Establishment of an Animal Model Scheme of Strongyloides stercoralis-Infected Meriones meridianus
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Parasite Maintenance and Culture
2.3. Gerbil and Dog Infection
2.4. Stool Examination and Necropsy Procedures
2.5. Histology
2.6. Morphology and Statistics
2.7. Haematological Assessments
3. Results
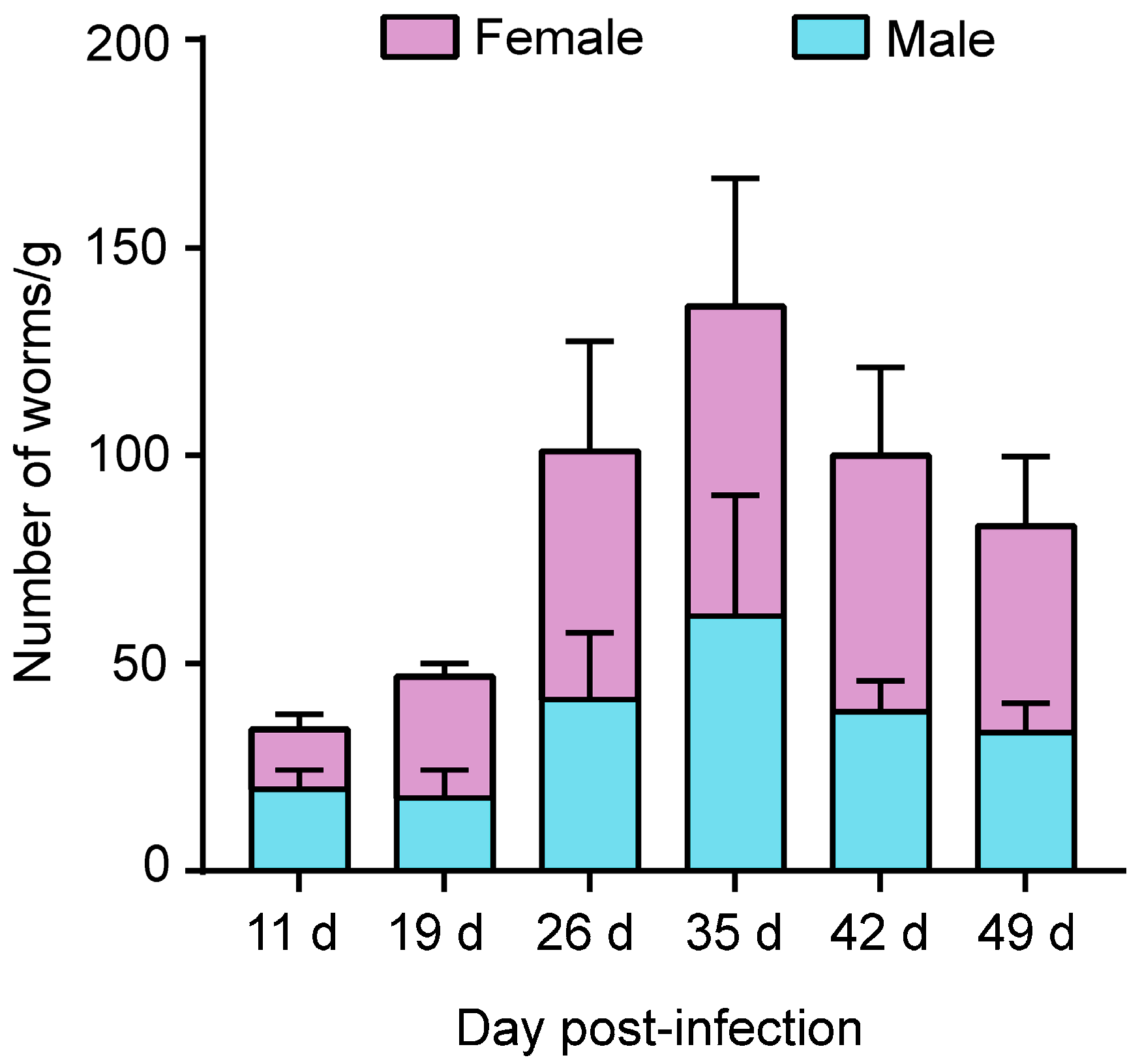
3.1. Both Female and Male M. meridianus Can Be Infected by S. stercoralis
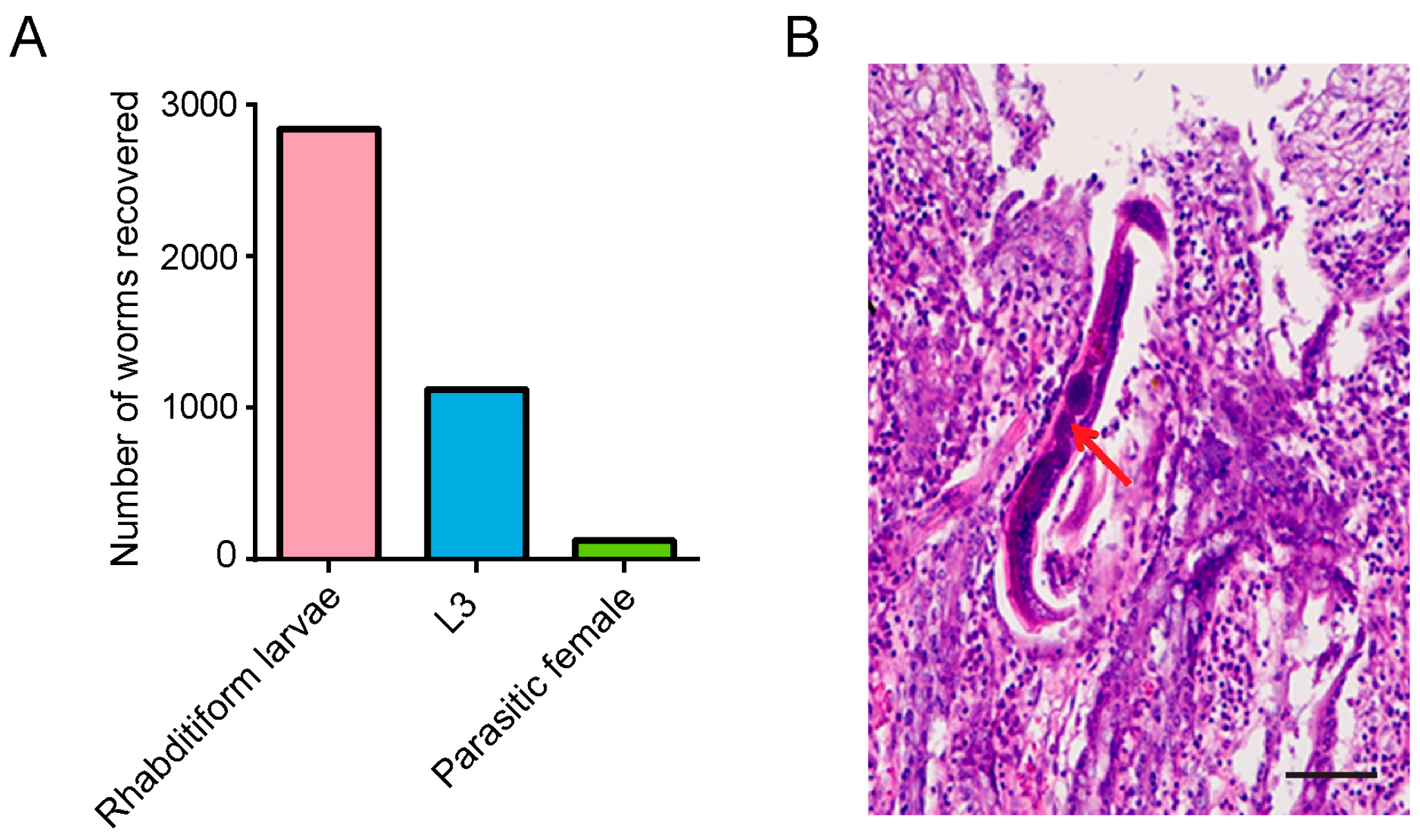
3.2. Strongyloides stercoralis Complete Their Life Cycle in M. meridianus
3.3. Morphometric Features of S. stercoralis from M. meridianus
3.4. Changes to Physiological and Biochemical Parameters of M. meridianus Infected with S. stercoralis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alvarado-Gonzalez, J.C.; Alvis-Zakzuk, N.R.; Castillo-Saavedra, D.E.; Lozada-Martinez, I.D.; Picon-Jaimes, Y.A.; Narvaez-Rojas, A.R.; Zakzuk, J. Impact of helminthiasis on gestational anemia in low- and middle-income countries: A systematic review and meta-analysis of more than 19,000 women. Le. Infezioni. In. Medicina. 2022, 31, 36–48. [Google Scholar] [PubMed]
- Charlier, J.; Rinaldi, L.; Musella, V.; Ploeger, H.W.; Chartier, C.; Vineer, H.R.; Hinney, B.; von Samson-Himmelstjerna, G.; Bacescu, B.; Mickiewicz, M.; et al. Initial assessment of the economic burden of major parasitic helminth infections to the ruminant livestock industry in Europe. Prev. Vet. Med. 2020, 182, 105103. [Google Scholar] [CrossRef] [PubMed]
- Schad, G.A.; Hellman, M.E.; Muncey, D.W. Strongyloides stercoralis: Hyperinfection in immunosuppressed dogs. Exp. Parasitol. 1984, 57, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.F.; Hadip, F.; Ngui, R.; Lim, Y.A.L.; Mahmud, R. Serological and molecular detection of Strongyloides stercoralis infection among an Orang Asli community in Malaysia. Parasitol. Res. 2013, 112, 2811–2816. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.L.Y.; Kennedy, B.; Nelson, R. Fatal Strongyloides hyperinfection syndrome in an immunocompetent adult with review of the literature. Intern. Med. J. 2018, 48, 872–875. [Google Scholar] [CrossRef]
