Biologically Relevant Murine Models of Chronic Pseudomonas aeruginosa Respiratory Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Biofilm Assay
2.3. Intra-Nasal Infection of Mice
2.4. Preparation of Pseudomonas Agar Beads and Oro-Tracheal Adminstration
2.5. Staphlococycuccus aureus Assessment
2.6. Antibiotic Treatment
2.7. Infection via the Drinking Water
2.8. Cytokine Measurements
2.9. Flow Cytometry
2.10. Assay for Alginate
2.11. Statistical Analysis
3. Results
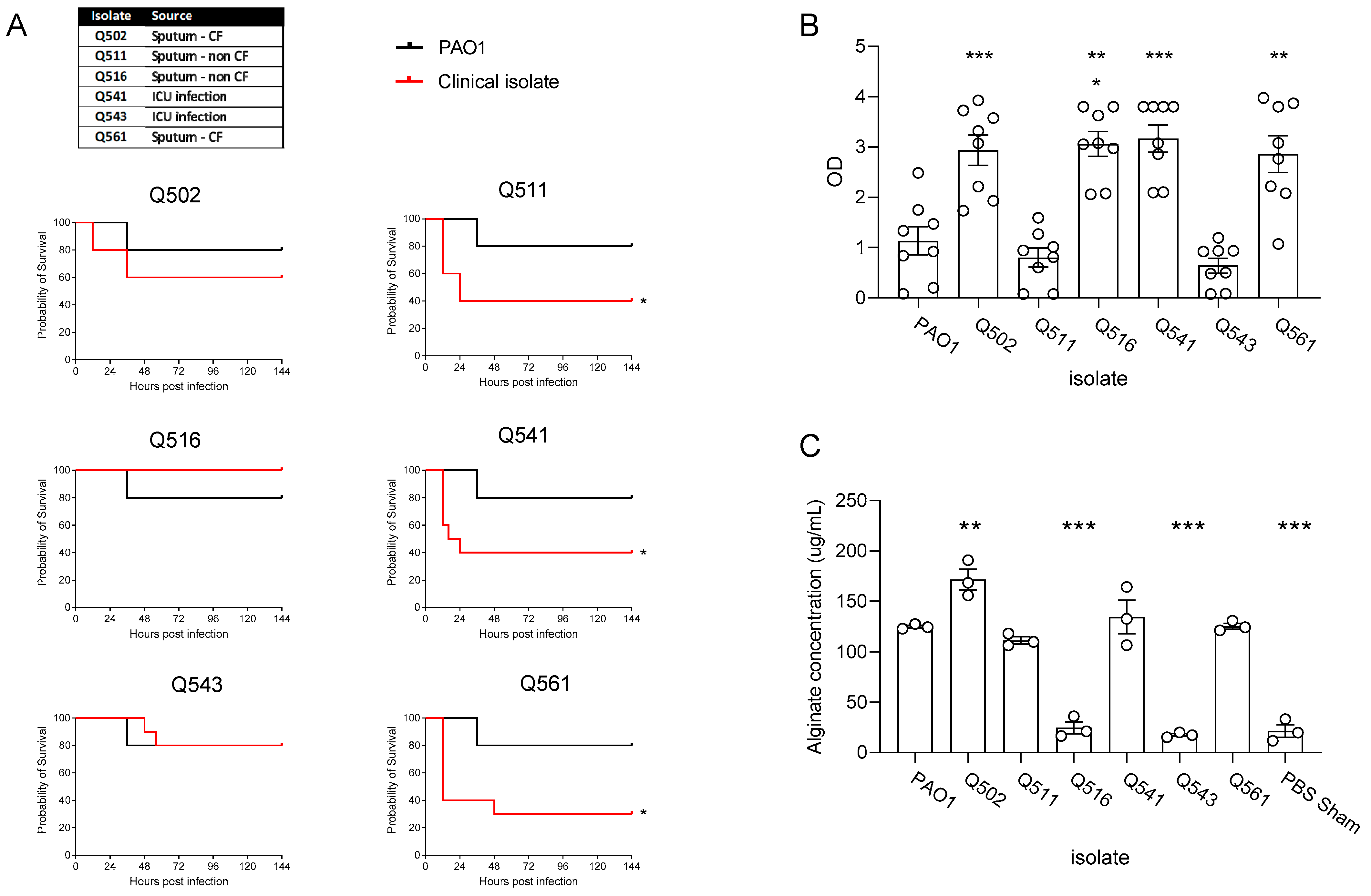
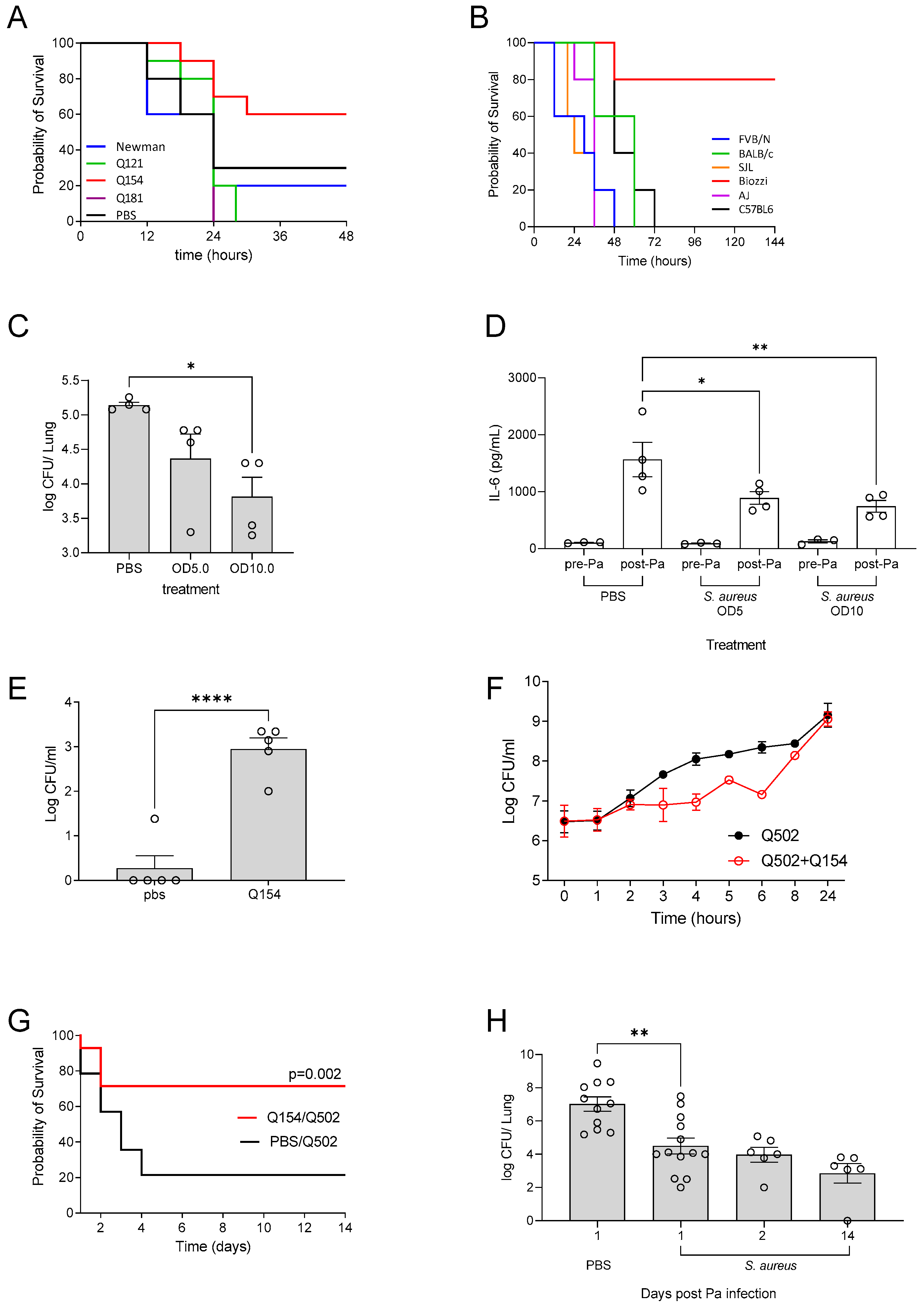
3.1. Clinical Isolate Chronic Infection Model
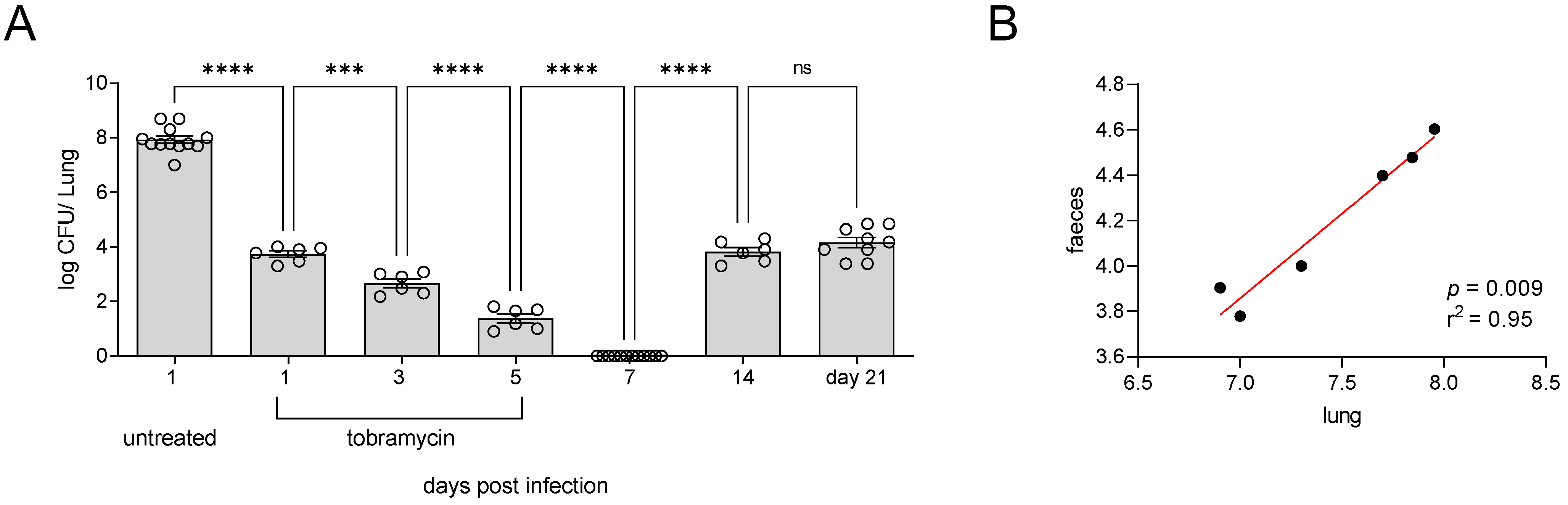
3.2. Incomplete Antibiotic Clearance Leading to Chronic Lung Infection
3.3. Water Bottle Chronic Infection Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhagirath, A.Y.; Li, Y.; Somayajula, D.; Dadashi, M.; Badr, S.; Duan, K. Cystic fibrosis lung environment and Pseudomonas aeruginosa infection. BMC Pulm. Med. 2016, 16, 174. [Google Scholar] [CrossRef] [PubMed]
- Oluyombo, O.; Penfold, C.N.; Diggle, S.P. Competition in Biofilms between Cystic Fibrosis Isolates of Pseudomonas aeruginosa Is Shaped by R-Pyocins. MBio 2019, 10, e01828-18. [Google Scholar] [CrossRef] [PubMed]
- Moser, C.; Jensen, P.; Thomsen, K.; Kolpen, M.; Rybtke, M.; Lauland, A.S.; Trøstrup, H.; Tolker-Nielsen, T. Immune Responses to Pseudomonas aeruginosa Biofilm Infections. Front. Immunol. 2021, 12, 625597. [Google Scholar] [CrossRef] [PubMed]
- Kukavica-Ibrulj, I.; Levesque, R.C. Animal models of chronic lung infection with Pseudomonas aeruginosa: Useful tools for cystic fibrosis studies. Lab. Anim. 2008, 42, 389–412. [Google Scholar] [CrossRef]
- Osbourn, M.; Rodgers, A.M.; Dubois, A.V.; Small, D.M.; Humphries, F.; Delagic, N.; Moynagh, P.N.; Weldon, S.; Taggart, C.C.; Ingram, R.J. Secretory Leucoprotease Inhibitor (SLPI) Promotes Survival during Acute Pseudomonas aeruginosa Infection by Suppression of Inflammation Rather Than Microbial Killing. Biomolecules 2022, 12, 1728. [Google Scholar] [CrossRef]
