First Molecular Evidence of Babesia vogeli, Babesia vulpes, and Theileria ovis in Dogs from Kyrgyzstan
Abstract
1. Introduction
2. Materials and Methods
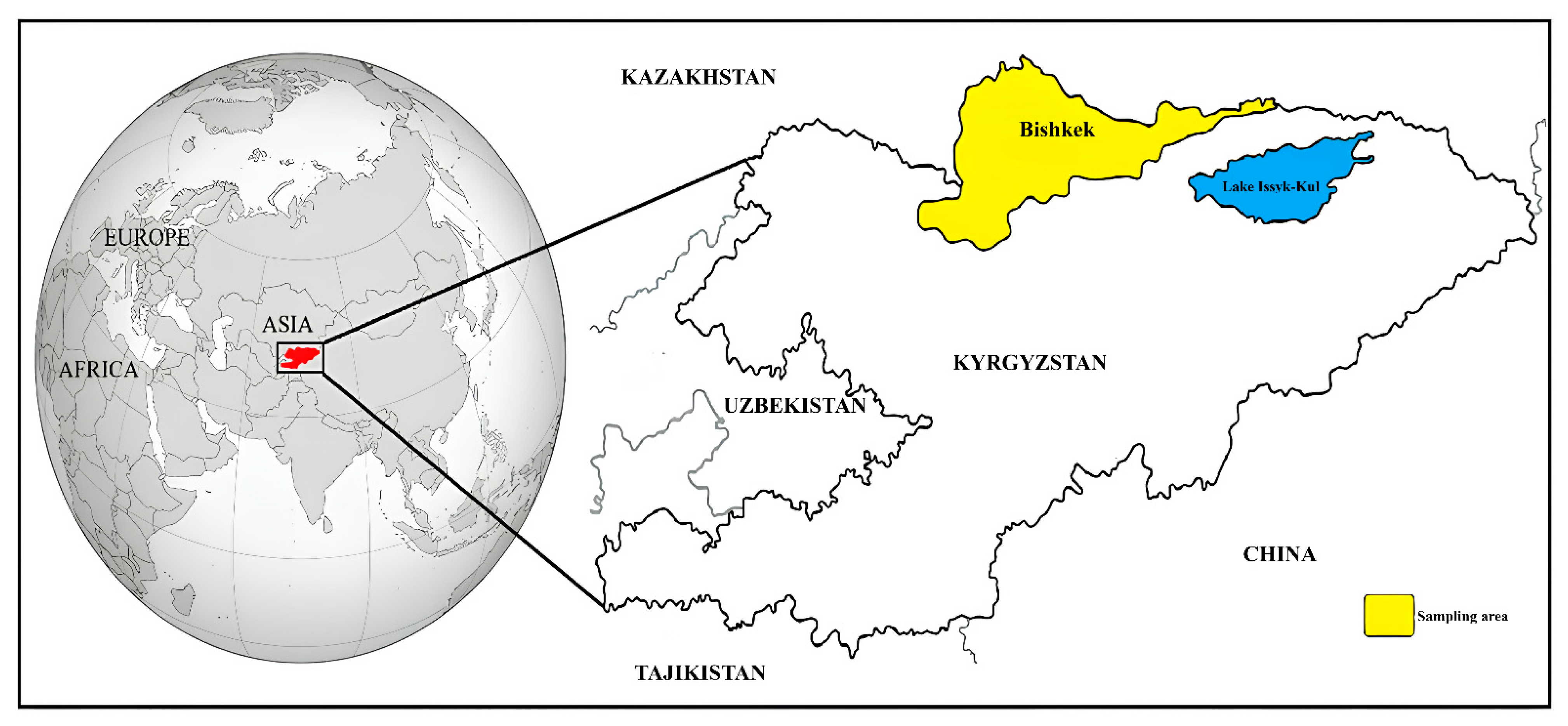
2.1. Study Area, Collection of Blood Samples, and DNA Extraction
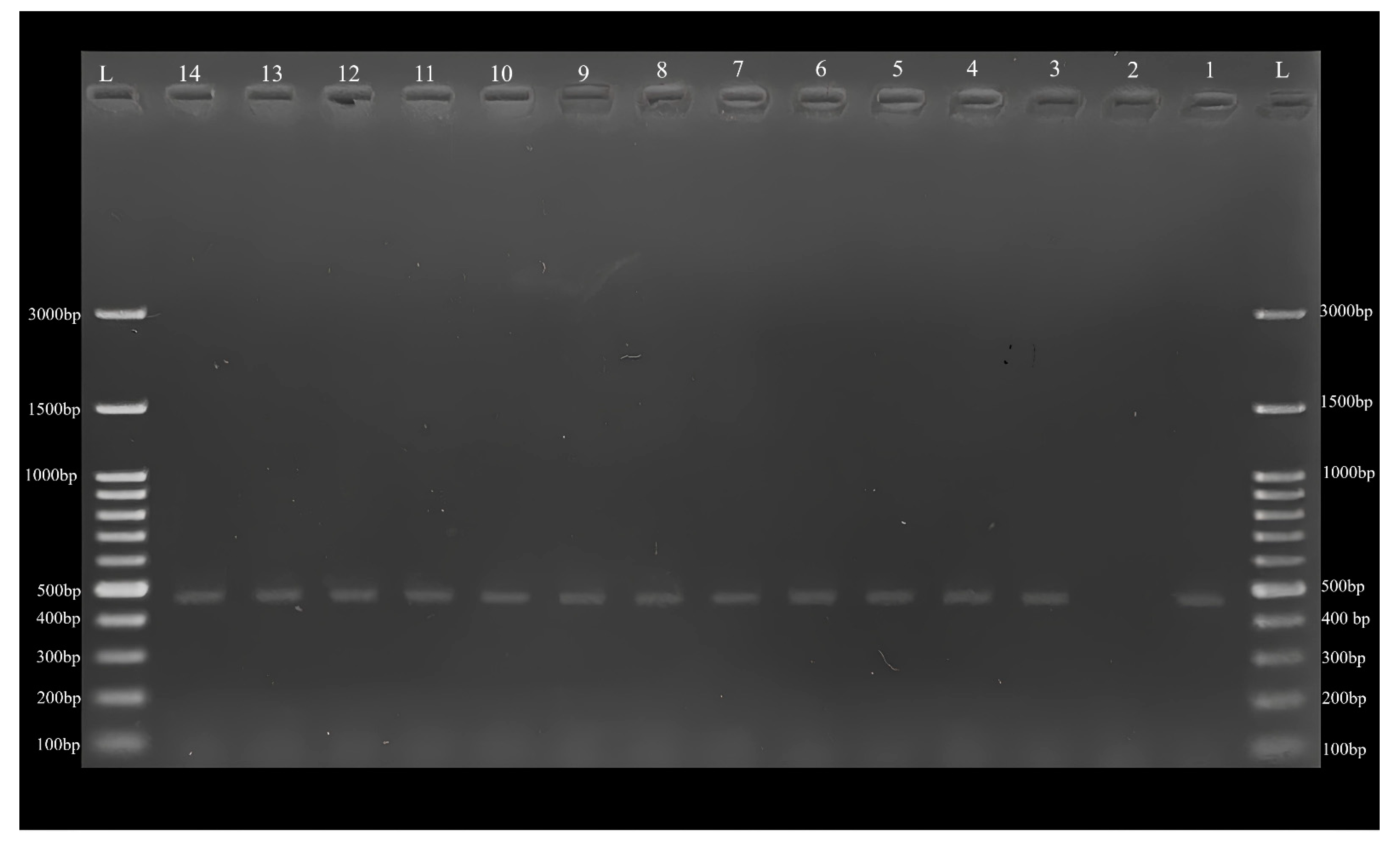
2.2. Polymerase Chain Reaction (PCR), Sequencing, and Phylogenetic Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baneth, G.; Florin-Christensen, M.; Cardoso, L.; Schnittger, L. Reclassification of Theileria annae as Babesia vulpes sp. nov. Parasite. Vector. 2015, 8, 207–214. [Google Scholar] [CrossRef]
- Beck, R.; Vojta, L.; Mrljak, V.; Marinculić, A.; Beck, A.; Živičnjak, T.; Cacciò, S.M. Diversity of Babesia and Theileria species in symptomatic and asymptomatic dogs in Croatia. Int. J. Parasitol. 2009, 39, 843–848. [Google Scholar] [CrossRef]
- Bigdeli, M.; Rafie, S.M.; Namavari, M.M.; Jamshidi, S. Report of Theileria annulata and Babesia canis infections in dogs. Comp. Clin. Path. 2012, 21, 375–377. [Google Scholar] [CrossRef]
- Gholami, S.; Laktarashi, B.; Shiadeh, M.M.; Spotin, A. Genetic variability, phylogenetic evaluation and first global report of Theileria luwenshuni, T. buffeli, and T. ovis in sheepdogs in Iran. Parasitol. Res. 2015, 115, 2125–2130. [Google Scholar] [CrossRef] [PubMed]
- Piana, G.P.; Galli-Valerio, B. Su di un’ infezione del cane con parasiti endoglobulari. Mod. Zooiatr. 1895, 6, 163–169. [Google Scholar]
- Matijatko, V.; Torti, M.; Schetters, T.P. Canine babesiosis in Europe: How many diseases? Trends Parasitol. 2012, 28, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Aktas, M.; Ozubek, S. A survey of canine haemoprotozoan parasites from Turkey, including molecular evidence of an unnamed Babesia. Comp. Immunol. Microbiol. Infect. Dis. 2017, 52, 36–42. [Google Scholar] [CrossRef]
- Uilenberg, G. Babesia—A historical overview. Vet. Parasitol. 2006, 138, 3–10. [Google Scholar] [CrossRef]
- Uilenberg, G.; Franssen, F.F.J.; Perie, N.M.; Spanjer, A.A.M. Three groups of Babesia canis distinguished and a proposal for nomenclature. Vet. Quart. 1989, 11, 33–40. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Baneth, G. Babesiosis in dogs and cats-expanding parasitological and clinical spectra. Vet. Parasitol. 2011, 181, 48–60. [Google Scholar] [CrossRef]
- Patton, W.S. Preliminary report on a new piroplasm (Piroplasma gibsoni sp. nov.) found in the blood of the hounds of the Madras Hunt and subsequently discovered in the blood of the jackal Canis aureus. Bull. Soc. Pathol. Exot. 1910, 3, 274–280. [Google Scholar]
