Abstract
Campylobacter jejuni is a Gram-negative bacterium which is considered as the most reported cause of foodborne infection, especially for poultry species. The object of this work is to evaluate the occurrence of C. jejuni in chicken meat as well its control via three types of sorghum extracts (white sorghum (WS), yellow sorghum (YS), and red sorghum (RS)); antibacterial activity, antioxidant power, and cytotoxicity of sorghum extracts were also assessed. It was found that C. jejuni is very abundant in chicken meat, especially breast and thigh. WS extract showed more effectiveness than both yellow and red ones. Lyophilized WS extract offered high total phenolic compounds (TPCs) and total flavonoid compounds (TFCs) of 64.2 ± 0.8 mg gallic acid equivalent (GAE/g) and 33.9 ± 0.4 mg catechol equivalent (CE)/g, respectively. Concerning the antibacterial and antioxidant activities, WS showed high and significant antibacterial activity (p < 0.001); hence, WS displayed a minimum inhibitory concentration (MIC) of 6.25%, and revealed an inhibition zone of 7.8 ± 0.3 mm; it also showed an IC50 at a concentration of 34.6 μg/mL. In our study, different samples of chicken fillet were collected and inoculated with pathogenic C. jejuni and stored at 4 °C. Inoculated samples were treated with lyophilized WS extract at (2%, 4%, and 6%), the 2% treatment showed a full reduction in C. jejuni on the 10th day, the 4% treatment showed a full reduction in C. jejuni on the 8th day, while the 6% treatment showed a full reduction in C. jejuni on the 6th day. Additionally, 2%, 4%, and 6% WS extracts were applied on un-inoculated grilled chicken fillet, which enhanced its sensory attributes. In sum, WS extract is a promising natural preservative for chicken meat with accepted sensory evaluation results thanks to its high antibacterial and antioxidant potentials.
1. Introduction
Campylobacter jejuni is a Gram-negative, microaerophilic bacterium that is regarded as a worldwide leading cause of foodborne illness [1]. It is responsible for most bacterial infections associated with poultry consumption, causing symptoms ranging from mild diarrhea to severe abdominal cramping and fever [2,3]. Campylobacter infections were found to cause several diarrheal diseases from 2 to 7 times as regularly as infections with Escherichia coli, Salmonella species, or Shigella species [4]. The bacterium is expected to be responsible for over 400 million cases of gastroenteritis annually; most of these cases are in developing countries [5].
Poultry is considered a primary host for C. jejuni, with up to 90% of chicken and turkey flocks being colonized with the bacterium. The contamination of poultry meat during processing is a considerable concern, as it can cause transmission of various bacteria to humans [6,7]. The occurrence of C. jejuni in poultry is influenced by a range of diverse factors, including the hygiene of the rearing environment, the use of antibiotics, and the presence of other microorganisms [8,9,10]. The high occurrence of Campylobacter species including C. jejuni that mainly invade poultry species has been reported to be due to its ability to attack birds’ intestinal tracts’ epithelium and multiply inside it, owing to their warm-blooded body nature [11,12]. Chicken ceca of age between 14 and 21 days are colonized by C. jejuni reaching 1 × 109 CFU/g [13]. The ability of the bacterium to form biofilms also contributes to its persistence in the poultry gut, as these structures provide a protective environment that can resist antimicrobial treatments [14].
Recently, consumers’ demands regarding healthy and sustainable food have increased and are currently more critical than before owing to the severe outcomes of epidemics, climate change, and conflicts [15]. Additionally, there is a serious need for efficient interventions to minimize the occurrence of pathogenic bacteria in poultry species, providing that C. jejuni contributes most of the pathogenic contamination in poultry species that causes dangerous public health consequences [16]. Lately, it was found that sorghum, which is a cereal crop which belongs to the family Graminae, has been found to possess anti-bacterial properties against a range of pathogenic bacterial species. Sorghum plants have been identified as potential sources of natural antimicrobial compounds due to the existence of high levels of phytochemicals, such as tannins and flavonoids [17]. Furthermore, sorghum extracts have been shown to have a prebiotic effect, promoting the growth of beneficial gut bacteria while inhibiting the growth of harmful pathogens [18].
In this paper, the extracts of three types of sorghum were employed as antibacterial agents against C. jejuni, as it has been reported recently that sorghum extract can be used as a natural alternative to antibiotics in poultry feed to control C. jejuni colonization in the intestinal tract of broiler chickens, which could ultimately minimize the risk of human infection [19]. Therefore, the aim of our study is the experimental application of sorghum extract on chicken fillet inoculated experimentally with C. jejuni, and assessment of its antibacterial activity, antioxidant activity, minimum inhibitory concentration (MIC), total phenolic compounds (TPCs), total flavonoid compounds (TFCs), and the cytotoxicity.
2. Materials and Methods
2.1. Growth Conditions of Bacterial Strains
The C. jejuni EMCC 1835 reference strain was brought from Microbial Resource Center (MIRCEN), Ain Shams University, Cairo, Egypt. The cell counts were accustomed to 106 CFU/mL as the infective dose is >105 CFU/g [20]. The strains were stored at a temperature of −80 °C in Brain Heart Broth (Merck, 1.10493.0500, Darmstadt, Germany), including both glycerol and lysed horse blood (LHB) (Oxoid, SR048C, Hampshire, UK), then they were sub-cultured at a temperature of 42 °C on Columbia agar base (CAB) (Oxoid, CM0331, Hampshire, UK) under certain conditions as follow: 5% O2, 85% N2, and 10% CO2.