- A Cash, H.; E Woods, D.; McCullough, B.; Johanson, W.G.; A Bass, J. A rat model of chronic respiratory infection with Pseudomonas aeruginosa. Am. Rev. Respir. Dis. 1979, 119, 453–459. [Google Scholar]
- Van Heeckeren, A.M.; Schluchter, M.D. Murine models of chronic Pseudomonas aeruginosa lung infection. Lab. Anim. 2002, 36, 291–312. [Google Scholar] [CrossRef]
- Hoffmann, N.; Rasmussen, T.B.; Jensen, P.; Stub, C.; Hentzer, M.; Molin, S.; Ciofu, O.; Givskov, M.; Johansen, H.K.; Høiby, N. Novel Mouse Model of Chronic Pseudomonas aeruginosa Lung Infection Mimicking Cystic Fibrosis. Infect. Immun. 2005, 73, 2504–2514. [Google Scholar] [CrossRef]
- Yanagihara, K.; Tomono, K.; Sawai, T.; Hirakata, Y.; Kadota, J.; Koga, H.; Tashiro, T.; Kohno, S. Effect of clarithromycin on lymphocytes in chronic respiratory Pseudomonas aeruginosa infection. Am. J. Respir. Crit. Care Med. 1997, 155, 337–342. [Google Scholar] [CrossRef]
- Bayes, H.K.; Ritchie, N.; Irvine, S.; Evans, T.J. A murine model of early Pseudomonas aeruginosa lung disease with transition to chronic infection. Sci. Rep. 2016, 6, 35838. [Google Scholar] [CrossRef]
- Christophersen, L.J.; Trøstrup, H.; Damlund, D.S.M.; Bjarnsholt, T.; Thomsen, K.; Jensen, P.; Hougen, H.P.; Høiby, N.; Moser, C. Bead-size directed distribution of Pseudomonas aeruginosa results in distinct inflammatory response in a mouse model of chronic lung infection. Clin. Exp. Immunol. 