Host Soluble Factors Cause Changes in Staphylococcus epidermidis Antibiotic Susceptibility and Biofilm Formation Ability
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Chemicals
2.2. Antibiotic Susceptibility Assays
2.3. Biofilm Formation Assays
2.4. Planktonic Growth Curves
2.5. Gene Expression Quantification by qPCR
2.6. Zeta Potential Measurements
2.7. Statistical Analysis
3. Results
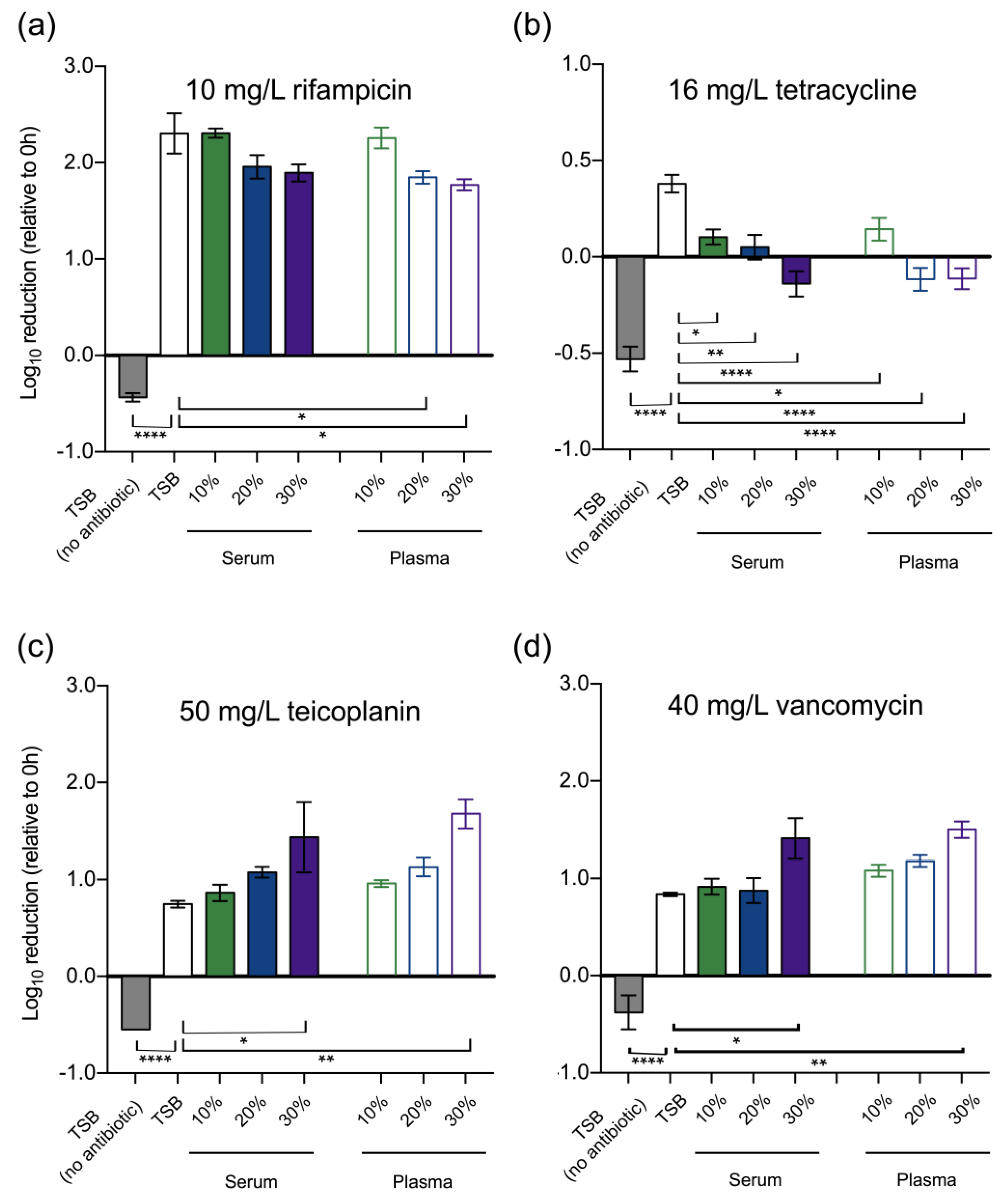
3.1. Effect of Host Serum Factors on Susceptibility to Antibiotics
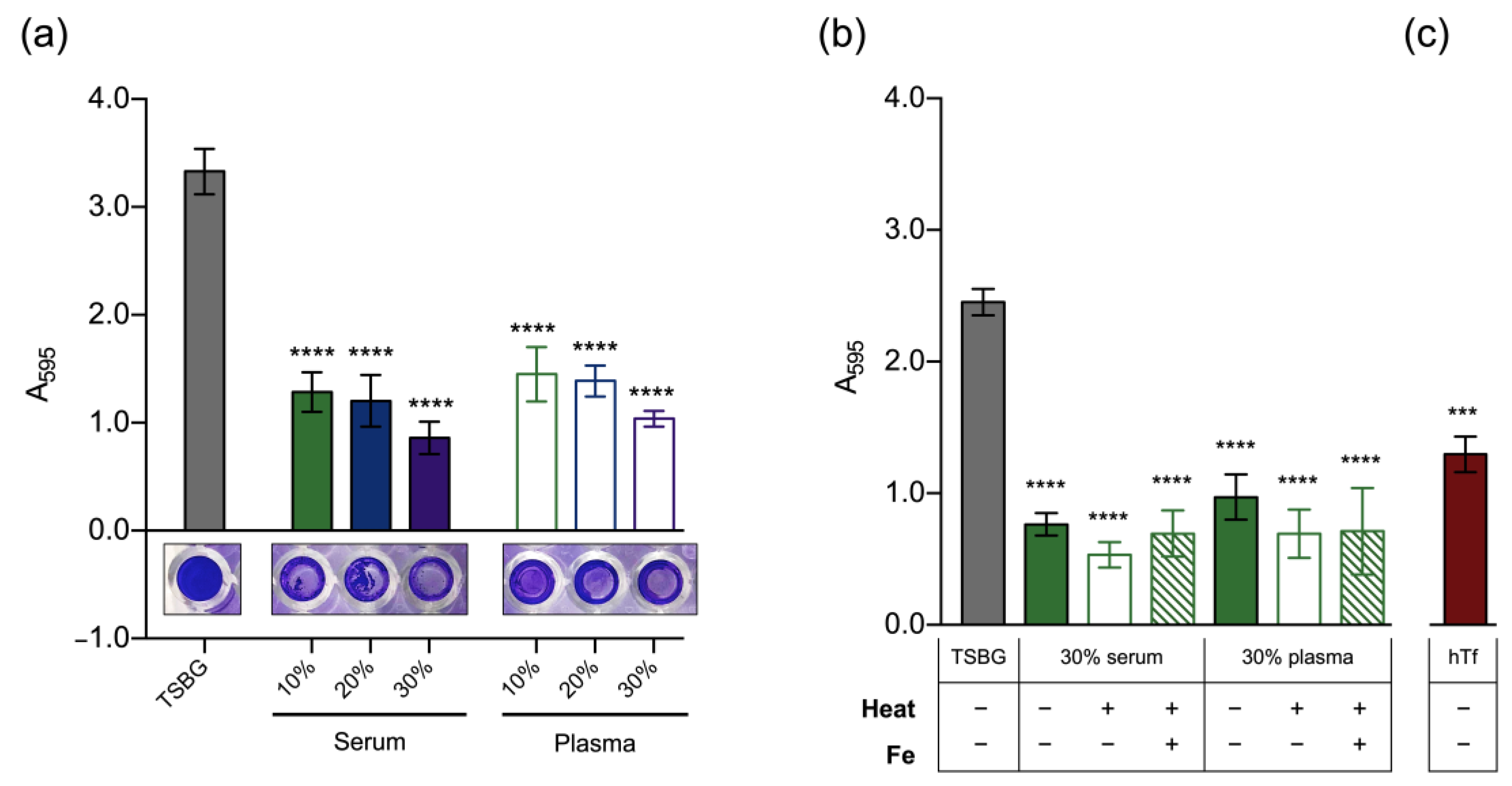
3.2. Effect of Host Serum Factors on Biofilm Formation
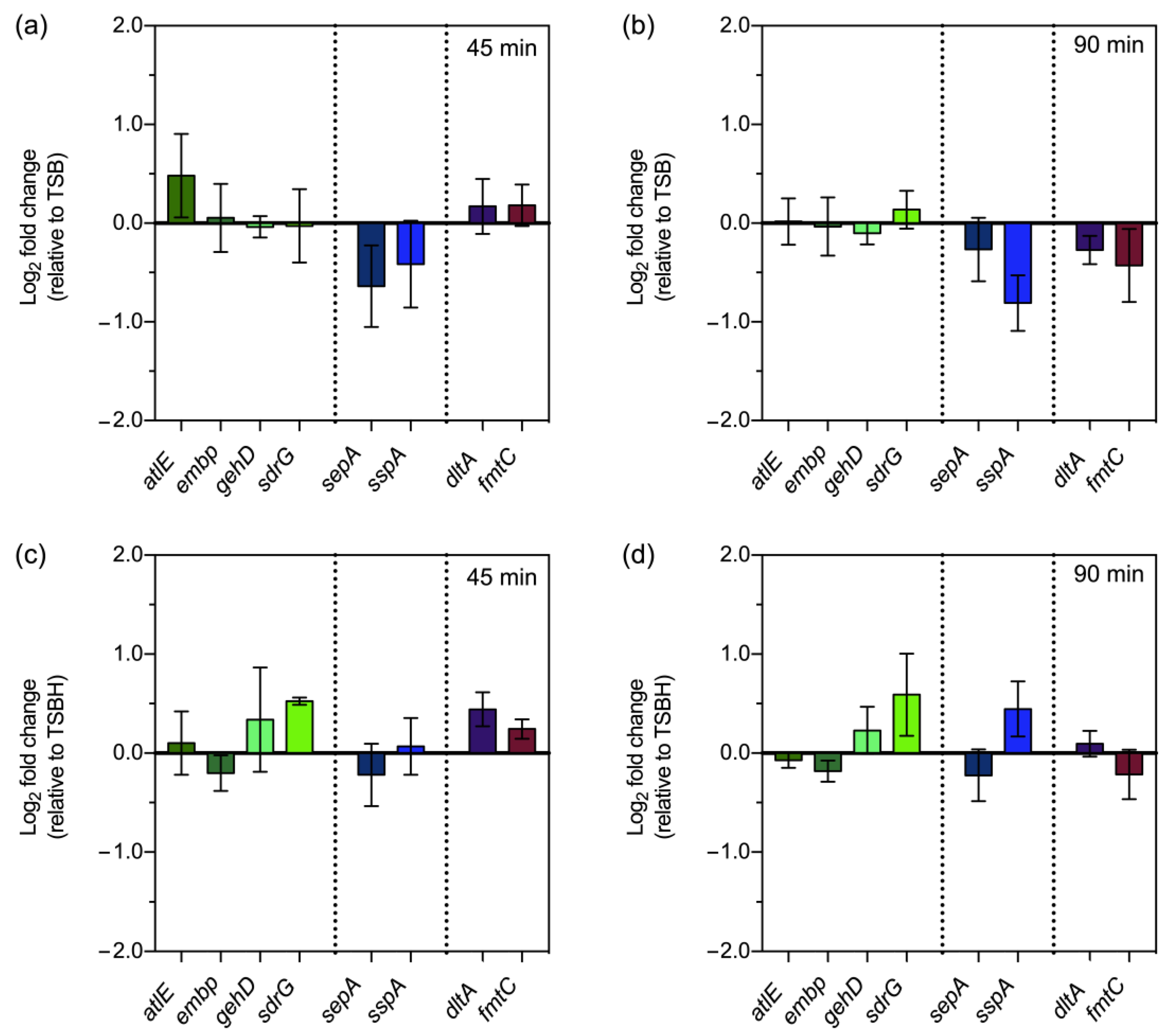
3.3. Gene Expression following Exposure to Host Serum Factors
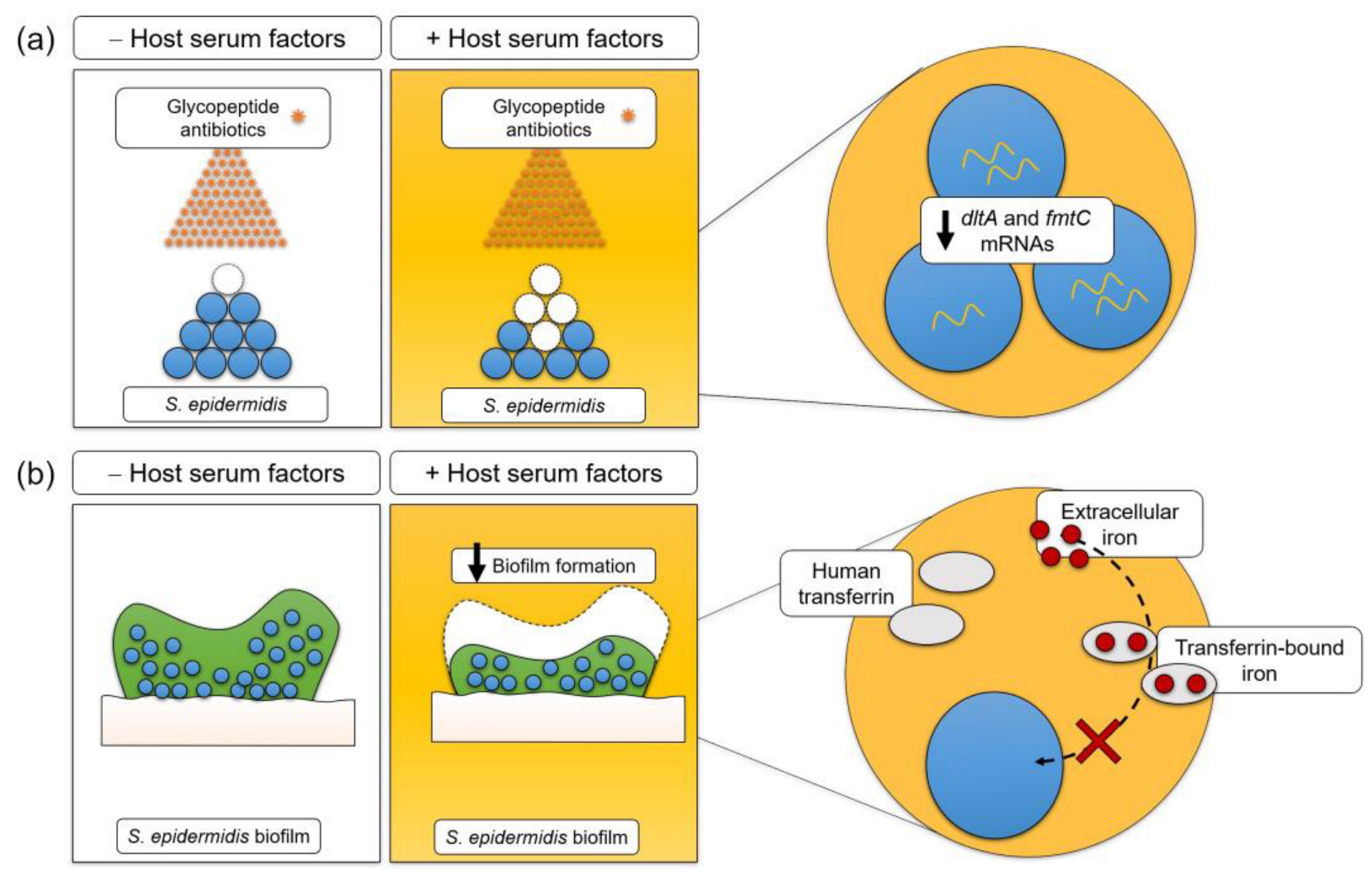
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Francolini, I.; Donelli, G. Prevention and Control of Biofilm-Based Medical-Device-Related Infections. FEMS Immunol. Med. Microbiol. 2010, 59, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcus Epidermidis: A Major Player in Bacterial Sepsis? Future Microbiol. 2017, 12, 1031–1033. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.H.; Monk, I.R.; Gonçalves da Silva, A.; Seemann, T.; Chua, K.Y.L.; Kearns, A.; Hill, R.; Woodford, N.; Bartels, M.D.; Strommenger, B.; et al. Global Spread of Three Multidrug-Resistant Lineages of Staphylococcus epidermidis. Nat. Microbiol. 2018, 3, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- França, A.; Carvalhais, V.; Maira-Litrán, T.; Vilanova, M.; Cerca, N.; Pier, G. Alterations in the Staphylococcus epidermidis Biofilm Transcriptome Following Interaction with Whole Human Blood. Pathog. Dis. 2014, 70, 444–448. [Google Scholar] [CrossRef] [PubMed]