2.2. Collection of Chicken Samples and Detection of C. jejuni
A hundred chicken breast, thigh, liver, and gizzard samples were grouped and gathered from various local markets located in Alexandria Governorate, Egypt, in 2022. During the collection process, the chicken meat that is sold in pieces was randomly collected from the local retail stores at refrigerated temperature and packaged in bags made of polyethylene. Immediately after collection, samples were transferred in a box containing ice to the laboratory to be examined bacteriologically. C. jejuni was isolated and applied according to the method illustrated by El-Khawas et al. [21].
2.3. Sorghum Materials and Extraction
Three local varieties of sorghum—red, white, and yellow seeds (see Figure 1)—were obtained and identified by the Shandawil Sohag Research Center (Sohag Governorate, Egypt) as follows: Shandaweel 1 (white sorghum), Giza 54 (yellow sorghum), Alsabeinaa (red sorghum). The collected grains were washed and dried, and 10 g were then extracted in an ethanolic solution (70% ethanol: deionized water v/v) of up to 100 mL with random shaking at 100 rpm. After incubation for 2 days, the attained extracts were centrifuged for 30 min at 2.147× g, then filtered by using normal filter papers. Finally, extract lyophilization took place at −50 °C (Telstar Model 50, Barcelona, Spain) and the resultant extracts were diluted in distilled H2O with specific recognized concentrations in mg/mL [22,23].

Figure 1.
Pictures of the investigated sorghum seeds in this study: red, yellow, and white sorghum seeds (Sorghum bicolor L.).
2.4. Antibacterial Activity
2.4.1. Antibacterial Potential of Sorghum Extract
The aptitude of sorghum extracts as a microbial agent contrary to C. jejuni EMCC 1835 reference strain (prepared in MIRCEN) was measured following the procedure of Hamad et al. [22] and Klančnik et al. [24]. The overnight C. jejuni cultures were enriched on Mueller hinton media (MHM) (Oxoid, UK) at 42 °C/48 h and then spread on MHM plates. The inhibition zone was then recorded (mm), observing the anti-C. jejuni power of the three types of sorghum extracts. Additionally, a comparative study was carried out between the results of the inhibition zone and those of 3 antibiotic disks which are Erythromycin (ERY), Gentamicin (GEN), and Amoxicillin (AMX) [25].
2.4.2. Assessment MICs of Sorghum Extract against C. jejuni
Sorghum extracts of minimum inhibitory concentrations against C. jejuni EMCC 1835 were evaluated using agar disk diffusion assay [22,25] with the following descending concentrations of white sorghum extract: 100, 50, 25, 12.5, 6.25, 3.12 µL. C. jejuni spread on MHM plates of grown Mueller Hinton Medium (MHM) plates and adjusted to a density of 106 CFU/mL [26] and the plates were kept at a temperature of 4 °C for 30 min and then incubated at 42 °C/24 h. A clear and sharp inhibition zone was noted in mm, taking in consideration the anti-C. jejuni potential of the different sorghum extracts.
2.5. Phytochemical Analysis of White Sorghum Extract
2.5.1. Total Phenolic Compounds (TPCs) of White Sorghum Extract
The total phenol compounds (TPCs) of sorghum extracts was evaluated by using the test method described by Hamad et al. [23]. Briefly, approximately 0.1 mL reconstituted extract was incorporated into a 100 µL Folin–Ciocalteu substance, the mixture then stood for fifteen min, and then we added 2 mL sodium carbonate (2%). After that, the mixture was left at ambient temperature for half hour, and gallic acid as a calibration was used in the evaluation of TPCs by using a spectrophotometer (T80, PG Instrument, Lutterworth, UK) at 760 nm. TPC is expressed as mg of gallic acid equivalents to each gram per sample.
2.5.2. Total Flavonoid Compounds (TFCs) of White Sorghum Extract
TFCs of sorghum extracts was assessed following the method illustrated by Hamad et al. [27]. Four milliliters of water was mixed with 1 mL of the examined sample in a volumetric flask. After that, 0.15 mL of aluminum chloride (10% AlCl3) and 0.75 mL of sodium nitrite (5% NaNO2) were added. Finally, and after 5 min, 500 µL of 1 M from NaOH was added. The TFCs was measured spectrophotometrically at 510 nm.
2.5.3. Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Assay
The ability of sorghum extracts to scavenge DPPH free radicals was examined [27,28] by applying some modifications. A stock solution of each extract in methanol to 1 mg/mL was prepared. Serialized dilutions of plant extracts were carried out by mixing about 1 mL of every dilution with 1 mL DPPH and then measured at 517 nm spectrophotometrically. The results were expressed as IC50 (the concentration of the extract that can suppress the 50% DPPH). Then we calculated inhibition % through this equation:
where: A: Absorbance.
