A Prior Usutu Virus Infection Can Protect Geese from Severe West Nile Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Geese
2.2. Viruses
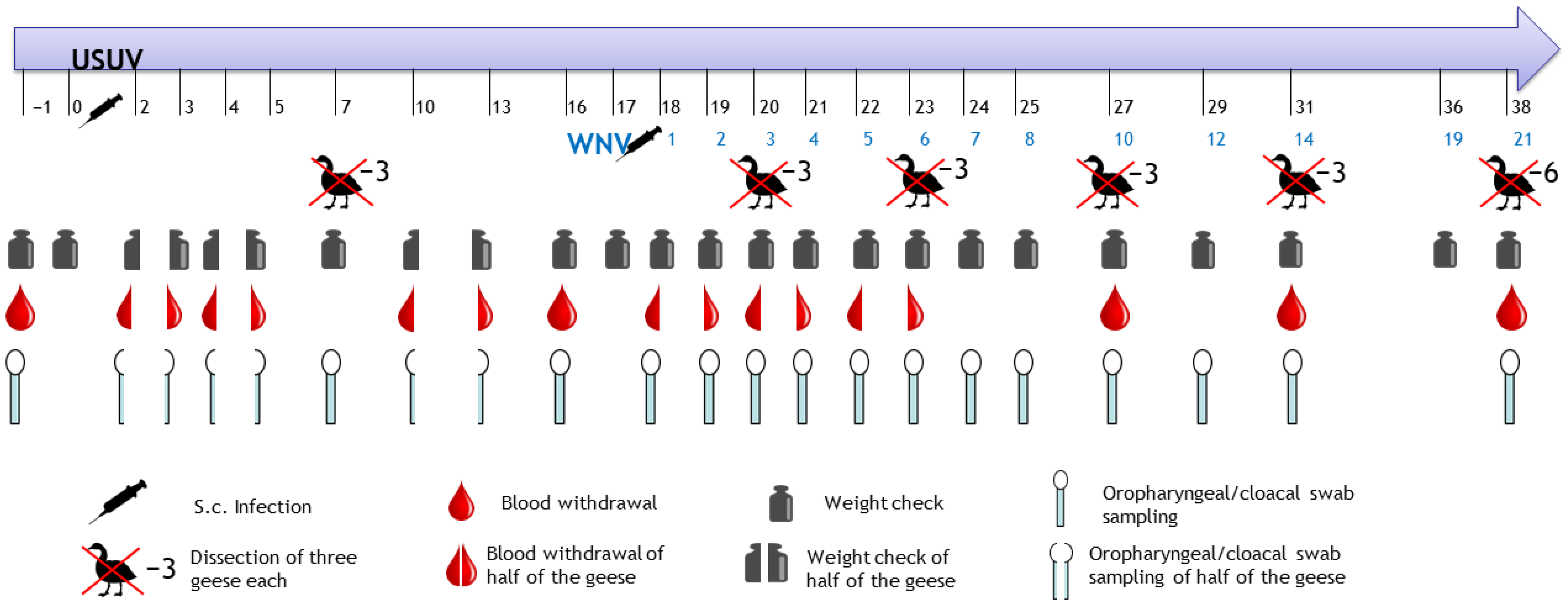
2.3. Procedure of the Animal Trial
2.4. Sampling Procedure
2.5. Reverse Transcription Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
2.6. Virus Titration
2.7. Serology
2.8. Histopathology and Immunohistochemistry
2.9. Statistics
2.10. Ethical Approval
3. Results
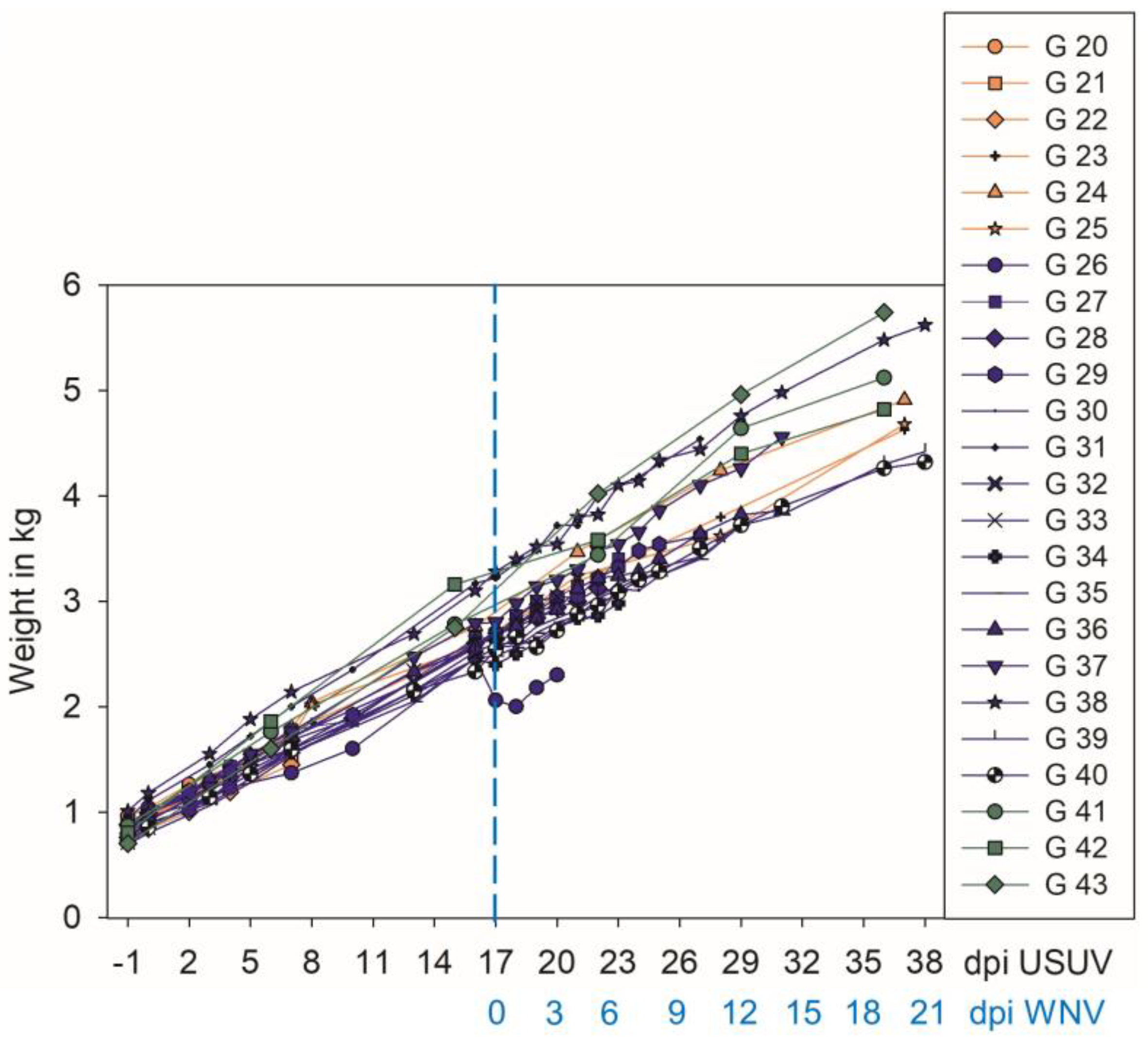
3.1. Weight Gain and Clinical Signs
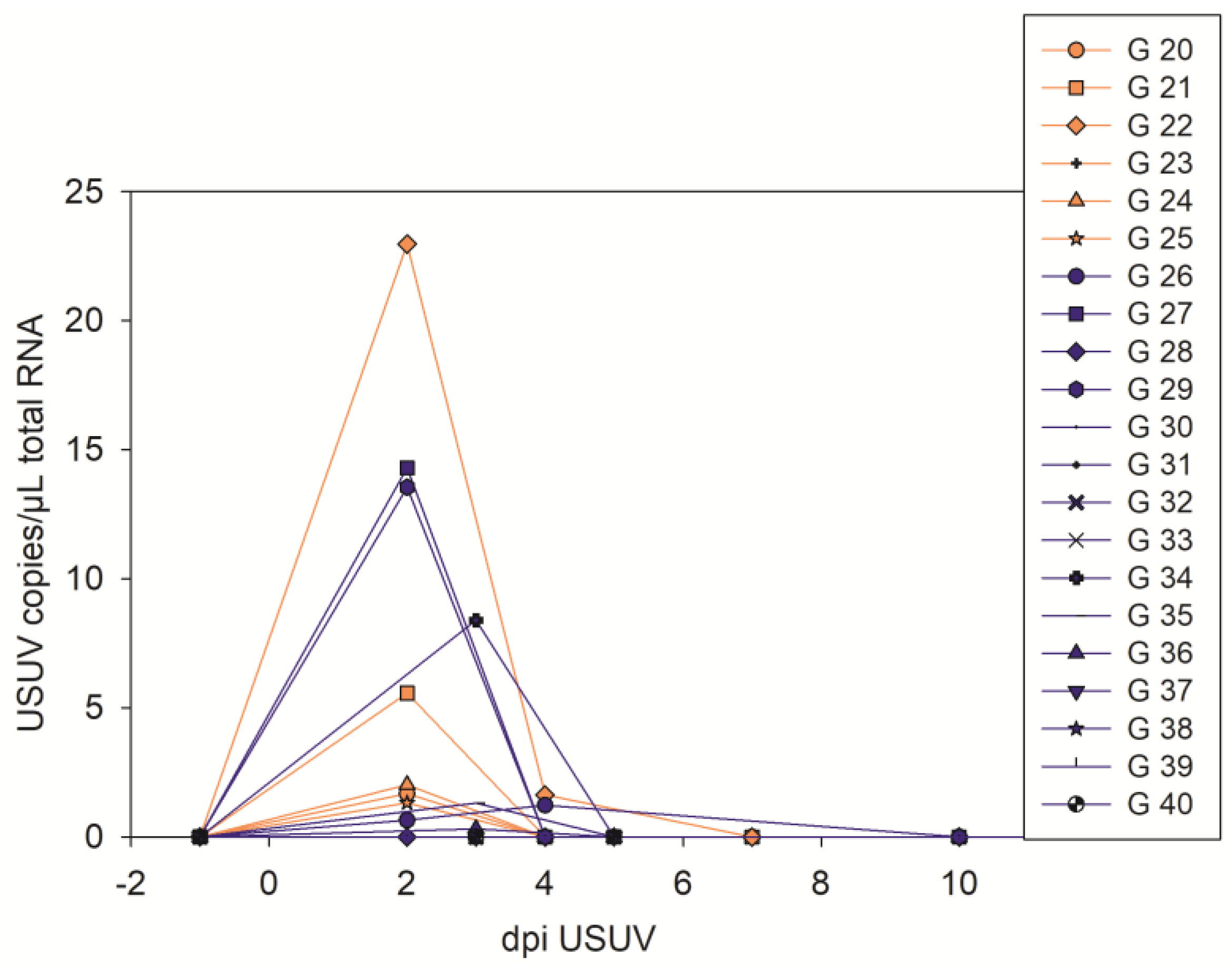
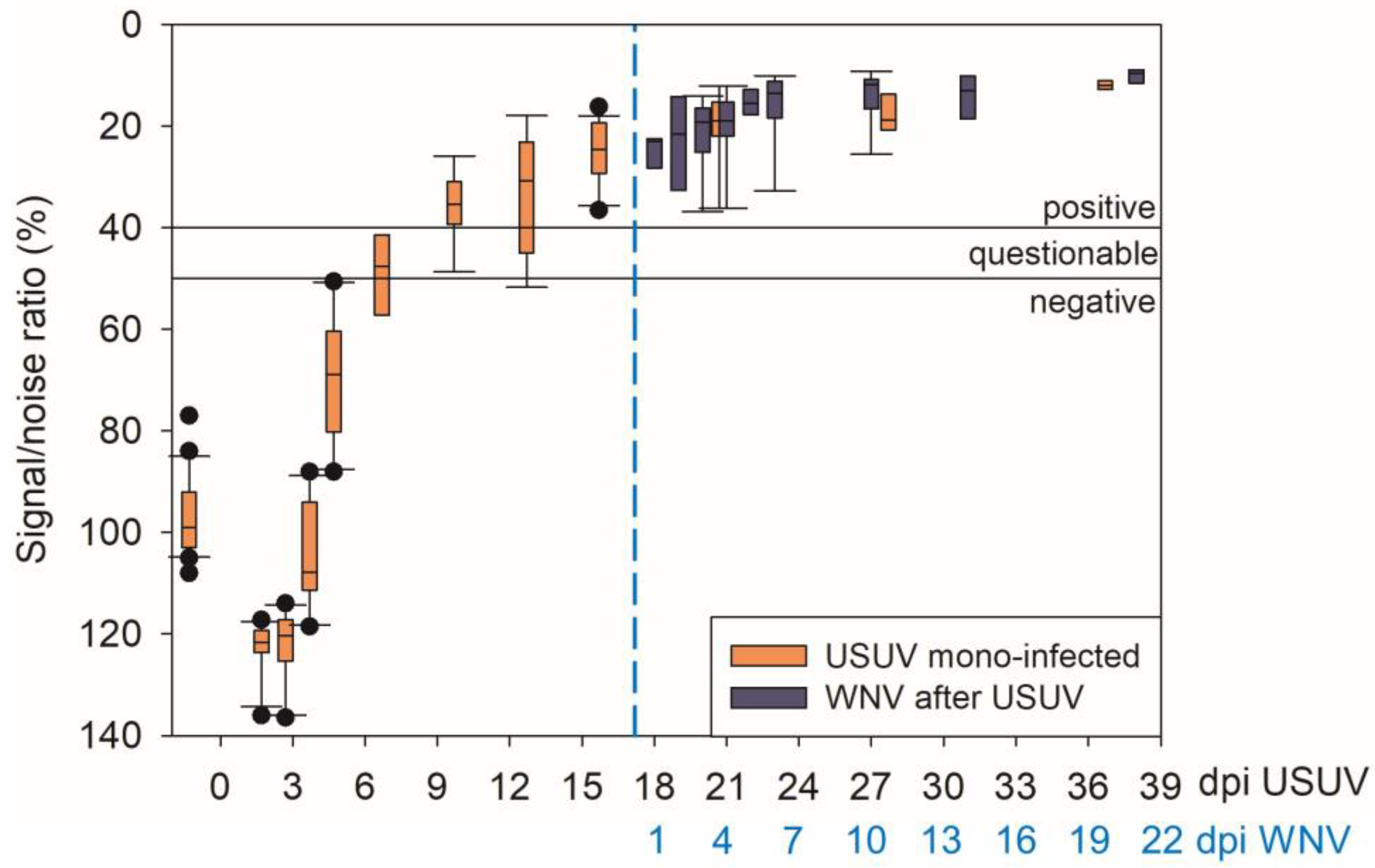
3.2. USUV Virological Results
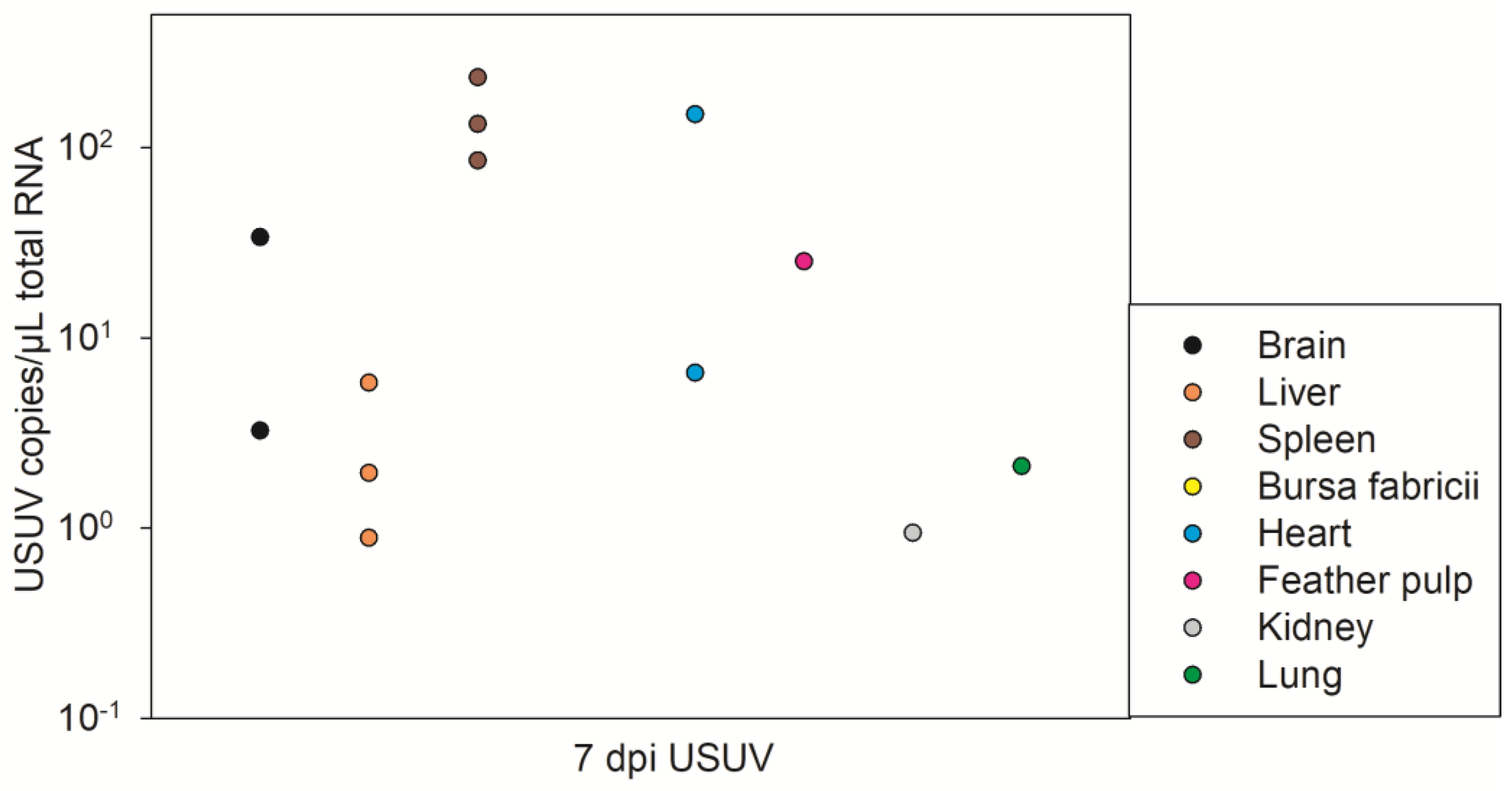
3.3. USUV Pathological Results
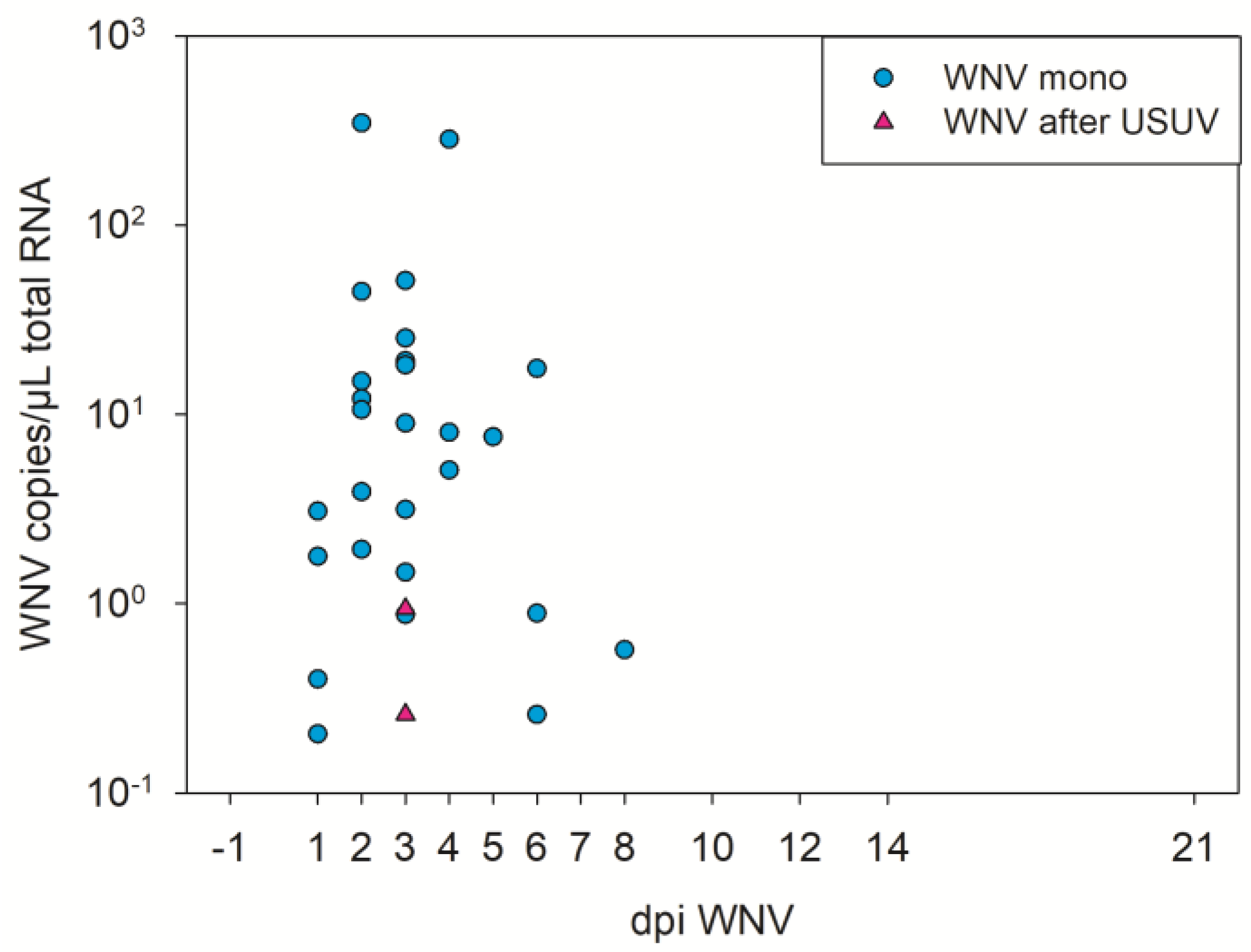
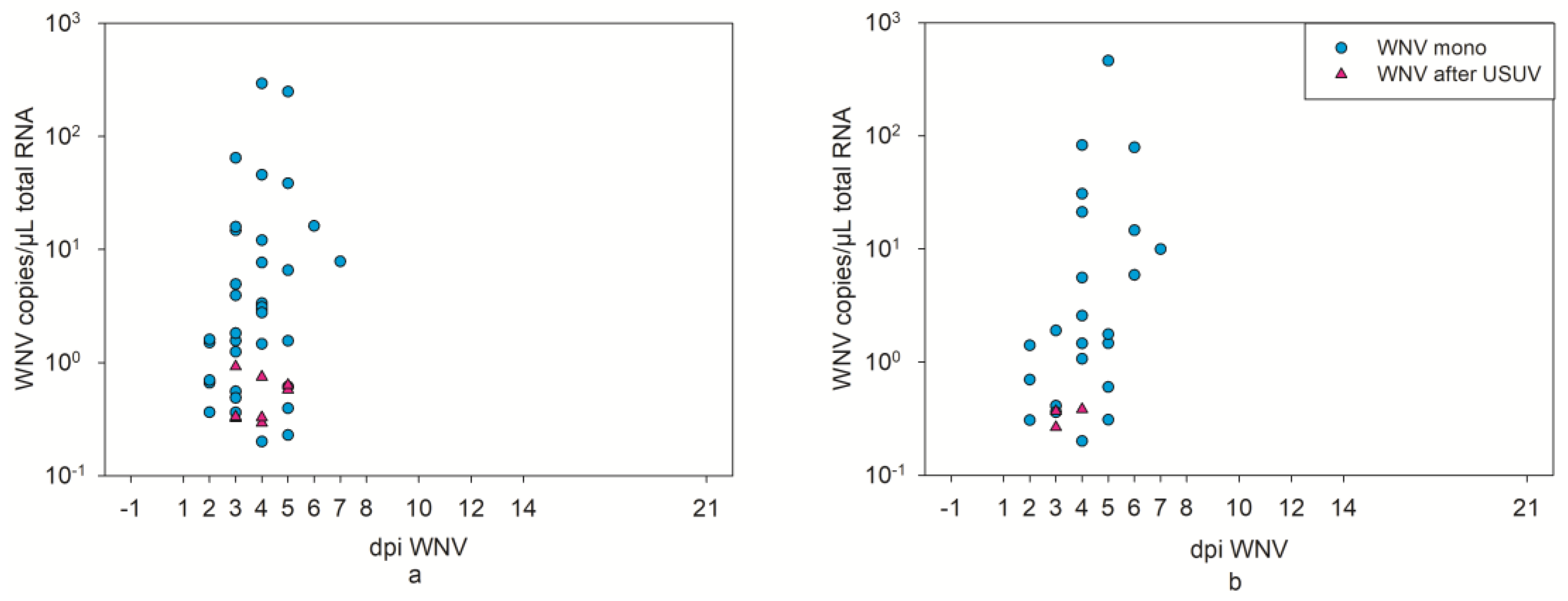
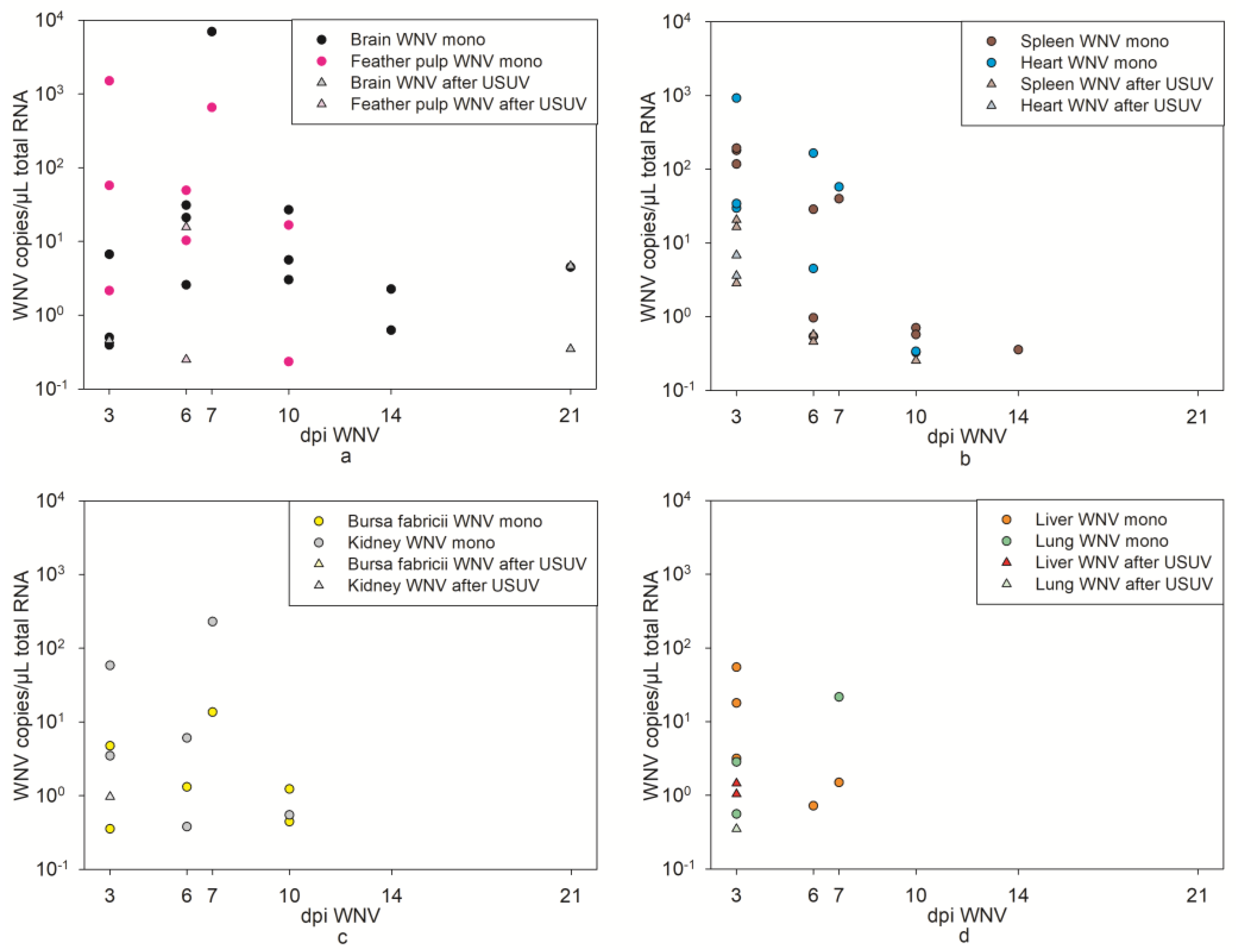
3.4. WNV Virological Results
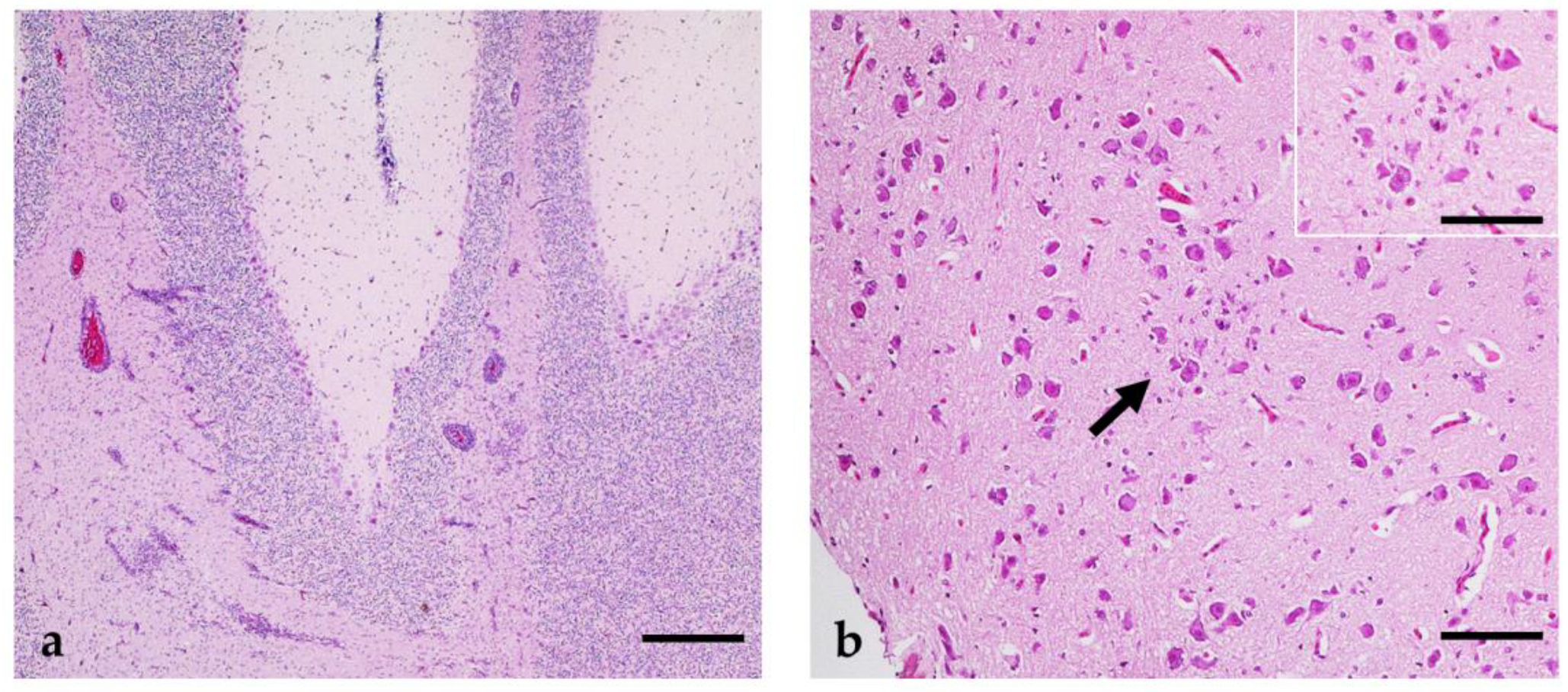
3.5. WNV Pathological Results
3.6. Controls’ Pathological Results
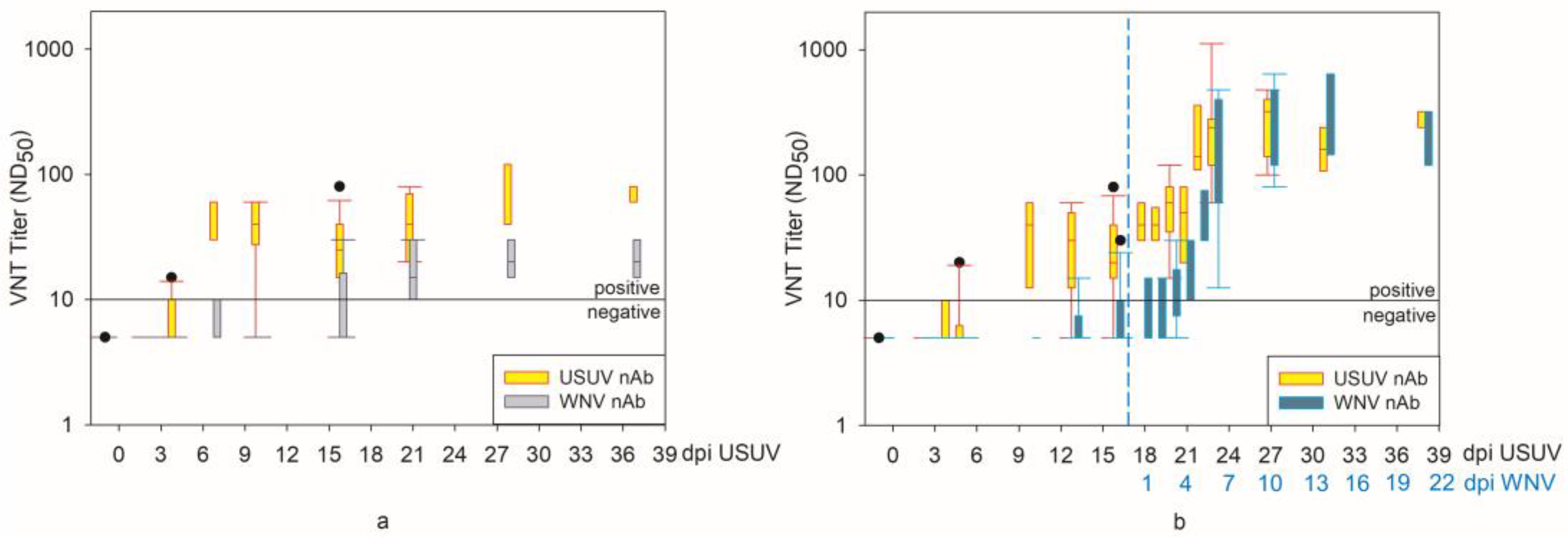
3.7. Serology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuno, G. Persistence of arboviruses and antiviral antibodies in vertebrate hosts: Its occurrence and impacts. Rev. Med. Virol. 2001, 11, 165–190. [Google Scholar] [CrossRef]
- Calisher, C.H.; Shope, R.E.; Brandt, W.; Casals, J.; Karabatsos, N.; Murphy, F.A.; Tesh, R.B.; Wiebe, M.E. Proposed antigenic classification of registered arboviruses I. Togaviridae, Alphavirus. INT 1980, 14, 229–232. [Google Scholar] [CrossRef]
- Hayes, E.B.; Komar, N.; Nasci, R.S.; Montgomery, S.P.; O’Leary, D.R.; Campbell, G.L. Epidemiology and transmission dynamics of West Nile virus disease. Emerg. Infect. Dis. 2005, 11, 1167–1173. [Google Scholar] [CrossRef]
- Clé, M.; Beck, C.; Salinas, S.; Lecollinet, S.; Gutierrez, S.; van de Perre, P.; Baldet, T.; Foulongne, V.; Simonin, Y. Usutu virus: A new threat? Epidemiol. Infect. 2019, 147, e232. [Google Scholar] [CrossRef]
- Weissenböck, H.; Bakonyi, T.; Rossi, G.; Mani, P.; Nowotny, N. Usutu virus, Italy, 1996. Emerg. Infect. Dis. 2013, 19, 274–277. [Google Scholar] [CrossRef]
- Weissenböck, H.; Kolodziejek, J.; Url, A.; Lussy, H.; Rebel-Bauder, B.; Nowotny, N. Emergence of Usutu virus, an African mosquito-borne flavivirus of the Japanese encephalitis virus group, central Europe. Emerg. Infect. Dis. 2002, 8, 652–656. [Google Scholar] [CrossRef]