- Schar, F.; Trostdorf, U.; Giardina, F.; Khieu, V.; Muth, S.; Marti, H.; Vounatsou, P.; Odermatt, P. Strongyloides stercoralis: Global distribution and risk factors. PLoS Neglect. Trop. Dis. 2013, 7, e2288. [Google Scholar] [CrossRef]
- Schar, F.; Giardina, F.; Khieu, V.; Muth, S.; Vounatsou, P.; Marti, H.; Odermatt, P. Occurrence of and risk factors for Strongyloides stercoralis infection in South-East Asia. Acta. Trop. 2016, 159, 227–238. [Google Scholar] [CrossRef]
- Toma, H.; Sato, Y.; Shiroma, Y.; Kobayashi, J.; Shimabukuro, I.; Takara, M. Comparative studies on the efficacy of three anthelmintics on treatment of human strongyloidiasis in Okinawa, Japan. Southeast Asian J. Trop. Med. Public Health 2000, 31, 147–151. [Google Scholar]
- Olsen, A.; van Lieshout, L.; Marti, H.; Polderman, T.; Polman, K.; Steinmann, P.; Stothard, R.; Thybo, S.; Verweij, J.J.; Magnussen, P. Strongyloidiasis—The most neglected of the neglected tropical diseases? T. Roy. Soc. Trop. Med. H. 2009, 103, 967–972. [Google Scholar] [CrossRef]
- Keiser, P.B.; Nutman, T.B. Strongyloides stercoralis in the immunocompromised population. Clin. Microbiol. Rev. 2004, 17, 208–217. [Google Scholar] [CrossRef]
- Myint, A.; Chapman, C.; Almira-Suarez, I.; Mehta, N. Strongyloides hyperinfection syndrome in an immunocompetent host resulting in bandemia and death. BMJ Case Rep. 2017, 2017, bcr2016217911. [Google Scholar] [CrossRef]
- Lok, J.B. Strongyloides stercoralis: A model for translational research on parasitic nematode biology. WormBook 2007, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Barrett, K.E.; Neva, F.A.; Gam, A.A.; Cicmanec, J.; London, W.T.; Phillips, J.M.; Metcalfe, D.D. The immune response to nematode parasites: Modulation of mast cell numbers and function during Strongyloides stercoralis infections in nonhuman primates. Am. J. Trop. Med. Hyg. 1988, 38, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Zhou, H.; Lok, J.B.; Lei, W.; He, S.; Gasser, R.B.; Zhou, R.; Fang, R.; Zhou, Y.; Zhao, J.; et al. Functional genomic exploration reveals that Ss-RIOK-1 is essential for the development and survival of Strongyloides stercoralis larvae. Int. J. Parasitol. 2017, 47, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhou, T.X.; Zhang, B.Y.; Lei, W.Q.; Yuan, W.; Shan, J.N.; Zhang, Y.; Gupta, N.; Hu, M. RIOK-2 protein is essential for egg hatching in a common parasitic nematode. Int. J. Parasitol. 2020, 50, 595–602. [Google Scholar] [CrossRef]
- Dawkins, H.J.; Grove, D.I. Attempts to establish infections with Strongyloides stercoralis in mice and other laboratory animals. J. Helminthol. 1982, 56, 23–26. [Google Scholar] [CrossRef]
- Nolan, T.J.; Bhopale, V.M.; Schad, G.A. Hyperinfective strongyloidiasis: Strongyloides stercoralis undergoes an autoinfective burst in neonatal gerbils. J. Parasitol. 1999, 85, 286–289. [Google Scholar] [CrossRef]
- Nolan, T.J.; Megyeri, Z.; Bhopale, V.M.; Schad, G.A. Strongyloides stercoralis: The first rodent model for uncomplicated and hyperinfective strongyloidiasis, the Mongolian gerbil (Meriones unguiculatus). J. Infect. Dis. 1993, 168, 1479–1484. [Google Scholar] [CrossRef]
- Kerlin, R.L.; Nolan, T.J.; Schad, G.A. Strongyloides stercoralis: Histopathology of uncomplicated and hyperinfective strongyloidiasis in the Mongolian gerbil, a rodent model for human strongyloidiasis [corrected]. Int. J. Parasitol. 1995, 25, 411–420. [Google Scholar] [CrossRef]
- Patton, J.B.; Bonne-Annee, S.; Deckman, J.; Hess, J.A.; Torigian, A.; Nolan, T.J.; Wang, Z.; Kliewer, S.A.; Durham, A.C.; Lee, J.J.; et al. Methylprednisolone acetate induces, and Δ7-dafachronic acid suppresses, Strongyloides stercoralis hyperinfection in NSG mice. Proc. Natl. Acad. Sci. USA 2018, 115, 204–209. [Google Scholar] [CrossRef]