- França, A.; Cerca, N. Plasma Is the Main Regulator of Staphylococcus epidermidis Biofilms Virulence Genes Transcription in Human Blood. Pathog. Dis. 2016, 74, ftv125. [Google Scholar] [CrossRef][Green Version]
- França, A.; Pier, G.B.; Vilanova, M.; Cerca, N. Transcriptomic Analysis of Staphylococcus epidermidis Biofilm-Released Cells upon Interaction with Human Blood Circulating Immune Cells and Soluble Factors. Front. Microbiol. 2016, 7, 1143. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Montelius, M.N.; Paulsson, M.; Gouda, L.; Larm, O.; Montelius, L.; Ljungh, Å. Adhesion of Coagulase-Negative Staphylococci and Adsorption of Plasma Proteins to Heparinized Polymer Surfaces. Biomaterials 1994, 15, 805–814. [Google Scholar] [CrossRef]
- Linnes, J.C.; Mikhova, K.; Bryers, J.D. Adhesion of Staphylococcus epidermidis to Biomaterials Is Inhibited by Fibronectin and Albumin. J. Biomed. Mater. Res.—Part A 2012, 100, 1990–1997. [Google Scholar] [CrossRef]
- Abraham, N.M.; Jefferson, K.K. A Low Molecular Weight Component of Serum Inhibits Biofilm Formation in Staphylococcus aureus. Microb. Pathog. 2010, 49, 388–391. [Google Scholar] [CrossRef] [PubMed]
- She, P.; Chen, L.; Qi, Y.; Xu, H.; Liu, Y.; Wang, Y.; Luo, Z.; Wu, Y. Effects of Human Serum and Apo-Transferrin on Staphylococcus epidermidis RP62A Biofilm Formation. Microbiologyopen 2016, 5, 957–966. [Google Scholar] [CrossRef]
- Zeitlinger, M.A.; Derendorf, H.; Mouton, J.W.; Cars, O.; Craig, W.A.; Andes, D.; Theuretzbacher, U. Protein Binding: Do We Ever Learn? Antimicrob. Agents Chemother. 2011, 55, 3067–3074. [Google Scholar] [CrossRef] [PubMed]
- Nishi, H.; Komatsuzawa, H.; Fujiwara, T.; McCallum, N.; Sugai, M. Reduced Content of Lysyl-Phosphatidylglycerol in the Cytoplasmic Membrane Affects Susceptibility to Moenomycin, as Well as Vancomycin, Gentamicin, and Antimicrobial Peptides, in Staphylococcus aureus. Antimicrob. Agents Chemother. 2004, 48, 4800–4807. [Google Scholar] [CrossRef]