DPPH inhibition % = [(A of control − A of the sample)/A of control] × 100
2.6. Safety and Cytotoxicity Assay of White Sorghum Extract
White sorghum extract was assessed for its impact on the peripheral blood mononuclear cell’s viability (PBMCs). Tested wells (150 µL PBMCs), control wells (150 µL PBMCs), and blank wells (150 µL PBS) were used. Several different concentrations of white sorghum extract were inoculated for test wells and incubated for one day, according to the approach of Popiołkiewicz et al. [29]. Using the spectrophotometer, the absorbance was observed at 540 nm; white sorghum extract inhibition % was calculated from the following equation. IC50 values were calculated through this portal www.aatbio.com/tools/ic50-calculator (accessed on 22 February 2023).
White sorghum extract inhibition % = 100 − (optical density (O.D) Control − O.D Treatment/O.D Control)
2.7. Experimental Application and Evaluation of the Antimicrobial Power of White Sorghum Extract against C. jejuni Experimentally Inoculated into Chicken Fillet
2.7.1. Microbes
C. jejuni EMCC 1835 was gained from MIRCEN. Bacterial strain was set, and its bacterial density was adjusted at a value of 1 × 107 CFU/mL [26,30].
2.7.2. Refrigerated Storage Study of Chicken Breast Fillets
Raw boneless chicken breast fillets were sliced with a sterile knife into 5 cm × 5 cm pieces. Before doing the experiment, chicken samples were sterilized according to Hamad et al. [22] and Morsy et al. [31]. Chicken samples were grouped into several treatments (6 treatments): T1, chicken samples without applying any handlings (negative control (1)); T2, chicken samples supplemented with white sorghum extract (2%) (negative control (2)); T3, chicken samples with 1 × 107 CFU/mL C. jejuni (positive control); T4, chicken samples with C. jejuni and white sorghum extract (2%); T5, chicken samples with C. jejuni and white sorghum extract (4%); T6, chicken samples with C. jejuni and white sorghum extract (6%).
Samples were kept for a time of 15 min at room temperature, and further were cooled at 4 °C and observed for C. jejuni occurrence every two days bacteriologically until complete reduction of the bacterial count. Chicken samples were assessed bacteriologically at various storage periods (i.e., 0, 2, 4, 6, 8, and 10 days) for determination of C. jejuni amount in the products [32].
2.7.3. Sensory Evaluation of the Acceptability of Chicken Fillet Fortified with White Sorghum Extract
To determine the acceptability of white sorghum extract as a food additive, organoleptic attributes were evaluated in chicken samples enriched with the sorghum extracts. The experiment was carried out on four groups, the first group is chicken fillet without any treatments (control); the other three groups are chicken samples with different concentrations of white sorghum extract of 2%, 4%, and 6%. Ten experienced panelists analyzed the samples. Panelists checked the degree of acceptability of chicken for the sensorial scores: texture, appearance, taste, odor, color, and overall acceptability, with a scale ranging from 1 to 10 (10 points/each item), where 10 is more accepted [22,33].
2.8. Statistical Analysis
R software, version 4.2.0 (Lucent Technologies, New Providence, NJ, United States), was employed for data analyses. One-way analysis of variance (ANOVA) of the means of three reads (mean ± SE) using Tukey’s test at p < 0.01 or p < 0.05 and regression analysis was used for continuous independent variables.
3. Results and Discussion
3.1. Occurrence of C. jejuni in Chicken Meat
Chicken meat makes up a high percentage of our diet, which surely leads to human infection. Unfortunately, the processing step is the main reason various poisoning bacteria contaminate chicken food products [22]. The occurrence of C. jejuni in chicken fillets can vary between countries and regions and has been the subject of numerous studies. C. jejuni affects human health negatively as it causes mild to severe symptoms such as diarrhea, fever, nausea, and vomiting. Symptoms typically develop 2–5 days after exposure to the bacteria and can last for up to 10 days [34].
In our study, the collected chicken meat samples showed a high presence of C. jejuni especially in thigh and breast meat with percentages of 88% and 80%, respectively, as illustrated in Table 1. These findings are in line with results shown by Walker et al. [35] who isolated E. coli and C. jejuni from chicken, pork offal, lamb, and beef collected from retail food outlets and showed a very high incidence of Campylobacter in chicken meat.

Table 1.
Incidence of C. jejuni in chicken collected from different local markets (n = 100).
The chicken meat samples in this study were collected from suppliers with poor hygiene levels and low sanitation levels for chicken cutting tools to observe the prevalence of C. jejuni. In addition, the high incidence of C. jejuni found in chicken samples may be caused by contamination from microbes anywhere along the supply chain. Therefore, this highlights the importance of implementing proper hygiene and sanitation practices to prevent contamination and the spread of foodborne illness. Additionally, consumers should be aware of the risks associated with improperly handled and cooked chicken meat and should follow proper food safety guidelines to minimize the risk of foodborne illness.
3.2. Antibacterial Activity of Lyophilized Sorghum Extracts
Three types of sorghum extracts were prepared and the antibacterial effect on C. jejuni was evaluated using agar disk diffusion assay as well as three types of antibiotics: Gentamicin, Erythromycin, and Amoxicillin. Then, results were obtained; they are shown in Figure 2 and tabulated in Table 2.