- Schnittger, L.; Rodriguez, A.E.; Florin-Christensen, M.; Morrison, D.A. Babesia: A world emerging. Infect. Genet. Evol. 2012, 12, 1788–1809. [Google Scholar] [CrossRef]
- Penzhorn, B.L.; Oosthuizen, M.C. Babesia species of domestic cats: Molecular characterization has opened pandora’s box. Front. Vet. Sci. 2020, 7, 134. [Google Scholar] [CrossRef] [PubMed]
- Birkenheuer, A.J.; Levy, M.G.; Breitschwerdt, E.B. Development and evaluation of a seminested PCR for detection and differentiation of Babesia gibsoni (Asian genotype) and B. canis DNA in canine blood samples. J. Clin. Microbiol. 2003, 41, 4172–4177. [Google Scholar] [CrossRef]
- Ozubek, S.; Aktas, M. Molecular evidence of a new Babesia sp. in goats. Vet. Parasitol. 2017, 233, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Aydın, M.F.; Altay, K.; Aytmirzakizi, A.; Dumanlı, N. First molecular detection of Dirofilaria immitis and D. repens in dogs from Kyrgyzstan. Acta Parasitol. 2020, 65, 949–953. [Google Scholar] [CrossRef]
- Altay, K.; Aydın, M.F.; Aytmirzakizi, A.; Jumakanova, Z.; Cunusova, A.; Dumanlı, N. First molecular evidence for Mycoplasma haemocanis and Candidatus Mycoplasma haematoparvum in asymptomatic shelter dogs in Kyrgyzstan. Kafkas Univ. Vet. Fak. Derg. 2020, 26, 143–146. [Google Scholar] [CrossRef]
- Altay, K.; Aydın, M.F.; Aytmirzakizi, A.; Jumakanova, Z.; Cunusova, A.; Dumanlı, N. First molecular detection and phylogenetic analysis of Mycoplasma wenyonii and Candidatus Mycoplasma haemobos in cattle in different parts of Kyrgyzstan. Biologia 2023, 78, 633–640. [Google Scholar] [CrossRef]
- Altay, K.; Aydın, M.F.; Aytmirzakizi, A.; Jumakanova, Z.; Cunusova, A.; Dumanlı, N. Molecular survey of hepatozoonosis in natural infected dogs: First detection and molecular characterisation of Hepatozoon canis in Kyrgyzstan. Kafkas Univ. Vet. Fak. Derg. 2019, 25, 77–81. [Google Scholar] [CrossRef]
- Aktaş, M.; Kısadere, İ.; Özübek, S.; Cihan, H.; Salıkov, R.; Cirak, V.Y. First molecular survey of piroplasm species in cattle from Kyrgyzstan. Parasitol. Res. 2019, 118, 2431–2435. [Google Scholar] [CrossRef] [PubMed]
- Ozubek, S.; Ulucesme, M.C.; Cirak, V.Y.; Aktas, M. Detection of Theileria orientalis genotypes from cattle in Kyrgyzstan. Pathogens 2022, 11, 1185. [Google Scholar] [CrossRef]
- Altay, K.; Erol, U.; Sahin, O.F.; Aytmirzakizi, A. First molecular detection of Anaplasma species in cattle from Kyrgyzstan; molecular identification of human pathogenic novel genotype Anaplasma capra and Anaplasma phagocytophilum related strain. Ticks Tick Borne Dis. 2022, 13, 101861. [Google Scholar] [CrossRef]