- Peschel, A.; Vuong, C.; Otto, M.; Gotz, F. The D-Alanine Residues of Staphylococcus aureus Teichoic Acids Alter the Susceptibility to Vancomycin and the Activity of Autolytic Enzymes. Antimicrob. Agents Chemother. 2000, 44, 2845–2847. [Google Scholar] [CrossRef] [PubMed]
- McKay, G.A.; Beaulieu, S.; Sarmiento, I.; Arhin, F.F.; Parr, T.R.; Moeck, G. Impact of Human Serum Albumin on Oritavancin in vitro Activity against Enterococci. Antimicrob. Agents Chemother. 2009, 53, 2687–2689. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically-Tenth Edition: Approved Standard M07-A10; CLSI, Ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Freitas, A.I.; Vasconcelos, C.; Vilanova, M.; Cerca, N. Optimization of an Automatic Counting System for the Quantification of Staphylococcus epidermidis Cells in Biofilms. J. Basic Microbiol. 2014, 54, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Stepanovic, S.; Vukovic, D.; Hola, V.; Di Bonaventura, G.; Djukic, S.; Cirkovi, I.; Ruzicka, F. Quantification of Biofilm in Microtiter Plates: Overview of Testing Conditions and Practical Recommendations for Assessment of Biofilm Production by Staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef] [PubMed]
- França, A.; Freitas, A.I.; Henriques, A.F.; Cerca, N. Optimizing a QPCR Gene Expression Quantification Assay for S. epidermidis Biofilms: A Comparison between Commercial Kits and a Customized Protocol. PLoS ONE 2012, 7, e37480. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.; França, Â.; Cerca, N. Staphylococcus Epidermidis Is Largely Dependent on Iron Availability to Form Biofilms. Int. J. Med. Microbiol. 