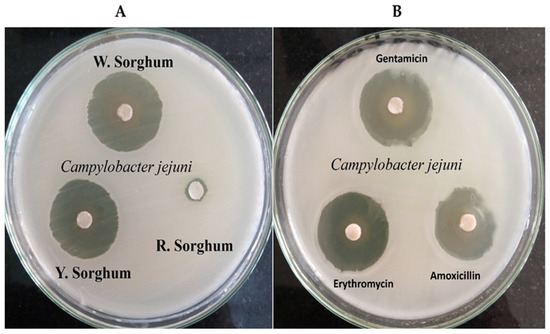
Figure 2.
Antibacterial activity of red (R. Sorghum), white (W. Sorghum), and yellow sorghum (Y. Sorghum) extracts against C. jejuni using agar disk diffusion assay vs. Erythromycin, Gentamicin, and Amoxicillin antibiotics. Inhibition zones are indicated in mm. (A) Antibacterial impact of three sorghum extracts; (B) Antibacterial power of 3 antibiotics compared to sorghum extracts.

Table 2.
Antibacterial activity of lyophilized sorghum extracts against C. jejuni using agar disk diffusion assay.
Results showed that red sorghum extract has a negative effect on C. jejuni, while the other sorghum extracts showed significant effects, especially white sorghum which exhibited an inhibitory zone of 39.1 ± 0.2 mm, even higher than the three antibiotic types (p < 0.001). White and yellow sorghum showed extremely high effects compared with Piskernik et al. [36], who used rosemary extract. A recent study by Chen et al. [37] reported that polyphenol extract of sweet sorghum displayed antibacterial activity against Staphylococcus aureus, Escherichia coli, Listeria spp., and Salmonella spp. Another report by Garzón et al. [38] confirmed that a higher antimicrobial potential of sorghum spent grain could be an important natural source of bioactive peptides with antimicrobial activity against Bacillus cereus growth.
3.3. Minimal Inhibitory Concentration (MIC) of Sorghum Plant Extract
In our study, the MIC of lyophilized white sorghum extract against C. jejuni in vitro and the antimicrobial potential at several ratios of white sorghum extracts were examined; the results are shown in Figure 3 and tabulated in Table 3. The results confirmed that the MIC value of white sorghum extract was 6.25% with an inhibition zone of 7.8 ± 0.3 mm. Additionally, the concentration of 3.12% showed negative results and the anti-C. jejuni bacterial activity increased gradually on increasing the extract concentration percentage (p < 0.001).
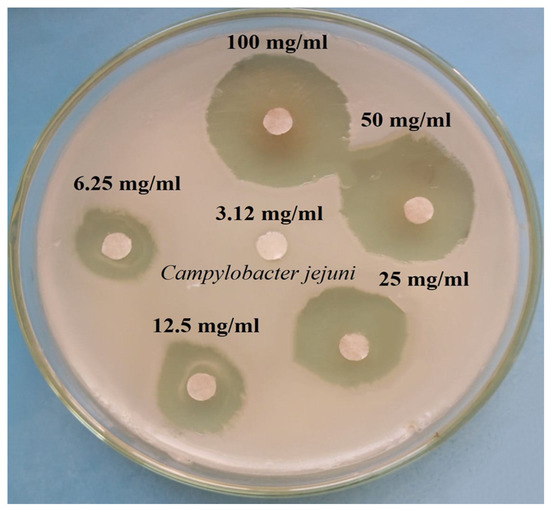
Figure 3.
Minimum inhibitory concentrations (MICs) of the white sorghum extracts against C. jejuni (mm).

Table 3.
Minimum inhibitory concentrations (MICs) of the white sorghum extracts against C. jejuni (mm).
3.4. Total Phenolic Compounds (TPCs) and Total Flavonoid Compounds (TFCs) of Lyophilized Sorghum Extract
Phenolic compounds and flavonoids are important antioxidant and antibacterial agents in plants that play a significant role in preventing many diseases such as cancer and promote human health and immunology [39].
According to the results in Table 4, TPC and TFC of white sorghum extract were 64.2 ± 0.8 mg GAE/g and 33.9 ± 0.4 mg CE/g, respectively, so it has the highest antioxidant activity. It showed significant results, followed by yellow sorghum extract then red sorghum extract. White sorghum extract showed the best results in treating C. jejuni in chicken fillets.

Table 4.
Total phenolic (mg GAE/g) and flavonoid contents (mg CE/g) of sorghum extracts.
TPC and TFC values may differ according to temperature, seasonal exchange, pH, polarity of used solvents in extraction process, light incidence, water nutrient composition, and salinity [22,40,41].
3.5. Antioxidant Potential and DPPH Radical Scavenging Ability
The antioxidant power can be estimated accurately via assessment of the DPPH radical scavenging ability. The antioxidant capacity of lyophilized sorghum extract was estimated according to the DPPH radical scavenging capacity and compared with a standard which is ascorbic acid. Results are presented in Table 5; it was observed that the IC50 values of the lyophilized extracts were 34.62 μg/mL for white sorghum, 51.5 μg/mL for yellow sorghum, and 65.8 μg/mL for red sorghum, while the IC50 value of ascorbic acid was 20.1 μg/mL. The highest free radical scavenging capacity of the extracts was 99.2% at a ratio of 100 μg/mL for white sorghum, 97.6% at a concentration of 100 μg/mL for yellow sorghum, and 89.2% at a concentration of 100 μg/mL for red sorghum. These findings contradict the findings of Pontieri et al. [42], who found that red sorghum extract has higher antioxidant activity than white sorghum.