- Altay, K.; Erol, U.; Sahin, O.F.; Aytmirzakizi, A.; Temizel, E.M.; Aydin, M.F.; Dumanli, N.; Aktas, M. The detection and phylogenetic analysis of Anaplasma phagocytophilum-like 1, A. ovis and A. capra in sheep: A. capra divides into two genogroups. Vet. Res. Commun. 2022, 46, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Oosthuizen, M.C.; Zweygarth, E.; Collins, N.E.; Troskie, M.; Penzhorn, B.L. Identification of a novel Babesia sp. from a sable antelope (Hippotragus niger Harris, 1838). J. Clin. Microbiol. 2008, 46, 2247–2251. [Google Scholar] [CrossRef] [PubMed]
- Casati, S.; Sager, H.; Gern, L.; Piffaretti, J.C. Presence of potentially pathogenic Babesia sp. for human in Ixodes ricinus in Switzerland. Ann. Agric. Environ. Med. 2006, 13, 65–70. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Schetters, T.P.; Kleuskens, J.A.G.M.; Scholtes, N.; Gorenflot, A. Parasite localization and dissemination in the Babesia-infected host. Ann. Trop. Med. Parasitol. 1998, 92, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Birkenheuer, A.J. Babesiosis. In Green’s Infectious Diseases of the Dog and Cat, 4th ed.; Greene, C.E., Ed.; Elsevier: St. Louis, MI, USA, 2012; pp. 771–784. [Google Scholar]
- Sahin, O.F.; Erol, U.; Altay, K. Buffaloes as new hosts for Anaplasma capra: Molecular prevalence and phylogeny based on gtlA, groEL, and 16S rRNA genes. Res. Vet. Sci. 2022, 152, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Aktas, M.; Altay, K.; Dumanli, N. PCR-based detection of Theileria ovis in Rhipicephalus bursa adult ticks. Vet. Parasitol. 2006, 140, 259–263. [Google Scholar] [CrossRef]
- Criado, A.; Martinez, J.; Buling, A.; Barba, J.C.; Merino, S.; Jefferies, R.; Irwin, P.J. New data on epizootiology and genetics of piroplasms based on sequences of small ribosomal subunit and cytochrome b genes. Vet. Parasitol. 2006, 142, 238–247. [Google Scholar] [CrossRef]
- Criado-Fornelio, A.; Martinez-Marcos, A.; Buling-Sarana, A.; Barba-Carretero, J.C. Molecular studies on Babesia, Theileria and Hepatozoon in southern Europe. Part II: Phylogenetic analysis and evolutionary history. Vet. Parasitol. 2003, 114, 173–194. [Google Scholar] [CrossRef]