- Jöst, H.; Bialonski, A.; Maus, D.; Sambri, V.; Eiden, M.; Groschup, M.H.; Günther, S.; Becker, N.; Schmidt-Chanasit, J. Isolation of usutu virus in Germany. Am. J. Trop. Med. Hyg. 2011, 85, 551–553. [Google Scholar] [CrossRef]
- Weissenböck, H.; Kolodziejek, J.; Fragner, K.; Kuhn, R.; Pfeffer, M.; Nowotny, N. Usutu virus activity in Austria, 2001–2002. Microbes Infect. 2003, 5, 1132–1136. [Google Scholar] [CrossRef]
- Becker, N.; Jöst, H.; Ziegler, U.; Eiden, M.; Höper, D.; Emmerich, P.; Fichet-Calvet, E.; Ehichioya, D.U.; Czajka, C.; Gabriel, M.; et al. Epizootic emergence of Usutu virus in wild and captive birds in Germany. PLoS ONE 2012, 7, e32604. [Google Scholar] [CrossRef]
- Ziegler, U.; Jöst, H.; Müller, K.; Fischer, D.; Rinder, M.; Tietze, D.T.; Danner, K.-J.; Becker, N.; Skuballa, J.; Hamann, H.-P.; et al. Epidemic Spread of Usutu Virus in Southwest Germany in 2011 to 2013 and Monitoring of Wild Birds for Usutu and West Nile Viruses. Vector Borne Zoonotic Dis. 2015, 15, 481–488. [Google Scholar] [CrossRef]
- Cadar, D.; Bosch, S.; Jöst, H.; Börstler, J.; Garigliany, M.-M.; Becker, N.; Schmidt-Chanasit, J. Putative Lineage of Novel African Usutu Virus, Central Europe. Emerg. Infect. Dis. 2015, 21, 1647–1650. [Google Scholar] [CrossRef]
- Ziegler, U.; Fast, C.; Eiden, M.; Bock, S.; Schulze, C.; Hoeper, D.; Ochs, A.; Schlieben, P.; Keller, M.; Zielke, D.E.; et al. Evidence for an independent third Usutu virus introduction into Germany. Vet. Microbiol. 2016, 192, 60–66. [Google Scholar] [CrossRef]
- Michel, F.; Sieg, M.; Fischer, D.; Keller, M.; Eiden, M.; Reuschel, M.; Schmidt, V.; Schwehn, R.; Rinder, M.; Urbaniak, S.; et al. Evidence for West Nile Virus and Usutu Virus Infections in Wild and Resident Birds in Germany, 2017 and 2018. Viruses 2019, 11, 674. [Google Scholar] [CrossRef]
- Bergmann, F.; Holicki, C.M.; Michel, F.; Bock, S.; Scuda, N.; Priemer, G.; Kenklies, S.; Siempelkamp, T.; Skuballa, J.; Sauerwald, C.; et al. Reconstruction of the molecular evolution of Usutu virus in Germany: Insights into virus emersion and circulation. bioRxiv 2023. [Google Scholar] [CrossRef]
- Lühken, R.; Jöst, H.; Cadar, D.; Thomas, S.M.; Bosch, S.; Tannich, E.; Becker, N.; Ziegler, U.; Lachmann, L.; Schmidt-Chanasit, J. Distribution of Usutu Virus in Germany and Its Effect on Breeding Bird Populations. Emerg. Infect. Dis. 2017, 23, 1994–2001. [Google Scholar] [CrossRef]