Table 5.
Antioxidant activity and DPPH radical scavenging capacity of the sorghum extracts.
Some sorghum species contain several types of phytochemicals that have the ability to neutralize free radicals. It has been confirmed that the high antioxidant activity of sorghum extract gives it a potential health effect as protection against cardiovascular disease, obesity, dyslipidemia, oxidative stress, and diabetes [42,43], as well as antimicrobial, anti-inflammatory, and anticancer activity [42,44].
3.6. Safety Assay and Cytotoxicity of White Sorghum Extract
The PBMC cytotoxicity method involves using a number of cells obtained from various individuals to assess the in vitro cytotoxicity of potential drugs in a high-throughput manner. Moreover, this assay can offer valuable insights into the response of immune cells from different donors to the compounds being developed. By utilizing this approach, researchers can obtain a more comprehensive understanding of the efficacy and safety of potential drugs [22]. That is why both the safety and the cytotoxicity of lyophilized white sorghum extract were assessed due to the high concern regarding evaluation of the safety of new antimicrobials applied on food. The estimated cytotoxic effects of white sorghum extract on peripheral blood mononuclear cell (PBMC) viability and IC50 are presented in Table 6 and exhibited a positive correlation with the concentration of sorghum extract. The concentration ranged from 19.5 µg/mL to 10,000 µg/mL. It was proven that the lyophilized extract is toxic to PBMCs at several concentrations as the minimum concentration showed 16% inhibition and 84% viability, while the maximum concentration showed 100% inhibition and zero viability. Furthermore, white sorghum showed a high IC50 at 482.4 µg/mL, which allowed its usage as a safe and promising food additive in chicken meat.

Table 6.
The safety and cytotoxicity assessment of white sorghum extract on the viability of PBMC cells.
3.7. Preparation of Chicken Fillets and Their Acceptability after the Fortification with Lyophilized White Sorghum Extract
Gaining insight into how consumers perceive the safety and quality of chicken meat can be a valuable resource for public health educators, as it is the most widely consumed meat [45]. To ensure the safety of chicken meat upon applying the white sorghum extract, experimentally inoculated chicken fillets with C. jejuni were prepared and treated with different concentrations of white sorghum extract to determine the antibacterial effect. In Table 7, the results are presented; they reveal that white sorghum extract has an anti-C. jejuni effect on chicken fillet meat stored at 4 °C. The 6% treatment showed a high chicken fillet treatment and a complete reduction of C. jejuni on the 6th day, while the 2% treatment showed a low chicken fillet treatment and a complete reduction of C. jejuni on the 10th day.

Table 7.
Antibacterial impact of several ratios from white sorghum extract against C. jejuni experimentally inoculated into chicken fillet stored at 4 °C (mean ± SE).
In addition to the above study, sensory attributions of grilled un-inoculated chicken fillet were assessed upon applying white sorghum extract. Results are tabulated in Figure 4 and showed that white sorghum of 2%, 4%, and 6% improved taste, color, appearance, odor, and texture of the grilled chicken fillet. In addition to being a good preservative for chicken fillet due to its high antibacterial activity as shown before, it was reported that sorghum could increase the nutritional value of food due to its enrichment with proteins, vitamins including riboflavin, vitamin B6, thiamin, and several minerals including sodium, potassium, zinc, and iron [46]. It is also reported that sorghum flour has a water-holding capacity and has the ability to lower meat fat content so it could improve the texture of chicken fillet meat and increase its juiciness [47].
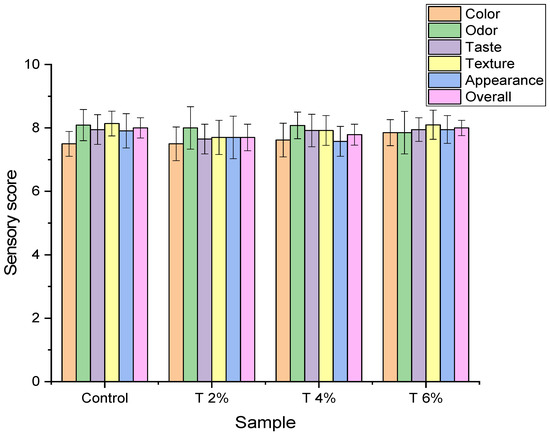
Figure 4.
Grilled un-inoculated chicken fillet’s acceptability supplemented with white sorghum extract based on organoleptic characteristics. Control: chicken fillet without any treatment; T 2%: chicken with white sorghum extract 2%; T 4%: chicken with white sorghum extract 4%; T 6%: chicken with white sorghum extract 6%.