2017, 307, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3-New Capabilities and Interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Zuker, M. Mfold Web Server for Nucleic Acid Folding and Hybridization Prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A Tool to Design Target-Specific Primers for Polymerase Chain Reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Mouton, J.W.; Muller, A.E.; Canton, R.; Giske, C.G.; Kahlmeter, G.; Turnidge, J. MIC-Based Dose Adjustment: Facts and Fables. J. Antimicrob. Chemother. 2018, 73, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Dykhuizen, R.S.; Harvey, G.; Stephenson, N.; Nathwani, D.; Gould, I.M. Protein Binding and Serum Bactericidal Activities of Vancomycin and Teicoplanin. Antimicrob. Agents Chemother. 1995, 39, 1842–1847. [Google Scholar] [CrossRef]
- Thummel, K.E.; Shen, D.D.; Isoherranen, N. Appendix II: Design and Optimization of Dosage Regimens: Pharmacokinetic Data. In Goodman And Gilman’s The Pharmacological Basis of Therapeutics; Brunton, L.L., Hilal-Dandan, R., Knollmann, B.C., Eds.; McGraw-Hill Education: New York, NY, USA, 2018; pp. 1369–1376. ISBN 978-1-25-958474-9. [Google Scholar]
- Büttner, H.; Mack, D.; Rohde, H. Structural Basis of Staphylococcus Epidermidis Biofilm Formation: Mechanisms and Molecular Interactions. Front. Cell. Infect. Microbiol. 2015, 5, 14. [Google Scholar] [CrossRef]
- Kelly, A.U.; McSorley, S.T.; Patel, P.; Talwar, D. Interpreting Iron Studies. BMJ 2017, 357, j2513. [Google Scholar] [CrossRef]
- Nussbaumer-Pröll, A.K.; Knotzer, S.; Eberl, S.; Reiter, B.; Stimpfl, T.; Jäger, W.; Poschner, S.; Zeitlinger, M. Impact of Erythrocytes on Bacterial Growth and Antimicrobial Activity of Selected Antibiotics. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 485–495. [Google Scholar] [CrossRef]