- Cadar, D.; Simonin, Y. Human Usutu Virus Infections in Europe: A New Risk on Horizon? Viruses 2022, 15, 77. [Google Scholar] [CrossRef]
- Ziegler, U.; Lühken, R.; Keller, M.; Cadar, D.; van der Grinten, E.; Michel, F.; Albrecht, K.; Eiden, M.; Rinder, M.; Lachmann, L.; et al. West Nile virus epizootic in Germany, 2018. Antivir. Res. 2019, 162, 39–43. [Google Scholar] [CrossRef]
- Kampen, H.; Holicki, C.M.; Ziegler, U.; Groschup, M.H.; Tews, B.A.; Werner, D. West Nile Virus Mosquito Vectors (Diptera: Culicidae) in Germany. Viruses 2020, 12, 493. [Google Scholar] [CrossRef]
- Ziegler, U.; Santos, P.D.; Groschup, M.H.; Hattendorf, C.; Eiden, M.; Höper, D.; Eisermann, P.; Keller, M.; Michel, F.; Klopfleisch, R.; et al. West Nile Virus Epidemic in Germany Triggered by Epizootic Emergence, 2019. Viruses 2020, 12, 448. [Google Scholar] [CrossRef]
- Ziegler, U.; Bergmann, F.; Fischer, D.; Müller, K.; Holicki, C.M.; Sadeghi, B.; Sieg, M.; Keller, M.; Schwehn, R.; Reuschel, M.; et al. Spread of West Nile Virus and Usutu Virus in the German Bird Population, 2019–2020. Microorganisms 2022, 10, 807. [Google Scholar] [CrossRef]
- Frank, C.; Schmidt-Chanasit, J.; Ziegler, U.; Lachmann, R.; Preußel, K.; Offergeld, R. West Nile Virus in Germany: An Emerging Infection and Its Relevance for Transfusion Safety. Transfus. Med. Hemother. 2022, 49, 192–204. [Google Scholar] [CrossRef]
- Santos, P.; Keller, M.; Homeier-Bachmann, T.; Beer, M.; Ziegler, U.; Höper, D.; Günther, A.; Groschup, M. An advanced sequence clustering and designation workflow reveals the enzootic maintenance of a dominant West Nile virus subclade in Germany. Virus Evol. 2023, 9, vead013. [Google Scholar] [CrossRef]
- Nikolay, B. A review of West Nile and Usutu virus co-circulation in Europe: How much do transmission cycles overlap? Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 609–618. [Google Scholar] [CrossRef]
- Zannoli, S.; Sambri, V. West Nile Virus and Usutu Virus Co-Circulation in Europe: Epidemiology and Implications. Microorganisms 2019, 7, 184. [Google Scholar] [CrossRef]
- Vilibic-Cavlek, T.; Savic, V.; Petrovic, T.; Toplak, I.; Barbic, L.; Petric, D.; Tabain, I.; Hrnjakovic-Cvjetkovic, I.; Bogdanic, M.; Klobucar, A.; et al. Emerging Trends in the Epidemiology of West Nile and Usutu Virus Infections in Southern Europe. Front. Vet. Sci. 2019, 6, 437. [Google Scholar] [CrossRef]
