cattle with clinical babesiosis in Sri Lanka. J. Clin. Microbiol. 2018, 56, 10–1128. [Google Scholar] [CrossRef]
- Kirvar, E.; Ilhan, T.; Katzer, F.; Wilkie, G.; Hooshmand-Rad, P.; Brown, D. Detection of Theileria lestoquardi (hirci) in ticks, sheep, and goats using the polymerase chain reaction. Ann. N. Y. Acad. Sci. 1998, 849, 52–62. [Google Scholar] [CrossRef]
- Yin, H.; Liu, Z.; Guan, G.; Liu, A.; Ma, M.; Ren, Q.; Luo, J. Detection and differentiation of Theileria luwenshuni and T. uilenbergi infection in small ruminants by PCR. Transbound. Emerg. Dis. 2008, 55, 233–237. [Google Scholar] [CrossRef]
- Altay, K.; Dumanli, N.; Holman, P.J.; Aktas, M. Detection of Theileria ovis in naturally infected sheep by nested PCR. Vet. Parasitol. 2005, 127, 99–104. [Google Scholar] [CrossRef]
- Inci, A.; Yildirim, A.; Duzlu, O.; Doganay, M.; Aksoy, S. Tick-borne diseases in Turkey: A review based on one health perspective. PLoS Negl. Trop Dis. 2016, 10, e0005021. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Ayllón, N.; de la Fuente, J. Impact of climate trends on tick-borne pathogen transmission. Front. Physiol. 2012, 3, 64. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Mu, L.M.; Zhang, K.; Yang, M.H.; Zhang, L.; Du, J.Y.; Liu, Z.Q.; Li, Y.X.; Lu, W.H.; Chen, C.F.; et al. A broad-range survey of ticks from livestock in Northern Xinjiang: Changes in tick distribution and the isolation of Borrelia burgdorferi sensu stricto. Parasit. Vectors 2015, 8, 449. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, O.; Uslu, A.; Ceylan, C.; Sevinc, F. Predominancy of Rhipicephalus turanicus in tick-infested sheep from Turkey: A large-scale survey. Pak. Vet. J. 2021, 41, 2074–7764. [Google Scholar] [CrossRef]
- Uilenberg, G. Babesia—A historical overview. Vet. Parasitol. 2006, 138, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Liu, G.; Liu, D.; Ren, J.; Li, X. Isolation and preliminary characterization of a large Babesia sp. from sheep and goats in the Eastern part of Gansu Province, China. Parasitol. Res. 2002, 88, S16–S21. [Google Scholar] [CrossRef]
- Sevinc, F.; Sevinc, M.; Ekici, O.D.; Yildiz, R.; Isik, N.; Aydogdu, U. Babesia ovis infections: Detailed clinical and laboratory observations in the pre-and post-treatment periods of 97 field cases. Vet. Parasitol. 2013, 191, 35–43. [Google Scholar] [CrossRef]
- Ceylan, O.; Sevinc, F. Endemic instability of ovine babesiosis in Turkey: A country-wide sero-epidemiological study. Vet. Parasitol. 2020, 278, 109034. [Google Scholar] [CrossRef]
- Altay, K.; Dumanli, N.; Aktas, M. A study on ovine tick-borne hemoprotozoan parasites (Theileria and Babesia) in the East Black Sea Region of Turkey. Parasitol. Res. 2012, 111, 149–153. [Google Scholar] [CrossRef]
- Zhou, M.; Cao, S.; Sevinc, F.; Sevinc, M.; Ceylan, O.; Ekici, S.; Jirapattharasate, C.; Moumouni, P.F.A.; Liu, M.; Wang, G.; et al. Molecular detection and genetic characterization of Babesia, Theileria and Anaplasma amongst apparently healthy sheep and goats in the Central Region of Turkey. Ticks Tick Borne Dis. 2017, 8, 246–252. [Google Scholar] [CrossRef]
- Aydin, M.F.; Aktas, M.; Dumanli, N. Molecular identification of Theileria and Babesia in ticks collected from sheep and goats in the Black Sea Region of Turkey. Parasitol. Res. 2015, 114, 65–69. [Google Scholar] [CrossRef]
- Friedhoff, K. Tick-borne diseases of sheep and goats caused by Babesia, Theileria or Anaplasma spp. Parasitologia 1997, 39, 99–109. [Google Scholar]
- Ozubek, S.; Aktas, M. Molecular evidence for a novel species of Babesia in unfed Rhipicephalus sanguineus sensu lato (Acari: Ixodidae). J. Med. Entomol. 2018, 55, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Hamid, P.H.; Cahyadi, M.; Wardhana, A.H.; Sawitri, D.H.; Setya, N.N.R.; Insyariati, T.; Kurnianto, H.; Hermosilla, C.R. First autochthonous report on cattle Babesia naoakii in Central Java, Indonesia, and identification of Haemaphysalis bispinosa ticks in the investigated area. Pathogens 2022, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Futse, J.E.; Ueti, M.W.; Knowles, D.P.; Palmer, G.H. Transmission of Anaplasma marginale by Boophilus microplus: Retention of vector competence in the absence of vector-pathogen interaction. J. Clin. Microbiol. 2003, 41, 3829–3834. [Google Scholar] [CrossRef] [PubMed]
- Ringo, A.E.; Aboge, G.O.; Adjou Moumouni, P.F.; Hun Lee, S.; Jirapattharasate, C.; Liu, M.; Gao, Y.; Guo, H.; Zheng, W.; Efstratiou, A.; et al. Molecular detection and genetic characterisation of pathogenic Theileria, Anaplasma and Ehrlichia species among apparently healthy sheep in Central and Western Kenya. Onderstepoort J. Vet. Res. 2019, 86, e1–e8. [Google Scholar] [CrossRef]
- Aktaş, M.; Altay, K.; Dumanli, N. Survey of Theileria parasites of sheep in eastern Turkey using polymerase chain reaction. Small Rumin. Res. 2005, 60, 289–293. [Google Scholar] [CrossRef]
- Ahmed, J.; Yin, H.; Bakheit, M.; Liu, Z.; Mehlhorn, H.; Seitzer, U. Small ruminant theileriosis. Prog. Parasitol. 2011, 2, 135–153. [Google Scholar] [CrossRef]
- Büscher, G.; Friedhoff, K.T.; El-Allawy, T.A.A. Quantitative Description of the development of Babesia ovis in Rhipicephalus bursa (hemolymph, ovary, eggs). Parasitol. Res. 1988, 74, 331–339. [Google Scholar] [CrossRef]
- Neitz, W.O. The experimental transmission of Theileria ovis by Rhipicephalus evertsi mimeticus and R. bursa. Onderstepoort J. Vet. Res. 1972, 39, 83–86. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).