- Nussbaumer-Pröll, A.K.; Eberl, S.; Reiter, B.; Stimpfl, T.; Jäger, W.; Poschner, S.; Zeitlinger, M. Impact of Thrombocytes, on Bacterial Growth and Antimicrobial Activity of Selected Antibiotics. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 593–597. [Google Scholar] [CrossRef]
- Dalhoff, A. Seventy-Five Years of Research on Protein Binding. Antimicrob. Agents Chemother. 2018, 62, e01663-17. [Google Scholar] [CrossRef]
- Burian, A.; Wagner, C.; Stanek, J.; Manafi, M.; Böhmdorfer, M.; Jäger, W.; Zeitlinger, M. Plasma Protein Binding May Reduce Antimicrobial Activity by Preventing Intra-Bacterial Uptake of Antibiotics, for Example Clindamycin. J. Antimicrob. Chemother. 2011, 66, 134–137. [Google Scholar] [CrossRef]
- Stanley, D.; McGrath, B.J.; Lamp, K.C.; Rybak, M.J. Effect of Human Serum on Killing Activity of Vancomycin and Teicoplanin against Staphylococcus aureus. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1994, 14, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Dalhoff, A.; Schubert, S. Evaluation of the Effect of Serum Proteins on the Antibacterial Activity and Pharmacodynamics of Ceftaroline against Staphylococcus aureus. Int. J. Antimicrob. Agents 2013, 42, 285–287. [Google Scholar] [CrossRef]
- Gold, M.J.; Calmon, J.; Wendeler, M.; Levison, M.E.; Johnson, C.C. Synergistic Bactericidal Activity of Rat Serum with Vancomycin against Enterococci. J. Infect. Dis. 1991, 163, 1358–1361. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.M.; Deitz, J.M.; Petersen, P.J.; Mikels, S.M.; Weiss, W.J. Therapeutic Efficacy of GAR-936, a Novel Glycylcycline, in a Rat Model of Experimental Endocarditis. Antimicrob. Agents Chemother. 2000, 44, 3022–3027. [Google Scholar] [CrossRef]