2012, 170, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Coleman, F.T.; Mueschenborn, S.; Meluleni, G.; Ray, C.; Carey, V.J.; Vargas, S.O.; Cannon, C.L.; Ausubel, F.M.; Pier, G.B. Hypersusceptibility of cystic fibrosis mice to chronic Pseudomonas aeruginosa oropharyngeal colonization and lung infection. Proc. Natl. Acad. Sci. USA 2003, 100, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Johannesson, B.; Hirtz, S.; Schatterny, J.; Schultz, C.; Mall, M.A. CFTR Regulates Early Pathogenesis of Chronic Obstructive Lung Disease in βENaC-Overexpressing Mice. PLoS ONE 2012, 7, e44059. [Google Scholar] [CrossRef] [PubMed]
- Harkema, J.R. Development of chronic bronchitis and emphysema in beta-epithelial Na+ channel-overexpressing mice: Role of airway surface dehydration in the pathogenesis of COPD. Int. J. Toxicol. 2010, 29. [Google Scholar] [CrossRef]
- Mall, M.A.; Grubb, B.R.; Harkema, J.R.; O′Neal, W.K.; Boucher, R.C. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat. Med. 2004, 10, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Duerr, J.; Johannesson, B.; Schubert, S.C.; Treis, D.; Harm, M.; Graeber, S.Y.; Dalpke, A.; Schultz, C.; Mall, M.A. The ENaC-overexpressing mouse as a model of cystic fibrosis lung disease. J. Cyst. Fibros. 2011, 10 (Suppl. S2), S172–S182. [Google Scholar] [CrossRef]
- Rodgers, A.M.; McCrudden, M.T.C.; Vincente-Perez, E.M.; Dubois, A.V.; Ingram, R.J.; Larrañeta, E.; Kissenpfennig, A.; Donnelly, R.F. Design and characterisation of a dissolving microneedle patch for intradermal vaccination with heat-inactivated bacteria: A proof of concept study. Int. J. Pharm. 2018, 549, 87–95. [Google Scholar] [CrossRef]
- Oleszycka, E.; Rodgers, A.M.; Xu, L.; Moynagh, P.N. Dendritic Cell–Specific Role for Pellino2 as a Mediator of TLR9 Signaling Pathway. J. Immunol. 2021, 207, 2325–2336. [Google Scholar] [CrossRef]
- Reyne, N.; McCarron, A.; Cmielewski, P.; Parsons, D.; Donnelley, M. To bead or not to bead: A review of Pseudomonas aeruginosa lung infection models for cystic fibrosis. Front. Physiol. 2023, 14, 1104856. [Google Scholar] [CrossRef]