- Percivalle, E.; Sassera, D.; Rovida, F.; Isernia, P.; Fabbi, M.; Baldanti, F.; Marone, P. Usutu Virus Antibodies in Blood Donors and Healthy Forestry Workers in the Lombardy Region, Northern Italy. Vector Borne Zoonotic Dis. 2017, 17, 658–661. [Google Scholar] [CrossRef]
- Faggioni, G.; de Santis, R.; Pomponi, A.; Grottola, A.; Serpini, G.F.; Meacci, M.; Gennari, W.; Tagliazucchi, S.; Pecorari, M.; Monaco, F.; et al. Prevalence of Usutu and West Nile virus antibodies in human sera, Modena, Italy, 2012. J. Med. Virol. 2018, 90, 1666–1668. [Google Scholar] [CrossRef]
- Constant, O.; Gil, P.; Barthelemy, J.; Bolloré, K.; Foulongne, V.; Desmetz, C.; Leblond, A.; Desjardins, I.; Pradier, S.; Joulié, A.; et al. One Health surveillance of West Nile and Usutu viruses: A repeated cross-sectional study exploring seroprevalence and endemicity in Southern France, 2016 to 2020. Eurosurveillance 2022, 27, 2200068. [Google Scholar] [CrossRef]
- Aberle, S.W.; Kolodziejek, J.; Jungbauer, C.; Stiasny, K.; Aberle, J.H.; Zoufaly, A.; Hourfar, M.K.; Weidner, L.; Nowotny, N. Increase in human West Nile and Usutu virus infections, Austria, 2018. Eurosurveillance 2018, 23, 1800545. [Google Scholar] [CrossRef]
- Sinigaglia, A.; Pacenti, M.; Martello, T.; Pagni, S.; Franchin, E.; Barzon, L. West Nile virus infection in individuals with pre-existing Usutu virus immunity, northern Italy, 2018. Eurosurveillance 2019, 24, 1900261. [Google Scholar] [CrossRef]
- Santos, P.D.; Michel, F.; Wylezich, C.; Höper, D.; Keller, M.; Holicki, C.M.; Szentiks, C.A.; Eiden, M.; Muluneh, A.; Neubauer-Juric, A.; et al. Co-infections: Simultaneous detections of West Nile virus and Usutu virus in birds from Germany. Transbound. Emerg. Dis. 2021, 69, 776–792. [Google Scholar] [CrossRef]
- Bergmann, F.; Fischer, D.; Fischer, L.; Maisch, H.; Risch, T.; Dreyer, S.; Sadeghi, B.; Geelhaar, D.; Grund, L.; Merz, S.; et al. Vaccination of Zoo Birds against West Nile Virus—A Field Study. Vaccines 2023, 11, 652. [Google Scholar] [CrossRef]
- Swayne, D.E.; Beck, J.R.; Smith, C.S.; Shieh, W.-J.; Zaki, S.R. Fatal Encephalitis and Myocarditis in Young Domestic Geese (Anser anser domesticus) Caused by West Nile Virus. Emerg. Infect. Dis. 2001, 7, 751–753. [Google Scholar] [CrossRef]
- Banet-Noach, C.; Simanov, L.; Malkinson, M. Direct (non-vector) transmission of West Nile virus in geese. Avian Pathol. 2003, 32, 489–494. [Google Scholar] [CrossRef]