- Stratton, C.W.; Weeks, L.S. Effect of Human Serum on the Bactericidal Activity of Daptomycin and Vancomycin against Staphylococcal and Enterococcal Isolates as Determined by Time-Kill Kinetic Studies. Diagn. Microbiol. Infect. Dis. 1990, 13, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Lamp, K.C.; Rybak, M.J. Teicoplanin and Daptomycin Bactericidal Activities in the Presence of Albumin or Serum under Controlled Conditions of PH and Ionized Calcium. Antimicrob. Agents Chemother. 1993, 37, 605–609. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bailey, E.M.; Rybak, M.J.; Kaatz, G.W. Comparative Effect of Protein Binding on the Killing Activities of Teicoplanin and Vancomycin. Antimicrob. Agents Chemother. 1991, 35, 1089–1092. [Google Scholar] [CrossRef]
- Skovdal, S.M.; Hansen, L.K.K.; Ivarsen, D.M.M.; Zeng, G.; Buttner, H.; Rohde, H.; Jorgensen, N.P.; Meyer, R.L.; Büttner, H.; Rohde, H.; et al. Host Factors Abolish the Need for Polysaccharides and Extracellular Matrix-Binding Protein Staphylococcus epidermidis Biofilm Formation. J. Med. Microbiol. 2021, 70, 001287. [Google Scholar] [CrossRef]
- Ikemoto, H.; Ventura, M.M. Differential Scanning Calorimetry of the Thermal Denaturation of Human Serotransferrin. An. Acad. Bras. Cienc. 1979, 51, 165–171. [Google Scholar] [PubMed]
- Ardehali, R.; Shi, L.; Janatova, J.; Mohammad, F.; Burns, G.L. The Effect of Apo-Transferrin on Bacterial Adhesion to Biomaterials. Artif. Organs 2002, 26, 512–520. [Google Scholar] [CrossRef]
- Artini, M.; Scoarughi, G.L.; Cellini, A.; Papa, R.; Barbato, G.; Selan, L. Holo and Apo-Transferrins Interfere with Adherence to Abiotic Surfaces and with Adhesion/Invasion to HeLa Cells in Staphylococcus Spp. BioMetals 2012, 25, 413–421. [Google Scholar] [CrossRef]