- Kukavica-Ibrulj, I.; Bragonzi, A.; Paroni, M.; Winstanley, C.; Sanschagrin, F.; O’Toole, G.A.; Levesque, R.C. In Vivo Growth of Pseudomonas aeruginosa Strains PAO1 and PA14 and the Hypervirulent Strain LESB58 in a Rat Model of Chronic Lung Infection. J. Bacteriol. 2008, 190, 2804–2813. [Google Scholar] [CrossRef]
- Camus, L.; Briaud, P.; Vandenesch, F.; Moreau, K. How Bacterial Adaptation to Cystic Fibrosis Environment Shapes Interactions Between Pseudomonas aeruginosa and Staphylococcus aureus. Front. Microbiol. 2021, 12, 617784. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.; Waters, V. Factors influencing the acquisition and eradication of early Pseudomonas aeruginosa infection in cystic fibrosis. J. Cyst. Fibros. 2021, 20, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.J.; Singh, S.B.; LaMarche, M.M.; Maakestad, L.J.; Kienenberger, Z.E.; Peña, T.A.; Stoltz, D.A.; Limoli, D.H. Sustained Coinfections with Staphylococcus aureus and Pseudomonas aeruginosa in Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2021, 203, 328–338. [Google Scholar] [CrossRef]
- Adam, L.; López-González, M.; Björk, A.; Pålsson, S.; Poux, C.; Wahren-Herlenius, M.; Fernández, C.; Spetz, A.-L. Early Resistance of Non-virulent Mycobacterial Infection in C57BL/6 Mice Is Associated With Rapid Up-Regulation of Antimicrobial Cathelicidin Camp. Front. Immunol. 2018, 9, 1939. [Google Scholar] [CrossRef]
- Fornefett, J.; Krause, J.; Klose, K.; Fingas, F.; Hassert, R.; Eisenberg, T.; Schrödl, W.; Grunwald, T.; Müller, U.; Baums, C.G. Comparative analysis of clinics, pathologies and immune responses in BALB/c and C57BL/6 mice infected with Streptobacillus moniliformis. Microbes Infect. 2018, 20, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Bernal, A.M.; Fernández-Brando, R.J.; Bruballa, A.C.; Fiorentino, G.A.; Pineda, G.E.; Zotta, E.; Vermeulen, M.; Ramos, M.V.; Rumbo, M.; Palermo, M.S. Differential Outcome between BALB/c and C57BL/6 Mice after Escherichia coli O157:H7 Infection Is Associated with a Dissimilar Tolerance Mechanism. Infect. Immun. 2021, 89, e00031-21. [Google Scholar] [CrossRef]