- Springer, L.E.; Patton, J.B.; Zhan, T.; Rabson, A.B.; Lin, H.C.; Manser, T.; Lok, J.B.; Hess, J.A.; Abraham, D. Strongyloides stercoralis and HTLV-1 coinfection in CD34+ cord blood stem cell humanized mice: Alteration of cytokine responses and enhancement of larval growth. PLoS Negl. Trop. Dis. 2021, 15, e0009559. [Google Scholar] [CrossRef] [PubMed]
- Charuchaibovorn, S.; Sanprasert, V.; Nuchprayoon, S. The experimental infections of the human isolate of Strongyloides stercoralis in a rodent model (The Mongolian gerbil, Meriones unguiculatus). Pathogens 2019, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Jiang, W.; Sato, J.J.; Zhen, Q.; Jiao, W.; Goto, K.; Sato, H.; Ishiwata, K.; Oku, Y.; Chai, J.J.; et al. Molecular phylogeny of the subfamily Gerbillinae (Muridae, Rodentia) with emphasis on species living in the Xinjiang-Uygur Autonomous Region of China and based on the mitochondrial cytochrome b and cytochrome c oxidase subunit II genes. Zool. Sci. 2010, 27, 269–278. [Google Scholar] [CrossRef]
- Shenbrot, G.I.; Krasnov, B.R.; Rogovin, K.A. Spatial Ecology of Desert Rodent Communities; Springer: Berlin, Germany; New York, NY, USA, 1999; Volume 8, pp. 203–242. [Google Scholar]
- Tchabovsky, A.; Savinetskaya, L.; Surkova, E. Breeding versus survival: Proximate causes of abrupt population decline under environmental change in a desert rodent, the midday gerbil (Meriones meridianus). Integr. Zool. 2019, 14, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Gol’tsman, M.E.; Popov, S.V.; Chabovskii, A.V.; Borisova, N.G. The sociality syndrome. A comparative study of the behavior of gerbils. Zh. Obshch. Biol. 1994, 55, 49–69. [Google Scholar]
- Ding, B.Y.; Chi, Q.S.; Liu, W.; Shi, Y.L.; Wang, D.H. Thermal biology of two sympatric gerbil species: The physiological basis of temporal partitioning. J. Therm. Biol. 2018, 74, 241–248. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, S.; Yan, H.; Xu, X.; Yuan, J.; Liao, L. Isolation and identification of respiratory tract and intestinal microflora of Meriones meridianus in a conventional animal facility. Anim. Model. Exp. Med. 2018, 1, 322–327. [Google Scholar] [CrossRef]