- Peschel, A.; Otto, M.; Jack, R.W.; Kalbacher, H.; Jung, G.; Götz, F. Inactivation of the Dlt Operon in Staphylococcus aureus Confers Sensitivity to Defensins, Protegrins, and Other Antimicrobial Peptides. J. Biol. Chem. 1999, 274, 8405–8410. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.; Cramton, S.E.; Götz, F.; Peschel, A. Key Role of Teichoic Acid Net Charge in Staphylococcus Aureus Colonization of Artificial Surfaces. Infect. Immun. 2001, 69, 3423–3426. [Google Scholar] [CrossRef] [PubMed]
- Ruzin, A.; Severin, A.; Moghazeh, S.L.; Etienne, J.; Bradford, P.A.; Projan, S.J.; Shlaes, D.M. Inactivation of MprF Affects Vancomycin Susceptibility in Staphylococcus aureus. Biochim. Biophys. Acta—Gen. Subj. 2003, 1621, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.L.; Gurusiddappa, S.; McCrea, K.W.; Perkins, S.; Höök, M. SdrG, a Fibrinogen-Binding Bacterial Adhesin of the Microbial Surface Components Recognizing Adhesive Matrix Molecules Subfamily from Staphylococcus epidermidis, Targets the Thrombin Cleavage Site in the Bβ Chain. J. Biol. Chem. 2001, 276, 27799–27805. [Google Scholar] [CrossRef]
- Sellman, B.R.; Timofeyeva, Y.; Nanra, J.; Scott, A.; Fulginiti, J.P.; Matsuka, Y.V.; Baker, S.M. Expression of Staphylococcus epidermidis SdrG Increases Following Exposure to an in vivo Environment. Infect. Immun. 2008, 76, 2950–2957. [Google Scholar] [CrossRef]
- Ohara-Nemoto, Y.; Ikeda, Y.; Kobayashi, M.; Sasaki, M.; Tajika, S.; Kimura, S. Characterization and Molecular Cloning of a Glutamyl Endopeptidase from Staphylococcus epidermidis. Microb. Pathog. 2002, 33, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Dubin, G.; Chmiel, D.; Mak, P.; Rakwalska, M.; Rzychon, M.; Dubin, A. Molecular Cloning and Biochemical Characterization of Proteases from Staphylococcus epidermidis. Biol. Chem. 2001, 382, 1575–1582. [Google Scholar] [CrossRef]