- Holicki, C.M.; Michel, F.; Vasić, A.; Fast, C.; Eiden, M.; Răileanu, C.; Kampen, H.; Werner, D.; Groschup, M.H.; Ziegler, U. Pathogenicity of West Nile Virus Lineage 1 to German Poultry. Vaccines 2020, 8, 507. [Google Scholar] [CrossRef]
- Reemtsma, H.; Holicki, C.M.; Fast, C.; Bergmann, F.; Eiden, M.; Groschup, M.H.; Ziegler, U. Pathogenesis of West Nile Virus Lineage 2 in Domestic Geese after Experimental Infection. Viruses 2022, 14, 1319. [Google Scholar] [CrossRef]
- Chvala, S.; Bakonyi, T.; Hackl, R.; Hess, M.; Nowotny, N.; Weissenböck, H. Limited pathogenicity of usutu virus for the domestic goose (Anser anser f. domestica) following experimental inoculation. J. Vet. Med. B Infect. Dis. Vet. Public Health 2006, 53, 171–175. [Google Scholar] [CrossRef]
- Chvala, S.; Bakonyi, T.; Hackl, R.; Hess, M.; Nowotny, N.; Weissenböck, H. Limited pathogenicity of Usutu virus for the domestic chicken (Gallus domesticus). Avian Pathol. 2005, 34, 392–395. [Google Scholar] [CrossRef]
- Vázquez-Calvo, Á.; Blázquez, A.-B.; Escribano-Romero, E.; Merino-Ramos, T.; Saiz, J.-C.; Martín-Acebes, M.A.; Jiménez de Oya, N. Zika virus infection confers protection against West Nile virus challenge in mice. Emerg. Microbes Infect. 2017, 6, e81. [Google Scholar]
- Blázquez, A.-B.; Escribano-Romero, E.; Martín-Acebes, M.A.; Petrovic, T.; Saiz, J.-C. Limited susceptibility of mice to Usutu virus (USUV) infection and induction of flavivirus cross-protective immunity. Virology 2015, 482, 67–71. [Google Scholar] [CrossRef]
- Zaccaria, G.; Malatesta, D.; Jurisic, L.; Marcacci, M.; Di Teodoro, G.; Conte, A.; Teodori, L.; Monaco, F.; Marini, V.; Casaccia, C.; et al. The envelope protein of Usutu virus attenuates West Nile virus virulence in immunocompetent mice. Vet. Microbiol. 2021, 263, 109262. [Google Scholar] [CrossRef]
- Jurisic, L.; Malatesta, D.; Zaccaria, G.; Di Teodoro, G.; Bonfini, B.; Valleriani, F.; Teodori, L.; Bencivenga, F.; Leone, A.; Ripà, P.; et al. Immunization with Usutu virus and with a chimeric West Nile virus (WNV) harboring Usutu-E protein protects immunocompetent adult mice against lethal challenges with different WNV lineage 1 and 2 strains. Vet. Microbiol. 2023, 277, 109636. [Google Scholar] [CrossRef]
- Escribano-Romero, E.; Jiménez de Oya, N.; Camacho, M.-C.; Blázquez, A.-B.; Martín-Acebes, M.A.; Risalde, M.A.; Muriel, L.; Saiz, J.-C.; Höfle, U. Previous Usutu Virus Exposure Partially Protects Magpies (Pica pica) against West Nile Virus Disease But Does Not Prevent Horizontal Transmission. Viruses 2021, 13, 1409. [Google Scholar] [CrossRef]