- Verevkin, M. Reproductive biology of the midday jird (Meriones meridianus). Zool. Zh. 1985, 64, 276–281. [Google Scholar]
- Popov, S.; Tchabovsky, A.; Shilova, S.; Shchipanov, N. Mechanisms of formation of the spatial-and-ethological population structure in the Midday gerbil. Fauna Ecol. Rodents. 1989, 17, 5–57. [Google Scholar]
- Strelkova, M.V. Duration and character of the course of cutaneous leishmaniasis in the midday gerbil (Meriones meridianus Pall.). Parazitologia 1975, 9, 532–534. [Google Scholar]
- Oku, Y.; Wei, J.; Chai, J.J.; Osman, I.; Wei, J.; Liao, L.F.; Asakawa, M.; Hagiwara, K.; Kobayashi, K.; Ito, M. Meriones meridianus and Lagurus lagurus as alternative definitive hosts of Echinococcus multilocularis and E. granulosus. Exp. Anim. Tokyo. 2002, 51, 27–32. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Osman, I.; Jiao, W.; Liao, L.; Chai, J. Comparative observation on experimental infection with Echinococcus multilocularis in Cricetulus Migratorius and Meriones meridianus. Chin. J. Parasitol. Parasitic Dis. 1998, 16, 130–132. [Google Scholar]
- Gang, S.S.; Castelletto, M.L.; Bryant, A.S.; Yang, E.; Mancuso, N.; Lopez, J.B.; Pellegrini, M.; Hallem, E.A. Targeted mutagenesis in a human-parasitic nematode. PLoS Pathog. 2017, 13, e1006675. [Google Scholar] [CrossRef]
- Kukurba, K.R.; Montgomery, S.B. RNA Sequencing and Analysis. Cold Spring Harb. Protoc. 2015, 2015, 951–969. [Google Scholar] [CrossRef]
- Zhao, L.; He, X.; Grevelding, C.G.; Ye, Q.; Li, Y.; Gasser, R.B.; Dissous, C.; Mughal, M.N.; Zhou, Y.Q.; Zhao, J.L.; et al. The RIO protein kinase-encoding gene Sj-riok-2 is involved in key reproductive processes in Schistosoma japonicum. Parasite Vector 2017, 10, 604. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.K. Strongyloidosis. Indian. Med. Gaz. 1923, 58, 155–158. [Google Scholar]
- Mondal, H.; Budh, D.P. Hematocrit; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Senior, J.R. Alanine aminotransferase: A clinical and regulatory tool for detecting liver injury-past, present, and future. Clin. Pharmacol. Ther. 2012, 92, 332–339. [Google Scholar] [CrossRef]