- Matjila, P.T.; Penzhorn, B.L.; Bekker, C.P.J.; Nijhof, A.M.; Jongejan, F. Confirmation of occurrence of Babesia canis vogeli in domestic dogs in South Africa. Vet. Parasitol. 2004, 122, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Inokuma, H.; Yoshizaki, Y.; Shimada, Y.; Sakata, Y.; Okuda, M.; Onishi, T. Epidemiological survey of Babesia species in Japan performed with specimens from ticks collected from dogs and detection of new Babesia DNA closely related to Babesia odocoilei and Babesia divergens DNA. J. Clin. Microbiol. 2003, 41, 3494–3498. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, R.; Ryan, U.M.; Muhlnickel, C.J.; Irwin, P.J. Two species of canine Babesia in Australia: Detection and characterization by PCR. J. Parasitol. 2003, 89, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Prakash, B.K.; Low, V.L.; Vinnie-Siow, W.Y.; Tan, T.K.; Lim, Y.A.L.; Morvarid, A.R.; AbuBakar, S.; Sofian-Azirun, M. Detection of Babesia spp. in dogs and their ticks from Peninsular Malaysia: Emphasis on Babesia gibsoni and Babesia vogeli infections in Rhipicephalus sanguineus sensu lato (Acari: Ixodidae). J. Med. Entomol. 2018, 55, 1337–1340. [Google Scholar] [CrossRef]
- de Oliveira Carieli, E.P.; da Silva, V.C.L.; de Lima, E.R.; Dias, M.B.D.M.C.; Fukahori, F.L.P.; de Azevedo Rêgo, M.S.; Júnior, J.W.P.; de Cássia Peixoto Kim, P.; Leitão, R.S.C.S.; Mota, R.A.; et al. Parasitological and molecular detection of Babesia canis vogeli in dogs of Recife, Pernambuco and evaluation of risk factors associated. Semina. Ciências. Agrárias. 2016, 37, 163–171. [Google Scholar] [CrossRef]
- Selim, A.; Megahed, A.; Ben Said, M.; Alanazi, A.D.; Sayed-Ahmed, M.Z. Molecular survey and phylogenetic analysis of Babesia vogeli in dogs. Sci. Rep. 2022, 12, 6988. [Google Scholar] [CrossRef] [PubMed]
- Badawi, N.M.; Yousif, A.A. Babesia canis spp. in dogs in Baghdad province, Iraq: First molecular identifcation and clinical and epidemiological study. Vet. World. 2020, 13, 579–585. [Google Scholar] [CrossRef]
- Ćoralić, A.; Gabrielli, S.; Zahirović, A.; Stojanović, N.M.; Milardi, G.L.; Jažić, A.; Zuko, A.; Čamo, D.; Otašević, S. First molecular detection of Babesia canis in dogs from Bosnia and Herzegovina. Ticks Tick Borne Dis. 2018, 9, 363–368. [Google Scholar] [CrossRef]
- Khanmohammadi, M.; Zolfaghari-Emameh, R.; Arshadi, M.; Razmjou, E.; Karimi, P. Molecular identifcation and genotyping of Babesia canis in dogs from Meshkin Shahr County, Northwestern Iran. J. Arthropod Borne Dis. 2021, 15, 97–107. [Google Scholar] [CrossRef]
- Obeta, S.S.; Ibrahim, B.; Lawal, I.A.; Natala, J.A.; Ogo, N.I.; Balogun, E.O. Prevalence of canine babesiosis and their risk factors among asymptomatic dogs in the federal capital territory, Abuja, Nigeria. Parasite Epidemiol. Control. 2020, 11, e00186. [Google Scholar] [CrossRef]