- Mayr, A.; Bachmann, P.A.; Bibrack, B.; Wittmann, G. Quantitative Bestimmung der Virusinfektiosität (Virustitration), 1st ed.; Gustav Fischer Verlag: Stuttgart, Germany, 1974. [Google Scholar]
- Eiden, M.; Vina-Rodriguez, A.; Hoffmann, B.; Ziegler, U.; Groschup, M.H. Two new real-time quantitative reverse transcription polymerase chain reaction assays with unique target sites for the specific and sensitive detection of lineages 1 and 2 West Nile virus strains. J. Vet. Diagn. Investig. 2010, 22, 748–753. [Google Scholar] [CrossRef]
- Pinho dos Reis, V. The Role of Integrins in Flavivirus Infection. Ph.D. Dissertation, Ernst-Moritz-Arndt-Universität, Mathematisch-Naturwissenschaftliche Fakultät, Greifswald, Germany, 2018. [Google Scholar]
- Seidowski, D.; Ziegler, U.; von Rönn, J.A.C.; Müller, K.; Hüppop, K.; Müller, T.; Freuling, C.; Mühle, R.-U.; Nowotny, N.; Ulrich, R.G.; et al. West Nile virus monitoring of migratory and resident birds in Germany. Vector-Borne Zoonotic Dis. 2010, 10, 639–647. [Google Scholar] [CrossRef]
- Mayr, A.; Bachmann, P.A.; Bibrack, B.; Wittmann, G. Neutraliationstest: Virologische Arbeitsmethoden (Serologie), 1st ed.; Gustav Fischer Verlag: Jena, Germany, 1977. [Google Scholar]
- RCore Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Enviroment for R (2022.12.0); PBC: Boston, MA, USA, 2022. [Google Scholar]
- Störk, T.; de Le Roi, M.; Haverkamp, A.-K.; Jesse, S.T.; Peters, M.; Fast, C.; Gregor, K.M.; Könenkamp, L.; Steffen, I.; Ludlow, M.; et al. Analysis of avian Usutu virus infections in Germany from 2011 to 2018 with focus on dsRNA detection to demonstrate viral infections. Sci. Rep. 2021, 11, 24191. [Google Scholar] [CrossRef]
- Agliani, G.; Giglia, G.; Marshall, E.M.; Gröne, A.; Rockx, B.H.; van den Brand, J.M. Pathological features of West Nile and Usutu virus natural infections in wild and domestic animals and in humans: A comparative review. One Health 2023, 16, 100525. [Google Scholar] [CrossRef]
- Kuchinsky, S.C.; Marano, J.; Hawks, S.A.; Loessberg, E.; Honaker, C.F.; Siegel, P.B.; Lahondère, C.; LeRoith, T.; Weger-Lucarelli, J.; Duggal, N.K. North American House Sparrows Are Competent for Usutu Virus Transmission. mSphere 2022, 7, e0029522. [Google Scholar] [CrossRef]