4. Conclusions
In conclusion, C. jejuni is very abundant in chicken meat, especially breast and thigh. WS extract showed more effectiveness than both yellow and red ones. WS extract showed a high antibacterial effect against C. jejuni due to its large TPC and TFC content, in addition to its high antioxidant activity that could scavenge free radicals. Inoculated samples were treated with lyophilized WS extract at several ratios; the 2% treatment showed a full reduction of C. jejuni on the 10th day, the 4% treatment showed a full reduction of C. jejuni on the 8th day, and the 6% treatment showed a full reduction of C. jejuni on the 6th day. Furthermore, WS extract showed a high acceptance upon application to chicken fillet as it does not affect negatively its sensory attributes. The current results confirmed that WS can be used as a promising food preservative because it is safe and is not toxic for humans; moreover, it increases the nutritional value of food and improves both the texture and the juiciness of meat.
Author Contributions
Conceptualization, G.M.H.; methodology, G.M.H.; software, T.M., G.M.H., M.G., S.M.H., M.E., R.G.T., Y.E.-H., A.M.M., E.E.H. and E.M.E.; validation, T.M., G.M.H., T.E. and M.G.; formal analysis, T.M., G.M.H., M.G., T.E., S.M.H., M.E., R.G.T., Y.H, A.M.M., E.E.H. and E.M.E.; investigation, T.M., G.M.H., T.E. and M.G.; resources, G.M.H., M.G.; data curation, T.M., G.M.H. and M.G.; writing—original draft preparation, G.M.H., M.G. and T.M.; writing—review and editing, T.M., G.M.H., T.E. and M.G.; visualization, G.M.H., M.G., T.M., T.E., S.M.H., M.E., R.G.T., Y.E.-H., A.M.M., E.E.H. and E.M.E.; supervision, G.M.H.; project administration, G.M.H., M.G., T.M., T.E. and E.E.H.; All authors have read and agreed to the published version of the manuscript.
Funding
This study received no funding. The open access publication of this article was supported by the Open Access Fund of Leibniz Universität Hannover.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Meurer, L.; Payne, W.; Guffey, J.S. Visible light as an inhibitor of Campylobacter jejuni. Int. J. Antimicrob. Agent. 2020, 55, 105818. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-C.; Oh, E.; Kim, J.; Jeon, B. Regulation of oxidative stress resistance in Campylobacter jejuni, a microaerophilic foodborne pathogen. Front. Microbiol. 2015, 6, 751. [Google Scholar] [CrossRef] [PubMed]
- Allos, B.M.; Blaser, M.J. Campylobacter jejuni and the expanding spectrum of related infections. Clin. Infect. Dis. 1995, 20, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Acheson, D.; Allos, B.M. Campylobacter jejuni infections: Update on emerging issues and trends. Clin. Infect. Dis. 2001, 32, 1201–1206. [Google Scholar] [CrossRef]
- Ruiz-Palacios, G.M. The Health Burden of Campylobacter Infection and the Impact of Antimicrobial Resistance: Playing Chicken; The University of Chicago Press: Chicago, IL, USA, 2007; Volume 44, pp. 701–703. [Google Scholar]
- Hermans, D.; Pasmans, F.; Heyndrickx, M.; Van Immerseel, F.; Martel, A.; Van Deun, K.; Haesebrouck, F. A tolerogenic mucosal immune response leads to persistent Campylobacter jejuni colonization in the chicken gut. Cri. Rev. Microbiol. 2012, 38, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Newell, D.; Elvers, K.; Dopfer, D.; Hansson, I.; Jones, P.; James, S.; Gittins, J.; Stern, N.; Davies, R.; Connerton, I. Biosecurity-based interventions and strategies to reduce Campylobacter spp. on poultry farms. Appl. Environ. Microbiol. 2011, 77, 8605–8614. [Google Scholar] [CrossRef]
- Sibanda, N.; McKenna, A.; Richmond, A.; Ricke, S.C.; Callaway, T.; Stratakos, A.C.; Gundogdu, O.; Corcionivoschi, N. A review of the effect of management practices on Campylobacter prevalence in poultry farms. Front. Microbiol. 2018, 9, 2002. [Google Scholar] [CrossRef]
- Abbas, S.G.E.; Karmi, M.; Mubarak, A.G.; Youseef, A.G. Prevalence and virulence genes profile of zoonotic Campylobacter species in chickens and human in Aswan governorate. SVU-Int. J. Vet. Sci. 2022, 5, 15–32. [Google Scholar] [CrossRef]
- Abdallah, M.; Abaza, M.A.; Fathy, R.R.; Youseef, A.G.; Sobhy, M.; Abd Elhamid, H.S.; Ahmed, W. Detection of Some Virulence and Antibiotic Resistance Genes in Campylobacter jejuni isolated from Poultry and Human. Egypt. J. Hosp. Med. 2022, 89, 6373–6381. [Google Scholar] [CrossRef]