- Mandal, M.; Banerjee, P.S.; Garg, R.; Ram, H.; Kundu, K.; Kumar, S.; Kumar, G.R. Genetic characterization and phylogenetic relationships based on 18S rRNA and ITS1 region of small form of canine Babesia spp. from India. Infect. Genet. Evol. 2014, 27, 325–331. [Google Scholar] [CrossRef]
- Camacho, A.T.; Pallas, E.; Gestal, J.J.; Guitián, F.J.; Olmeda, A.S.; Telford III, S.R.; Spielman, A. Ixodes hexagonus is the main candidate as vector of Theileria annae in Northwest Spain. Vet. Parasitol. 2003, 112, 157–163. [Google Scholar] [CrossRef]
- Lledó, L.; Giménez-Pardo, C.; Dominguez-Penafiel, G.; Sousa, R.; Gegúndez, M.I.; Casado, N.; Criado, A. Molecular detection of hemoprotozoa and Rickettsia species in arthropods collected from wild animals in the Burgos Province, Spain. Vector Borne Zoonotic Dis. 2010, 10, 735–738. [Google Scholar] [CrossRef]
- Hodžić, A.; Zörer, J.; Duscher, G.G. Dermacentor reticulatus, a putative vector of Babesia cf. microti (syn. Theileria annae) piroplasm. Parasitol. Res. 2017, 116, 1075–1077. [Google Scholar] [CrossRef] [PubMed]
- Iori, A.; Gabrielli, S.; Calderini, P.; Moretti, A.; Pietrobelli, M.; Tampieri, M.P.; Galuppi, R.; Cancrini, G. Tick reservoirs for piroplasms in central and northern Italy. Vet. Parasitol. 2010, 170, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Akyshova, B.; Chen, Y.N.; Chen, J. Abundance of ectoparasitic ticks and mites (Acari: Ixodida, Mesostigmata, Trombidiformes) on rodents in the Alamedin Gorge of Kyrgyz Range, Kyrgyzstan. Syst. Appl. Acarol. 2022, 27, 1120–1131. [Google Scholar] [CrossRef]
- Aknazarov, B.; Jetigenov, E.; Atabekova, N.; Suerkulov, U.; Abdumanap, N. Spread of arthropod-borne infections in Kyrgyzstan. E3S Web Conf. 2023, 380, 01027. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Sainz, Á.; Roura, X.; Estrada-Peña, A.; Miró, G. A review of canine babesiosis: The European perspective. Parasit. Vectors. 2016, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Checa, R.; López-Beceiro, A.M.; Montoya, A.; Barrera, J.P.; Ortega, N.; Gálvez, R.; Marino, V.; González, J.; Olmeda, Á.S.; Fidalgo, L.E.; et al. Babesia microti-like piroplasm (syn. Babesia vulpes) infection in red foxes (Vulpes vulpes) in NW Spain (Galicia) and its relationship with Ixodes hexagonus. Vet. Parasitol. 2018, 252, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, L.; Cortes, H.C.E.; Reis, A.; Rodrigues, P.; Simões, M.; Lopes, A.P.; Vila-Viçosa, M.J.; Talmi-Frank, D.; Eyal, O.; Solano-Gallego, L.; et al. Prevalence of Babesia microti-like infection in red foxes (Vulpes vulpes) from Portugal. Vet. Parasitol. 2013, 196, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Hodžić, A.; Mrowietz, N.; Cezanne, R.; Bruckschwaiger, P.; Punz, S.; Habler, V.E.; Tomsik, V.; Lazar, J.; Duscher, G.G.; Glawischnig, W.; et al. Occurrence and diversity of arthropod-transmitted pathogens in red foxes (Vulpes vulpes) in western Austria, and possible vertical (transplacental) transmission of Hepatozoon canis. Parasitology 2018, 145, 335–344. [Google Scholar] [CrossRef]