- Sardelis, M.R.; Turell, M.J.; Dohm, D.J.; O’Guinn, M.L. Vector competence of selected North American Culex and Coquillettidia mosquitoes for West Nile virus. Emerg. Infect. Dis. 2001, 7, 1018–1022. [Google Scholar] [CrossRef]
- Komar, N.; Langevin, S.; Hinten, S.; Nemeth, N.; Edwards, E.; Hettler, D.; Davis, B.; Bowen, R.; Bunning, M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg. Infect. Dis. 2003, 9, 311–322. [Google Scholar] [CrossRef]
- Ben-Nathan, D.; Huitinga, I.; Lustig, S.; van Rooijen, N.; Kobiler, D. West Nile virus neuroinvasion and encephalitis induced by macrophage depletion in mice. Arch. Virol. 1996, 141, 459–469. [Google Scholar] [CrossRef]
- Shrestha, B.; Diamond, M.S. Role of CD8+ T cells in control of West Nile virus infection. J. Virol. 2004, 78, 8312–8321. [Google Scholar] [CrossRef]
- Calisher, C.H.; Karabatsos, N.; Dalrymple, J.M.; Shope, R.E.; Porterfield, J.S.; Westaway, E.G.; Brandt, W.E. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J. Gen. Virol. 1989, 70 Pt 1, 37–43. [Google Scholar] [CrossRef]
- Heinz, F.X.; Stiasny, K. Flaviviruses and flavivirus vaccines. Vaccine 2012, 30, 4301–4306. [Google Scholar] [CrossRef]
- Hu, T.; Wu, Z.; Wu, S.; Chen, S.; Cheng, A. The key amino acids of E protein involved in early flavivirus infection: Viral entry. Virol. J. 2021, 18, 136. [Google Scholar] [CrossRef]
- Fiacre, L.; Pagès, N.; Albina, E.; Richardson, J.; Lecollinet, S.; Gonzalez, G. Molecular Determinants of West Nile Virus Virulence and Pathogenesis in Vertebrate and Invertebrate Hosts. Int. J. Mol. Sci. 2020, 21, 9117. [Google Scholar] [CrossRef]
- Vaughan, A.T.; Roghanian, A.; Cragg, M.S. B cells--masters of the immunoverse. Int. J. Biochem. Cell Biol. 2011, 43, 280–285. [Google Scholar] [CrossRef]
- Roehrig, J.T.; Hombach, J.; Barrett, A.D.T. Guidelines for Plaque-Reduction Neutralization Testing of Human Antibodies to Dengue Viruses. Viral Immunol. 2008, 21, 123–132. [Google Scholar] [CrossRef]
- Lim, S.M.; Geervliet, M.; Verhagen, J.H.; Müskens, G.J.D.M.; Majoor, F.A.; Osterhaus, A.D.M.E.; Martina, B.E.E. Serologic evidence of West Nile virus and Usutu virus infections in Eurasian coots in the Netherlands. Zoonoses Public Health 2017, 65, 96–102. [Google Scholar] [CrossRef]
- Vatti, A.; Monsalve, D.M.; Pacheco, Y.; Chang, C.; Anaya, J.-M.; Gershwin, M.E. Original antigenic sin: A comprehensive review. J. Autoimmun. 2017, 83, 12–21. [Google Scholar] [CrossRef]