- Burnham, P.M.; Hendrixson, D.R. Campylobacter jejuni: Collective components promoting a successful enteric lifestyle. Nature Rev. Microbiol. 2018, 16, 551–565. [Google Scholar] [CrossRef]
- Fonseca, B.B.; Fernandez, H.; Rossi, D.A. Campylobacter Spp. and Related Organisms in Poultry; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Al Hakeem, W.G.; Fathima, S.; Shanmugasundaram, R.; Selvaraj, R.K. Campylobacter jejuni in Poultry: Pathogenesis and Control Strategies. Microorganisms 2022, 10, 2134. [Google Scholar] [CrossRef] [PubMed]
- Siringan, P.; Connerton, P.L.; Payne, R.J.; Connerton, I.F. Bacteriophage-mediated dispersal of Campylobacter jejuni biofilms. Appl. Environ. Microbiol. 2011, 77, 3320–3326. [Google Scholar] [CrossRef] [PubMed]
- Mehany, T.; Siddiqui, S.A.; Olawoye, B.; Olabisi Popoola, O.; Hassoun, A.; Manzoor, M.F.; Punia Bangar, S. Recent innovations and emerging technological advances used to improve quality and process of plant-based milk analogs. Crit. Rev. Food Sci. Nutr. 2023, 2023, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Gashaw, G.; Fassil, A.; Redi, F. Evaluation of the antibacterial activity of Pleurotus spp. cultivated on different agricultural wastes in Chiro, Ethiopia. Int. J. Microbiol. 2020, 2020, 9312489. [Google Scholar] [CrossRef]
- Mehany, T.; Rashad, Y.M.; Olawoye, B.; Cacciotti, I.; Johnson, E.O.; Popoola, O.O.; Han, Z.; Fekry, W.M. Pigmented Sorghum: Functional Properties and Bioactive Diversity. In Pigmented Cereals and Millets: Bioactive Profile and Food Applications; Royal Society of Chemistry: London, UK, 2023; pp. 109–143. [Google Scholar]
- Chen, W.; Zhang, T.; Ma, Q.; Zhu, Y.; Shen, R. Structure Characterization and Potential Probiotic Effects of Sorghum and Oat Resistant Dextrins. Foods 2022, 11, 1877. [Google Scholar] [CrossRef]
- Navarro, M.; Stanley, R.; Cusack, A.; Sultanbawa, Y. Combinations of plant-derived compounds against Campylobacter in vitro. J. Appl. Poultry Res. 2015, 24, 352–363. [Google Scholar] [CrossRef]
- Tribble, D.R.; Baqar, S.; Carmolli, M.P.; Porter, C.; Pierce, K.K.; Sadigh, K.; Guerry, P.; Larsson, C.J.; Rockabrand, D.; Ventone, C.H. Campylobacter jejuni strain CG8421: A refined model for the study of Campylobacteriosis and evaluation of Campylobacter vaccines in human subjects. Clin. Infect. Dis. 2009, 49, 1512–1519. [Google Scholar] [CrossRef]
- El-Khawas, K.M.; Hendy, B.A.S. Assessment and improvement of hygienic status of chicken fillet from slaughterhouses using organic acids from natural sources. Assiut Vet. Med. J. 2015, 61, 8–17. [Google Scholar]
- Hamad, G.; Amer, A.; Kirrella, G.; Mehany, T.; Elfayoumy, R.A.; Elsabagh, R.; Elghazaly, E.M.; Esatbeyoglu, T.; Taha, A.; Zeitoun, A. Evaluation of the Prevalence of Staphylococcus aureus in Chicken Fillets and Its Bio-Control Using Different Seaweed Extracts. Foods 2023, 12, 20. [Google Scholar] [CrossRef]
- Hamad, G.; Ombarak, R.A.; Eskander, M.; Mehany, T.; Anees, F.R.; Elfayoumy, R.A.; Omar, S.A.; Lorenzo, J.M.; Abou-Alella, S.A.E. Detection and inhibition of Clostridium botulinum in some Egyptian fish products by probiotics cell-free supernatants as bio-preservation agents. LWT 2022, 163, 113603. [Google Scholar] [CrossRef]
- Klančnik, A.; Piskernik, S.; Jeršek, B.; Možina, S.S. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J. Microbiol. Methods 2010, 81, 121–126. [Google Scholar] [CrossRef]
- Solís-Soto, L.; Prabhakarankutty, L.K.; García, S.; Ortíz-Reyes, Y.; Heredia, N. Controlling Campylobacter jejuni in vitro and in chicken using combinations of citrus-based and trisodium phosphate formulations. J. Food Saf. 2021, 41, e12938. [Google Scholar] [CrossRef]
- Eldin, R.M.B.; Talaat, D.; Elbaba, A.H.; Ibrahim, M.S. Antibacterial activity y of some plant extracts on different bacteria in chicken fillet. Eur. J. Pharm. Med. Res. 2020, 7, 84–95. [Google Scholar]
- Hamad, G.M.; Mohdaly, A.A.A.; El-Nogoumy, B.A.; Ramadan, M.F.; Hassan, S.A.; Zeitoun, A.M. Detoxification of aflatoxin B1 and ochratoxin A using Salvia farinacea and Azadirachta indica water extract and application in meat products. Appl. Biochem. Biotechnol. 2021, 193, 3098–3120. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.; Saraiva, S.C.; Sobral, A.J.; Cardoso, S.M. Characterization of phenolic constituents and evaluation of antioxidant properties of leaves and stems of Eriocephalus africanus. Arab. J. Chem. 2018, 11, 62–69. [Google Scholar] [CrossRef]