- Thach, D.C.; Kimura, T.; Griffin, D.E. Differences between C57BL/6 and BALB/cBy Mice in Mortality and Virus Replication after Intranasal Infection with Neuroadapted Sindbis Virus. J. Virol. 2000, 74, 6156–6161. [Google Scholar] [CrossRef]
- Mantero, M.; Gramegna, A.; Pizzamiglio, G.; D’adda, A.; Tarsia, P.; Blasi, F. Once daily aerosolised tobramycin in adult patients with cystic fibrosis in the management of Pseudomonas aeruginosa chronic infection. Multidiscip. Respir. Med. 2017, 12, 2. [Google Scholar] [CrossRef]
- Boucher, R. An overview of the pathogenesis of cystic fibrosis lung disease. Adv. Drug Deliv. Rev. 2002, 54, 1359–1371. [Google Scholar] [CrossRef]
- Velzen, A.J.; Uges, J.W.; Heijerman, H.G.; Arets, B.G.; Nuijsink, M.; Wiel-Kooij, E.C.; Maarseveen, E.M.; Zanten, G.A.; Pullens, B.; Touw, D.J.; et al. Pharmacokinetics and safety of tobramycin nebulization with the I-neb and PARI-LC Plus in children with cystic fibrosis: A randomized, crossover study. Br. J. Clin. Pharmacol. 2019, 85, 1984–1993. [Google Scholar] [CrossRef]
- Tucker, S.L.; Sarr, D.; Rada, B. Neutrophil extracellular traps are present in the airways of ENaC-overexpressing mice with cystic fibrosis-like lung disease. BMC Immunol. 2021, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Vyas, H.K.N.; Xia, B.; Mai-Prochnow, A. Clinically relevant in vitro biofilm models: A need to mimic and recapitulate the host environment. Biofilm 2022, 4, 100069. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.C.; Hall, P.R.; Daly, S.M. Nonconformity of biofilm formation in vivo and in vitro based on Staphylococcus aureus accessory gene regulator status. Sci. Rep. 2022, 12, 1251. [Google Scholar] [CrossRef]