- Merino-Ramos, T.; Blázquez, A.-B.; Escribano-Romero, E.; Cañas-Arranz, R.; Sobrino, F.; Saiz, J.-C.; Martín-Acebes, M.A. Protection of a single dose west nile virus recombinant subviral particle vaccine against lineage 1 or 2 strains and analysis of the cross-reactivity with Usutu virus. PLoS ONE 2014, 9, e108056. [Google Scholar] [CrossRef]
- Bin, H.; Grossman, Z.; Pokamunski, S.; Malkinson, M.; Weiss, L.; Dudevani, P.; Banet, C.; Weisman, Y.; Annies, E.; Gandaku, D.; et al. West Nile fever in Israel 1999-2000: From geese to humans. Ann. N. Y. Acad. Sci. 2001, 951, 127–142. [Google Scholar] [CrossRef]
- Lobigs, M.; Diamond, M.S. Feasibility of cross-protective vaccination against flaviviruses of the Japanese encephalitis serocomplex. Expert Rev. Vaccines 2012, 11, 177–187. [Google Scholar] [CrossRef]


| Goose Number | Virus 1 | Virus 2 | Date of Euthanasia |
|---|---|---|---|
| G 20–22 | USUV | No virus | 7 dpi USUV |
| G 23–25 | USUV | No virus | 37 dpi USUV |
| G 26, 32, 33 | USUV | WNV | 20 dpi USUV and 3 dpi WNV |
| G 27, 28, 34 | USUV | WNV | 23 dpi USUV and 6 dpi WNV |
| G 29–31 | USUV | WNV | 27 dpi USUV and 10 dpi WNV |
| G 35–37 | USUV | WNV | 31 dpi USUV and 14 dpi WNV |
| G 38–40 | USUV | WNV | 38 dpi USUV and 21 dpi WNV |
| G 41–43 | No virus/controls | No virus/controls | 36 d after start of the experiment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reemtsma, H.; Holicki, C.M.; Fast, C.; Bergmann, F.; Groschup, M.H.; Ziegler, U. A Prior Usutu Virus Infection Can Protect Geese from Severe West Nile Disease. Pathogens 2023, 12, 959. https://doi.org/10.3390/pathogens12070959
Reemtsma H, Holicki CM, Fast C, Bergmann F, Groschup MH, Ziegler U. A Prior Usutu Virus Infection Can Protect Geese from Severe West Nile Disease. Pathogens. 2023; 12(7):959. https://doi.org/10.3390/pathogens12070959
Chicago/Turabian StyleReemtsma, Hannah, Cora M. Holicki, Christine Fast, Felicitas Bergmann, Martin H. Groschup, and Ute Ziegler. 2023. "A Prior Usutu Virus Infection Can Protect Geese from Severe West Nile Disease" Pathogens 12, no. 7: 959. https://doi.org/10.3390/pathogens12070959
APA StyleReemtsma, H., Holicki, C. M., Fast, C., Bergmann, F., Groschup, M. H., & Ziegler, U. (2023). A Prior Usutu Virus Infection Can Protect Geese from Severe West Nile Disease. Pathogens, 12(7), 959. https://doi.org/10.3390/pathogens12070959