- Moon, J.L.; Banbula, A.; Oleksy, A.; Mayo, J.A.; Travis, J. Isolation and Characterization of a Highly Specific Serine Endopeptidase from an Oral Strain of Staphylococcus epidermidis. Biol. Chem. 2001, 382, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, S.; Iwamoto, T.; Takada, K.; Okuda, K.I.; Tajima, A.; Iwase, T.; Mizunoe, Y. Staphylococcus epidermidis Esp Degrades Specific Proteins Associated with Staphylococcus aureus Biofilm Formation and Host-Pathogen Interaction. J. Bacteriol. 2013, 195, 1645–1655. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, F.; Gaio, V.; Brás, S.; Oliveira, S.; França, A. Host Soluble Factors Cause Changes in Staphylococcus epidermidis Antibiotic Susceptibility and Biofilm Formation Ability. Pathogens 2023, 12, 1064. https://doi.org/10.3390/pathogens12081064
Oliveira F, Gaio V, Brás S, Oliveira S, França A. Host Soluble Factors Cause Changes in Staphylococcus epidermidis Antibiotic Susceptibility and Biofilm Formation Ability. Pathogens. 2023; 12(8):1064. https://doi.org/10.3390/pathogens12081064
Chicago/Turabian StyleOliveira, Fernando, Vânia Gaio, Susana Brás, Sofia Oliveira, and Angela França. 2023. "Host Soluble Factors Cause Changes in Staphylococcus epidermidis Antibiotic Susceptibility and Biofilm Formation Ability" Pathogens 12, no. 8: 1064. https://doi.org/10.3390/pathogens12081064
APA StyleOliveira, F., Gaio, V., Brás, S., Oliveira, S., & França, A. (2023). Host Soluble Factors Cause Changes in Staphylococcus epidermidis Antibiotic Susceptibility and Biofilm Formation Ability. Pathogens, 12(8), 1064. https://doi.org/10.3390/pathogens12081064