- Cigana, C.; Bianconi, I.; Baldan, R.; De Simone, M.; Riva, C.; Sipione, B.; Rossi, G.; Cirillo, D.M.; Bragonzi, A. Staphylococcus aureus Impacts Pseudomonas aeruginosa Chronic Respiratory Disease in Murine Models. J. Infect. Dis. 2017, 217, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Michelsen, C.F.; Christensen, A.-M.J.; Bojer, M.S.; Høiby, N.; Ingmer, H.; Jelsbak, L. Staphylococcus aureus Alters Growth Activity, Autolysis, and Antibiotic Tolerance in a Human Host-Adapted Pseudomonas aeruginosa Lineage. J. Bacteriol. 2014, 196, 3903–3911. [Google Scholar] [CrossRef]
- Oakley, J.L.; Weiser, R.; Powell, L.C.; Forton, J.; Mahenthiralingam, E.; Rye, P.D.; Hill, K.E.; Thomas, D.W.; Pritchard, M.F. Phenotypic and Genotypic Adaptations in Pseudomonas aeruginosa Biofilms following Long-Term Exposure to an Alginate Oligomer Therapy. Msphere 2021, 6, e01216-20. [Google Scholar] [CrossRef]
- Singh, R.; Mackay, A.J.; Patel, A.R.; Garcha, D.S.; Kowlessar, B.S.; E Brill, S.; E Donnelly, L.; Barnes, P.J.; Donaldson, G.C.; A Wedzicha, J. Inflammatory thresholds and the species-specific effects of colonising bacteria in stable chronic obstructive pulmonary disease. Respir. Res. 2014, 15, 114. [Google Scholar] [CrossRef]
- Ghanem, M.K.; Makhlouf, H.A.; Hasan, A.A.; Rashed, H.G.; Khalifa, H.S. Bacteriological profile of critically ill patients with chronic obstructive pulmonary disease in respiratory intensive care unit in Assuit University Hospital. Egypt. J. Bronchol. 2019, 13, 343–348. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Almagro, P.; Romani, V.; Rodriguez-Carballeira, M.; Cuchi, E.; Canales, L.; Blasco, D.; Heredia, J.L.; Garau, J. Pseudomonas aeruginosa in patients hospitalised for COPD exacerbation: A prospective study. Eur. Respir. J. 2009, 34, 1072–1078. [Google Scholar] [CrossRef]