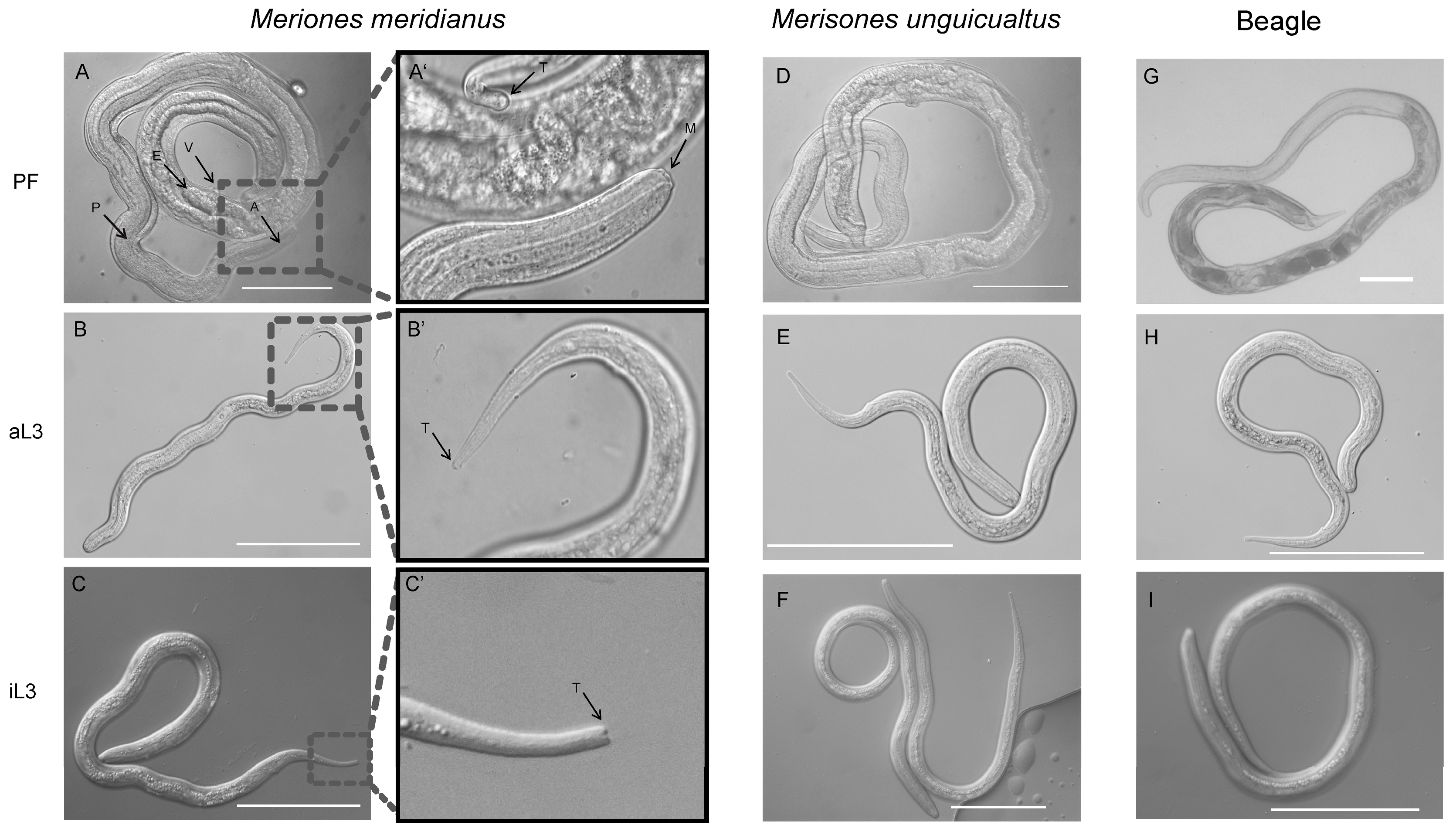
| Hosts | Stage | Body Length (mm) 2 | Maximum Diameter (mm) 2 |
|---|---|---|---|
| M. meridianus | PF | 2.14 ± 0.263 | 0.039 ± 0.031 |
| PP L3 | 0.544 ± 0.181 | 0.019 ± 0.028 1 | |
| M. unguiculatus | PF | 2.12 ± 0.142 | 0.042 ± 0.016 |
| PP L3 | 0.521 ± 0.142 | 0.022 ± 0.032 1 | |
| Beagle | PF | 2.09 ± 0.212 | 0.042 ± 0.016 |
| PP L3 | 0.513 ± 0.162 | 0.018 ± 0.057 1 |
| Indicators | Meriones meridianus | |
|---|---|---|
| Group 1 (Unsuccessfully Infected) | Group 4 (Successfully Infected) | |
| ALT (U/L) | 46 | 201 |
| TG (mmol/L) | 0.84 | 16.69 |
| HCT (%) | 28.3 | 31.6 |
| RBC (1012/L) | 6.13 | 4.68 |
| Bas (%) | 0.1 | 0.3 |
| Eos (%) | 0.4 | 0.8 |
| WBC (109/L) | 3.8 | 4.5 |
| PLT (109/L) | 268 | 1555 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Hu, J.; Zhou, T.; Zhang, Y.; Qin, P.; Zhang, B.; Wang, R.; Luo, X.; Hu, M. Establishment of an Animal Model Scheme of Strongyloides stercoralis-Infected Meriones meridianus. Pathogens 2023, 12, 1285. https://doi.org/10.3390/pathogens12111285
Zhou H, Hu J, Zhou T, Zhang Y, Qin P, Zhang B, Wang R, Luo X, Hu M. Establishment of an Animal Model Scheme of Strongyloides stercoralis-Infected Meriones meridianus. Pathogens. 2023; 12(11):1285. https://doi.org/10.3390/pathogens12111285
Chicago/Turabian StyleZhou, Huan, Jinyang Hu, Taoxun Zhou, Ying Zhang, Peixi Qin, Biying Zhang, Rui Wang, Xiaoping Luo, and Min Hu. 2023. "Establishment of an Animal Model Scheme of Strongyloides stercoralis-Infected Meriones meridianus" Pathogens 12, no. 11: 1285. https://doi.org/10.3390/pathogens12111285
APA StyleZhou, H., Hu, J., Zhou, T., Zhang, Y., Qin, P., Zhang, B., Wang, R., Luo, X., & Hu, M. (2023). Establishment of an Animal Model Scheme of Strongyloides stercoralis-Infected Meriones meridianus. Pathogens, 12(11), 1285. https://doi.org/10.3390/pathogens12111285