- Popiołkiewicz, J.; Polkowski, K.; Skierski, J.S.; Mazurek, A.P. In vitro toxicity evaluation in the development of new anticancer drugs—Genistein glycosides. Cancer Lett. 2005, 229, 67–75. [Google Scholar] [CrossRef]
- Salem, A.; Abou El Roos, N.; Nassar, Y. Antimicrobial Effects of some Essential Oils on the Foodborne Pathogen Campylobacter jejuni. Benha Vet. Med. J. 2019, 36, 65–70. [Google Scholar] [CrossRef]
- Morsy, M.K.; Elsabagh, R.; Trinetta, V. Evaluation of novel synergistic antimicrobial activity of nisin, lysozyme, EDTA nanoparticles, and/or ZnO nanoparticles to control foodborne pathogens on minced beef. Food Control. 2018, 92, 249–254. [Google Scholar] [CrossRef]
- Grant, F.S. Fish and Fishery Products Hazards and Controls Guidance, 4th ed.; United States Department of Health and Human Services: Washington, DC, USA, 2011. [Google Scholar]
- Hamad, G.M.; Abdelmotilib, N.M.; Darwish, A.M.; Zeitoun, A.M. Commercial probiotic cell-free supernatants for inhibition of Clostridium perfringens poultry meat infection in Egypt. Anaerobe 2020, 62, 102181. [Google Scholar] [CrossRef]
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global epidemiology of Campylobacter infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef]
- Walker, L.J.; Wallace, R.L.; Smith, J.J.; Graham, T.; Saputra, T.; Symes, S.; Stylianopoulos, A.; Polkinghorne, B.G.; Kirk, M.D.; Glass, K. Prevalence of Campylobacter coli and Campylobacter jejuni in retail chicken, beef, lamb, and pork products in three Australian states. J. Food Prot. 2019, 82, 2126–2134. [Google Scholar] [CrossRef] [PubMed]
- Piskernik, S.; Klančnik, A.; Riedel, C.T.; Brøndsted, L.; Možina, S.S. Reduction of Campylobacter jejuni by natural antimicrobials in chicken meat-related conditions. Food Control 2011, 22, 718–724. [Google Scholar] [CrossRef]
- Chen, H.; Xu, Y.; Chen, H.; Liu, H.; Yu, Q.; Han, L. Isolation and identification of polyphenols from fresh sweet sorghum stems and their antibacterial mechanism against foodborne pathogens. Front. Bioeng. Biotechnol. 2022, 9, 770726. [Google Scholar] [CrossRef]
- Garzón, A.G.; Veras, F.F.; Brandelli, A.; Drago, S.R. Purification, identification and in silico studies of antioxidant, antidiabetogenic and antibacterial peptides obtained from sorghum spent grain hydrolysate. LWT 2022, 153, 112414. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Neto, A.I.; Baptista, J. Seasonal variability of the biochemical composition and antioxidant properties of Fucus spiralis at two Azorean Islands. Mar. Drugs 2018, 16, 248. [Google Scholar] [CrossRef]
- Nazarudin, M.; Yasin, I.; Mazli, N.; Saadi, A.; Azizee, M.; Nooraini, M.; Saad, N.; Ferdous, U.; Fakhrulddin, I. Preliminary screening of antioxidant and cytotoxic potential of green seaweed, Halimeda opuntia (Linnaeus) Lamouroux. Saudi J. Biol. Sci. 2022, 29, 2698–2705. [Google Scholar] [CrossRef]
- Pontieri, P.; Pepe, G.; Campiglia, P.; Merciai, F.; Basilicata, M.G.; Smolensky, D.; Calcagnile, M.; Troisi, J.; Romano, R.; Del Giudice, F. Comparison of Content in Phenolic Compounds and Antioxidant Capacity in Grains of White, Red, and Black Sorghum Varieties Grown in the Mediterranean Area. ACS Food Sci. Technol. 2021, 1, 1109–1119. [Google Scholar] [CrossRef]
- De Morais Cardoso, L.; Pinheiro, S.S.; Martino, H.S.D.; Pinheiro-Sant’Ana, H.M. Sorghum (Sorghum bicolor L.): Nutrients, bioactive compounds, and potential impact on human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 372–390. [Google Scholar] [CrossRef]
- Stefoska-Needham, A.; Beck, E.J.; Johnson, S.K.; Tapsell, L.C. Sorghum: An underutilized cereal whole grain with the potential to assist in the prevention of chronic disease. Food Rev. Int. 2015, 31, 401–437. [Google Scholar] [CrossRef]
- Daghir, N.; Diab-El-Harake, M.; Kharroubi, S. Poultry production and its effects on food security in the Middle Eastern and North African region. J. Appl. Poult. Res. 2021, 30, 100110. [Google Scholar] [CrossRef]
- Mohamed, H.I.; Fawzi, E.M.; Basit, A.; Lone, R.; Sofy, M.R. Sorghum: Nutritional Factors, Bioactive Compounds, Pharmaceutical and Application in Food Systems: A Review. Phyton 2022, 91, 1303. [Google Scholar] [CrossRef]
- Huang, J.C.; Zayas, J.F.; Bowers, J.A. Functional properties of sorghum flour as an extender in ground beef patties 1. J. Food Qual. 1999, 22, 51–61. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).