- Martínez-Solano, L.; Macia, M.D.; Fajardo, A.; Oliver, A.; Martinez, J.L. Chronic Pseudomonas aeruginosa Infection in Chronic Obstructive Pulmonary Disease. Clin. Infect. Dis. 2008, 47, 1526–1533. [Google Scholar] [CrossRef]
- Botha, P.; Archer, L.; Anderson, R.L.; Lordan, J.; Dark, J.H.; Corris, P.A.; Gould, K.; Fisher, A.J. Pseudomonas aeruginosa Colonization of the Allograft After Lung Transplantation and the Risk of Bronchiolitis Obliterans Syndrome. Transplantation 2008, 85, 771–774. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodgers, A.M.; Lindsay, J.; Monahan, A.; Dubois, A.V.; Faniyi, A.A.; Plant, B.J.; Mall, M.A.; Ekkelenkamp, M.B.; Elborn, S.; Ingram, R.J. Biologically Relevant Murine Models of Chronic Pseudomonas aeruginosa Respiratory Infection. Pathogens 2023, 12, 1053. https://doi.org/10.3390/pathogens12081053
Rodgers AM, Lindsay J, Monahan A, Dubois AV, Faniyi AA, Plant BJ, Mall MA, Ekkelenkamp MB, Elborn S, Ingram RJ. Biologically Relevant Murine Models of Chronic Pseudomonas aeruginosa Respiratory Infection. Pathogens. 2023; 12(8):1053. https://doi.org/10.3390/pathogens12081053
Chicago/Turabian StyleRodgers, Aoife M., Jaime Lindsay, Avril Monahan, Alice V. Dubois, Aduragbemi A. Faniyi, Barry J. Plant, Marcus A. Mall, Miquel B. Ekkelenkamp, Stuart Elborn, and Rebecca J. Ingram. 2023. "Biologically Relevant Murine Models of Chronic Pseudomonas aeruginosa Respiratory Infection" Pathogens 12, no. 8: 1053. https://doi.org/10.3390/pathogens12081053
APA StyleRodgers, A. M., Lindsay, J., Monahan, A., Dubois, A. V., Faniyi, A. A., Plant, B. J., Mall, M. A., Ekkelenkamp, M. B., Elborn, S., & Ingram, R. J. (2023). Biologically Relevant Murine Models of Chronic Pseudomonas aeruginosa Respiratory Infection. Pathogens, 12(8), 1053. https://doi.org/10.3390/pathogens12081053