- Najm, N.A.; Meyer-Kayser, E.; Hoffmann, L.; Herb, I.; Fensterer, V.; Pfister, K.; Silaghi, C. A molecular survey of Babesia spp. and Theileria spp. in red foxes (Vulpes vulpes) and their ticks from Thuringia, Germany. Ticks Tick Borne Dis. 2014, 5, 386–391. [Google Scholar] [CrossRef]
- Mierzejewska, E.J.; Dwużnik, D.; Koczwarska, J.; Stańczak, Ł.; Opalińska, P.; Krokowska-Paluszak, M.; Wierzbicka, A.; Górecki, G.; Bajer, A. The red fox (Vulpes vulpes), a possible reservoir of Babesia vulpes, B. canis and Hepatozoon canis and its association with the tick Dermacentor reticulatus occurrence. Ticks Tick Borne Dis. 2021, 1, 101551. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.L.; McCarthy, K.P.; Fuller, T.K.; McCarthy, T.M. Assessing variation in wildlife biodiversity in the Tien Shan Mountains of Kyrgyzstan using ancillary camera-trap photos. Mt. Res. Develop. 2010, 30, 295–301. [Google Scholar] [CrossRef]
- Barash, N.R.; Thomas, B.; Birkenheuer, A.J.; Breitschwerdt, E.B.; Lemler, E.; Qurollo, B.A. Prevalence of Babesia spp. and clinical characteristics of Babesia vulpes infections in North American dogs. J. Vet. Intern. Med. 2019, 33, 2075–2081. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, A.C.; Foley, P.M.; Clancey, N.P. Babesia vulpes in a dog from Prince Edward Island, Canada. Can. Vet. J. 2022, 63, 589–592. [Google Scholar] [PubMed]
- Unterköfler, M.S.; Pantchev, N.; Bergfeld, C.; Wülfing, K.; Globokar, M.; Reinecke, A.; Fuehrer, H.P.; Leschnik, M. Case report of a fatal Babesia vulpes infection in a splenectomised dog. Parasitologia 2023, 3, 59–68. [Google Scholar] [CrossRef]
- Radyuk, E.; Karan, L. A case of Babesia vulpes infection in a dog in Russia. Vet. Parasitol. Reg. Stud. 2020, 22, 100467. [Google Scholar] [CrossRef]
- Tabar, M.D.; Francino, O.; Altet, L.; Sánchez, A.; Ferrer, L.; Roura, X. PCR survey of vectorborne pathogens in dogs living in and around Barcelona, an area endemic for leishmaniosis. Vet. Record. 2009, 164, 112–116. [Google Scholar] [CrossRef]
- Ozubek, S.; Aktas, M. Molecular and parasitological survey of ovine piroplasmosis, including the first report of Theileria annulata (Apicomplexa: Theileridae) in sheep and goats from Turkey. J. Med. Entomol. 2017, 54, 212–220. [Google Scholar] [CrossRef]
- Laus, F.; Spaterna, A.; Faillace, V.; Veronesi, F.; Ravagnan, S.; Beribé, F.; Cerquetella, M.; Meligrana, M.; Tesei, B. Clinical investigation on Theileria equi and Babesia caballi infections in Italian donkeys. BMC Vet. Res. 2015, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Matjila, P.T.; Leisewitz, A.L.; Oosthuizen, M.C.; Jongejan, F.; Penzhorn, B.L. Detection of a Theileria species in dogs in South Africa. Vet. Parasitol. 2008, 157, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Fritz, D. A PCR study of piroplasms in 166 dogs and 111 horses in France (March 2006 to March 2008). Parasitol. Res. 2010, 106, 1339–1342. [Google Scholar] [CrossRef] [PubMed]
- Simões, P.B.; Cardoso, L.; Araújo, M.; Yisaschar-Mekuzas, Y.; Baneth, G. Babesiosis due to the canine Babesia microti-like small piroplasm in dogs-first report from Portugal and possible vertical transmission. Parasit. Vectors. 2011, 4, 50. [Google Scholar] [CrossRef]

| Primer Name | Sequence (5′-3′) | Amplicon Size | PCR Conditions | References | |
|---|---|---|---|---|---|
| Outer PCR | Nbab_1F | AAGCCATGCATGTCTAAGTATAAGCTTTT | 1600 bp | −94 °C 5 min. −94ºC 1 min., 56 °C 1 min., and 72 °C 1 min. (35 cycle) −72 °C 7 min. | [24] |
| Nbab_1R | CTTCTCCTTCCTTTAAGTGATAAGGTTCAC | ||||
| Inner PCR | BJ1 | GTCTTGTAATTGGAATGATGG | ~500 bp | −94 °C 5 min. −94 °C 1 min., 56 °C 1 min., and 72 °C 1 min. (35 cycle) −72 °C 7 min. | [25] |
| BN2 | TAGTTTATGGTTAGGACTACG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altay, K.; Erol, U.; Sahin, O.F.; Aydin, M.F.; Aytmirzakizi, A.; Dumanli, N. First Molecular Evidence of Babesia vogeli, Babesia vulpes, and Theileria ovis in Dogs from Kyrgyzstan. Pathogens 2023, 12, 1046. https://doi.org/10.3390/pathogens12081046
Altay K, Erol U, Sahin OF, Aydin MF, Aytmirzakizi A, Dumanli N. First Molecular Evidence of Babesia vogeli, Babesia vulpes, and Theileria ovis in Dogs from Kyrgyzstan. Pathogens. 2023; 12(8):1046. https://doi.org/10.3390/pathogens12081046
Chicago/Turabian StyleAltay, Kursat, Ufuk Erol, Omer Faruk Sahin, Mehmet Fatih Aydin, Ayperi Aytmirzakizi, and Nazir Dumanli. 2023. "First Molecular Evidence of Babesia vogeli, Babesia vulpes, and Theileria ovis in Dogs from Kyrgyzstan" Pathogens 12, no. 8: 1046. https://doi.org/10.3390/pathogens12081046
APA StyleAltay, K., Erol, U., Sahin, O. F., Aydin, M. F., Aytmirzakizi, A., & Dumanli, N. (2023). First Molecular Evidence of Babesia vogeli, Babesia vulpes, and Theileria ovis in Dogs from Kyrgyzstan. Pathogens, 12(8), 1046. https://doi.org/10.3390/pathogens12081046






